Abstract
Arthrography is commonly performed in most radiology departments with a musculoskeletal section. When executed correctly, it provides valuable diagnostic information by optimizing visualization of the internal joint structures. This chapter examines the common technical and nontechnical pitfalls that occur during the direct arthrographic procedure, with emphasis on the fluoroscopic approach, which can lead to a suboptimal outcome. Various methods to prevent or minimize these occurrences on both a general and joint-specific level are also discussed in detail.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Arthrographic Procedure
- Direct Arthrography
- Long Head Of Biceps Tendon (LHBT)
- Contrast Agent Extravasation
- Triangular Fibrocartilage Complex (TFCC)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Arthrography is the process of introducing contrast material into a joint to optimize visualization of the internal anatomy on cross-sectional imaging with magnetic resonance imaging (MRI) or computed tomography (CT). It is a common procedure performed in many hospitals with a musculoskeletal radiology section within the radiology department. In this chapter, we will discuss the technical and nontechnical pitfalls during the direct arthrographic procedure, with emphasis on the fluoroscopic approach.
2 General Considerations and Pitfalls
This section discusses the general pitfalls that pertain to all arthrographic procedures, regardless of joint location.
2.1 Communication and Consent
It is vital that the patient understands the nature of the procedure, the indications for performing the procedure, and the potential risks and complications. Radiology departments should provide explanatory documentation, but it is always good practice to reiterate the key points during patient consultation. Consent may be verbal or written and is dependent on local hospital policy. Communication is key to maintaining good patient-doctor relationships.
2.2 Anticoagulation
Review of anticoagulant therapy, in particular warfarin, reduces complication rates of bleeding. A generally accepted standard threshold for joint arthrography in most institutions is an international normalized ratio (INR) of less than 2, although this could be potentially extended safely to an INR <3 for even large joints such as the knee (Conway et al. 2013). A good arthrographic technique is unlikely to result in significant hemarthrosis.
2.3 Infection
The risk of joint infection is small with an incidence of 0.003% for septic arthritis and cellulitis in a large retrospective cohort of 126,000 arthrographic procedures (Newberg et al. 1985). However, there have been case series demonstrating much higher incidence clusters of more than 0.6% (Vollman et al. 2013), possibly related to cross contamination from the fluoroscopic image intensifier or contaminated arthrographic trays. All arthrographic procedures should be performed with careful sterile technique to minimize the risk of infection. A sterile arthrographic tray should be prepared (Fig. 6.1). Confusion can be avoided between transparent solutions in syringes, either by labeling or following a standardized preference, e.g., 10 ml syringe for local anesthetic and 5 ml syringe for iodinated contrast agent.
It is also good practice to ensure a thorough wipe down of the fluoroscopic image intensifier and bed in-between patients, particularly in cases of potential splash contamination or in patients with a known infectious pathology. Direct arthrography should never be performed if the patient has soft tissue infection overlying the site of needle puncture or is known to have an active septic arthritis. Joint injection in patients with reflex sympathetic dystrophy can reactivate or aggravate symptoms (Hodler 2008).
2.4 Adverse Reactions and/or Allergies
Minor adverse reactions to the procedure are much more common than true contrast agent allergies, and this includes vasovagal episodes, nausea, and localized pain from a sterile chemical synovitis. True allergies which include laryngeal or angioedema, severe urticaria, and bronchospasm are rare and only applicable to iodinated intra-articular contrast agents. There is currently no documented evidence in the literature of a true allergic reaction to intra-articular gadolinium (Gd)-based contrast agents. There are also no cases of nephrogenic systemic fibrosis attributed to intra-articular Gd administration.
Obtaining a history of such reactions is useful in risk assessment and pre-procedural planning to minimize potential pitfalls and complications. In patients with a known allergic reaction to iodinated contrast agents, the procedure can be performed under fluoroscopic guidance relying on needle guidance alone. Alternatively, ultrasound (US) imaging is an alternative and safe approach to confirm intra-articular placement of the needle. Prophylactic pre-procedural oral steroid cover can also be implemented, depending on local policy. Patients with needle phobia are more likely to experience an exaggerated vasovagal response. They are also more likely to occur in young burly men (Newberg et al. 1985). Positioning the patient in a lying position is recommended, in case of loss of consciousness. Obscuring the arthrographic tray and the needle puncture site from the patient can help reduce anxiety and subsequent incidence of vasovagal reactions (Cerezal et al. 2005).
2.5 Pre-procedural Scan
Non-contrast imaging may be required in some CT arthrographic studies. Iodinated contrast material can obscure small calcified intra-articular bodies, and a pre-contrast scan can be helpful when this is a clinical concern. This is not an issue with MR arthrographic studies, although CT may be preferred because it is usually more sensitive for detection of small intra-articular bodies.
3 General Concepts of Periprocedural Arthrographic Technique Pitfalls
3.1 Patient Positioning
Proper patient positioning optimizes the success rate of the arthrographic procedure, irrespective of joint location. A position which maximizes the articular surfaces for access makes the procedure easier and more comfortable for the patient. Individual positioning for each joint is discussed later on in this chapter.
3.2 Joint Line Versus Articular Surface
Although the joint line may provide a landmark for direct needle access in some arthrographic procedures, anatomic restraints may require modification of technique. For example, in arthrography of the radiocarpal joint, the overhanging dorsal lip of the radius will prevent direct access into the joint, unless an oblique approach is utilized (Hodler 2008). In large joints with capacious joint recesses, such as the hip and shoulder, the articular surface can be targeted rather than the joint line. When the tip of the needle is in direct contact with articular cartilage, it must by definition be intra-articular.
3.3 Needle Selection and Bevel Positioning
Needle selection depends on the target joint and patient body habitus. Typically, small or superficial joints, such as the wrist, can be performed utilizing a short 23–25 gauge needle. Larger and deeper joints, such as the hip, can be assessed by a 20–22 gauge spinal needle, typically 7–12 cm in length. Longer needles are required in patients of large body habitus, and manipulating and directing the needle becomes technically more difficult. Gentle abutment of the needle against the articular surface is commonly employed to confirm intra-articular location. In such instances, resistance to contrast injection may be encountered as the needle tip lies within the hyaline cartilage. Rotating the needle can move the bevel out of the cartilage, whereby a “give” in the syringe pressure will be felt, and there will be unhindered flow into the joint (Fig. 6.2). Similarly, the needle can be withdrawn slightly to displace the bevel out of the cartilage. Needle withdrawal of more than 1–2 mm risks extra-articular positioning and contrast extravasation (Jacobson et al. 2003).
3.4 Contrast Agent and Mixture
In CT arthrography, iodinated contrast material should be diluted to 150–300mgI/ml (Winalski and Alparslan 2008). Lower concentrations may improve identification of small intra-articular bodies but will reduce delineation of articular cartilage. Double contrast with air and iodinated contrast material instillation has been historically advocated (Haynor and Schuman 1984) as a method to better delineate the capsulolabral complex. However, this has largely been abandoned as modern CT technology provides high-resolution multi-planar reconstructed images. Single contrast studies using air alone may occasionally be useful in patients with allergic or adverse reactions to contrast agents.
Proprietary Gd solutions are available for intra-articular usage. Although Gd-based contrast agents have not been licensed for intra-articular usage by the Food and Drug Administration (Peh and Cassar-Pullicino 1999; Steinbach et al. 2002), its excellent safety profile has led to a general and wide acceptance by radiologists for this off-label purpose. If proprietary intra-articular Gd preparations are not available, then it is possible to prepare a dilution of intravenous Gd contrast. The injectate should be diluted to ~2 mmol/L (Kalke et al. 2012; Rhee et al. 2012) using normal saline to achieve satisfactory contrast on T1-weighted sequences. If too concentrated, there is marked T1 and T2 shortening, resulting in low signal intensity of fluid and accompanying susceptibility artifact which will result in a nondiagnostic study (Grainger et al. 2000) (Fig. 6.3).
Susceptibility artifact in the wrist from undiluted gadolinium injectate. Coronal (a) fat-suppressed T2-W and (b) T1-W MR arthrographic images show marked susceptibility artifact from excessive T1 and T2 shortening (white arrows) from the undiluted gadolinium preparation. As a result, soft tissue structures are obscured with fluid appearing hypointense, resulting in a nondiagnostic study
Iodine can be substituted for saline in the dilution for Gd, as this is useful for confirmation of intra-articular placement, as well as detecting pathology from the pattern of contrast flow during the fluoroscopic procedure. It is also useful for patients who require both CT and MRI, as both arthrographic studies may be acquired at the same attendance. However, iodine produces a greater decrease in signal across T1- and T2-weighted sequences, when compared with saline dilution (Montgomery et al. 2002) and therefore should only be implemented when necessary. A small amount of iodinated contrast agent in a separate syringe can be used to confirm intra-articular location of the needle prior to injection of the Gd preparation, or a small amount can be drawn up within the extension tube attached to the syringe. If there is anticipated delay from injection time to scanning time, epinephrine can be added to the solution to delay resorption of gadolinium, but this is rarely required.
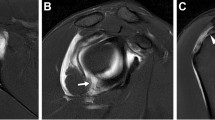
Substitution of Gd-based agents with saline solution alone may be used for MR arthrography, particularly in patients with a history of previous allergic-like reaction to Gd. The technique of saline arthrography requires the use of T2-weighted sequences only. It can be difficult to distinguish periarticular fluid and native effusions from injected saline, which is not an issue with Gd contrast agents (Grainger et al. 2000). Abnormal communications into the extra-articular bursae are also more conspicuous with gadolinium-based agents which often isolate the underlying pathology (Fig. 6.4). Smaller abnormal communications may be missed on saline arthrograms. Both T1-weighted and fat-suppressed T2-weighted sequences should be obtained in at least one plane in all studies, to help distinguish pathologic fluid collections in or around the joint such as paralabral cysts (Fig. 6.5) or subacromial-subdeltoid bursitis (Fig. 6.6).
Fluid in the subacromial-subdeltoid bursa accompanied with bursal-sided signal partial tear and fraying of the supraspinatus tendon. These findings are not appreciated on the (a) coronal fat-suppressed T1-W MR arthrographic image (white arrow) but are easily visualized on the (b) coronal fat-suppressed T2-W MR arthrographic image (yellow arrow) (Courtesy of Dr. A. Grainger)
3.5 Inadvertent Air Instillation
Gas bubble artifacts occur due to the unintentional introduction of air into the joint during the arthrographic procedure. This can mimic intra-articular bodies on both CT and MRI (Fig. 6.7) or create susceptibility artifacts on MRI that can obscure adjacent structures (Hodler 2008). Air bubbles tend to lie in the nondependent uppermost regions of the joint and can also create more pronounced blooming artifacts on certain MRI images, such as gradient-echo sequences. This pitfall can be minimized by employing a technique of filling the needle hub with contrast agent prior to connection to the extension tube. It is also helpful to ensure that all connecting tubes and syringes have been flushed out of any residual bubbles.
3.6 Joint Fluid
Joint effusions should be aspirated before the administration of intra-articular contrast agent. This helps to preserve optimum joint opacification by preventing over-dilution of the contrast material and also avoids layering between contrast material and native joint fluid.
3.7 Joint Distension and Manipulation
Adequate joint distension is required to optimize visualization of intra-articular structures. The contrast agent improves diagnostic quality by filling all the normal joint recesses and outlining normal and abnormal structures. It can also help to identify abnormal communication between anatomical spaces (Morrison 2005). Joint recesses may not be distended if there is inadequate joint distension and may result in false-negative examinations. This is especially true for CT arthrography where the inherent soft tissue contrast is poor. For example, a lack of separation of opposing articular surfaces will obscure chondral defects. Conversely, overdistension can result in unintended capsular rupture with extravasation of contrast material, either obscuring or mimicking pathology.
The optimal volume of injectate required is specific to each joint and varies enormously. The knee is able to accommodate up to 50 ml of injectate (Chung et al. 2005), while the distal radioulnar joint (DRUJ) typically requires only 1.5 ml (Table 6.1). These are general estimates, and other factors may determine the required volume of injectate. For example, adhesive capsulitis will significantly limit the volume of contrast agent that can be injected in a shoulder joint (Fig. 6.8), whereas a full-thickness rotator cuff tear will communicate with the subacromial bursa, and a much greater volume of contrast agent may need to be injected. Other examples include normal anatomical communications, such as that often exists between the ankle joint and subtalar joint which effectively increases the overall joint capacity. Some joints, particularly the wrist and knee, may require post-procedural manipulation in order to distribute the contrast material throughout the joint capsule and to encourage the contrast material to pass through small areas of abnormal communication.
Restrictive pattern of contrast agent flow in the shoulder joint. Fluoroscopic image taken after intra-articular contrast administration through the anterior rotator cuff interval approach shows an abnormal pattern of contrast agent flow with evidence of axillary synovitis and a tight capsule (white arrows). The patient was found to have a frozen shoulder
3.8 Logistical Issues
Imaging of the joint should take place soon after completion of the arthrogram procedure. Delays in imaging can result in suboptimal studies due to contrast resorption and imbibition, leading to decrease in signal-to-noise and joint distention. Different joints have different tolerances for imaging delay, with successful acquisitions of the knee obtained up to 3.5 h (Wagner et al. 2001), 1.5 h for the shoulder and hip, and 45 min for the wrist postinjection (Andreisek et al. 2007). As a general rule, imaging should ideally be performed relatively soon after the arthrogram. Communication with the CT/MRI sections of the radiology department will help anticipate any potential delays and which can be managed accordingly. There are also financial implications. Direct arthrography is more expensive than indirect arthrography, requiring additional time, trained personnel, and procedural rooms. Clinical history will help to determine the most appropriate imaging technique.
3.9 Ultrasound Imaging Guidance
Ultrasound (US) is a useful alternative modality to guide needle placement when fluoroscopy is not available. It is particularly applicable for joints where the articular surface can be visualized, such as in the shoulder or hip. The tip of the needle can hence be guided onto the chondral surface. It is important to identify a technique where the needle can be kept as horizontal as possible to the probe face, to optimize visualization of the needle tip. Angulation beyond 45° to the probe face will result in very poor visualization of the needle and will increase the incidence of contrast agent extravasation. Joint-specific techniques for US guidance are beyond the scope of this text.
The contrast agent should not pool at the tip of the needle, and if this is identified, the needle should be repositioned. However, it may be difficult to visualize the contrast agent entering the joint, and extravasation may not be appreciated until after cross-sectional imaging has occurred. Furthermore, it may be difficult to estimate the required volume of injectate in patients with extra-compartmental communication into other joints, which can potentially result in a lack of joint distension.
4 Joint-Specific Considerations and Pitfalls
4.1 Shoulder
The common indications for shoulder arthrography include assessment of the glenolabral complex, rotator cuff and long head of biceps tendon (LHBT), and evaluation of the postoperative shoulder. Shoulder arthrography is performed either via an anterior or posterior approach. The anterior approach became popular with the Schneider technique (Schneider et al. 1975). Over the years, discussion focused on potential iatrogenic injury and disruption of the anterior stabilizing tendons and anteroinferior labrum, when this method is used. This initiated the development of the modified anterior approach, targeting the rotator cuff interval (Depelteau et al. 2004) and the posterior approach, as typically practiced by orthopedic surgeons during arthroscopy (Farmer and Hughes 2002). More recently, a posterior overhead approach has been used as an alternative to a conventional posterior approach, if access is limited.
4.1.1 Anterior Approach
Needle placement with the Schneider technique is at the medial border of the junction of the middle and lower third of the glenohumeral joint. The modified anterior approach targets the triangular space of the rotator cuff interval between the subscapularis tendon and the intra-articular portion of the LHBT and supraspinatus tendons, where there is relative paucity of important anatomical structures (Fig. 6.9). While the patient is in the supine position, the arm remains in external rotation (palm facing upwards) during the procedure as this relocates the tendon laterally, minimizing unintended biceps needle perforation. Both anterior techniques have a common pitfall involving needle malpositioning with contrast agent extravasation, most commonly into the subscapularis tendon (Fig. 6.10) or adjacent bursa and soft tissues. This can obscure underlying anterior capsular abnormalities. Other pitfalls include a long coracoid process which can obscure entry into the rotator cuff interval. If the needle is placed too medially, the capsuloglenolabral complex may be subject to iatrogenic perforation and injury. In patients with proven or suspected rotator cuff injuries, there is a higher risk of needle perforation into a medially displaced LHBT.
(a) Schematic diagram shows the rotator cuff interval (red triangle) with the labral cartilage (blue outline) in relation to the long head of biceps and subscapularis tendons (black lines). (b) Postinjection radiograph shows the needle position at the rotator interval and contrast agent flowing away from the joint
4.1.2 Posterior Approach
This method avoids potential instrumentation trauma and contrast agent extravasation around the anterior stabilizing structures which are most typically the area of primary interest (Chung et al. 2001; Farmer and Hughes 2002). The patient lies in a prone position with the arm in neutral or external rotation position, with the palm facing medially or toward the bed. The shoulder may be elevated to a slight anterior oblique position to achieve a tangent orientation of the joint space and the X-ray beam. This approach is subject to variations in position and anatomy, which can complicate the procedure. A morphologically normal acromion or posterior glenoid can often overhang the humeral head, obscuring a direct pathway to the joint capsule (Fig. 6.11). In this case, an oblique needle approach under the acromion may be required. Otherwise, a more direct approach can be employed directly onto the articular surface of the posterior humeral head (Fig. 6.12). Repositioning and applying downward traction on the arm can sometimes increase the target area, but this is limited by the mobility of the patient. Alternatively, a posterior overhead approach with the arm above the head may be used (Fig. 6.13). However, this may not be tolerated by the patient if there is significant restriction of shoulder movement or pain. Contrast agent extravasation may occur around the infraspinatus tendon and can potentially mimic tendon pathology. Recognizing this pitfall minimizes interpretative error (Fig. 6.14).
Extravasation of contrast agent into the infraspinatus muscle tendon. (a) Fluoroscopic and corresponding (b) sagittal fat-suppressed T1-W MR arthrographic images show a large amount of contrast agent extravasation into and around the infraspinatus tendon and muscle belly (white arrows). This can mimic or obscure true pathology
4.2 Elbow
Elbow arthrography is most useful for identifying intra-articular bodies, ligamentous injury, and subtle chondral abnormalities (Delport and Zoga 2012). The lateral radiocapitellar approach is the most common approach. The patient is seated next to the fluoroscopic table with the upper arm elevated and the elbow flexed at 90° in a true lateral position. If there is a risk of a vasovagal episode, then the procedure can be performed with the patient lying prone. This lateral approach is considered safe as it avoids neurovascular bundles. However, contrast agent extravasation around the lateral stabilizing structures can mimic pathology (Fig. 6.15) and should not be mistaken for tendinosis or pathological ligamentous injuries. A posterior medial approach is an alternative technique, with the needle introduced into the olecranon fossa (Masala et al. 2010). The risk of ulnar nerve injury is increased with this method but can be mitigated by ensuring at least a 1 cm clearance lateral to the medial epicondyle (Fig. 6.16).
(a) Fluoroscopic image shows the direct lateral approach for elbow arthrography. (b–c) Corresponding coronal fat-suppressed T1-W MR arthrographic images show signal hyperintensity in and around the radial collateral ligament and the common extensor tendons (white arrows). This can be misinterpreted as ligamentous or tendon injuries
4.3 Wrist
Wrist arthrography is most usually performed for instability or triangular fibrocartilage complex (TFCC) tears. It can incorporate one or more of the three joint compartments – the radiocarpal joint, the DRUJ, and the midcarpal joint (Fig. 6.17). Practice varies considerably between institutions and is also dependent on the clinical situation. Screening during the procedure can help identify abnormal communication between joint compartments, which may negate the need to perform a second compartment injection. Postinjection wrist manipulation encourages contrast material to pass through small ligament or TFCC defects. A combined radiocarpal and DRUJ injection for evaluation of the TFCC may be preferred, if no abnormal communication is seen on the initial radiocarpal injection. Opacification of the DRUJ can help to demonstrate small partial proximal surface and foveal attachment tears of the TFCC (Fig. 6.18). Radiocarpal and midcarpal injections may be used for assessment of the intrinsic scapholunate and lunotriquetral ligaments (Cerezal et al. 2005). However, a single radiocarpal injection in most situations will provide adequate detail to exclude the most significant TFCC and intrinsic ligament injuries.
Wrist arthrography can be performed with the patient sitting with the arm resting palm down on the fluoroscopic table. Alternatively, the patient can lie prone with the arm overhead, elbow partially flexed, and hand in pronated position – the “superman position” – or supine with the arm by the side. The dorsal lip of the radius overlies the radiocarpal joint, limiting joint access on a straight posteroanterior projection (Fig. 6.19). This pitfall can be minimized by using minor wrist flexion with a small wedge placed beneath the wrist. Applying slight ulnar deviation will also maximize access to the radiocarpal joint space. Alternatively, a similar result can be achieved by applying cranial tilt on the imaging intensifier (Cerezal et al. 2012). The path of the needle should subsequently run approximately 5–10 degrees off-tangent to the distal radial articular surface (Fig. 6.20). A similar pitfall can be encountered with injection of the DRUJ, because the ulnar head lies within the concave articular fossa of the distal radius. The DRUJ is best injected at its proximal margin. The midcarpal joint is accessible via a direct puncture, usually at the junction between the capitate, hamate, lunate, and triquetrum.
The wrist joints require only a small amount of contrast agent, and extravasation can occur very early during injection. It often occurs along the extensor tendon sheaths and should not be misinterpreted as pathology, e.g., tenosynovitis (Fig. 6.21). It is important in MR arthrography not to fill the joint with iodinated contrast agent to confirm intra-articular location of the needle, as this will largely obscure or dilute the Gd contrast material. This pitfall can be avoided with use of a single syringe that contains both iodinated contrast agent and Gd of the appropriate concentration and will also permit dynamic evaluation under fluoroscopy. Observing contrast material flow during the dynamic wrist arthrography procedure can immediately demonstrate abnormal communication between joint compartments (Figs. 6.22 and 6.23). This may negate the need to perform a second joint injection. Small ligamentous and TFCC tears may not initially demonstrate transcompartmental flow of contrast material. However, this can be achieved by manipulation of the wrist postinjection and therefore helps to avoid a second unnecessary joint injection (Fig. 6.24). Digital subtraction techniques can be applied to make the radiological findings more conspicuous.
(a) Post-arthrographic spot fluoroscopic image of the wrist shows contrast material tracking along the extensor carpi radialis brevis/longus and extensor pollicis longus tendon sheaths (white arrows). (b) Corresponding axial fat-suppressed T1-W MR arthroscopic image shows hyperintense contrast material in the tendon sheaths (white arrows)
(a) Initial spot fluoroscopic image of the wrist taken during radiocarpal arthrographic injection shows contrast agent initially contained within the proximal carpal row. (b) Later spot fluoroscopic image shows contrast agent extravasation through the distal radioulnar joint (white arrow) indicating an underlying TFC tear or perforation
(a) Digital subtraction acquisition of a radiocarpal arthrographic puncture. Contrast material is contained on the initial fluoroscopic image. (b) Spot fluoroscopic image taken following wrist manipulation after the injection shows extensive contrast material flow into the distal carpal row (white arrows) indicating proximal row ligamentous injury and/or perforation
4.4 Hip
The main indications for hip arthrography are identification of acetabular labral tears and articular cartilage abnormalities. The patient is placed in a supine position. The hip is ideally placed in minor internal rotation and flexion. This position minimizes tension on the anterior joint capsule and may reduce the incidence of contrast agent extravasation. Needle placement should avoid the neurovascular bundle, and clinical palpation of the femoral artery can be performed. However, unless there is skeletal deformity or hip dysplasia requiring a medial approach, this pitfall is rarely encountered.
There are two main target areas for needle positioning, namely: (1) the femoral neck and (2) superior to the femoral head-neck junction along the lateral edge. A greater rate of contrast agent extravasation occurs with the neck approach, due to the thick underlying annular ligament or zona orbicularis, which encircles the femoral neck (Duc et al. 2006). The lateral aspect of the femoral head is preferred (Fig. 6.25). An oblique needle approach rather than a straight-down perpendicular puncture may also reduce contrast agent extravasation (Llopis et al. 2012), allowing the tip of the needle to slide under the joint capsule. Contrast agent extravasation may occur just around the femoral neck but may also be seen in the iliopsoas tendon sheath. Normal communication between the hip joint and bursa occurs in up to 15% of the population (Llopis et al. 2012) (Fig. 6.26).
4.5 Knee
Knee arthrography is occasionally utilized for evaluating osteochondral injuries and postoperative menisci (Kalke et al. 2012). This is especially true where MRI is contraindicated and CT arthrography is the only option. The patient is placed in a supine position, with the knee in extension to minimize tension in the extensor mechanism. The joint is usually accessed via a retropatellar approach, either laterally or medially, into the patellofemoral articulation (Shortt et al. 2009). The presence of underlying osteoarthritic change will dictate the approach taken, as large osteophytes may obscure joint access.
The main pitfall is an approach that is too cranial in location which can result in injection into the prefemoral fat pad rather than the joint space (Kalke et al. 2012) (Fig. 6.27). Alternative techniques include a direct anterior approach down to the medial femoral condyle (Shortt et al. 2009) or the anterolateral approach down to the lateral femoral condyle (Moser et al. 2008). This may be of benefit in obese patients where the patella is not easily palpated. Contrast material may pool within the suprapatellar recess, reducing overall joint distension. This effect can be minimized by the application of a tourniquet around the thigh above the patella following joint injection (Grainger et al. 2000). Otherwise, gentle pressure can be applied over the suprapatellar region with the free hand during contrast agent injection.
(a) Fluoroscopic image shows abnormal pooling and concentration of contrast material along the lateral edge of the distal femur (white arrow). Corresponding (b) coronal and (c) axial CT images with bone windows show contrast extravasation (white arrows) into the lateral aspect of the prefemoral fat pad
4.6 Ankle
Ankle arthrography is occasionally used to assess osteochondral and cartilage lesions, especially with CT where orthopedic metalware limits the use of MRI. Arthrography is much less commonly used for ankle ligament deficiency. The joint is accessed primarily through the anteromedial or anterolateral approach (Chandnani et al. 1994; Fox et al. 2013) (Fig. 6.28), with the patient in a supine position with mild plantar flexion. Clinical palpation (or US imaging guidance) can help avoid puncture of the dorsalis pedis artery. An oblique approach is required to avoid the lip of the anterior tibial plafond and bring the needle tip onto the articular surface of the dome of the talus. Cranial tilt of the X-ray tube (2–5°) may also be helpful.
Schematic diagrammatic overlays overlying an AP radiograph of the ankle. Common sites of needle puncture include the medial and lateral clear spaces or anterior puncture into the tibiotalar joint (white circles and white oval). The extensor tendons (black lines) and the dorsalis pedis artery (red line) should be avoided during needle puncture. The talofibular/lateral stabilizing ligaments are also shown (white lines)
Large anterior osteophytes can result in a failed procedure, regardless of technique. These may be difficult to appreciate on frontal fluoroscopic images. Lateral fluoroscopy can be utilized when difficulties are encountered. Contrast agent extravasation into the flexor hallucis and digitorum tendon sheaths, and the posterior subtalar joint, is often encountered as a normal variation in up to 25% of cases (Cerezal et al. 2005) (Fig. 6.29). Contrast agent extension into the distal tibiofibular syndesmotic recess should not be misinterpreted as being a syndesmotic injury.
Conclusion
In summary, arthrography is widely practiced in most radiology departments with musculoskeletal sections and serves to optimize diagnostic images by better visualization of the internal structures of the joint. As with any procedure, there are technical pitfalls that can result in inadequate outcomes. Understanding these technical pitfalls will help optimize the procedure, maximizing image quality as well as patient comfort.
Abbreviations
- CT:
-
Computed tomography
- Gd:
-
Gadolinium
- MRI:
-
Magnetic resonance imaging
References
Andreisek G, Duc SR, Froehlich JM (2007) MR arthrography of the shoulder, hip, and wrist: evaluation of contrast dynamics and image quality with increasing injection-to-imaging time. AJR Am J Roentgenol 188:1081–1088
Cerezal L, Abascal F, Garcia-Valtuille R, Canga A (2005a) Ankle MR arthrography: how, why, when. Radiol Clin N Am 43:693–707
Cerezal L, Abascal F, Garcia-Valtuille R, Del Pinal F (2005b) Wrist MR arthrography: how, why, when. Radiol Clin N Am 43:709–731
Cerezal L, Berna-Mestre JD, Canga A et al (2012) MR and CT arthrography of the wrist. Semin Musculoskelet Radiol 16:27–41
Chandnani VP, Harper MT, Ficke JR et al (1994) Chronic ankle instability: evaluation with MR arthrography, MR imaging, and stress radiography. Radiology 192:189–194
Chung CB, Dwek JR, Feng S, Resnick D (2001) MR arthrography of the glenohumeral joint: a tailored approach. AJR Am J Roentgenol 177:217–219
Chung CB, Isaza IL, Angulo M et al (2005) MR arthrography of the knee: know, why, when. Radiol Clin N Am 43:733–746
Conway R, O’Shea FD, Cunnane G, Doran MF (2013) Safety of joint and soft tissue injections in patients on warfarin anticoagulation. Clin Rheumatol 32:1811–1814
Delport AG, Zoga AC (2012) MR and CT arthrography of the elbow. Semin Musculoskelet Radiol 16:15–26
Depelteau H, Bureau NJ, Cardinal E et al (2004) Arthrography of the shoulder: a simple fluoroscopically guided approach for targeting the rotator cuff interval. AJR Am J Roentgenol 182:329–332
Duc SR, Hodler J, Schmid M et al (2006) Prospective evaluation of two different injection techniques for MR arthrography of the hip. Eur Radiol 16:473–478
Farmer KD, Hughes PM (2002) MR arthrography of the shoulder: fluoroscopically guided technique using a posterior approach. AJR Am J Roentgenol 178:433–434
Fox MG, Wright PR, Alford B et al (2013) Lateral mortise approach for therapeutic ankle injection: an alternative to the anteromedial approach. AJR Am J Roentgenol 200:1096–1100
Grainger AJ, Elliott JM, Campbell RSD et al (2000) Direct MR arthrography: a review of current use. Clin Radiol 55:163–176
Haynor DR, Schuman WP (1984) Double contrast CT arthrography of the glenoid labrum and shoulder girdle. Radiographics 4:411–421
Hodler J (2008) Technical errors in MR arthrography. Skeletal Radiol 37:9–18
Jacobson JA, Lin J, Jamadar DA, Hayes CW (2003) Aids to successful shoulder arthrography performed with a fluoroscopically guided anterior approach. Radiographics 23:373–379
Kalke RJ, Di Primio GA, Schweitzer ME (2012) MR and CT arthrography of the knee. Semin Musculoskelet Radiol 16:57–68
Llopis E, Fernandez E, Cerezal L (2012) MR and CT arthrography of the hip. Semin Musculoskelet Radiol 16:42–56
Masala S, Fiori R, Bartolucci DA et al (2010) Diagnostic and therapeutic joint injections. Semin Interv Radiol 27:160–171
Montgomery DD, Morrison WB, Schweitzer ME et al (2002) Effects of iodinated contrast and field strength on gadolinium enhancement: implications for direct MR arthrography. J Magn Reson Imaging 15:334–343
Morrison WB (2005) Indirect MR arthrography: concepts and controversies. Semin Musculoskelet Radiol 9:124–134
Moser T, Moussaoui A, Dupuis M et al (2008) Anterior approach for knee arthrography: tolerance evaluation and comparison of two routes. Radiology 246:193–197
Newberg AH, Munn CS, Robbins AH (1985) Complications of arthrography. Radiology 155:605–606
Peh WCG, Cassar-Pullicino VN (1999) Magnetic resonance arthrography: current status. Clin Radiol 54:575–587
Rhee RB, Chan KK, Lieu JG et al (2012) MR and CT arthrography of the shoulder. Semin Musculoskelet Radiol 16:3–14
Schneider R, Ghelman B, Kaye JJ (1975) A simplified injection technique for shoulder arthrography. Radiology 114:738–739
Shortt CP, Morrison WB, Roberts CC et al (2009) Shoulder, hip, and knee arthrography needle placement using fluoroscopic guidance: practice patterns of musculoskeletal radiologists in North America. Skeletal Radiol 38:377–385
Steinbach LS, Palmer WE, Schweitzer ME (2002) Special focus session. MR arthrography. Radiographics 22:1223–1246
Vollman AT, Craig JG, Hulen R et al (2013) Review of three magnetic resonance arthrography related infections. World J Radiol 5:41–44
Wagner SC, Schweitzer ME, Weishaupt D (2001) Temporal behaviour of intra-articular gadolinium. J Comput Assist Tomogr 25:661–670
Winalski CS, Alparslan L (2008) Imaging of articular cartilage injuries of the lower extremity. Semin Musculoskelet Radiol 12:283–301
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Chin, T.Y., Campbell, R.S.D. (2017). Arthrographic Technique Pitfalls. In: Peh, W. (eds) Pitfalls in Musculoskeletal Radiology. Springer, Cham. https://doi.org/10.1007/978-3-319-53496-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-53496-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53494-7
Online ISBN: 978-3-319-53496-1
eBook Packages: MedicineMedicine (R0)