Abstract
Dendritic cells (DCs) are specialized bone marrow-derived leukocytes that orchestrate both innate and adaptive immunity. DCs reside in all tissues where they continuously survey the local environment and inform cells of the immune system to modulate their responses. Under inflammatory conditions, DCs undergo terminal maturation and activation to become fully immunogenic. DC heterogeneity and differential activation states ultimately determine the type and quality of immune responses. DCs initiate an immune response by capturing and presenting antigen in the form of peptide–major histocompatibility complex (MHC) molecule complexes to naive T cells in lymphoid tissues. DCs share most features of antigen-processing cells and class I and II MHC-restricted presentation with other antigen-presenting cells (APCs). DCs, however, are endowed with the capacity to cross-present exogenous antigens on their own class I MHC molecules to autologous T cells regardless of the MHC alleles expressed by the antigen source. When compared with other APCs, like macrophages, DCs are much more efficient and can elicit responses from very low numbers of T cells. In this chapter we review such and other mechanisms of DCs in the setting of hematopoietic cell transplantation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Human Dendritic Cells
1.1 Dendritic Cells: Critical Regulators of Immunity
Dendritic cells (DCs) are specialized bone marrow-derived leukocytes that orchestrate both innate and adaptive immunity. DCs reside in all tissues where they continuously survey the local environment and inform cells of the immune system to modulate their responses (Banchereau and Steinman 1998; Steinman and Banchereau 2007; Steinman 2012). Under physiologic steady-state conditions, DCs are predominantly immature or semi-mature and efficiently process self-antigens to induce and maintain self-tolerance (Hawiger et al. 2001; Bonifaz et al. 2002; Lutz and Schuler 2002). Under inflammatory conditions, DCs undergo terminal maturation and activation to become fully immunogenic. DC heterogeneity and differential activation states ultimately determine the type and quality of immune responses.
DCs initiate an immune response by capturing and presenting antigen in the form of peptide–major histocompatibility complex (MHC) molecule complexes to naive T cells in lymphoid tissues (Banchereau and Steinman 1998; Steinman and Banchereau 2007; Steinman 2012). DCs share most features of antigen-processing cells and class I and II MHC-restricted presentation with other antigen-presenting cells (APCs). DCs, however, are endowed with the capacity to cross-present exogenous antigens on their own class I MHC molecules to autologous T cells regardless of the MHC alleles expressed by the antigen source (Albert et al. 1998; Guermonprez et al. 2003; Ackerman et al. 2005). When compared with other APCs, like macrophages, DCs are much more efficient and can elicit responses from very low numbers of T cells (Steinman 2012).
In peripheral tissues, DCs capture antigens by different complementary mechanisms (Trombetta and Mellman 2005) and then migrate through the afferent lymphatics to draining lymph nodes. DCs process proteins into peptides that bind to both MHC class I and class II molecules. Lipid antigens are processed differently through nonclassical MHC molecules of the CD1 family (Banchereau et al. 2000). Antigens also reach lymph node-resident DCs directly through the lymph (Itano and Jenkins 2003). On interaction with DCs, naive CD4+ and CD8+ T cells differentiate into antigen-specific effector cells with diverse functions. CD4+ T cells can become T helper 1 (TH1) cells, TH2 cells, TH17 cells, or T follicular helper (TFH) cells, as well as regulatory T (TReg) cells that suppress the function of other lymphocytes. Naive CD8+ T cells can give rise to effector cytotoxic T lymphocytes (CTLs). The type of CD4+ or CD8+ T-cell response is at least in part dependent on the DC subset presenting the antigen (Banchereau and Steinman 1998). DCs also interact with cells of the innate immune system, including natural killer (NK) cells and mast cells (Banchereau and Steinman 1998; Steinman and Banchereau 2007; Steinman 2012). DCs control humoral immunity, directly by interacting with B cells and indirectly by inducing CD4+ helper T-cell expansion and differentiation (Jego et al. 2005; Qi et al. 2006).
1.2 DC Maturation and Activation
DC maturation and activation is pivotal to the control of immune responses. Immature or non-activated DCs in peripheral tissues induce immune tolerance either through T-cell deletion or TReg expansion (Steinman et al. 2003). Immature DCs efficiently capture antigens, accumulate MHC class II molecules in the late endosome–lysosomal compartment, express low levels of co-stimulatory molecules, and have a limited capacity for cytokine secretion (Trombetta and Mellman 2005). In response to environmental signals, immature DCs differentiate into mature forms that efficiently induce immune responses. Maturation is associated with decreased antigen-capture activity, increased expression of surface MHC class II and co-stimulatory molecules, increased cytokine secretion (Trombetta and Mellman 2005), increased CCR7 expression to enable migration to draining lymph nodes (Trombetta and Mellman 2005), and upregulation of CD83, the prototypical marker of DC maturation (Zhou and Tedder 1995). DCs also upregulate the immune-modulatory enzyme, indoleamine 2,3-dioxygenase, with maturation to induce TRegs, thus providing an intrinsic brake to counter otherwise unrestrained immune responses (Munn et al. 2002; Chung et al. 2009).
Microbial products provide a physiologic activation stimulus to DCs via pathogen-associated molecular patterns (PAMPs), including toll-like receptors. Although most microbes activate DCs, a few can block DC maturation (Steinman and Banchereau 2007; Pulendran et al. 2001; Palucka and Banchereau 2002). Tissue-localized DCs can also be polarized into distinct phenotypes by products released from neighboring immune cells responding to injury, including interferon (IFN)-γ from γδ-T cells and NK cells, preformed interleukin (IL)-4 and TNF from mast cells, IFNα from pDCs, and IL-15 and thymic stromal lymphopoietin (TSLP) from stromal cells (Ueno et al. 2010; Cheng et al. 2010). A combination of pro-inflammatory cytokines that include IL-1-β, IL-6, prostaglandin E2, and tumor necrosis factor (TNF)-α (Jonuleit et al. 1997) simulates physiologic stimulation and is often used to mature DCs for study in vitro and use in clinical vaccine trials. In addition, adjuvant components of many vaccines trigger DC activation through distinct molecular pathways, resulting in varied T-cell responses (Maldonado-Lopez et al. 1999; Pulendran et al. 1999).
1.3 Human DC Subsets
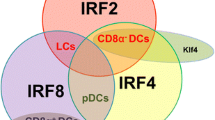
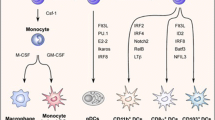
Comparative phenotypic and functional studies have identified distinct DC subsets present in all mammals. Human DCs express MHC class II (HLA-DR) at high levels but lack T cell (CD3), B cell (CD19/CD20), and NK cell (CD56) lineage markers. The classical descriptions of DCs as HLA-DR+ lineage− cells have been refined to include additional positive DC lineage markers that categorize DCs as either “myeloid” (or “conventional”) or “plasmacytoid” (Ziegler-Heitbrock et al. 2010). Human DC subsets in the blood can be distinguished by the differential expression of the cell-surface molecules CD303 (BDCA2 and CLEC4C), CD1C (BDCA1), and CD141 (BDCA3 and thrombomodulin) (Dzionek et al. 2000).
Myeloid DCs (mDCs) express the myeloid antigens CD11c, CD13, CD33, and CD11b, corresponding to mouse CD11c+ “classical” or “conventional” DCs. In humans, both monocytes and mDCs express CD11c, but DCs lack CD14 or CD16 and can be subclassified into two populations by the reciprocal expression of CD1c and CD141. These two subpopulations share homology with mouse classical DCs expressing either CD11b (CD1c+ DCs) or CD8/CD103 (CD141+ DCs). Human CD141+ mDCs are adept at taking up dead or necrotic cells via CLEC9A, sensing viral nucleic acids via TLR3 and TLR8, and cross-presenting antigen to CD8+ T cells in vitro (Collin et al. 2013). Thus, human CD141+ DCs are well equipped to stimulate CD8+ T-cell-mediated immune responses. It is important to note that other human DCs, such as epidermal Langerhans cells (Klechevsky et al. 2008), also cross-present antigens effectively. Whether CD141+ blood mDCs are related to DC subsets in peripheral tissues is unknown. CD1c+ mDCs are the major population of human mDCs in blood, tissues, and lymphoid organs, and defining their unique function(s) remains an area of active research. Overall, the division of labor between CD141+ DCs and other myeloid DCs in humans appears less sharply demarcated than in the mouse, underscoring the imprecise nature of cross-species comparisons.
Langerhans cells (LCs) and dermal interstitial DCs (dermal DCs) are the two primary subsets of mDCs present in the skin. LCs express high levels of langerin, a C-type lectin, and CD1a, a non-polymorphic class I MHC molecule, and are superior stimulators of cytotoxic T lymphocytes (CTLs) in vitro, at least against recall viral antigen and cross-presented tumor antigen (Ratzinger et al. 2004). Dermal DCs can be further subdivided into CD1a+ DCs and CD14+ DCs (Valladeau and Saeland 2005; Nestle et al. 1993). CD14+ DCs appear to be specialized participants in humoral responses (Klechevsky et al. 2008; Ueno et al. 2007), as they can directly help activated B cells and induce naive T cells to differentiate into cells with TFH cell-like properties (Klechevsky et al. 2008; Caux et al. 1997).
Plasmacytoid DCs (pDCs) secrete copious amounts of type I interferon (IFN-α/β) in response to foreign nucleic acids and thereby mediate antiviral immunity (Siegal et al. 1999). pDCs are distinguished by the absence of myeloid antigens and expression of CD123 (IL-3R), CD303, and CD304. Freshly isolated plasmacytoid DCs express much lower levels of MHC and co-stimulatory molecules than their conventional DC counterparts (Grouard et al. 1997). Non-activated pDCs capture, process, and load antigens onto MHC molecules less effectively and are therefore relatively poor stimulators of T cells. In their resting state, pDCs participate in immune tolerance, including oral tolerance (Reizis et al. 2011). IL-3, in combination with CD40L or microbial products, leads to full pDC activation, abundant type I IFN secretion, and more potent lymphocyte stimulation (Siegal et al. 1999; Cella et al. 1999; Fonteneau et al. 2003). Activated pDCs also induce the maturation of activated B cells into plasma cells through cytokines and surface signaling (Jego et al. 2003; Shaw et al. 2010).
1.4 DC Receptors
DCs sense the environment with a diverse repertoire of surface and intracellular receptors, including toll-like receptors (TLRs), C-type lectins (CLRs), and helicases. TLRs recognize specific components conserved among microorganisms, and ligand binding to TLRs on DCs initiates the entire range of innate and acquired immunity (Takeda et al. 2003). TLR ligands include peptidoglycan (TLR2), viral dsRNA (TLR3), LPS (TLR4), viral ssRNA (TLR7), and unmethylated bacterial CpG DNA motif (TLR9). Myeloid DCs express various combinations of TLR1–TLR6 and TLR8, depending on the subset and activation state (Kadowaki et al. 2001; Jarrossay et al. 2001). pDCs are the only human DC subtype with TLR7 and TLR9 expression (Kadowaki et al. 2001; Jarrossay et al. 2001). CLRs bind carbohydrate moieties of glycoprotein self-antigens and pathogens, as well as many noncarbohydrate ligands such as lipids and proteins, by mechanisms that are not yet fully understood, to variably trigger pro-inflammatory or anti-inflammatory reactions (Reis e Sousa 2006). CLRs can synergize with, antagonize, or regulate signals from other receptors, thereby fine-tuning responses to infection or damage. CLR expression by DCs varies with activation status (Valladeau et al. 2000; Ebner et al. 2004; Bonifaz et al. 2004) and includes DEC-205 (CD205), DC-SIGN (CD209), BDCA-2, Dectin-1, Langerin (CD207), and CLEC9A. Helicases are members of a large family of molecules, including retinoic acid-inducible gene I (RIGI), which recognize nucleic acids. Activation of helicases can differentially affect DC function to yield distinct immune responses (Takeuchi and Akira 2010; Zhang et al. 2011). DCs also express a combination of activating (CD16, CD32a, and CD64) and inhibitory (CD32b) Fc-γ receptors that influence processing and presentation of antigens in the steady state and during inflammation (Guilliams et al. 2014).
2 Human DCs in Clinical Hematopoietic Cell Transplantation
2.1 DC Antigen Presentation and Chimerism After Allogeneic Hematopoietic Cell Transplantation
DCs initiate T-cell responses to MHC and minor histocompatibility antigens (miHAs) and are both initiators and targets of graft–host interactions in hematopoietic cell transplantation (HCT). Specifically, DCs participate in the induction of graft-versus-tumor (GVT) activity and graft-versus-host disease (GvHD), two distinct but overlapping syndromes. The cytokine storm associated with pretransplantation conditioning and the early peri-transplant period can activate DCs to present MHC and miHAs through two separate pathways in an immunogenic manner (Hill and Ferrara 2000). Persistent host DCs present antigen by direct ligation of the donor T-cell receptor (TCR) by MHC molecules on recipient DCs. Engrafting donor DCs use an indirect pathway to cross-present host antigens. In both cases, antigens are presented to engrafting donor T cells. Polymorphic residues in the MHC binding groove, which themselves are not accessible to TCRs, affect binding of peptides recognized by allogeneic T cells. This intensifies the antigenic effect of MHC polymorphisms and explains the much higher frequency of T cells (1–10%) reactive with allogeneic MHC compared with those that react with miHAs presented by MHC-identical individuals (Sherman and Chattopadhyay 1993). Donor T cells use an indirect pathway to recognize miHAs, which are peptides derived from polymorphic genes unique to the host but recognized because they are presented by shared MHC molecules in matched allogeneic HCT (allo-HCT) (Bleakley and Riddell 2004). The ultimate goal in clinical transplantation is to stimulate T cells against miHAs unique to a malignancy that are absent from normal tissues, thus achieving GVT activity without GvHD.
DCs are terminally differentiated and, due to their nonproliferating state, are resistant to myeloablative regimens that target dividing cells, including total body irradiation. This results in the persistence of host DCs that coexist with new donor-derived DCs after allo-HCT. Most chimerism studies have evaluated conventional or myeloid DCs, identifying rapid conversion to donor type even though small numbers of residual host DCs may persist for extended periods, especially after reduced-intensity conditioning. In one study, approximately 80% of peripheral blood DCs were of donor origin by day +14 after allo-HCT, increasing to >95% by day +56 (Auffermann-Gretzinger et al. 2002). The kinetics of DC chimerism in peripheral tissues varies by conditioning regimen. A study of epidermal LC chimerism after allo-HCT found an average 97% donor-derived LCs with full-intensity conditioning, but only 36.5% donor-derived LCs with reduced-intensity conditioning 40 days after allo-HCT; at least 90% of LCs were donor-derived by day +100 (Collin et al. 2006). Donor chimerism is delayed in the presence of acute GvHD (Collin et al. 2006; Auffermann-Gretzinger et al. 2006), but the presence of residual host DCs is also seen in the absence of acute GvHD (Andani et al. 2014).
2.2 DCs and GvHD
GvHD is a frequent complication of allo-HCT and causes significant morbidity and mortality. At its root, GvHD is an inflammatory process mediated by both the innate and adaptive arms of the immune system (Ball and Egeler 2008; Ferrara et al. 2009). Residual host- and donor-derived DCs participate in GvHD pathogenesis. In murine models, host-derived DCs are essential for the induction of acute GvHD, whereas donor-derived DCs amplify acute GvHD and may be involved in the development of chronic GvHD (Shlomchik et al. 1999; Matte et al. 2004). During the effector phase of GvHD, tissue-resident, host-derived macrophages and DCs control the migration of alloreactive donor T cells into the tissues and subsequent local development of GvHD in mice (Zhang et al. 2002).
Mouse models of GvHD demonstrate that DC homeostasis after transplant influences GvHD outcome. LCs can self-renew in the skin of parabiotic mice from local precursors and remain of host origin for a prolonged period (Merad et al. 2004). LCs and dermal DCs can survive myeloablative radiation and persist for months after transplantation of purified stem cells or T-cell-depleted bone marrow in the absence of GvHD (Merad et al. 2004; Bogunovic et al. 2006). The presence or absence of GvHD is crucial to DC composition. In the absence of GvHD, trace populations of low-level cycling precursors in the skin can replace LCs or dermal DCs that exit to secondary lymphoid tissues, thus maintaining DCs of host origin. In contrast, in the setting of GvHD, the loss of DCs exceeds the capacity of local precursors to replenish host populations, allowing for circulating donor marrow-derived DC precursors to fill the resulting void. Elimination of host LCs and replacement by donor DCs prevent cutaneous GvHD in MHC-mismatched allo-HCT (Merad et al. 2004). In addition, residual allogeneic T cells from donor marrow, once primed against host MHC or miHA, eliminate host DCs from GvHD target organs, with subsequent replacement by donor marrow-derived DCs (Merad et al. 2004). Previous acute GvHD of the skin in humans correlates with complete donor LC chimerism, again supporting a role of allogeneic T cells in promoting donor LC engraftment (Collin et al. 2006).
Resident populations of DCs in peripheral tissues may be more relevant to acute GvHD, as addressed in the murine studies cited above. In humans, host LCs decreased and then recovered with donor LCs more rapidly after myeloablative conditioning than with reduced-intensity conditioning, although the nadirs were comparable between days 14 and 21 (Collin et al. 2006). Donor LC recovery to pretransplant levels was more brisk in the absence of acute GvHD but more complete in the presence of acute GvHD, indicating a role for donor T cells in promoting LC engraftment as in mice (Merad et al. 2002, 2004). Dermal DC reconstitution can exhibit similarly rapid turnover by about day +100 (Auffermann-Gretzinger et al. 2006), although some host dermal DCs persist, especially after reduced-intensity conditioning (Bogunovic et al. 2006). Co-expression of the activation marker, CMRF-44, by conventional or myeloid CD11c+ DCs in peripheral blood precedes the onset of clinically significant acute GvHD (Lau et al. 2007), suggesting the predictive value of monitoring such subsets in the blood.
Prophylactic and therapeutic immunosuppressive agents for GvHD affect DC numbers. During clinically significant acute GvHD, circulating DC levels decline, reflecting the effects of therapy (especially steroids), more rapid turnover and migration into tissues, or both (Reddy et al. 2004). Alemtuzumab rapidly depletes circulating host DCs but does not alter donor DC engraftment or deplete other DCs that lack the CD52 target epitope (LCs or dermal DCs) (Ratzinger et al. 2003; Collin et al. 2005; Klangsinsirikul et al. 2002). Cyclosporin A and tacrolimus can impair antigen processing by DCs (Lee et al. 2005) but, like steroids, are nonselective and also exert broad effects on T cells by calcineurin inhibition. Sirolimus (rapamycin), which blocks the signal transduction resulting from ligation of the IL-2, IL-4, and IL-6 receptors in T cells, also suppresses DC immunogenicity (Hackstein et al. 2003). Thus, drugs that block DC function should modulate immune interactions in allo-HCT. More targeted reagents are still required, however, especially if the goal is to maintain viral immunity and GVT effects while eliminating GvHD and avoiding overly global immune suppression and its attendant complications. The use of tolerogenic recipient DCs to pretreat donor stem cell sources and minimize allogeneic T-cell responses is an alternative consideration.
2.3 DCs and GVT Responses
Host DCs presenting tumor antigen(s) either directly or by cross-presentation should induce at least a portion of the GVT response. The precise role of the different human DC subtypes in GVT responses after allo-HCT, however, remains poorly understood. Mouse studies have shown that host DCs may play an important role in GVT effects (Mapara et al. 2002), especially those that are able to cross-present tumor-specific antigen(s) to donor T cells (Toubai et al. 2013). The participation of host DCs in GVT in humans was supported by a study where the combination of donor T cells and mixed chimerism in DC subsets stimulated a potent GVL effect in association with GvHD, whereas donor lymphocyte infusions in patients with donor chimerism in both T cells and DC subsets resulted in GVL reactivity without GvHD (Levenga et al. 2007). Whether DC subtypes separately direct GVH or GVT reactions at the level of antigen presentation to responding T cells is incompletely understood. In the absence of concomitant tissue damage, persistent host LCs migrating from the skin to draining lymph nodes can stimulate potent graft responses against host antigens, thus supporting GVT without GvHD in an MHC-matched murine allo-HCT model (Durakovic et al. 2006). This finding is also relevant to the immunologic effects mediated by donor lymphocyte infusions to treat relapsed/recurrent disease. The ultimate goal is to preferentially target miHAs that are only expressed by tumor cells and not shared with normal tissue to avoid the overlapping development of GvHD (Bleakley and Riddell 2004). Maintaining DCs in an immature or semi-mature state to preserve graft–host tolerance while promoting GVT is an area of ongoing study.
2.4 DC Vaccines: General Considerations
Methods for the large-scale generation of human DCs have enabled their clinical evaluation as vaccines. Although early phase I and II trials of DC-based vaccines showed limited success, many studies used immature DCs that were insufficiently immunogenic, suboptimal routes and schedules of vaccination, and patients with advanced disease in whom there was inadequate time to respond. Importantly, numerous other studies have demonstrated the feasibility of DC-based immunization to induce both immune and objective clinical responses against tumors (Palucka and Banchereau 2012), although primarily in the non-transplant setting.
DC precursors can be obtained from several sources, including nondividing peripheral blood monocytes or cycling CD34+ progenitors in cord blood, granulocyte colony-stimulating factor-elicited peripheral blood, or bone marrow. Regardless of source, precursor cells require recombinant cytokine support in vitro to generate DCs, with subsequent terminal maturation to ensure optimal stimulation of T-cell immunity. A potential advantage of using CD34+-derived DCs, especially in the setting of cord blood transplantation, is their capacity for expansion prior to DC differentiation to generate larger numbers of DCs from a limited pool of precursor cells. In addition to cytokine-supported methods, preformed circulating DCs can also be isolated from blood by density gradient (Hsu et al. 1996; Timmerman et al. 2002) or direct immunoselection (Dzionek et al. 2000; Lopez et al. 2003).
Almost all previous DC vaccine trials have used monocyte-derived DCs (moDCs), in large part because monocyte precursors are easier to obtain and culture in vitro than CD34+-derived subsets, including LCs. LCs, however, are superior to moDCs and other conventional DC subsets at inducing antigen-specific CTLs against viral and tumor antigens in vitro (Klechevsky et al. 2008; Ratzinger et al. 2004). When compared with moDCs, LCs secrete more IL-15 (Klechevsky et al. 2008; Ratzinger et al. 2004; Munz et al. 2005), which in turn reduces IL-2-induced T-cell apoptosis and decreases TReg expansion during LC-mediated CTL generation (Romano et al. 2012). LCs can overcome tolerance against tumor-associated antigens by an IL-15Rα/IL-15/pSTAT5-dependent mechanism (Romano et al. 2012). Clinical trial data have shown greater efficacy of DC vaccines that contain LCs (Banchereau et al. 2001), as well as greater tetramer reactivity stimulated by LCs when compared with moDCs (Romano et al. 2011). Thus, selection of DC subtype for use in vaccine formulations is an important consideration.
Optimizing antigen loading is another key parameter of DC vaccine preparation. The simplest and most often used approach is “peptide pulsing,” which is the incubation of DCs with synthetic peptides of limited length and defined HLA restrictions, most commonly HLA-A*0201. DNA (Yuan et al. 2006)- or RNA (Gilboa and Vieweg 2004)-based methods of antigen delivery offer the potential advantage of facilitating the processing and presentation of a broad repertoire of multiple class I and II MHC-restricted epitopes from the translated protein (Romano et al. 2011), together with more sustained antigen expression than peptide pulsing. Other approaches have used tumor lysates for uptake and cross-presentation (Ratzinger et al. 2004; Berard et al. 2000; Palucka et al. 2006), DC receptor targeting for antigen delivery in vivo (Bonifaz et al. 2004; Birkholz et al. 2010), and systemic delivery of antigen-encoding RNA lipoplexes to DCs in vivo (Kranz et al. 2016). The majority of DC vaccine studies have been limited to single antigen and restricted epitope targets. Simultaneously targeting more than one antigen, however, offers the potential to improve the breadth of immune responses and clinical response rates (Karan et al. 2011; Walter et al. 2012).
Different routes of immunization have been tested, with subcutaneous administration by far the most common method. Other approaches include intradermal, intravenous, intranodal, and intratumoral injections. Although direct comparisons are generally lacking, intradermal vaccination may be more effective than subcutaneous vaccination due to the rich lymphatics at the epidermal–dermal junction. Intravenous administration does not compare favorably with the intradermal route in animal and limited clinical comparisons. Intranodal vaccination removes considerable uncertainty but cannot be widely implemented. The ideal frequency and duration for vaccination is unknown, but maintaining an ongoing vaccination schedule in responding patients may provide benefit (Palucka et al. 2006).
Early-phase trials often rely on proxy measurements in vitro of responses to vaccines. These include antigen-driven assays for measurement of IFN-γ secretion, intracellular cytokine secretion assays, T-cell reactivity with tetramers/pentamers of defined peptides with known MHC restrictions, CTL assays against antigen-expressing targets, and, more recently, next-generation deep sequencing of the TCR-V-beta CDR3 region to assess changes in T-cell clonal diversity. Clinical responses remain mostly anecdotal in the relatively small numbers of patients among the many treated in the presence of persistent systemic disease.
2.5 DC Vaccination After Autologous Stem Cell Transplantation
DC-based vaccination to induce or restore antitumor immunity offers a promising approach to target residual malignancy and to improve clinical outcomes after autologous HCT (auto-HCT). The minimal residual disease state and lymphopenia after auto-HCT afford a unique platform to induce antitumor immune responses by limiting tumor-driven immunosuppression (Kim et al. 2006), eliminating cytokine sinks (Gattinoni et al. 2005), and transiently depleting TRegs (Zhang et al. 2005; Chung et al. 2016). Importantly, CD8+ T cells can respond to autologous DCs presenting tumor antigen in vitro as early as day +12 posttransplant, becoming antigen-specific cytolytic T-lymphocyte effectors and thereby demonstrating preservation of cellular reactivity after transplant (Chung et al. 2016). DC-based vaccination in this setting therefore offers one approach to redirect recovering T cells toward specific MHC-restricted antigen(s).
The feasibility of DC vaccines in the setting of auto-HCT has been demonstrated in multiple myeloma, the most common indication for auto-HCT (Table 11.1). DCs from patients with multiple myeloma are functionally intact, comparable to those from healthy donors, and induce autologous antigen-specific T cells with lytic activity in vitro (Chung et al. 2016). Clinical validation of this approach was shown with an idiotype-pulsed autologous DC vaccine for multiple myeloma after auto-HCT that induced idiotype-specific T-cell responses in a subset of patients (Reichardt et al. 1999). Subsequently, posttransplant vaccination with an idiotype-pulsed cellular product containing DCs was associated with improved progression-free survival compared with a historical control cohort (Lacy et al. 2009). More recently, vaccination with a DC–myeloma fusion vaccine following autologous transplant was associated with an increase in myeloma-specific T cells and conversion from partial response to complete response in a subset of patients (Rosenblatt et al. 2013). Vaccination in these three studies was well tolerated without evidence of significant autoimmunity or adverse effect on posttransplant engraftment.
Posttransplant maintenance therapy with lenalidomide improves progression-free and overall survival after auto-HCT (McCarthy et al. 2012; Attal et al. 2012; Palumbo et al. 2014). In addition, lenalidomide has immunostimulatory properties (Benson Jr et al. 2011; Luptakova et al. 2013; Noonan et al. 2012) that could further augment vaccine-induced immunity. This is being assessed in two DC vaccine studies that include lenalidomide maintenance in the treatment regimen. A phase I study is examining early posttransplant vaccination using autologous LCs electroporated with mRNA encoding three MM-associated antigens followed by lenalidomide maintenance (NCT01995708). A phase II, multicenter trial will study posttransplant lenalidomide maintenance alone or in conjunction with serial vaccination with DC–myeloma fusions (NCT02728102).
Immune checkpoint inhibitors enhance vaccine-induced antitumor immune responses in various preclinical models. Both CD4+ and CD8+ T cells from multiple myeloma patients express the negative regulatory molecules, CTLA-4, PD-1, LAG-3, and TIM-3, before and after auto-HCT (Chung et al. 2016). In addition, a subpopulation of hyporesponsive, exhausted/senescent PD-1-expressing CD8+ T cells that characterize immune impairment and relapse after auto-HCT can be revived with PD-1 blockade in vitro (Chung et al. 2016). The combination of DC vaccines with checkpoint blockade to “prime and boost” antitumor immune responses warrants investigation.
2.6 DC Vaccination After Allogeneic Stem Cell Transplantation
As in the auto-HCT setting, DC-based vaccination after allo-HCT offers similar advantages in exploiting a minimal residual disease state and the autoreactive potential of recovering T-cell populations, relatively devoid of TRegs. In contrast to auto-HCT, however, allo-HCT regimens often include immunosuppressant agents for GvHD prophylaxis, which could impede responses to vaccines. Nonetheless, DC vaccination after allo-HCT has been well tolerated with evidence of both clinical and immunological antiviral and antitumor responses without increases in adverse events, albeit in a limited number of patients (Table 11.2).
Virus-specific immunity against human cytomegalovirus (CMV) can be induced by DC vaccination after allo-HCT. Patients at high risk for developing CMV disease, defined as a CMV-seropositive patient and a CMV-seronegative donor and/or receipt of a T-cell-depleted graft, received donor CD14-derived peptide-loaded DCs after allo-HCT, with induction of measureable CMV-specific T-cell responses and evidence of clinical benefit, without the stimulation or expansion of allo-reactive T cells (Grigoleit et al. 2007). Vaccination with CMV pp65-pulsed DCs induced antigen-specific CD4 and CD8 cells and sustained CMV viral clearance in a patient with recurrent CMV viremia resistant to standard antiviral therapies (Feuchtinger et al. 2010).
Tumor-specific immunity elicited by donor-derived DCs loaded with irradiated tumor cells was observed in three of four patients with hematologic malignancies relapsed after allo-HCT (Fujii et al. 2001). Additional reports have demonstrated antigen-specific immune responses in patients immunized with DC-based vaccines after allo-HCT (Tatsugami et al. 2004; Kitawaki et al. 2008; Levenga et al. 2010).
Because allo-HCT patients may not be sufficiently immune reconstituted to respond to direct immunization, alternative approaches merit consideration. Donors could be vaccinated with DCs bearing their recipients’ tumor antigen(s) before stem cell collection and transplantation. Donor DCs could also be used to stimulate donor lymphocytes ex vivo against specific tumor antigens for adoptive immunotherapy, with less off-target effects that could trigger GvHD.
3 Expert Point of View
DCs comprise a complex system of bone marrow-derived leukocytes that are critical to the onset and modulation of immunity. The divisions of labor among distinct human DC subsets maintain an equilibrium between steady-state tolerance and stimulation of antigen-specific immunity against pathogens, tumors, and other insults. Maintenance of tolerance in the steady state is an active process mediated by resting or semi-mature DCs. Under inflammatory conditions, this homeostasis is disrupted, leading to the maturation and activation of DCs and triggering a cascade of events leading to an immune response. In the setting of HCT, the mechanisms that regulate DC homeostasis offer potential targets to fine-tune graft–host interactions. It is not yet known whether a particular subtype of DC is more or less responsible for initiating or being targeted (or both) by GvHD reactions. The precise role of the different DC subtypes in GVT responses after HCT also remains poorly understood. Animal models are providing important data about distinct DC precursors, homeostasis of tissue-resident DCs, and turnover of DCs in response to inflammatory stimuli and pathological conditions like GvHD. Ultimately, therapeutic interventions that use or specifically target defined DC subtypes to selectively induce both the innate and adaptive arms of immunity, either in combination or in a prime-boost sequence, may provide optimal clinical utility by harnessing both effector cell compartments.
4 Future Directions
Advancing our knowledge of how different DC subsets are related, their roles in graft–host interactions and disease pathogenesis, and their most favorable therapeutic implementation are among the key issues for future studies. Progress in systems immunology is expected to lend insights into the molecular pathways that determine DC-guided immunity. Thus, an integrated approach combining transcriptional profiling, genetic and small-molecule screening, and proteomics will further our understanding of DC biology and thereby enable the discovery of novel adjuvants and strategies to induce protective immune responses while minimizing the risk of autoimmunity or GvHD. In turn, this will yield more rational and refined clinical applications of DC-based therapies in HCT.
References
Ackerman AL, Kyritsis C, Tampe R, Cresswell P (2005) Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol 6:107–113
Albert ML, Sauter B, Bhardwaj N (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86–89
Andani R et al (2014) Origin of Langerhans cells in normal skin and chronic GVHD after hematopoietic stem-cell transplantation. Exp Dermatol 23:75–77
Attal M et al (2012) Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 366:1782–1791
Auffermann-Gretzinger S et al (2002) Rapid establishment of dendritic cell chimerism in allogeneic hematopoietic cell transplant recipients. Blood 99:1442–1448
Auffermann-Gretzinger S et al (2006) Fast appearance of donor dendritic cells in human skin: dynamics of skin and blood dendritic cells after allogeneic hematopoietic cell transplantation. Transplantation 81:866–873
Ball LM, Egeler RM (2008) Acute GvHD: pathogenesis and classification. Bone Marrow Transplant 41(Suppl 2):S58–S64
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Banchereau J et al (2000) Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811
Banchereau J et al (2001) Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 61:6451–6458
Benson DM Jr et al (2011) IPH 2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood 118:6387–6391
Berard F et al (2000) Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med 192:1535–1544
Birkholz K et al (2010) Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood 116:2277–2285
Bleakley M, Riddell SR (2004) Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer 4:371–380
Bogunovic M et al (2006) Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med 203:2627–2638
Bonifaz L et al (2002) Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 196:1627–1638
Bonifaz LC et al (2004) In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 199:815–824
Caux C et al (1997) CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood 90:1458–1470
Cella M et al (1999) Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med 5:919–923
Cheng P, Zhou J, Gabrilovich D (2010) Regulation of dendritic cell differentiation and function by Notch and Wnt pathways. Immunol Rev 234:105–119
Chung DJ et al (2009) Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 114:555–563
Chung DJ et al (2016) T-cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immunotherapy. Cancer Immunol Res 4:61–71
Collin M, McGovern N, Haniffa M (2013) Human dendritic cell subsets. Immunology 140:22–30
Collin MP et al (2005) In vitro depletion of tissue-derived dendritic cells by CMRF-44 antibody and alemtuzumab: implications for the control of graft-versus-host disease. Transplantation 79:722–725
Collin MP et al (2006) The fate of human Langerhans cells in hematopoietic stem cell transplantation. J Exp Med 203:27–33
Durakovic N et al (2006) Host-derived Langerhans cells persist after MHC-matched allografting independent of donor T cells and critically influence the alloresponses mediated by donor lymphocyte infusions. J Immunol 177:4414–4425
Dzionek A et al (2000) BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 165:6037–6046
Ebner S et al (2004) Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int Immunol 16:877–887
Ferrara JL, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. Lancet 373:1550–1561
Feuchtinger T et al (2010) Dendritic cell vaccination in an allogeneic stem cell recipient receiving a transplant from a human cytomegalovirus (HCMV)-seronegative donor: induction of a HCMV-specific T (helper) cell response. Cytotherapy 12:945–950
Fonteneau JF et al (2003) Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 101:3520–3526
Fujii S et al (2001) Treatment of post-transplanted, relapsed patients with hematological malignancies by infusion of HLA-matched, allogeneic-dendritic cells (DCs) pulsed with irradiated tumor cells and primed T cells. Leuk Lymphoma 42:357–369
Gattinoni L et al (2005) Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202:907–912
Gilboa E, Vieweg J (2004) Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev 199:251–263
Grigoleit GU et al (2007) Dendritic cell vaccination in allogeneic stem cell recipients: induction of human cytomegalovirus (HCMV)-specific cytotoxic T lymphocyte responses even in patients receiving a transplant from an HCMV-seronegative donor. J Infect Dis 196:699–704
Grouard G et al (1997) The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 185:1101–1111
Guermonprez P et al (2003) ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425:397–402
Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN (2014) The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol 14:94–108
Hackstein H et al (2003) Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood 101:4457–4463
Hawiger D et al (2001) Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 194:769–779
Hill GR, Ferrara JL (2000) The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 95:2754–2759
Hsu FJ et al (1996) Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 2:52–58
Itano AA, Jenkins MK (2003) Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol 4:733–739
Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A (2001) Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol 31:3388–3393
Jego G, Pascual V, Palucka AK, Banchereau J (2005) Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun 8:124–139
Jego G et al (2003) Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19:225–234
Jonuleit H et al (1997) Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 27:3135–3142
Kadowaki N et al (2001) Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194:863–869
Karan D et al (2011) Dual antigen target-based immunotherapy for prostate cancer eliminates the growth of established tumors in mice. Immunotherapy 3:735–746
Kim R, Emi M, Tanabe K, Arihiro K (2006) Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res 66:5527–5536
Kitawaki T et al (2008) Potential of dendritic-cell immunotherapy for relapse after allogeneic hematopoietic stem cell transplantation, shown by WT1 peptide- and keyhole-limpet-hemocyanin-pulsed, donor-derived dendritic-cell vaccine for acute myeloid leukemia. Am J Hematol 83:315–317
Klangsinsirikul P, Carter GI, Byrne JL, Hale G, Russell NH (2002) Campath-1G causes rapid depletion of circulating host dendritic cells (DCs) before allogeneic transplantation but does not delay donor DC reconstitution. Blood 99:2586–2591
Klechevsky E et al (2008) Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 29:497–510
Kranz LM et al (2016) Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534:396–401
Lacy MQ et al (2009) Idiotype-pulsed antigen-presenting cells following autologous transplantation for multiple myeloma may be associated with prolonged survival. Am J Hematol 84:799–802
Lau J et al (2007) Activated circulating dendritic cells after hematopoietic stem cell transplantation predict acute graft-versus-host disease. Transplantation 83:839–846
Lee YR et al (2005) Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood 105:3951–3955
Levenga H et al (2007) Dynamics in chimerism of T cells and dendritic cells in relapsed CML patients and the influence on the induction of alloreactivity following donor lymphocyte infusion. Bone Marrow Transplant 40:585–592
Levenga H et al (2010) Partial T cell-depleted allogeneic stem cell transplantation following reduced-intensity conditioning creates a platform for immunotherapy with donor lymphocyte infusion and recipient dendritic cell vaccination in multiple myeloma. Biol Blood Marrow Transplant 16:320–332
Lopez JA et al (2003) Single step enrichment of blood dendritic cells by positive immunoselection. J Immunol Methods 274:47–61
Luptakova K et al (2013) Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 62:39–49
Lutz MB, Schuler G (2002) Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 23:445–449
Maldonado-Lopez R et al (1999) CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med 189:587–592
Mapara MY et al (2002) Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood 100:1903–1909
Matte CC et al (2004) Donor APCs are required for maximal GVHD but not for GVL. Nat Med 10:987–992
McCarthy PL et al (2012) Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 366:1770–1781
Merad M et al (2002) Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol 3:1135–1141
Merad M et al (2004) Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med 10:510–517
Munn DH et al (2002) Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297:1867–1870
Munz C et al (2005) Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood 105:266–273
Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ (1993) Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol 151:6535–6545
Noonan K et al (2012) Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res 18:1426–1434
Palucka AK et al (2006) Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother 29:545–557
Palucka K, Banchereau J (2002) How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol 14:420–431
Palucka K, Banchereau J (2012) Cancer immunotherapy via dendritic cells. Nat Rev Cancer 12:265–277
Palumbo A et al (2014) Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 371:895–905
Pulendran B, Palucka K, Banchereau J (2001) Sensing pathogens and tuning immune responses. Science 293:253–256
Pulendran B et al (1999) Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A 96:1036–1041
Qi H, Egen JG, Huang AY, Germain RN (2006) Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312:1672–1676
Ratzinger G, Reagan JL, Heller G, Busam KJ, Young JW (2003) Differential CD52 expression by distinct myeloid dendritic cell subsets: implications for alemtuzumab activity at the level of antigen presentation in allogeneic graft-host interactions in transplantation. Blood 101:1422–1429
Ratzinger G et al (2004) Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol 173:2780–2791
Reddy V et al (2004) Low dendritic cell count after allogeneic hematopoietic stem cell transplantation predicts relapse, death, and acute graft-versus-host disease. Blood 103:4330–4335
Reichardt VL et al (1999) Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma--a feasibility study. Blood 93:2411–2419
Reis e Sousa C (2006) Dendritic cells in a mature age. Nat Rev Immunol 6:476–483
Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V (2011) Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 29:163–183
Romano E et al (2011) Peptide-Loaded Langerhans cells, despite increased IL15 secretion and T-cell activation in vitro. Elicit antitumor T-cell responses comparable to peptide-loaded monocyte-derived dendritic cells in vivo. Clin Cancer Res 17:1984–1997
Romano E et al (2012) Human Langerhans cells use an IL-15R-alpha/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood 119:5182–5190
Rosenblatt J et al (2013) Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res 19:3640–3648
Shaw J, Wang YH, Ito T, Arima K, Liu YJ (2010) Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood 115:3051–3057
Sherman LA, Chattopadhyay S (1993) The molecular basis of allorecognition. Annu Rev Immunol 11:385–402
Shlomchik WD et al (1999) Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285:412–415
Siegal FP et al (1999) The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835–1837
Steinman RM (2012) Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30:1–22
Steinman RM, Banchereau J (2007) Taking dendritic cells into medicine. Nature 449:419–426
Steinman RM, Hawiger D, Nussenzweig MC (2003) Tolerogenic dendritic cells. Annu Rev Immunol 21:685–711
Takeda K, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21:335–376
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
Tatsugami K et al (2004) Dendritic-cell therapy after non-myeloablative stem-cell transplantation for renal-cell carcinoma. Lancet Oncol 5:750–752
Timmerman JM et al (2002) Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood 99:1517–1526
Toubai T et al (2013) Host-derived CD8+ dendritic cells are required for induction of optimal graft-versus-tumor responses after experimental allogeneic bone marrow transplantation. Blood 121:4231–4241
Trombetta ES, Mellman I (2005) Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23:975–1028
Ueno H et al (2007) Dendritic cell subsets in health and disease. Immunol Rev 219:118–142
Ueno H et al (2010) Harnessing human dendritic cell subsets for medicine. Immunol Rev 234:199–212
Valladeau J, Saeland S (2005) Cutaneous dendritic cells. Semin Immunol 17:273–283
Valladeau J et al (2000) Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12:71–81
Walter S et al (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 18(8):1254–1261
Yuan J et al (2006) Langerhans-type dendritic cells genetically modified to express full-length antigen optimally stimulate CTLs in a CD4-dependent manner. J Immunol 176:2357–2365
Zhang H et al (2005) Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med 11:1238–1243
Zhang Y et al (2002) APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J Immunol 169:7111–7118
Zhang Z et al (2011) The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol 12:959–965
Zhou LJ, Tedder TF (1995) Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol 154:3821–3835
Ziegler-Heitbrock L et al (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chung, D.J. (2019). Dendritic Cells in Hematopoietic Cell Transplantation. In: Perales, MA., Abutalib, S., Bollard, C. (eds) Cell and Gene Therapies. Advances and Controversies in Hematopoietic Transplantation and Cell Therapy. Springer, Cham. https://doi.org/10.1007/978-3-319-54368-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-54368-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54367-3
Online ISBN: 978-3-319-54368-0
eBook Packages: MedicineMedicine (R0)