Abstract
The study of pediatric skin of color encompasses a variety of beautiful birthmarks, reactive pigmentary changes, and altered disease morphologies and prevalences compared to patients with fair skin. This chapter discusses noteworthy congenital and acquired conditions in children with darker skin, including their psychosocial impact.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Pediatric dermatology
- Pigmentary birthmarks and disorders
- Secondary dyspigmentation
- Atopic dermatitis
- Pediatric malignancy
- Psychosocial issues in pediatric dermatology
A major theory regarding the evolution of skin pigmentation is defense against folate photoproteolysis, as folate is necessary for normal nucleic acid biosynthesis [1]. Thousands of years of migration from our origins in Africa, the distance from the equator, and different environmental parameters are believed to play roles in genetic selection and variations in skin color [2,3,4,5]. This chapter will discuss skin conditions that are commonly observed in patients with skin of color or that phenotypically differ compared to presentations in light skin; for example, erythema is not as easily distinguished or might have a violaceous or brown hue in dark-skinned patients. Also, pigmented skin may demonstrate variant responses to skin disease, including pigment lability, follicular vs hyperkeratotic presentations, mesenchymal (granulomatous or keloidal) reactions, and bullous lesions [6, 7]. Normal pigmented skin findings, birthmarks, and pediatric hair disorders will also be discussed. We end with a brief review of the psychosocial impact of congenital and acquired skin conditions, a fundamental aspect of the patient and family experience in pediatric dermatology.
Birthmarks
Congenital pigmentary lesions vary according to race, sex, and type of birthmark. Birthmarks are either localized and limited to having potential psychosocial impact (discussed later in this chapter) or are extensive and might signify systemic involvement [8,9,10,11]. In this chapter, we will review birthmarks that are due to pigment changes, as they are more common among dark-skinned newborns. There is generally no treatment available for pigmented birthmarks, although laser therapy is sometimes helpful.
Dermal Melanocytosis
Between the 11th and 14th weeks of gestation, dermal melanocytes migrate from the neural crest to the epidermis or undergo apoptosis; however, some dermal melanocytes persist at the time of birth and produce melanin within the dermis [12]. Hepatocyte growth factor and other gene mutations, i.e., GNA11 (G protein subunit alpha 11) and GNAQ (G protein subunit alpha G), are believed to play a role in dermal melanocyte survival in head and neck, distal extremities, and sacral skin area resulting in Nevus of Ota, Nevus of Ito, blue nevus, and Mongolian spots. The Tyndall effect, an optical phenomenon, results from reflection of dermal pigment giving the bluish appearance of dermal melanin [12,13,14].
Mongolian Spots
The most common form of congenital dermal melanocytosis is usually referred to as Mongolian spots, occurring in approximately 90% of infants of color all over the world [15, 16]. They typically are observed at birth as blue-gray or slate gray patches on the lumbosacral region, and fade partially or completely over a period of years. “Aberrant” Mongolian spots can be found in extra-sacral locations. Superimposed Mongolian spots have been reported (a darker macule or patch overlying a lighter colored patch) [13]. Extensive Mongolian spots at birth or persistent Mongolian spots in older children may be associated with lysosomal storage diseases, i.e., Hurler disease, GM1(monosialotetrahexosylganglioside) gangliosidosis, Mucopolysaccharidosis type II, and Niemann–Pick disease [17]. Extensive Mongolian spots are also seen in phakomatosis pigmentovascularis; careful physical exam is crucial to identify coexisting vascular birthmarks, which might be difficult to appreciate in darkly pigmented patients [13, 16, 17]. It is important to note uniformity of color and chronicity, to avoid confusing these birthmarks with ecchymoses due to child abuse or neglect (Fig. 17.1).
Nevus of Ota (Oculodermal Melanocytosis/Nevus Fuscoceruleus Ophthalmomaxillaris)
Nevus of Ota is common among Asians and other females with skin of color [18]. It is usually a unilateral blue-gray or brown mottled patch, located on skin and mucous membranes innervated by the ophthalmic and maxillary branches of the trigeminal nerve. Approximately 5% are bilateral [19]. Up to 70% of patients have ipsilateral scleral involvement. It presents shortly after birth and darkens around puberty or may not be obvious until puberty, when it is thought that hormones stimulate pigmentation [18, 20,21,22,23]. Although rare, cutaneous, choroidal or leptomeningeal melanoma has been reported within nevus of Ota [24, 25].
Nevus of Ito (Nevus Fuscoceruleus Acromiodeltoideus)
Clinically and histologically similar to nevus of Ito, this form of dermal melanocytosis is located on the posterior supraclavicular, scapular, and deltoid areas (lateral cutaneous nerve distribution) [19, 26]. Malignant melanoma transformation within nevus of Ito has been reported [27]. Coexistence of nevus of Ito with nevus spilus [26] or nevus of Ito with nevus of Ota [19] have been described.
Blue Nevus (Dermal Melanocytoma)
Blue nevi are congenital in 25% of cases, and 75% are acquired during childhood or later in life. They are usually solitary, blue or blue-black papules on the scalp, sacral, and extensor distal extremities [23, 28]. Giant congenital blue nevi are rare, commonly seen on the scalp, with risk of malignant transformation during childhood or later in life [28, 29]. Blue nevi are categorized as common (1 cm or less, often located on the dorsal hand), cellular (1–3 cm, most often on the buttock and sacral area), or epithelioid (sporadic or in association with Carney complex, usually on trunk and extremities) [23, 28,29,30]. It is challenging to differentiate atypical cellular blue nevi from malignant blue nevi histologically.
Café-Au-Lait Macules
Café-au-lait macules (CALMs) are a very common finding in the general population; prevalence of CALMs in large cohort studies range from 0.3 to 2.7% of newborns [11, 31, 32]. In one large cohort study, 18.3% of black infants versus 0.3% of white infants were found to have CALMs at birth [11]. These are solitary or multiple, well-defined, evenly pigmented brown macules or patches. Smooth borders are described as “coast of California” while serrated borders are described as “coast of Maine.” Average size is 0.2–4 cm in newborns and 1.5–30 cm in older children [32]. Histopathologic examination reveals increased melanin content in the basal layer as well as in melanocytes, with no evidence of increased melanocytic infiltration. Multiple CALMs could be a normal finding with no other systemic disease association (1.9% of normal children with skin of color reportedly have more than 2 CALMs) or might signify systemic involvement in the context of several genetic disorders. Neurofibromatosis 1, neurofibromatosis 2, ring chromosome syndrome, and Watson syndrome are most closely associated with multiple CALMs, and McCune Albright syndrome with large/segmental CALMs [32]. Other systemic disorders with questionable association with CALMs include tuberous sclerosis, ataxia-telangiectasia, Bloom, and Silver–Russell Syndromes [31, 32]. Full discussion of associated genodermatoses is beyond the scope of this chapter.
Patterned Pigmentation (Pigmentary Mosaicism)
Patterned pigmentation refers to hyper- or hypopigmentation that results from two different cell clones (chromosomal mosaicism) [33,34,35]. A wide range of clinically distinct presentations include linear Blaschkoid, broad checkerboard (flag-like), and phylloid (leaf-like) hyper- and hypopigmentation. Patterned pigmentation is rarely associated with cutaneous, skeletal, and neurological abnormalities. Cases are sporadic; however, a few familial cases due to functional (epigenetic) modification of autosomal genes have been described [34, 35].
Linear Nevoid Hypomelanosis Versus Linear and Whorled Nevoid Hypermelanosis
Linear nevoid hypomelanosis, also known as hypomelanosis of Ito (HI), describes hypopigmented Blaschkoid linear swirls and streaks [36, 37]. It presents at birth or during childhood as a result of somatic mosaicism with two clones of cells, where one clone produces less melanin. It affects males and females with no racial predilection; however, it is easier to appreciate in darker skinned patients [36, 38]. Reported association with neurologic, ocular, and musculoskeletal abnormalities varies widely, between 30 and 94% of patients; it is now believed that the lower estimates are more likely [37].
Conversely, linear and whorled nevoid hypermelanosis (LWNH) involves hyperpigmented linear swirls that follow Blaschko’s lines due to pigmentary mosaicism causing increased melanin in one clone. Similar to HI, it has been associated with ocular, musculoskeletal, and neurological abnormalities, but in both conditions the risk of extracutaneous abnormalities should not be overemphasized to patients and parents. In one case review of 16 cases with LWNH, only one out of six patients with extensive LWNH had extracutaneous abnormalities [39]. Dermoscopic exam shows linear and circular streak-like pigmentation organized in a parallel linear manner [40]. Differential diagnoses include incontinentia pigmenti and macular/early epidermal nevus. Histopathology reveals increased basal layer hyperpigmentation with no melanophages [33].
Pigmentary Demarcation Lines
Pigmentary demarcation lines, also known as Futcher’s lines or Voight’s lines, are normal in skin of color and persist through adulthood [41]. They represent an abrupt transition of pigmentation established during fetal development [42,43,44]. The most commonly observed lines are found on the anterolateral upper arms and posteromedial legs (Fig. 17.2a, b).
Transient Infantile Patterned Hyperpigmentation
An often-overlooked diagnosis, transient infantile patterned hyperpigmentation is a benign condition that fades over a few months [45]. Skin examination reveals multiple transverse, parallel pigmented thin lines on the extremities and/or torso. In contrast to hormonal hyperpigmentation of the genitals and areolar skin, transient infantile patterned hyperpigmentation is believed to be due to intrauterine fetal position and friction [45, 46].
Pigmented Nevi
Many pigmented lesions are benign and more frequently observed in children of color. Mucosal hyperpigmentation has been reported in 72–100% of darker skinned populations, with 25% onset before the age of 10. Positive family history is usually elicited. Scleral hyperpigmentation is reported in 78%, 8% before the age of 10 [6]. Hyperpigmented palms, melanonychia (pigmented nail plate), and congenital melanocytic nevi are also common.
Melanonychia and Nevi of the Nail Matrix
Longitudinal melanonychia, also known as melanonychia striata, represents melanin deposition within the nail plate. While 5–10% of longitudinal melanonychia in adults are due to malignant melanoma, no cases of invasive melanoma have been reported in association with pediatric longitudinal melanonychia [47]. Melanonychia presents as a longitudinal hyperpigmented band with variable widths (up to complete involvement of the nail plate) affecting fingernails, especially thumbs, more often than toenails [47, 48]. Color can vary from bluish-gray, brown, to black. Melanonychia of childhood is predominantly caused by benign lentigines and melanocytic nevi, much less commonly due to atypical melanocytic nevi or malignant melanoma in situ. Biopsy or surgical excision is recommended only for lesions with concerning signs: irregularity of pigmentation or borders, width of the pigmentation exceeding 3 mm, hazy border, Hutchinson’s sign (pigmented proximal or lateral nail bed), irregular pigmented lines on dermoscopy, and rapid evolution [48].
Congenital Melanocytic Nevi (CMN)
Congenital melanocytic nevi are melanocytic neural crest-derived hamartomas, common in skin of color. They occur as a result of unregulated melanoblast growth between the 5th and 24th weeks of gestation. Approximately 1% of newborns are affected, with slightly higher female prevalence [49]. Giant CMN have different histological elements because they originate from pluripotent stem cells and are difficult to evaluate using dermoscopy, as the cells extend deeper into the dermis and subcutaneous tissue [49, 50]. Hypertrichosis, perifollicular hypo/hyperpigmentation, pseudomilia, and vascular structures are common dermoscopic findings of CMN [50]. Neurocutaneous melanosis (NCM) is a melanocytic proliferation of the leptomeninges (brain and/or spinal cord) that occurs in association with larger and axial CMN [50]. Diagnosis and management is the same for all skin phototypes.
Disorders of Late-Onset and Secondary Hyperpigmentation
Hyperpigmented lesions occurring beyond infancy include entities of congenital origin. Dermal melanocytosis can manifest later in life, i.e., Nevus of Ito, Ota-like (Hori nevus), and acquired dermal melanocytosis of the hands [51, 52]. Also, late-onset linear and whorled nevoid hypermelanosis in a zosteriform distribution has been reported [53].
Becker’s Nevus (Pigmented Hairy Epidermal Nevus)
Becker’s nevus is thought to be a hamartomatous congenital lesion of ectodermal and mesodermal origin where the pigmentation usually presents years later, although congenital cases have been described [54]. A pigmented patch may appear during childhood then darken during puberty along with coarse hair development. Males are more often affected. Androgen is believed to play a major role in its development. It is usually unilateral, on chest, shoulder, lateral arm, and scapular regions. Hypertrichosis and acneiform lesions may be seen within Becker’s nevus. Rare cases have been reported involving hands, feet, and segmental face with mucosa [55]. Becker’s nevus syndrome is characterized by association with musculoskeletal abnormalities such as ipsilateral mammary hypoplasia or odontomaxillary dysplasia [56, 57]. Treatment is mainly reassurance and camouflage. Laser treatment of the hypertrichosis may be attempted with low-fluence high-repetition-rate diode lasers (808–810 nm) [58], and the pigmentation has been treated with long-pulsed ruby laser [59] and fractional resurfacing [60], although greater risk of dyspigmentation exists for patients of color.
Postinflammatory Hyperpigmentation
Many dermatologic diseases heal with hyperpigmentation or hypopigmentation, especially in darker skinned patients [61, 62]. The exact mechanism is not fully understood; however, many inflammatory mediators have been found to increase melanogenesis, such as interleukin 1 and 6 [62]. Other studies have suggested that epithelial–mesenchymal interactions via growth factors such as keratinocyte growth factor and fibroblast-derived growth factor modulate melanogenesis [63].
Friction Hypermelanosis
Friction hypermelanosis is an acquired hyperpigmentation resulting from continuous rubbing of the skin [64, 65]. Friction hypermelanosis is common in darker skin but it is not commonly observed in infants or young children. Unique curvilinear hyperpigmented patches were described in infants wearing tight socks and mittens for a prolonged length of time, also known as sock-line and mitten-line hyperpigmentation. The differential diagnosis includes child abuse and amniotic band syndrome [66]. Vertically oriented, round, discrete, hyperpigmented patches overlying the lumbosacral area have been described in an institutionalized orphan as a result of repetitive backward and forward movement, inspiring the name “orphan rocker tracks” [67]. A similar hyperpigmentation, Davener’s dermatosis, has been described among young orthodox Jewish males who perform repetitive rocking motions during religious rituals [65].
Idiopathic Eruptive Macular Pigmentation
This condition presents in children and adolescents with sudden onset of multiple brown nonconfluent macules on the trunk, neck, face, and proximal extremities with no preceding inflammatory skin disease or medication administration [68, 69]. The pathogenesis in unknown, although hormonal etiology, has been suggested [68]. Histopathologic exam reveals basal cell layer hyperpigmentation and occasional dermal melanophages without structural changes in the basal layer or lichenoid inflammatory infiltrate. It is important to differentiate it from ashy dermatosis in which the macules are gray, sometimes with erythema, and basal layer vacuolization. Other differential diagnoses include pityriasis versicolor, urticaria pigmentosa, café-au-lait macules, and postinflammatory hyperpigmentation [68, 70]. It resolves over the course of a few months to years [68].
Reticulate Hyperpigmentation
Skin disorders with reticulate hyperpigmentation (net-like pattern) are not very common, and they can be idiopathic, genetic, or drug-induced. Family history, age of onset, race, sex, and distribution as well as full hair and skin exam are important when approaching the patient [71]. Confluent and reticulated papillomatosis (CARP) usually affects peripubertal females, presenting with reticulated hyperpigmented patches, papules, and plaques on the chest and upper back, sometimes axillae [72]. Prurigo pigmentosa, which has a similar distribution to CARP but affects young Japanese females, presents with pruritic inflammatory papules that heal with reticulate hyperpigmentation [73]. Both disorders have shown response to oral minocycline [74] and other tetracyclines.
Genetic disorders with reticulate hyperpigmentation are rare [75]. They can be generalized, flexural, and acral. Genital reticulate hyperpigmentation in association with vitiligo on the same skin area has been described [71, 75].
Disorders with Later Onset Hypopigmentation and Depigmentation
Postinflammatory Hypopigmentation and Depigmentation
Although acquired partial or total pigment loss affects all skin types, it is more noticeable and troublesome for darker skinned patients. Certain skin diseases have a tendency to resolve with postinflammatory hypopigmentation, including lichen striatus and pityriasis lichenoides chronica; infections such as impetigo, pityriasis versicolor, and chicken pox; and skin injuries such as burns and cryotherapy [76]. Leukotriene C4 released during inflammation was found to reduce melanogenesis [62].
Vitiligo
Vitiligo is a common disorder of acquired depigmentation, affecting 1–2% of the world population [77]. About half of the cases occur before the age of 20 and about one-fourth of total cases occur before the age of 8. No gender or skin phototype predilection is known [77, 78]. The underlying pathogenesis includes autoimmunity, genetic, neurologic, and biochemical events resulting in destruction of melanocytes [77,78,79]. Hypopigmented and depigmented macules or patches with focal, generalized, or segmental distribution is observed [77]. Trichrome vitiligo represents the combination of hypopigmentation, depigmentation, and normal skin pigment in the same patient [80]. Treatment of childhood vitiligo remains challenging; about one-third of patients do not respond to treatment [77,78,79]. Topical treatment is preferred in childhood vitiligo if less than 20% of body surface area (BSA) is involved, and includes topical steroids, topical calcineurin inhibitors, and calcipotriol. A combination of these may yield a better response in patients who have failed monotherapy [78]. Camouflage is a good option for small lesions or when side effects are a concern. Other treatment options include narrow-band UVB, excimer laser, surgical approaches including epidermal autotransplantation, intralesional steroid injection, and total depigmentation for the most extensive involvement [78, 79]. Many of these are difficult to perform on children without sedation.
Pityriasis Alba
Pityriasis alba is a common skin disorder affecting children 3–16 years of age, with higher incidence reported in patients of color and the atopic population [81,82,83]. It may be a mild form of atopic dermatitis, with spontaneous relapse and remission. Clinically, it can present with subtly erythematous and scaly, poorly defined small patches, typically on the cheeks, then hypopigmented patches with minimal to no scale [83]. Most patients present with poorly defined hypopigmentation and deny preceding inflammation. Differential diagnosis includes postinflammatory hypopigmentation and pityriasis versicolor. Photoprotection, emollients, topical tacrolimus [84], and low potency topical steroids may improve pityriasis alba [81].
Progressive Macular Hypomelanosis
Progressive macular hypomelanosis manifests as hypopigmented, non-scaly macules coalescing over the trunk. It usually affects adolescents, with no gender predilection [85]. Pathology shows only reduced melanin content compared to normal skin. The etiology remains unknown, although a Propionibacterium species is thought to play a role, causing red fluorescence under Wood’s lamp and in some cases improving after treatment with benzoyl peroxide 5% gel with or without clindamycin 1% lotion [86,87,88]. Other treatment options include psoralen and Ultraviolet A (PUVA), narrow-band Ultraviolet B, and oral tetracycline derivatives [85, 87]. It is important to counsel the patient regarding the course and the outcome of the disease, as recurrence after successful treatment is often reported, and it may take up to 5 years before spontaneous regression, if any, is observed [85].
Clear Cell Papulosis
Clear cell papulosis is a rare entity predominantly affecting Asian children, between 4 months and 5 years of age. It presents with multiple asymptomatic non-scaly hypopigmented macules or thin papules on the lower abdomen and tends to follow mammary lines [89,90,91,92]. Pathology shows normal basal keratinocytes admixing with larger keratinocytes characterized by pale cytoplasm that stain positive for cytokeratin 7, EMA, CEA, and AE; therefore, some authors consider it a benign form of extramammary Paget disease [93]. Not all cases display reduced basal pigmentation, so the exact etiology of hypopigmentation remains unknown. Spontaneous regression has been reported [93].
Dermatitis
Atopic Dermatitis
Atopic dermatitis (AD) is the most common pediatric skin disorder; more than two-thirds of patients present before the age of 5 years [94, 95]. Genetic predisposition, barrier dysfunction, and cytokine signaling along with immunoglobulin switching are thought to contribute to etiology, and interestingly, recent studies have concluded that pediatric AD differs from adult AD in their pathophysiology [96, 97]. For example, pediatric AD patients display increased expression of CD69 on T cells soon after CD4 and CD8 are activated, but lower levels of IL-22, which increases susceptibility to infection [97]. IL-22 is believed to induce epidermal hyperplasia, suggesting that it plays an important role in chronic lesions, seen more in adults than in children with AD. About two-thirds of pediatric patients outgrow their AD within 10 years, likely due to upregulation of TH1 and TH17, resulting in decreased TH2 and TH22 levels [97].
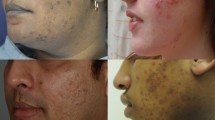
African American (AA) patients reportedly have a higher incidence rate of AD, and of greater severity, compared to Asians and Caucasians [96]. Although studies on the structure and function of ethnic skin are limited and somewhat controversial, with conflicting results, some have shown that AA patients have the lowest ceramide-to-cholesterol ratio in the stratum corneum, higher transepidermal water loss, lower response to capsaicin, and decreased itch signaling due to lower pH levels in their skin [95, 98]. Genetic susceptibility also seems to differ in AA patients. Fewer AA patients have filaggrin mutations than their Caucasian counterparts, where filaggrin mutation is linked to AD susceptibility and severity [95]. Caucasian AD patients tend to develop eczematous plaques on extremity flexor surfaces while AA patients tend to alternatively or additionally develop discrete follicular papules, prurigo nodularis and lichen simplex chronicus or lichen planus-like lesions on the extensor extremities (Fig. 17.3). The infraorbital crease (commonly referred to as Dennie–Morgan lines) is a minor feature of AD often seen in AA and Asian patients, but is common among darker skinned children regardless of atopy, so its presence should not be overemphasized in diagnosing AD in these patients [99].
Clinicians should tailor management toward individual symptoms and severity of skin findings (including degree of lichenification or presence of prurigo nodules), as well as comorbidities such as food and environmental allergies or superinfections. First-line therapy always involves optimization of baseline skin care using bland, hypoallergenic emollients, and cleansing regimens, and the safe but effective use of topical corticosteroids. Alternative or adjunctive strategies include wet wraps, antihistamines, calcineurin inhibitors, other nonsteroidal anti-inflammatory topicals, nb-UVB phototherapy, and systemic immunosuppressants [94, 95]. Phototherapy and systemic medications are reserved for severe, recalcitrant cases. Cyclosporine is often used as a first-line systemic agent due to its relatively rapid effect, and stopped upon resolution of clinical flaring or after transitioning to safer long-term systemic agents such as methotrexate, azathioprine, or mycophenolate mofetil [100, 101]. Several biologics are in clinical trials at the time of this printing.
Treatment of pediatric AD patients is the same regardless of skin pigmentation; however, while managing AA patients with AD, a few points should be considered. Hypopigmentation is usually postinflammatory but patient/parent concerns over corticosteroid-related hypopigmentation may affect compliance. Thicker plaques usually require more potent corticosteroids or topical treatment under occlusion [95]. Risk of azathioprine toxicity is higher in the AA population due to altered dosing requirements; there is reportedly a 20–30% decreased oral bioavailability and 17% reduction in thiopurine methyltransferase activity in these patients [102]. And finally, higher doses of nb-UVB may be needed to treat AA patients with AD [95].
Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) in children is not uncommon and may even occur in early infancy [103]. Females are affected more than males [104]. Nickel remains the number one antigenic culprit; other common allergens include fragrance mix, lanolin, thimerosal, and neomycin [103, 104]. ACD in skin of color may be missed by clinicians unfamiliar with cultural practices, such as henna body art and early skin piercing, and undertreated by those unfamiliar with lichenification or lichenoid dermatitis as features of ACD in skin of color, or those who do not look for subtle erythema in pigmented skin [6]. Intricate skin art using traditional red henna (leaves of the plant Lawsonia inermis crushed into essential oils) is common in the Indian subcontinent and the Middle East, but ACD to red henna is rare; allergic contact dermatitis is more often caused by adulterated products termed ‘black henna’ in which p-phenylenediamine (PPD) is admixed with red henna [105]. Keloidal lesions, an erythema multiforme-like targetoid reaction, bullous, and generalized dermatitis due to black henna have been reported [105]. Patch testing may be needed to confirm a contact allergen [104], but patients should be cautioned about dyspigmentation in patch test sites. Education, allergen avoidance, and topical corticosteroids or steroid sparing agents for active dermatitis are all important in the management of ACD [106].
Seborrheic Dermatitis
Seborrheic dermatitis (SD) is a very common skin disorder that affects patients of all ages [107]. Incidence in infants reaches 42% [108]. Malassezia furfur overgrowth and/or hypersensitivity are thought to contribute to the etiology of SD, especially in adults, but the etiology of infantile SD remains uncertain; metabolic disturbance of essential fatty acids is assumed to play a role in the pathogenesis [109] while other studies have concluded that Pityrosporum ovale is likely to be the cause of infantile SD [110]. SD presents with nonpruritic, scaly, erythematous lesions on the scalp, face (eyebrows and paranasal skin), retro-auricular folds, flexures, and diaper region [107]. In dark-skinned patients, the erythema is not easily distinguished, and patients may present with scalp dandruff and hyperkeratosis that can be mistaken for tinea capitis, atopic dermatitis, or scalp psoriasis [111]. Also, they may present with hyper- or hypopigmented scaly lesions. Topical preparations are usually used to treat infantile SD, e.g., mineral oil or bland emollients to loosen scale, 2% ketoconazole shampoo, and topical steroids used with caution to prevent systemic absorption or atrophy [110].
Papulosquamous Disorders
Psoriasis
Psoriasis is a common chronic inflammatory skin disorder that affects children with an annual incidence of 40.8/100,000 found in one study of the US Midwest [112]. Similar to adults, the most common type of psoriasis in children is plaque-type psoriasis [113, 114]. Guttate, inverse, palmoplantar, and pustular psoriasis are also seen in pediatric patients [114, 115]. Guttate psoriasis is more often seen in children, tends to follow streptococcal infection more often than in plaque-type pediatric psoriasis, and tends to predict more severe psoriatic disease [116]. Scalp psoriasis was found to be more prevalent in girls while nail psoriasis was more common among boys. Nail psoriasis does not correlate with disease severity in pediatric patients, in contrast to what is observed in adults [113, 115]. Darker skin pigmentation may complicate the assessment of psoriasis severity beyond the greater difficulty observing erythema; one study found thicker plaques and more dyspigmentation in psoriasis in patients of color as compared to Caucasians [117]. Also, scalp psoriasis lesions in dark-skinned children may be more hyperkeratotic than in patients with lighter skin [111].
Pediatric psoriasis is generally treated with emollients, keratolytics, corticosteroids, and steroid sparing agents [118]. No standard guidelines exist for phototherapy, systemic, and biologic drugs for pediatric psoriasis [118]; many protocols are derived from adult psoriasis treatment regimens and other specialists’ experience with biologics and systemic drugs used to treat children with rheumatoid arthritis, hematological malignancies, and Crohn’s disease [118,119,120]. Psychological support is crucial for children with psoriasis, given that it is associated with obesity, hypertension, diabetes mellitus, and psychosocial stress [118, 121].
Pityriasis Rosea
Pityriasis rosea (PR) is a papulosquamous skin disorder of uncertain etiology that affects young individuals worldwide [122, 123]. It may be associated with human herpes virus 6 and 7. PR typically manifests as mildly pruritic erythematous thin papules and plaques with few scales on the trunk, erupting within a few weeks of the appearance of a herald patch—a single, relatively larger patch or plaque with raised scaly margins [122]. Atypical variants have been described, including inverse, unilateral, erythema multiforme-like, and generalized papular and papulovesicular PR [124,125,126,127]. Case reports and studies of dark-skinned individuals with this eruption have reported reduced or absent erythema, thicker scale, and significant itching in comparison to PR in light-skinned patients [123]. In addition, generalized papular, follicular, and erythema multiforme-like variants were noticed to be more prevalent among dark-skinned and Indian patients [124, 126, 127]. Facial distribution is also observed among African Americans with pityriasis rosea [123, 125]. About two-thirds of dark-skinned patients with PR heal with hyperpigmentation while postinflammatory hypopigmentation tends to be noticed after the papular variant resolves [123]. Pityriasis rosea tends to heal spontaneously; treatment is mainly to control the itching using antihistamines, emollients, and topical steroids. Phototherapy, systemic antibiotics, and antivirals have been used in severe and recalcitrant cases with different results [123, 128, 129]. The dyspigmentation left by this benign condition can be very obvious and quite upsetting to patients and parents.
Lichen Planus
Lichen planus (LP) is a chronic pruritic skin disorder that mainly affects adults [130]; however, studies from different countries have shown increasing rates among children, reaching ~11% of cases [130,131,132,133,134]. In one study of 36 children from the United States, childhood LP affected females twice as often as males, and 72% were African American [135]. Childhood lichen planus is not uncommon in India, Middle East, and Mexico [131, 133, 135]; a retrospective study in the United Kingdom showed that more than two-thirds of the children with lichen planus came from Indian descent [134]. The exact etiology remains unknown and many theories including autoimmune, infectious, genetic, Hepatitis B infection, and/or vaccination triggers have been suggested [130, 136]. Classic LP is the most common presentation of childhood lichen planus [135], but linear, annular, mucosal, bullous, and lichen planopilaris subtypes are also reported [133, 135, 137, 138]. In darker skinned patients, lichen planus lesions tend to be gray in color rather than violaceous, with persistent and severe postinflammatory hyperpigmentation [139]. Actinic lichen planus predominates in tropical areas [130, 132]. Treatment of childhood lichen planus is mainly achieved by the use of topical corticosteroids under occlusion and topical tacrolimus. Systemic drugs and phototherapy are reserved for eruptive, bullous, and recalcitrant cases [130, 138].
Acneiform Eruptions
Acne Vulgaris
Acne vulgaris is one of the most common skin disorders, affecting patients from all ethnic backgrounds and skin pigmentation [7, 140,141,142]. Due to the practice of applying heavy oils and thick pomades to their hair, African American patients tend to have more comedonal acne on the forehead, also known as pomade acne [143]. Newer trends in African American hair care practices such as chemical relaxation led to a reduction in the use of thick hair oils, eventually decreasing the incidence of pomade acne [139]. Postinflammatory hyperpigmentation is very common among patients with darker skin, and the application of cosmetic products used to cover the hyperpigmentation can lead to acne cosmetica among those groups [140,141,142]. Treatment of acne vulgaris in skin of color does not differ from that for other ethnic groups; however, patient education and counseling are crucial, as well as sensitivity toward the psychological impact of the hyperpigmentation and/or acne-related keloidal scarring [141, 144].
Periorificial Dermatitis
Periorificial dermatitis is an eruption of erythematous, flesh-colored papules in association with skin desquamation and rare pustules around the mouth, eyes, nose, and occasionally the vulva or perineum [145,146,147]. Topical corticosteroid application may play a role in the pathogenesis [145]. Histology may reflect a granulomatous dermatitis, as seen in reports of childhood granulomatous periorificial dermatitis, sarcoid-like granulomatous dermatitis, and facial Afro-Caribbean childhood eruption [145, 147]. Treatment includes discontinuing steroid application, topical 0.75% metronidazole [148], topical calcineurin inhibitors, and mupirocin [145]. Oral antibiotics such as tetracycline, doxycycline, and erythromycin have been used [145, 147].
Hair Disorders
Human hair can be categorized based on sulfur protein content, keratin type, and amino acid composition, but it is generally classified based on ethnic background, differentiating Caucasian, Asian and African American (AA) hair. AA hair shafts are elliptical in cross section, with a variable diameter along the hair shaft, and also characterized by random reversals and twisting. AA hair follicles are curved, with less elastic fiber content attaching it to the dermis, resulting in spiral, curly hair and higher risk for hair shedding and breakage [149]. AA hair dryness is due to decreased sebaceous gland activity and lower moisture content compared with other ethnic groups [149].
Trichorrhexis Nodosa
Trichorrhexis Nodosa is a congenital or acquired hair shaft disorder. Congenital cases are seen among various genetic diseases including trichothiodystrophy and Menkes kinky hair syndrome, while acquired cases are caused by excessive heat, forceful combing and prolonged chemical use. Patients usually present with complaints of brittle, dull hair appearance, and white spots along the hair shaft. Microscopic hair shaft exam reveals what looks like two paintbrushes facing each other, which represent the whitish dots along the hair shaft. Treatment of acquired trichorrhexis nodosa is mainly through reducing chemical and physical hair shaft trauma [149].
Traction Alopecia
Traction is a common cause of hair loss that occurs as a result of prolonged tension applied on the hair, leading to clinical or subclinical inflammation of the hair follicles. It usually starts during childhood and might progress to permanent loss of the hair follicles. Traction alopecia predominantly affects AA females, with onset reportedly as young as 8 months of age. In one clinic population the prevalence of traction alopecia in AA females between the ages of 5 and 14.5 years was 18% [150]. Another study showed that African schoolchildren with traction alopecia increased from 8.6% in the first year of school to 21.7% in the last year of high school [151]. Traction alopecia is usually marginal frontal and/or temporal, with the presence of fringe hair. Hair casts in a biopsy indicate ongoing traction alopecia. Treatment is mainly through prevention and education.
Tinea Capitis
Tinea capitis is the most common fungal infection affecting the pediatric population. AA children have the highest incidence rate, in males more than females. Trichophyton tonsurans is an anthropophilic organism; it remains the most common causative agent of tinea capitis in North America. Microsporum canis is the most frequent cause of zoophilic tinea capitis [152]. In AA patients, tinea capitis may manifest as non-inflammatory disease with patches of hair loss, hyperkeratosis, or breakage of the hair shaft at the scalp surface (black dot tinea). Another clinical presentation is kerion, a tender, boggy, sometimes purulent plaque in a patient who may also have fever, malaise, and occipital lymphadenopathy. Bright green fluorescence with Wood’s lamp is seen only in ectothrix fungi such as M. canis. It is negative in the presence of T. tonsurans, an endothrix infection. Dermoscopic examination reveals comma-shaped and corkscrew hair shafts. Hair culture is negative in up to 50% of cases [111, 153] so clinical features and history are important. Differential diagnoses of non-inflammatory tinea capitis with scalp hyperkeratosis include seborrheic dermatitis and atopic dermatitis, or rarer cause such as collagen vascular diseases and Langerhans cell histiocytosis, where erythema is difficult to appreciate in dark skin [111].
Tinea capitis is frequently undertreated and physicians tend to prescribe low doses of antifungal medication, resulting in frequent relapses once treatment is discontinued. While terbinafine, fluconazole, and pulsed itraconazole can be used for shorter or more convenient regimens, griseofulvin is still widely used by pediatric dermatologists due to its cost and safety profile. In cases of M. canis fungal infection, a longer duration of therapy is usually required [153].
Piedra
Piedra is a superficial fungal infection characterized by easily detachable gritty white or yellowish nodules (white piedra) or black nodules (black piedra) on hair shafts, usually of the axilla or scalp [154–156]. Black piedra is caused by Piedraia hortae while white piedra is caused by Trichosporon species. Most reported cases in the United States have been in immigrants from tropical regions [155]. Piedra affects females more than males, although in genital hair white piedra is more prevalent in black males [156]. Humid climate, poor hygiene, and the practice of veil hair covering have been implicated in the pathogenesis [157, 158]. They present easily detachable gritty nodules on the scalp, facial, or genital hair [154,155,156]. Diagnosis is based on the clinical presentation, KOH, and fungal cultures [152, 153]. Treatment is mainly achieved through hair shaving, although this is usually not desirable for scalp involvement. Oral azoles and topical antifungal shampoos have been successfully used with variable reported recurrence rates [154,155,156,157].
Vesicular and Pustular Diseases in Newborns
Transient neonatal pustular melanosis and infantile acropustulosis are seen more often in skin of color. Conversely, miliaria is less common, due to more efficient body temperature regulation with less sweating compared to white skin [6].
Transient Neonatal Pustular Melanosis
Transient neonatal pustular melanosis (TNPM) predominantly affects black newborns with a 4.4–15% incidence rate [6, 159, 160]. It usually presents at birth as innumerable, superficial pustules without surrounding erythema. Pustular lesions last up to 2 days, and then heal leaving delicate collarettes of scale around hyperpigmented macules, which take from 2 weeks to months to fade [159]. Pustules may not be seen due to disruption perinatally. An infant born with multiple hyperpigmented macules that fade away over weeks to months (unlike true lentigines that do not fade) can be presumed to have had TNPM pustules in utero [160]. A smear of pustular contents yields abundant neutrophils with rare eosinophils and Gram’s stain is negative (ruling out staphylococcus bullous impetigo) [6]. Biopsy is rarely necessary for diagnosis but shows corneal and subcorneal pustules with abundant neutrophils and rare eosinophils, while hyperpigmented macular lesions show basal layer hyperpigmentation and hyperkeratosis [6, 159]. The main differential diagnosis is erythema toxicum neonatorum (ETN), which can be differentiated by the presence of erythema surrounding the pustules, although this may be difficult to recognize in darkly pigmented infants [6]. In ETN, eosinophils predominate on smear and histopathologic exams [6, 160].
Infantile Acropustulosis
Infantile acropustulosis (IA) is a rare disorder that primarily affects black males 2–10 months of age [6, 159]. It presents with recurrent 1–3 mm acral vesicles and pustules that appear in crops, occasionally at non-acral sites [160]. Lesions are intensely pruritic, last for 1–2 weeks, and then spontaneously remit until the next crop of lesions appears. IA eventually resolves within 2–3 years [159]. It is frequently misdiagnosed as scabies; other differential diagnoses include dyshidrotic eczema, hand–foot–mouth disease, transient neonatal pustular melanosis, and erythema toxicum neonatorum [159, 161]. If biopsied, histopathologic exam reveals subcorneal and intraepidermal neutrophilic pustules, mild perivascular lymphohistiocytic infiltrate and rare eosinophils [160]. Treatment includes topical steroids and antihistamines for intense pruritus [160, 162]. IA has been successfully treated with dapsone 2 mg/kg/day with tapering over the following few months to prevent relapse [6, 162].
Pediatric Malignancies
Skin cancers are rare in children. Most of the malignancies in darker skinned patients are described in the literature as case reports. Hypopigmented mycosis fungoides (MF) is more prevalent in patients of color. It has a chronic course that rarely progresses and responds well to topical steroids and phototherapy; however, recurrence after successful treatment is often seen [163]. Malignant melanoma has been reported within nevus of Ito [27] and nevus of Ota [25]. Malignant blue nevus arising within giant blue nevus in an infant has been described [28]. Childhood subungual malignant melanoma in situ is rare; no case of invasive subungual melanoma has been reported in children [47]. It has been argued that these reported cases of childhood subungual malignant melanoma in situ may in fact have been atypical melanocytic nevi because pediatric pigmented lesions in general display more atypia compared to lesions in adults [48].
Conclusion and Psychosocial Considerations
For the pediatric patient of color, skin conditions that alter pigmentation primarily, such as vitiligo or albinism [164, 165] and birthmarks (Figs. 17.4 and 17.5), or secondary to inflammation, as in atopic dermatitis, psoriasis, or ichthyosis, can significantly impair quality of life [165]. Devastating effects have been recorded cross-culturally and interracially. Children with visible differences are vulnerable to discrimination and bullying [166]. They often face other social challenges involving peer relationships during games and sports, as well as reduced academic performance [165, 167, 168]. These incidents influence future social engagement; fear of reliving a traumatizing event sometimes results in “safety-seeking behaviors” such as concealing the affected area with clothing or camouflage make-up, and avoiding activities that require skin exposure, such as swimming [169]. In addition, these conditions have an impact upon confidence, self-consciousness, shyness, and general happiness [165, 167, 168, 170, 171]. If prolonged, negative social and psychological experiences may precipitate mental illness; Picardi et al. found that 25% of patients with diverse skin conditions experience significant psychological distress and psychiatric morbidity [172]. While some children and young adults with skin conditions are remarkably confident and resilient, others may experience depressive symptoms, true clinical depression, anxiety, and suicidal ideation [165, 168, 173,174,175,176,177,178]. It is important to keep in mind that a clinician’s objective assessment of a patient’s skin condition does not correlate with the patient’s self-perception and distress [179].
In children with atopic dermatitis there is a direct relationship between dermatologic disease severity and psychological disturbances [176]. Maladaptive effects may be due to a combination of the physical lesions, the signature pruritus, and secondary dyspigmentation. In some cultures, postinflammatory hyperpigmentation on the neck is sometimes called “atopic dirty neck,” which adds the misperception of poor hygiene to the already challenging social experience of this condition [180]. Chronic pruritus can also cause fussiness, irritability, and significant problems with sleep and concentration [176, 181].
Appearance can represent one’s public representation of his or her personal self in society, and it is often heuristically linked to membership within social and cultural groups [182]. This association creates another challenge for children of color: among African Americans with vitiligo, the hypopigmentation can be misinterpreted as “turning white” [183]. Vitiligo and albinism can thus threaten racial identity and cultural membership.
Psychiatric comorbidities can complicate dermatologic disease when each condition exacerbates the other [178]. Furthermore, parental coping, mental health, and skin caretaker ability are critical in pediatric dermatology [184, 185]. Thus, multidisciplinary healthcare initiatives and patient- and family-centered interventions that reduce psychosocial stress, increase self-acceptance, and strengthen protective relationships with peers and family have the potential to significantly improve health outcomes.
References
Jablonski NG. The evolution of human skin colouration and its relevance to health in the modern world. J R Coll Physicians Edinb. 2012;42(1):58–63.
Chaplin G. Geographic distribution of environmental factors influencing human skin coloration. Am J Phys Anthropol. 2004;125(3):292–302.
Campbell MC, Tishkoff SA. The evolution of human genetic and phenotypic variation in Africa. Curr Biol. 2010;20(4):R166–73.
Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–33.
Bryc K, et al. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96(1):37–53.
Brauner GJ. Cutaneous disease in black children. Am J Dis Child. 1983;137(5):488–96.
Henderson MD, et al. Skin-of-color epidemiology: a report of the most common skin conditions by race. Pediatr Dermatol. 2012;29(5):584–9.
Shih IH, et al. A birthmark survey in 500 newborns: clinical observation in two northern Taiwan medical center nurseries. Chang Gung Med J. 2007;30(3):220–5.
McLaughlin MR, O’Connor NR, Ham P. Newborn skin: Part II. Birthmarks. Am Fam Physician. 2008;77(1):56–60.
Kahana M, et al. The incidence of birthmarks in Israeli neonates. Int J Dermatol. 1995;34(10):704–6.
Alper JC, Holmes LB. The incidence and significance of birthmarks in a cohort of 4,641 newborns. Pediatr Dermatol. 1983;1(1):58–68.
Bolognia J, Orlow SJ. Melanocyte biology. In: Bolognia J, Jorizzo JL, Schaffer JV, editors. Dermatology. Philadelphia: Elsevier Saunders; 2012.
Leung AKC, Robson WLM. Superimposed Mongolian Spots. Pediatr Dermatol. 2008;25(2):233–5.
Dermal melanocytosis (nevus of Ito) and concurrent cellular neurothekeoma. J Am Acad Dermatol. 2015;72(5, Supplement 1):AB83.
Chaithirayanon S, Chunharas A. A survey of birthmarks and cutaneous skin lesions in newborns. J Med Assoc Thai. 2013;96(Suppl 1):S49–53.
Gupta D, Thappa DM. Mongolian Spots—a prospective study. Pediatr Dermatol. 2013;30(6):683–8.
Mimouni-Bloch, A., et al. Extensive Mongolian Spots and lysosomal storage diseases. J Pediatr. 2016;170: 333-333.e1.
Yang T, Jiang X. Three cases of symmetrical nevus of Ota and a brief literature review. Int J Dermatol. 2016;55(7):e404–6.
Bilateral nevus of Ota and nevus of Ito. J Am Acad Dermatol. 2013;68(4, Supplement 1):AB150.
Shields JA, et al. Choroidal melanoma in a black patient with oculodermal melanocytosis. Retina. 2002;22(1):126–8.
Infante de German-Ribon R, et al. Choroidal melanoma with oculodermal melanocytosis in Hispanic patients. Am J Ophthalmol. 1999;128(2):251–3.
Goldman-Levy G, et al. Primary melanoma of the leptomeninges with bap1 expression-loss in the setting of a nevus of ota: a clinical, morphological and genetic study of 2 cases. Brain Pathol. 2016;26(4):547–50.
Franceschini D, Dinulos JG. Dermal melanocytosis and associated disorders. Curr Opin Pediatr. 2015;27(4):480–5.
Bisceglia M, et al. Nevus of Ota. Presentation of a case associated with a cellular blue nevus with suspected malignant degeneration and review of the literature. Pathologica. 1997;89(2):168–74.
Lindsey SF, et al. Malignant melanoma from a nevus of Ota in a pediatric patient with fatal outcome. J Am Acad Dermatol. 2013;69(4):e195–7.
Trindade F, Santonja C, Requena L. Bilateral nevus of Ito and nevus spilus in the same patient. J Am Acad Dermatol. 2008;59(2, Supplement):S51–2.
Wise SR, et al. Malignant melanoma transformation within a nevus of Ito. J Am Acad Dermatol. 2010;62(5):869–74.
Nakamura Y, et al. Malignant blue nevus arising in a giant congenital cellular blue nevus in an infant. Pediatr Dermatol. 2012;29(5):651–5.
Popovic M, et al. Childhood malignant blue nevus of the ear associated with two intracranial melanocytic tumors-metastases or neurocutaneous melanosis? Hum Pathol. 2004;35(10):1292–6.
Rabinovits HS, Barnhill RL. Benign Melanocytic Neoplasm. In Bolognia J, Jorizzo JL, Schaffer JV, editors. Dermatology. Philadelphia: Elsevier Saunders. 2012.
Shah KN. The diagnostic and clinical significance of cafe-au-lait macules. Pediatr Clin North Am. 2010;57(5):1131–53.
Landau M, Krafchik BR. The diagnostic value of café-au-lait macules. J Am Acad Dermatol. 1999;40(6):877–90.
Kalter DC, Griffiths WA, Atherton DJ. Linear and whorled nevoid hypermelanosis. J Am Acad Dermatol. 1988;19(6):1037–44.
Torchia D, Happle R. Segmental hypomelanosis and hypermelanosis arranged in a checkerboard pattern are distinct naevi: flag-like hypomelanotic naevus and flag-like hypermelanotic naevus. J Eur Acad Dermatol Venereol. 2015;29(11):2088–99.
Happle R, Franco-Guío MF, Santacoloma-Osorio G. Phylloid hypermelanosis: a cutaneous marker of several different disorders? Pediatr Dermatol. 2014;31(4):504–6.
Ruiz-Maldonado R, et al. Hypomelanosis of Ito: diagnostic criteria and report of 41 cases. Pediatr Dermatol. 1992;9(1):1–10.
Ruggieri M, Pavone L. Hypomelanosis of Ito: clinical syndrome or just phenotype? J Child Neurol. 2000;15(10):635–44.
Sybert VP, et al. Pigmentary abnormalities and mosaicism for chromosomal aberration: association with clinical features similar to hypomelanosis of Ito. J Pediatr. 1990;116(4):581–6.
Di Lernia V. Linear and whorled hypermelanosis. Pediatr Dermatol. 2007;24(3):205–10.
Ertam I, et al. Linear and whorled nevoid hypermelanosis: dermatoscopic features. J Am Acad Dermatol. 2009;60(2):328–31.
James WD, Carter JM, Rodman OG. Pigmentary demarcation lines: a population survey. J Am Acad Dermatol. 1987;16(3 Pt 1):584–90.
Somani VK, Razvi F, Sita VN. Pigmentary demarcation lines over the face. Indian J Dermatol Venereol Leprol. 2004;70(6):336–41.
Sarma N, Chakraborty S, Bhattacharya SR. Acquired, idiopathic, patterned facial pigmentation (AIPFP) including periorbital pigmentation and pigmentary demarcation lines on face follows the Lines of Blaschko on face. Indian J Dermatol. 2014;59(1):41–8.
Cho E, et al. Type B pigmentary demarcation lines of pregnancy involving the anterior thighs and knees. Ann Dermatol. 2012;24(3):348–50.
Garg G, Bhalla M, Thami GP. Transient infantile patterned hyperpigmentation. Pediatr Dermatol. 2012;29(3):372–3.
Fosse N, Itin P. Pigmentary lines of the newborn: a case report and review of the literature. Dermatology. 2014;228(3):198–201.
Antonovich DD, Grin C, Grant-Kels JM. Childhood subungual melanoma in situ in diffuse nail melanosis beginning as expanding longitudinal melanonychia. Pediatr Dermatol. 2005;22(3):210–2.
Cooper C, et al. A clinical, histopathologic, and outcome study of melanonychia striata in childhood. J Am Acad Dermatol. 2015;72(5):773–9.
Viana AC, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. An Bras Dermatol. 2013;88(6):863–78.
Ibrahimi OA, Alikhan A, Eisen DB. Congenital melanocytic nevi: Where are we now?: Part II. Treatment options and approach to treatment. J Am Acad Dermatol. 2012;67(4):515.e1–13.
Permatasari F, Zhou BR, Luo D. Late-onset acquired dermal melanocytosis on the hand of a Chinese woman. Indian J Dermatol Venereol Leprol. 2013;79(2):269.
Mataix J, et al. Late-onset Ito’s nevus: an uncommon acquired dermal melanocytosis. J Cutan Pathol. 2007;34(8):640–3.
Choi JC, et al. Progressive cribriform and zosteriform hyperpigmentation—the late onset linear and whorled nevoid hypermelanosis. J Eur Acad Dermatol Venereol. 2005;19(5):638–9.
Book SE, Glass AT, Laude TA. Congenital Becker’s nevus with a familial association. Pediatr Dermatol. 1997;14(5):373–5.
Pahwa P, Sethuraman G. Segmental Becker’s Nevi with mucosal involvement. Pediatr Dermatol. 2012;29(5):670–1.
Jones AC, Ford MJ. Simultaneous occurrence of segmental odontomaxillary dysplasia and Becker’s nevus. J Oral Maxillofac Surg. 1999;57(10):1251–4.
Baeta IG, et al. Becker’s nevus syndrome: case report. An Bras Dermatol. 2010;85(5):713–6.
Lapidoth M, et al. Hypertrichosis in Becker’s nevus: effective low-fluence laser hair removal. Lasers Med Sci. 2014;29(1):191–3.
Nanni CA, Alster TS. Treatment of a Becker’s nevus using a 694-nm long-pulsed ruby laser. Dermatol Surg. 1998;24(9):1032–4.
Glaich AS, et al. Fractional resurfacing: a new therapeutic modality for Becker’s nevus. Arch Dermatol. 2007;143(12):1488–90.
Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(2 Suppl Understanding):S41–62.
Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(6, Supplement):S143–8.
Cardinali G, Kovacs D, Picardo M. Mechanisms underlying post-inflammatory hyperpigmentation: lessons from solar lentigo. Ann Dermatol Venereol. 2012;139(Suppl 4):S148–52.
Magaña M, Herrera-Goepfert R. Friction hypermelanosis: other variants. J Am Acad Dermatol. 2002;47(3):454.
Naimer SA, et al. Davener’s dermatosis: a variant of friction hypermelanosis. J Am Acad Dermatol. 2000;42(3):442–5.
Ciliberto H, et al. Heel-line hyperpigmentation: a variant of sock-line hyperpigmentation after the use of heel-length socks. Pediatr Dermatol. 2013;30(4):473–5.
Diamond G, Ben D. Amitai, Orphan rocker tracks: a variant of friction melanosis in an institutionalized child. Pediatr Dermatol. 2013;30(6):e198–9.
Jang K-A, et al. Idiopathic eruptive macular pigmentation: report of 10 cases. J Am Acad Dermatol. 2001;44(2, Part 2):351–3.
Rodríguez VG, et al. Idiopathic eruptive macular pigmentation: a diagnostic challenge. J Am Acad Dermatol. 2011;64(2, Supplement 1):AB132.
Abbas O. Asymptomatic hyperpigmented macules. Pediatr Dermatol. 2015;32(5):733–4.
Chang MW. Disorders of hyperpigmentation. In: Bolognia J, Jorizzo JL, Schaffer JV, editors. Dermatology. Philadelphia: Elsevier Saunders. 2012. p. 1 online resource (2 v. in 1).
Wiesenborn A, Hengge U, Megahed M. Confluent and reticulated papillomatosis. Gougerot-Carteaud disease. Hautarzt. 2004;55(10):976–8.
Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16(6):533–43.
Ilkovitch D, Patton TJ. Is prurigo pigmentosa an inflammatory version of confluent and reticulated papillomatosis? J Am Acad Dermatol. 2013;69(4):e193–5.
Alfadley A, et al. Reticulate acropigmentation of Dohi: a case report of autosomal recessive inheritance. J Am Acad Dermatol. 2000;43(1, Part 1):113–7.
Vachiramon V, Thadanipon K. Postinflammatory hypopigmentation. Clin Exp Dermatol. 2011;36(7):708–14.
Phiske MM. Childhood vitiligo. Curr Rheumatol Rev. 2015.
Tamesis MEB, Morelli JG. Vitiligo treatment in childhood: a state of the art review. Pediatr Dermatol. 2010;27(5):437–45.
Ezzedine K, Silverberg N. A practical approach to the diagnosis and treatment of vitiligo in children. Pediatrics. 2016;138(1).
Hann SK, et al. Clinical and histopathologic characteristics of trichrome vitiligo. J Am Acad Dermatol. 2000;42(4):589–96.
Miazek N, et al. Pityriasis alba—common disease, enigmatic entity: up-to-date review of the literature. Pediatr Dermatol. 2015;32(6):786–91.
Jadotte YT, Janniger CK. Pityriasis alba revisited: perspectives on an enigmatic disorder of childhood. Cutis. 2011;87(2):66–72.
Blessmann Weber M, et al. Pityriasis alba: a study of pathogenic factors. J Eur Acad Dermatol Venereol. 2002;16(5):463–8.
Rigopoulos D, et al. Tacrolimus ointment 0.1% in pityriasis alba: an open-label, randomized, placebo-controlled study. Br J Dermatol. 2006;155(1):152–5.
Martínez-Martínez ML, et al. Progressive macular hypomelanosis. Pediatr Dermatol. 2012;29(4):460–2.
Cavalcanti SM, et al. The use of lymecycline and benzoyl peroxide for the treatment of progressive macular hypomelanosis: a prospective study. An Bras Dermatol. 2011;86(4):813–4.
Kim MB, et al. Narrowband UVB treatment of progressive macular hypomelanosis. J Am Acad Dermatol. 2012;66(4):598–605.
Cavalcanti SM, et al. Investigation of Propionibacterium acnes in progressive macular hypomelanosis using real-time PCR and culture. Int J Dermatol. 2011;50(11):1347–52.
Taylor S, Kircik L. Community-based trial results of combination acne therapy in subjects with skin of color: postinflammatory hyperpigmentation. J Am Acad Dermatol. 2008;58(2, Supplement 2):AB13.
Wysong A, Sundram U, Benjamin L. Clear-cell papulosis: a rare entity that may be misconstrued pathologically as normal skin. Pediatr Dermatol. 2012;29(2):195–8.
Tseng FW, et al. Long-term follow-up study of clear cell papulosis. J Am Acad Dermatol. 2010;63(2):266–73.
Kim SW, Roh J, Park CS. Clear cell papulosis: a case report. J Pathol Transl Med. 2016.
Sim JH, Do JE, Kim YC. Clear cell papulosis of the skin: acquired hypomelanosis. Arch Dermatol. 2011;147(1):128–9.
Eichenfield LF, et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–51.
Vachiramon V, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29(4):395–402.
Shaw TE, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73.
Czarnowicki T, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol. 2015;136(4):941–951.e3.
Wang H, et al. Ethnic differences in pain, itch and thermal detection in response to topical capsaicin: African Americans display a notably limited hyperalgesia and neurogenic inflammation. Br J Dermatol. 2010;162(5):1023–9.
Williams HC, Pembroke AC. Infraorbital crease, ethnic group, and atopic dermatitis. Arch Dermatol. 1996;132(1):51–4.
Slater NA, Morrell DS. Systemic therapy of childhood atopic dermatitis. Clin Dermatol. 2015;33(3):289–99.
Galli E, et al. Consensus conference on clinical management of pediatric atopic dermatitis. Ital J Pediatr. 2016;42:26.
McLeod HL, et al. Thiopurine methyltransferase activity in American white subjects and black subjects. Clin Pharmacol Ther. 1994;55(1):15–20.
Sharma VK, Asati DP. Pediatric contact dermatitis. Indian J Dermatol Venereol Leprol. 2010;76(5):514–20.
Mortz CG, et al. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br J Dermatol. 2001;144(3):523–32.
Almeida PJ, et al. Quantification of p-phenylenediamine and 2-hydroxy-1,4-naphthoquinone in henna tattoos. Contact Dermatitis. 2012;66(1):33–7.
Lee PW, Elsaie ML, Jacob SE. Allergic contact dermatitis in children: common allergens and treatment: a review. Curr Opin Pediatr. 2009;21(4):491–8.
Sampaio AL, et al. Seborrheic dermatitis. An Bras Dermatol. 2011;86(6):1061–71; quiz 1072–4.
Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol. 2015;3(2).
Tollesson A, Frithz A, Stenlund K. Malassezia furfur in infantile seborrheic dermatitis. Pediatr Dermatol. 1997;14(6):423–5.
Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18(1):13–26.
Silverberg NB. Scalp hyperkeratosis in children with skin of color: diagnostic and therapeutic considerations. Cutis. 2015;95(4):199–204, 207.
Tollefson MM, et al. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62(6):979–87.
Mercy K, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30(4):424–8.
Arese V, et al. Juvenile psoriasis: an epidemiological study of 69 cases in the universitary dermatology clinic in Turin. G Ital Dermatol Venereol. 2016 Jun 30 (Epub ahead of print).
Morris A, et al. Childhood psoriasis: a clinical review of 1262 cases. Pediatr Dermatol. 2001;18(3):188–98.
Conrado LA, et al. Body dysmorphic disorder among dermatologic patients: prevalence and clinical features. J Am Acad Dermatol. 2010;63(2):235–43.
McMichael AJ, et al. Psoriasis in African-Americans: a caregivers’ survey. J Drugs Dermatol. 2012;11(4):478–82.
Bronckers IM, et al. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr Drugs. 2015;17(5):373–84.
Napolitano M, et al. Systemic treatment of pediatric psoriasis: a review. Dermatol Ther (Heidelb). 2016;6(2):125–42.
Saikaly SK, Mattes M. Biologics and pediatric generalized pustular psoriasis: an emerging therapeutic trend. Cureus. 2016;8(6):e652.
Gutmark-Little I, Shah KN. Obesity and the metabolic syndrome in pediatric psoriasis. Clin Dermatol. 2015;33(3):305–15.
Gündüz Ö, Ersoy-Evans S, Karaduman A. Childhood pityriasis rosea. Pediatric Dermatol. 2009;26(6):750–1.
Amer A, Fischer H, Li X. The natural history of pityriasis rosea in black American children: how correct is the “classic” description? Arch Pediatr Adolesc Med. 2007;161(5):503–6.
Zawar V, Chuh A. Follicular pityriasis rosea. A case report and a new classification of clinical variants of the disease. J Dermatol Case Rep. 2012;6(2):36–9.
Vano-Galvan S, et al. Atypical Pityriasis rosea in a black child: a case report. Cases J. 2009;2:6796.
Sinha S, Sardana K, Garg VK. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29(4):538–40.
Das A, et al. A case series of erythema multiforme-like pityriasis rosea. Indian Dermatol Online J. 2016;7(3):212–5.
Jairath V, et al. Narrowband UVB phototherapy in pityriasis rosea. Indian Dermatol Online J. 2015;6(5):326–9.
Drago F, Rebora A. Treatments for pityriasis rosea. Skin Therapy Lett. 2009;14(3):6–7.
Kumar V, et al. Childhood lichen planus (LP). J Dermatol. 1993;20(3):175–7.
Nanda A, et al. Childhood lichen planus: a report of 23 cases. Pediatr Dermatol. 2001;18(1):1–4.
Luis-Montoya P, Dominguez-Soto L, Vega-Memije E. Lichen planus in 24 children with review of the literature. Pediatr Dermatol. 2005;22(4):295–8.
Kanwar AJ, De D. Lichen planus in children. Indian J Dermatol Venereol Leprol. 2010;76(4):366–72.
Balasubramaniam P, Ogboli M, Moss C. Lichen planus in children: review of 26 cases. Clin Exp Dermatol. 2008;33(4):457–9.
Walton KE, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27(1):34–8.
Limas C, Limas CJ. Lichen planus in children: a possible complication of hepatitis B vaccines. Pediatr Dermatol. 2002;19(3):204–9.
Kabbash C, et al. Lichen planus in the lines of Blaschko. Pediatr Dermatol. 2002;19(6):541–5.
Cohen DM, et al. Childhood lichen planus pemphigoides: a case report and review of the literature. Pediatr Dermatol. 2009;26(5):569–74.
Nnoruka EN. Lichen planus in African children: a study of 13 patients. Pediatr Dermatol. 2007;24(5):495–8.
Taylor SC, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 Suppl Understanding):S98–106.
Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3(4):24–38.
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80(5):387–94.
Arfan ul, Bari, Khan MB. Dermatological disorders related to cultural practices in black Africans of Sierra Leone. J Coll Physicians Surg Pak. 2007;17(5):249–52.
Shah SK, Alexis AF. Acne in skin of color: practical approaches to treatment. J Dermatolog Treat. 2010;21(3):206–11.
Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55(5):781–5.
Kuflik JH, Janniger CK, Piela Z. Perioral dermatitis: an acneiform eruption. Cutis. 2001;67(1):21–2.
Knautz MA, Lesher JL. Childhood granulomatous periorificial dermatitis. Pediatr Dermatol. 1996;13(2):131–4.
Rodriguez-Caruncho C, et al. Childhood granulomatous periorificial dermatitis with a good response to oral metronidazole. Pediatr Dermatol. 2013;30(5):e98–9.
Rodney IJ, et al. Hair and scalp disorders in ethnic populations. J Drugs Dermatol. 2013;12(4):420–7.
Mirmirani P, Khumalo NP. Traction alopecia: how to translate study data for public education—closing the KAP gap? Dermatol Clin. 2014;32(2):153–61.
Khumalo NP, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157(1):106–10.
Mirmirani P, Tucker L-Y. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California. J Am Acad Dermatol. 2013;69(6):916–21.
Gupta AK, et al. Tinea capitis: an overview with emphasis on management. Pediatr Dermatol. 1999;16(3):171–89.
Bonifaz A, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28(2):140–5.
Kiken DA, et al. White piedra in children. J Am Acad Dermatol. 2006;55(6):956–61.
Kalter DC, et al. Genital white piedra: epidemiology, microbiology, and therapy. J Am Acad Dermatol. 1986;14(6):982–93.
Viswanath V, et al. White piedra of scalp hair by Trichosporon inkin. Indian J Dermatol Venereol Leprol. 2011;77(5):591–3.
Desai DH, Nadkarni NJ. Piedra: an ethnicity-related trichosis? Int J Dermatol. 2014;53(8):1008–11.
Van Praag MCG. et al. Diagnosis and Treatment of Pustular Disorders in the Neonate. Pediatric Dermatology. 1997;14(2):131–143.
Ghosh S. Neonatal pustular dermatosis: an overview. Indian J Dermatol. 2015;60(2):211.
Jennings JL, Burrows WM. Infantile acropustulosis. J Am Acad Dermatol. 1983;9(5):733–8.
Mancini AJ, Frieden IJ, Paller AS. Infantile Acropustulosis Revisited: History of Scabies and Response to Topical Corticosteroids. Pediatric Dermatology. 1998;15(5):337–341.
Castano E, et al. Hypopigmented mycosis fungoides in childhood and adolescence: a long-term retrospective study. J Cutan Pathol. 2013;40(11):924–34.
Westhoff W. A psychosocial study of albinism in a predominately mulatto Caribbean community. Psychol Rep. 1993;73(3):1007–10.
Dertlioğlu SB, Cicek D, Balci DD, Halisdemir N. Dermatology life quality index scores in children with vitiligo: comparison with atopic dermatitis and healthy control subjects. Int J Dermatol. 2012;52(1):96–101.
Magin P, Adams J, Heading G, Pond D, Smith W. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22(3):430–6.
Noor Aziah MS, Rosnah T, Mardziah A, Norzila MZ. Atopic dermatitis: a measurement of quality of life and family impact. Med J Malaysia. 2002;57(3):329–39.
Ganemo A, Lindholm C, Lindberg M, Sjoden P-O, Vahlquist A. Quality of life in adults with congenital ichthyosis. J Adv Nurs. 2003;44(4):412–9.
Thompson AR. Body issues in dermatology. In: Cash TF, Smolak L, editors. Body image, a handbook of science, practice, and prevention. New York: Guilford Press; 2012.
Thompson A, Kent G. Adjusting to disfigurement: processes involved in dealing with being visibly different. Clin Psychol Rev. 2001;21(5):663–82.
Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33(2):129–35.
Picardi A, Abeni D, Melchi C, Puddu P, Pasquini P. Psychiatric morbidity in dermatological outpatients: an issue to be recognized. Br J Dermatol. 2000;143(5):983–91.
Bilgiç Ö, Bilgiç A, Akiş HK, Eskioğlu F, Kiliç EZ. Depression, anxiety and health-related quality of life in children and adolescents with vitiligo. Clin Exp Dermatol. 2010;36(4):360–5.
Alghamdi KM. Beliefs and perceptions of Arab vitiligo patients regarding their condition. Int J Dermatol. 2010;49(10):1141–5.
Mattoo S, Handa S, Kaur I, Gupta N, Malhotra R. Psychiatric morbidity in vitiligo: prevalence and correlates in India. J Eur Acad Dermatol Venereol. 2002;16(6):573–8.
Absolon CM, Cottrell D, Eldridge SM, Glover MT. Psychological disturbance in atopic eczema: the extent of the problem in school-aged children. Br J Dermatol. 1997;137(2):241–5.
Kimata H. Prevalence of suicidal ideation in patients with atopic dermatitis. Suicide Life Threat Behav. 2006;36:120–4.
Tareen RS, Tareen AN. Psychiatric disorders frequently encountered in dermatology practices. In: Tareen RS, Gredanus DE, Jafferany M, Patel DR, Merrick J, editors. Pediatric psychodermatology: a clinical manual of child and adolescent psychocutaneous disorders. Boston: De Gruyter; 2013.
Richards HL, Fortune DG, Weidmann A, Sweeney SK, Griffiths CE. Detection of psychological distress in patients with psoriasis; low consensus between dermatologist and patient. Br J Dermatol. 2004;151:1227–33.
Seghers AC, et al. Atopic dirty neck or acquired atopic hyperpigmentation? An epidemiological and clinical study from the National Skin Centre in Singapore. Dermatology. 2014;229(3):174–82.
Chamlin SL, Frieden IJ, Williams ML, Chren M. Effects of atopic dermatitis on young american children and their families. Pediatrics. 2004;114(3):607–11.
Naqvi H, Saul K. Culture and ethnicity. In: Rumsey N, Harcourt D, editors. The Oxford handbook of the psychology of appearance. Oxford: Oxford University Press; 2012.
Papadopoulos L, Bor R, Walker C, Flaxman P, Legg C. Different shades of meaning: illness beliefs among vitiligo sufferers. Psychol Health Med. 2002;7(4):425–33.
Ersser SJ, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev. 2015;4:CD009660.
Mitchell AE, et al. Childhood atopic dermatitis: a cross-sectional study of relationships between child and parent factors, atopic dermatitis management, and disease severity. Int J Nurs Stud. 2015;52(1):216–28.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Suaiti, L.H., Damji, Y.A., Lee, M.S. (2017). Pediatric Dermatology. In: Vashi, N., Maibach, H. (eds) Dermatoanthropology of Ethnic Skin and Hair. Springer, Cham. https://doi.org/10.1007/978-3-319-53961-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-53961-4_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53960-7
Online ISBN: 978-3-319-53961-4
eBook Packages: MedicineMedicine (R0)