Abstract
The field of wastewater management and alternative energy are one of the most unexplored fields of environmental biotechnology. The biomethane is considered as renewable natural gas which can be derived from organic waste and sewage treatment. In recent past biomethane is emerging as a promising gaseous fuel utilized in a cogeneration or trigeneration power plant. Biomethane usually produced through anaerobic digestion in oxygen-deficient environment where a series of microorganisms convert the complex waste to biogas via liquefaction through a cascade of enzymes. Biomethane produced in anaerobic digester can be utilized for decentralized power generation, and additionally revenue can be gained in the form of a CO2 credits and/or other greenhouse gas emission credits. In the present book chapter, the technical know-how of anaerobic digestion and biocatalyst associated with digester has been depicted. A thorough understanding of the fundamental principles of anaerobic digestion would help to perceive new aspects of bioenergy conversions. The book chapter highlights the concise of biochemistry for biomethane production as well as important major factors involved in the process toward the realization of a stable biomethane-based economy. Successful application of anaerobic digester was found in pilot-scale potential wastewater treatment along with renewable energy production. The proper configuration anaerobic digester and efficiently pretreated “feedstock” are key to maximize the production of methane. Therefore, basics of reactor design designs based on process economy have been discussed.
† Vinay Patel and Soumya Pandit have equal contribution.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Anaerobic digestion
- Methanogenesis
- Anaerobic Reactor design
- Wastewater management
- Renewable energy generation
- Acetogens
- Acidogens
- Methogens
1 Introduction
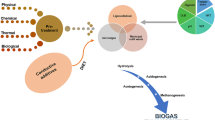
The anaerobic digestion is a sustainable process which is gaining popularity in the realm of increasing demand of constant energy sources and concern of global warming due to emission of greenhouse gases. The anaerobic digester utilizes the waste to extract renewable biofuels and by-products. The anaerobic digestion process can be proved as a boon to the developing and underdeveloped countries. The widespread application of the process can help these countries to eradicate the major problem of waste management, sanitation, health, bio-fertilizer production, and renewable energy. One of the major products of anaerobic digester (AD) is methane (Li et al. 2015). As biomethane is the greenest of all the biofuels, it is going to be reclassified as “super low-carbon fuel” from “low-carbon fuels” for decentralized system (Fig. 1) (Nguyen et al. 2015).
In 1776, Volta identifies that the anaerobic digestion can potentially convert organic matter to methane (Sowers 2014). In 1981, Cosmos a French journal cited “Mouras Automatic Scavenger,” the first anaerobic chamber to treat domestic wastewater. In 1895, Donald Cameron built a septic tank based on the model of Mouras Automatic Scavenger in Exter, England. The application of anaerobic process was limited till 1950, due to lack of the essential knowledge of the anaerobic process. In 1950, Stander identified the relevance of solid retention time (SRT) for anaerobic digestion of wastewater. This development has directed to the advancement of the high-rate anaerobic reactor. The progress of high-rate anaerobic reactor established the application of anaerobic process, particularly for biogas recovery and wastewater treatment (Li et al. 2015).
Methanogenesis is the process of formation of methane by some microorganisms commonly known as methanogens under anaerobic conditions (Venkata Mohan et al. 2011, 2013). Methanogens belong to the kingdom Archaea and are the most diverse group among the known members of the domain. These microorganisms drew the attention of the whole world due to their significant contribution to the global methane emissions and their wastewater treatment ability. Methanogens can produce methane using different organic and inorganic compounds such as carbon dioxide, acetate, etc. (Romero-Güiza et al. 2016). Methanogens can also utilize the waste products of different bacteria to produce methane. Methane production is a very versatile process which can utilize various organic and inorganic compounds to produce methane and carbon dioxide under anaerobic conditions (Kumar et al. 2014). The breakdown of the organic compounds is facilitated by a cascade of bacterial degradation enzymes by various groups of bacteria such as acidogens, acetogens, methanogens, etc. The methanogens are the most common microbes responsible for the reduction of the carbonaceous material to methane. They are the microbes which can thrive in anaerobic conditions such as bottom of the water bodies, paddy fields, dumping grounds, or in specialized environments—rumen bacteria to produce methane using organic matter (Kumar et al. 2016). The rumen bacteria form a symbiotic environment to a staggering amount of methane from cellulose. These bacteria produces 4 × 105 tonnes of methane daily (Ge et al. 2016).
2 Biochemistry of Methane Generation
The isolation and culturing of methanogens is very difficult as most of them are strict anaerobes. So, even a very small contamination of oxygen can ruin the culture. The anaerobic culture requires specific techniques such as pressurized culture vessels for liquid cultures and roll tubes for solid cultures (Ahring 2003). These techniques were compiled by Balch et al. (1979) in his review paper (Balch et al. 1979).
The primary fermentation, i.e., breakdown of macromolecules such as carbohydrates, proteins, lipids, etc. results in the formation of acetate, formate, carbon dioxide, and hydrogen (Chandrasekhar et al. 2015a, b). The methanogens produce methane by mainly two pathways: carbon dioxide reduction and acetate conversion. But methanogens can also convert formate, methanol, methylamines, and CO into methane (Chandrasekhar and Venkata Mohan 2014a, b; Stamatelatou et al. 2014).
2.1 Carbon Dioxide (CO2) Reduction
The reduction of CO2 is studied extensively by the researchers. The pathway uses many C1 coenzymes which are unique to methanogens. The conversion of carbon dioxide to methane occurs through many intermediate stages which begin with the conversion of carbon dioxide to formyl, methenyl, and methylene and ends with the formation of methane from methyl stage. The conversion of carbon dioxide to methane is facilitated by many C1 coenzymes which are mostly unique to methanogens. These coenzymes are tetrahydromethanopterin (THMP), methanofuran, and coenzyme M. THMP is similar to the eukaryotic pterins, while the latter two are unique to methanogens (Sebola et al. 2014).
The C1 group is passed in a bucket bridge fashion to a coenzyme as it is consecutively reduced. The first stable compound in the pathway is formyl-methanofuran. The formyl-methanofuran transfers the formyl group to TMHP in the presence of a formyltransferase. The formyl group is then converted to methenyl group by a cyclohydrolase named as 5,10-methenyltetrahydromethanopterin cyclohydrolase (Zinder 1990). The reduced coenzyme F420 donates two electrons to the methenyl group which results in formation of methylene group. The methylene group conversion is facilitated by an oxidoreductase enzyme named as methylene-tetrahydromethanopterin: coenzyme F420 oxidoreductase. The methylene is further converted to methyl group which forms a complex with coenzyme M. The conversion of methyl coenzyme to methane involves four enzyme complexes out of which only two are known till date. The known enzyme complexes involved are CoM-S-S-HTP and N-7-mercaptoheptanoyl-O-phospho-l-threonine (H-S-HTP). The reactions intricate in the pathway are mentioned in Table 1. The conversion of carbon dioxide to methane accounts of a net Gibbs free energy of −130.4 kJ/mol (O’Flaherty et al. 2006).
2.2 Acetate Conversion
Early researcher proposed that the acetate is first completely oxidized to carbon dioxide. The carbon dioxide produced was then converted to CH4. The carbon dioxide reduction theory was proved false by using 14C-labeled acetate. The results showed that most of the methane was resulting from the methyl group (–CH3) and little from the carboxyl (–COOH) carbon. Further, the hydrogen atom of –CH3 was replaced by the deuterium atoms in other study to show that the intact –CH3 is transferred to CH4. The methanogens cleave acetate into –CH3 which is reduced to CH4 (Choong et al. 2016).The electron is derived from the oxidation of –COOH to CO2. The pathway is mainly studied in Methanosarcina and can also be used to explain the acetate conversion in Methanothrix with some modifications. The first step involves the activation of acetate by converting it into acetyl-CoA with the expense of an ATP. The reaction is nonspontaneous with a Gibbs free energy value of +13 kJ/mol. The activation to acetyl-coA is followed by decarbonylation which is catalyzed by carbon monoxide dehydrogenase (CODH). CODH catalyzes the oxidation of carbonyl group to carbon dioxide and a molecule of ferredoxin is reduced in the process. The methyl group is transferred in the complex to corrinoid/iron-sulfur component and subsequently to coenzyme M (HS-CoM). The formed complex undergoes reduction to give methane (Gorris and van der Drift 1994). The last step involves the electrons from the reduction of ferredoxin but the electron transport chain is still unknown.
2.3 Other Pathways
The other pathway involves the conversion of CH3OH and methylamines to CH4 and CO2. The CH3OH is converted to CH4 and CO2. The –CH3 of CH3OH is transferred to coenzyme M (HS-CoM). The complex (methyl-coenzyme M) undergoes demethylation with 7-mercaptoheptanoyl-l-threonine phosphate (HS-HTP) which produces methane and the heterodisulfide (CoM-S-S-HTP). The electrons of the heterodisulfide are replenished from the oxidation of CH3OH. The conversion of methylamines is not studied in details, but the researchers suggest that the conversion is analogous to CH3OH (Gujer and Zehnder 1983).
3 Microbiology of Methane Generation in AD
The anaerobic digestion process is the sequential degradation of complex substrates to CH4 (Table 1). It can be broadly divided into three steps on the basis of the different microbial consortium. The microbial consortiums involved in the process are acidogenic bacteria, obligate hydrogen-producing acetogens, and two groups of methanogenic Archaeas (Gerardi 2003).
3.1 Fermentative Bacteria
The first stage of anaerobic digestion process is responsible for degradation of complex substrates such as carbohydrates, lipids, and proteins into simpler substances by facultative and obligate fermentation. The first step is facilitated by the anaerobic species which belongs to the family of Enterobacteriaceae and Streptococcaceae and the genera of Clostridium, Eubacterium, Lactobacillus, Bacteroides, Butyrivibrio, and Bifidobacterium (Novaes 1986). Bacillaceae is the predominant in digestive sludge along with the others such as Enterobacteriaceae and Lactobacillaceae (Kumar et al. 2015). Clostridia ferments the hydrolyzed products of proteins such as amino acids and peptides into volatile fatty acids (VFAs), hydrogen, ammonia, and carbon dioxide (Narihiro and Sekiguchi 2007).
This first step of fermentation consists of hydrolysis and acidogenesis. This step converts the complex substrates into oligomers or monomers, for example, proteins are converted to smaller peptides or amino acids. The hydrolysis of complex substrate is followed by the further degradation of these simpler substances into VFAs and other products such as methanol, CO2, formate, etc. The common volatile fatty acids generated are acetate and butyrate (Venkata Mohan and Chandrasekhar 2011a, b; Chandrasekhar and Mohan 2012; Kumar et al. 2012). This is generally the fastest step in the process. The cell count of acidogens in the digesters is between 106–108 per ml (Noike et al. 1985). A low partial pressure of hydrogen favors the production of desired precursors of methanogenesis such as acetates, carbon dioxide, and hydrogen. While high partial pressure of hydrogen shifts the process toward formation of other organic intermediates such as acetate, butyrate, formate, propionate, etc.
3.2 Hydrogen-Producing Acetogenic Bacteria (2) and Acidogenesis
This bacterial group is responsible for the degradation of higher organic acids such as propionic acid, butyric acid, etc. and aromatic compounds such as benzoate, ethanol, and other products to carbon dioxide, hydrogen, and acetate (Eqs. 1–3). These reactions are not thermodynamically favorable with pure culture. However, with coculture it is possible to convert C3 or higher organic acid to acetate at certain condition.
In 1967, Hungate studied the relevance of production and consumption of hydrogen in anaerobic processes. The process of hydrogen consumption by methanogens maintains a low partial pressure of hydrogen which favors the acetogenesis, i.e., breakdown of organic compounds into hydrogen, carbon dioxide, and acetate. Acetogenesis is the secondary fermentation process which converts the VFAs and other intermediates such as alcohols, hydrogen, and CO2 into methanogenic precursors (hydrogen and acetate). So, this is a very critical step for the methanogenesis (Hungate 1967). The acetogens can be further classified into two types on the basis of their prevalence in the reactor: obligate hydrogen-producing acetogens (OHPAs) and homoacetogens. OHPAs are the microbes which convert intermediate compounds like fatty acids, alcohols into acetate, CO2, and H2. But the oxidation of OHPAs is inhibited by the presence of the hydrogen, a metabolic product. The Gibbs free energy of the reaction is highly positive in the presence of hydrogen (Kotelnikova and Pedersen 1997). So, the process efficiency can be increased by the microbial consortium which can utilize the produced hydrogen. The best available option for syntrophy is methanogens, which can consume the produced H2. Hence most of the OHPAs grow in syntrophic environment with methanogens.
3.3 Homoacetogens
Homoacetogenesis is an interesting biochemical pathway in anaerobic digestion. The acetate is the major end product in homoacetogenesis, which is considered as an essential precursor for CH4 production. The answerable microorganisms are either autotrophs or heterotrophs. The autotrophic homoacetogens, the second group of acetogens, convert CO2 and H2 produced into acetate (Eq. 4). This group of microbes also helps in maintenance of little partial pressure of H2 in the reactor. Homoacetogens can also utilize other substrates as a carbon such as carbon monoxide (Eq. 5).
The heterotrophic homoacetogens can utilize methanol and formate as a carbon source and produce acetate as an end product (Eqs. 6 and 7). In 1986, Novaes isolated two mesophilic homoacetogens: Acetobacterium woodii and Clostridium aceticum. In 1981, Zeikus observed that there is no accumulation of H2 and CO2 during the growth of homoacetogens. The studies have shown that the homoacetogenesis is a rapid process (Diekert and Wohlfarth 1994). Even the Gibbs free energy that was found close to methanogenesis suggests competition for hydrogen and other electron donors (Eqs. 8 and 9).
3.4 Methanogen and Methanogenesis
Methanogenesis is considered as one of the major rate-limiting steps in the anaerobic digestion. The methanogens are found abundantly in anaerobic surroundings such as ponds, marine sediments, swamps, and lakes. The methanogenic precursors produced in the second step are utilized by the methanogens to produce methanogens (Archer and Harris 1986). The methanogens are obligate anaerobes which are further categorized into two types based on the substrate utilization: acetoclastic and hydrogen-utilizing methanogens. Acetoclastic methanogens are the major producers of methane which is close to 70% of the methane produced. They belong to mainly two genres: Methanosaeta and Methanosarcina. Generally, Methanosarcina, a spherical shaped methanogen, forms large packets which consist of coccoid (spherical) cell units. Methanosarcina can utilize various substrates such as H2/CO2 (eq), CH3OH (eq), and methylamines (eq). The doubling time of Methanosarcina on acetate is 1–2 days (Eq. 11), while Methanosaeta, a rod-shaped methanogen, can only grow on acetate and their doubling time is 4–9 days (Conklin et al. 2006). So, CH3COOH concentration greater than 1 mM favors the growth of Methanosarcina, and lower concentration favors Methanosaeta’s growth. As compared to other bacteria, the growth rates of acetotrophic methanogens are slow which limits the anaerobic process and may result in the acetic acid accumulation.
Hydrogen-utilizing methanogens consume hydrogens for the reduction of the process intermediates such as methanol, formate, carbon dioxide, and methyl amines to CH4, the final product of anaerobic digestion. These microbes constitute the remaining 30% of the methane produced by the anaerobic process (Eq. 12). These are chemolithotrophic autotrophs, which derives both energy and carbon from inorganic chemicals (Ferry 2012).
Although biomethane production occurs via two major pathways—hydrogenotrophic methanogenesis via CO2 reduction and acetotrophic/acetoclastic methanogenesis—there exists a third one, methylotrophic pathways. Methanol; mono-, di-, and trimethylamine; and dimethyl sulfide (reaction is a major substrate for methylotrophic methane generation where –CH3 is transferred to a methyl carrier and reduced to CH4 (Eqs. 13–15)).
4 Environmental Factors
As mentioned in the previous sections, anaerobic process is strongly impacted by the environmental factors. The anaerobic process requires a stricter control over the environmental parameters than the aerobic process. The impact of environmental parameters on the anaerobic process is measured by methane yield as methanogenesis is the rate-determining step of the anaerobic process. Methanogenesis, a biological process, is strongly impacted by environmental factors such as toxicity, temperature, pH, type and concentration of feed, nutrients, metal toxicity, and others. A brief outline of the impact of the abovementioned factors is described here.
4.1 Temperature
Methanogens can produce methane in a temperature range of 10–60 °C. The rate of methane production is very slow at 10 °C which attains saturation at a temperature of 37 °C. The anaerobic digestion can be segregated in three groups: psychrophilic (10–20 °C), mesophilic (20–40 °C), and thermophilic (40–60 °C) digestion. The microbial growth and the conversion process are strongly influenced by temperature. So, psychrophilic digestion requires a long retention which results in large reactor volume, and mesophilic digestion requires a small reactor as compared to the psychrophilic. Thermophilic, being a special case, can be utilized for wastewater treatment which is discharged at high temperature. The only methanogen which shows higher specific methanogenic activity is hydrogenotrophic methanogens with no change in the most probable number (MPN) at 65 °C, compared to 55 °C. While the number and activities of other microorganisms were considerably reduced (Sowers 2014).
The temperature requirement can be expressed mathematically using Arrhenius expression:
where “t” is the temperature in °C and r t , r 30 are rates of digestion at temperature t and 30○C, respectively. On the basis of the above equation, the optimum digestion rate decreases by 11% for every 1 °C decrease in optimum temperature. Temperature severely impacts the decay rate, maximum specific growth rate, growth yield, and half-velocity rate constant. So, the temperature effect on methane yield is the cumulative impact on different growth kinetics parameters. The rate of methane generation in thermophiles is independent of temperature between 50–70 °C. Hence, Water Pollution Control Federation (WPCF) recommended that the anaerobic process should be designed in such a way that the variation in process temperature should not exceed 0.6–1.2 °C/day (Griffin et al. 1998).
4.2 Operating pH
The pH in an anaerobic digester changes with the onset of the production of VFAs by acidogenic bacteria in the early stage of the process. But a further decrease in pH is observed in the latter stage due to the consumption of VFAs by the methanogens. Most of the anaerobic methanogens grows in neutral pH range (6.5–7.5). The methane production is inhibited at low pH which develops in anaerobic reactors due to the generation of volatile fatty acid intermediates (Venkata Mohan and Chandrasekhar 2011a). So, it is essential to maintain the pH for optimal productivity. Hydrogen bicarbonate is used to keep the pH in the neutral pH range. The optimal pH for the growth of acidogens lies in the range of 5.5–6.5, while that of methanogens ranges in 7.8–8.2. So, the challenge is to select a pH range at which both the microbial groups can coexist. The optimal pH for the coexistence of both the microbial groups is 6.8–7.4. Hence, it is advisable to uphold the pH close to the neutral (Griffin et al. 1998).
4.3 Alkalinity
The organic waste which contains high nitrogen can significantly contribute to the alkalinity. The primary constituent of this wastewater is proteins which on degradation generates ammonia. The ammonia reacts with the carbon dioxide present in the digester to generate bicarbonate which is mainly responsible for alkalinity in the digester. The biochemical reaction is as mentioned below (Eqs. 17 and 18):
However, treatment of sulfate-/sulfite-rich wastewater under anaerobic conditions also generates alkalinity due to sulfate/sulfite reduction (Eqs. 19 and 20). Theoretically, 1000 mg of SO4 reduction generates 1040 mg of alkalinity as calcium carbonate (CaCO3) (Sambo et al. 1995).
The biogas produced from sulfate-/sulfite-rich wastewater has a significant amount of H2S in it. H2S or hydrogen sulfide is hazardous and corrosive by nature due to its highly acidic property, and therefore it is imperative to quench this “sour gas.” This process is known as “gas sweetening.” The gas sweeting is done using an iron sponge which has a bed of wood chips and hydrated oxide. This bed removes the H2S present in the biogas streams. Amine plants can remove both H2S and CO2 from liquid hydrocarbons and natural gas. The process involves both chemical reactions and adsorption. The H2S removal process should meet the “pipeline quality gas” standards which is less than 0.25 grains of H2S (Díaz and Fdz-Polanco 2012).
4.4 Oxidation-Reduction Potentials
Many researchers have reported that the growth of obligate anaerobes requires a culture with an ORP value in the range −200 to −350 mV at pH 7. The methanogens can grow well in an extremely reducing environment, which has an ORP of −400 mV. Hence, the culture media for methanogens has a good amount of reducing agents such as cysteine, sulfide, or titanium III to maintain the ORP at a suitable value (Prasad and Prasad 2012).
4.5 Type and Concentration of Feed
The anaerobic digesters have evolved a lot with the research in the last decades. The present digesters are compatible to various types of feed such as agricultural wastes, industrial wastes, algal biomass, etc. as compared to the trivial digesters which can use only sewage sludge and animal wastes (Tijani et al. 2015). The constituents of feed vary with the type of waste used, for example, paper industry waste is quite rich in organic matter, food waste is rich in soluble organics, etc. The anaerobic digestion is seriously affected by the presence of many materials such as sand, plastics, and glass which results in the process failure if present in high concentration (Kimari et al. 2015).
The concentration of feed also plays an imperative role to decide the productivity of the reactor. The increase in solid content of the slurry decreases the rate of CH4 production. The study done by Fernandez et al. shows that the CH4 production was reduced by 17% with the increase of solid content from 20% to 30% (Ge et al. 2016).
4.6 Nutrients
The major nutrients required by the microbes are carbon, hydrogen, nitrogen, phosphorus, and sulfur. Most of the waste materials lack nitrogen and phosphorus. The waste is characterized mainly by C/N ratio which is the ratio of carbon and nitrogen present in the waste. The productivity of the digester can be enhanced by adding appropriate nutrients in optimum amount. We can also mix different waste to optimize the nutrient constituents of the digester (Jain et al. 2015).
4.7 Metal Toxicity
All microbes need trace elements and nutrients for waste stabilization, but these elements are not directly responsible for waste stabilization. These elements are the essential components for growth and synthesis of cells. These elements also provide optimal physicochemical conditions for microbial growth. Some metals such as Ni, Fe, Mo, and Co are also used by methanogens for growth on H2 and CO2 (Choong et al. 2016). The metals are required by the microbes for growth in an optimum amount. But these metals can also be toxic to microbes if present in high amounts. The presence of metals such as Na+, K+, Ca2+, etc. in high concentrations is toxic to methanogens. These metal salts can disturb the tonicity of the bacterial membrane which results in very deleterious effects (Schmidt et al. 2014).
5 Reactor Design
The selection of optimum reactor configuration is the most important part of any process. The slow reaction rate of biomethanation demands strict control parameters for reactor design. The rate-limiting step of any process determines the optimum reactor design which is methanogenesis in anaerobic digestion. The selection of reactor configuration is by high HRT/SRT ratio, which prevents the washout of methanogens. The most crucial parameter is sludge retention time (SRT) which directly impacts growth rate of the methanogens and hence productivity of the process. There are many other parameters which need attention for stable methanogenesis. The other process parameters of an anaerobic process are hydraulic retention time (HRT), process temperature, C/N ratio, organic loading, pH, mixing, etc.
5.1 Sludge Retention Time (SRT)
The SRT is defined as the ratio active biomass in the system to the production of active biomass in the system.
where V is the volume of reactor, X is the reactor cell mass, and X out is the cell mass flowing out of the reactor with a flow rate Q out. If SRT is less, the methanogenesis will not take place which results in pH decrease due to accumulation of VFAs. While very high SRT can result in a nutrient deficiency in the reactor. So, an optimum SRT of 15 days is required for the production of methane in an anaerobic digester.
5.2 Hydraulic Retention Time (HRT)
HRT is defined as the ratio of reactor volume (V) and inflow rate in the reactor (Q).
HRT of the process depends on the feed type and concentration and temperature. High HRT can be used for low feed concentration, while a high HRT is required for high feed concentration. If process temperature is high, then the rate of reaction increases and HRT decreases for the same yield and vice versa.
5.3 Temperature
As described in Sect. 4.1, methanogens can grow in a range of temperature 10–60 °C. The optimal temperature for methane production is 37 °C. The conventional reactors are designed to function in mesophilic conditions. The temperature in such reactors is maintained in a range between 37 °C ± 2 °C. The reactors operating under mesophilic conditions require less energy input and are more stable than thermophilic reactors (Wang et al. 2014). While the CH4 production rate of mesophilic is more than the psychrophilic reactors. So, the process temperature in an AD has to be strictly maintained throughout the operation time (Abdelgadir et al. 2014).
5.4 Carbon/Nitrogen Ratio
The C/N ratio gives the relative amount of nitrogen and carbon present in the reactor. The carbon is required by all the cells, but nitrogen is also a crucial factor for synthesis of proteins in the cell. The optimal C/N ratio is 20–30. The substrates with a very high C/N ratio are deficient in nitrogen and vice versa. The imbalance in C/N ratio can result in high VFA and total ammonia formation. Both the intermediates are the potential inhibitors of the methanogens. So, it is very essential to maintain the C/N ratio in the anaerobic digester (Wang et al. 2014).
5.5 Organic Loading Rate
Organic loading rate (OLR) defines the amount of organic substrate loaded per unit volume of the reactor.
where C VS is the concentration of volatile solids. Lower OLR results in a large reactor volume, while a higher OLR requires a small reactor. But higher OLR requires higher HRT and it can also lead to the overloading of the reactor. So, the reactor design should balance the HRT and OLR (Chandrasekhar and Venkata Mohan 2012).
5.6 pH
The pH of the digester lowers due to generation of many intermediates such as VFAs during digestion. The AD also involves a consortium of microbes which grows at different temperatures, for example, acidogens grow optimally in the pH range of 5.5–6.5 while acidic pH is toxic to methanogens. The biggest reason of the anaerobic digester failure is acid accumulation. So, the reactor has to be well equipped to maintain the pH in the desired range (Abdelgadir et al. 2014).
5.7 Mixing
The fermentation media should be mixed properly to maximize the degradation of the substrate by enzymes produced by the microbes. A proper mixing regulates a better temperature and pH control. It also maintains a uniform heat and mass transfer throughout the reactor. Mixing can be done in various ways such as recirculation of different reactor’s contents, mechanical mixing, etc. The mixing time and speed have to be optimized because overmixing can led to the degradation of microbes present in the anaerobic digester (Jain et al. 2015).
5.8 Ideal Anaerobic Reactor
Hence the ideal anaerobic digester should have following characteristics: optimal temperature and pH control, optimum balance between HRT and OLR, and a proper mixing time and speed. The disruption in the anaerobic digestion process due to acid accumulation can be solved using two stage anaerobic digestions. The digestion process is divided into two processes in two separate digesters. The first process involves hydrolysis and acidogenesis, while the second process involves acetogenesis and methanogenesis. The anaerobic digesters can be classified in three categories on the basis of the reactor design: low-rate (long hydraulic retention time), high-rate (short hydraulic retention time), and tubular reactor (Skiadas et al. 2003).
5.8.1 Low-Rate Reactors
The long hydraulic time is required for the waste treatment from streams such as solid wastes and slurry, while wastewater treatment requires short hydraulic retention time. The low-rate systems are batch, plug flow, and continuous reactors. The low-rate digestion process can be further classified into two types on the basis of number of steps in the process: one step and multiple steps mainly two steps. The digesters can be categorized in two categories on the basis of the dry matter present in the slurry: wet (dry matter less than 20%) and dry digester (dry matter between 20% and 40%).
5.8.2 High-Rate Reactors
The high-rate systems are further categorized into systems with fixed beds and systems with suspension culture. The fixed-bed system consists of fixed bacterial biofilms on a solid support, while in the latter the microbial mass is retained by settling (internal or external). The internal settling can be done by simply with the help of gravity, while external settling can be done by using external means such as a filter at the outlet. The low-rate systems are plug flow, batch, and continuous stirred tank reactors, while the high-rate systems are fluidized bed and upflow anaerobic sludge bed (UASB) reactors.
The UASB reactors are the most common reactor used by both industries and research. The UASB reactors function at solid content of 4–15% and a HRT of 0.5–12 days. The UASB reactors can be used at higher organic loading rate as compared to other high-rate reactors (Bal and Dhagat 2001).
5.8.3 Tubular Reactors
These reactors are used to produce methane at high altitude at process temperature between 10 and −20 °C (psychrophilic conditions). These are mainly household digester of volume ranges between 2.4 and −7.5 m3. These reactors are used to treat animal wastes with a HRT of 60–90 days. The low-cost tubular reactors have following problems: HRT and biogas pressure. The HRT is calculated by the reactor size not by the pit dimensions. So, the HRT changes during the process depending on the change of volume of the cylindrical bag used as a reactor. Similar is the case with the biogas pressure which also decreases due to reduction in HRT (Lettinga 1995).
The anaerobic digester has following basic components: digester vessel, biogas utilization system, premixing tank, effluent spreading, or distributing system.
The digesters can be classified in two categories: batch and continuous. The batch digesters are very simple to build and operate. The operation of batch digester is having the following steps: (1) loading the digester with the waste material to be digested, (2) the digestion process, (3) and effluent removal. The process is repeated after the completion of these steps. On the other side, the continuous digesters are fed regularly with the waste material. The material is infused into the digester either by flow of the feed or by mechanical methods such as pumps. These digesters can provide a continuous supply of biogas without any lag time due to loading and unloading of the effluent. The continuous digester can be further classified into: horizontal tanks, vertical tanks, and plug flow systems (plug flow digesters and multiple tank systems) (Abdelgadir et al. 2014). Continuous digesters can be used for large-scale production of biogas, but they require high maintenance and operating cost and a more sophisticated design as compared to the batch digesters. Even though anaerobic digesters have been used for sewage stabilization for many years, the use of these digesters is a recent phenomenon. The increasing use of the anaerobic digesters is due to the new reactor designs and better fundamental understanding of the anaerobic process. These new digesters can retain a much higher biomass which has substantially decreased the lag time of the process. The biomass retention technology can be further categorized into two groups: suspended growth (sludge blanket reactors) and attached-based growth (fluidized digesters) (Bal and Dhagat 2001). Different types of anaerobic reactor types used in industries are tabulated elsewhere (Table 2). Schematic representation of these reactors is illustrated in Fig. 2 (Skiadas et al. 2003).
6 Feedstock Used in AD
With the advancement of new anaerobic technology, the biogas can be produced using various feedstock such as plant extract like jatropa, poplar, miscanthus, seashore, sorghum, spartina, switch grass, camelina, etc. The feedstock mentioned above can be grown on marginal lands with very little fertilizer and water (Kimari et al. 2015). Apart from the agricultural feedstock, biogas can be produced from food processing wastes such as corn cobs, fruit waste, cheese whey, nut shells, rice hulls, restaurants waste, sugar waste, etc. Biogas can also be produced from the agricultural wastes (Ge et al. 2016).
6.1 Environmental Advantages of AD
Anaerobic digestion is a sustainable process to get rid of different waste generated and energy recovery from the waste. The bio-CH4 can be utilized as a potential fuel source for an onsite power plant (Fig. 1). This can also benefit the facility for additional revenue streams in various forms such as carbon dioxide credits, renewable energy credits, and/or greenhouse gas emission credits. The anaerobic process can reduce the BOD and COD by 50% approximately. Anaerobic digestion also reduces the phosphorous and nitrogen content of the wastewater which are mainly responsible for eutrophication in lakes or ponds. The process also reduces odor, surface water pollution, and groundwater pollution (Li et al. 2015).
6.2 Bottleneck of AD
Even though the anaerobic digestion has many benefits, it cannot treat all types of wastewater. The anaerobic digester is a good option for biomethane production, but they also have few bottlenecks such as the slow start-up time, large reactor volume, hydrogen sulfide generation, and ammonia inhibition (Ferry 2012).
6.2.1 Ammonia Inhibition
Ammonia is formed in anaerobic digestion due to degradation of nucleic acid, proteins, and urea. The ammonia is present as two forms in aqueous solutions: ammonium ions (NH4 +) and free ammonia (NH3). The optimum amount of ammonia (concentrations less than 200 mg/L) is helpful to anaerobic digestion process that helps to maintain the pH of the digester. But in most of the digesters the excess ammonia is the main inhibitor of the methanogenesis. The indicators of excess ammonia are increased production of intermediates such as VFAs and decrease in production rate of methane. There are many mechanisms proposed by researchers to explain ammonia inhibition: enzymatic reaction inhibition, change in pH, and adding further burden to maintenance energy. The methanogens are highly sensitive to increase in ammonia concentration among all the microbes present in anaerobic digesters. But the methanogens can be adapted to the change in ammonium concentration. This tolerance can be enhanced by change in metabolic pathways or shift in the methanogenic population (Abouelenien et al. 2010).
Free ammonia is highly permeable to the bacterial cell and hence the main cause of ammonia inhibition. Free ammonia diffuses into the microbial cells resulting into potassium deficiency or proton imbalance. The free ammonia changes to ammonium ion inside the cell and absorbs hydrogen ions present in the cell. Hence, free ammonia compels cell to expend more energy to maintain the ionic balance inside the cell. This can cause the inhibition of enzymatic reaction and increases the energy expenditure (Strik et al. 2006). Ammonia can inhibit the anaerobic digestion process at different levels: steady-state inhibition and accumulation of intermediates such as VFAs which can decrease the pH to very low value. The decrease in pH can further aggravate the problem (Wang et al. 2014).
6.2.2 H2S Generation
Sulfate is a very commonly found in industrial and other wastewater. The sulfate present can be reduced by the sulfate-reducing bacteria (SRB) present in the anaerobic digester. There are the two types of SRB: complete oxidizers and partial oxidizers. The complete oxidizers reduce CH3COOH to CO2 and bicarbonates, while the partial oxidizers convert other compounds such as lactate to CO2 and acetate. SRB inhibition can be divided into two stages: primary and secondary inhibition. Primary is a type of competitive inhibition in which SRB competes with other microbes for substrate. This competitive inhibition results in the suppression of methane production (Peu et al. 2012). Secondary inhibition is due to the toxic effect of sulfide on SRB and other microbial groups including methanogens (Chattanathan et al. 2014).
SRB can compete for substrate with almost all kind of microbes present in the anaerobic digester except microbes present in hydrolytic stage. As SRB are not equipped to degrade the complex substrates such as glycogen, lipids, starch, protein etc., SRB have a very high affinity toward propionate which is a very crucial intermediate formed by the acetogens. SRB can also compete for other intermediates such as butyrate and ethanol.
The studies done to establish the toxic effect of sulfides on microbes and its nature are not very conclusive. Some studies supports that H2S is a highly toxic form of sulfides, which can diffuse into the cell and forms disulfide and sulfide cross-links between the polypeptide chains which result in denaturation of the proteins. H2S can also affect the sulfur metabolism and sulfur linkages in various coenzymes. Despite of so many toxic effects, sulfur is a required nutrient by the microbes. The optimal concentration of sulfur in anaerobic digestion differs from 0.001 to 0.025 g/L. However, an inhibitory concentration also varies in a very broad range from 0.1 to −0.8 g/L dissolved sulfur and 0.05 and −0.4 g/L of undissociated hydrogen sulfide approximately (Kang et al. 2010).
7 Conclusions
The technology of anaerobic digestion has highly matured in the past decades. Now, production of methane by anaerobic digestion is used everywhere. The anaerobic digesters have also evolved to use various substrates such as industrial and agricultural wastewater, sewage sludge, household waste, kitchen waste, etc. The energy extraction from the waste can be further maximized by using efficient ways of conversion of waste to biomethane, and it will also depend on the efficient ways of energy utilization. The anaerobic digestion process requires less space and energy. The methane production by anaerobic digestion can be done using simple technology. The process also leaves less amount of stable sludge which can be further used as manure or can be dewatered to form compact leftovers. The fuel gas produced as the final product is a mixture of CH4 (55–75% v/v) and CO2 (25–45% v/v). The calorific value of the mixture produced ranges between 22 and −30 MJ/Kg. The amount of gas produced by the process depends on the concentration of the digestible organics present in the waste and the process parameters such as pH, temperature, etc. The anaerobic digestion process has many applications such as disposal of different industrial and agricultural wastes, sewage purification, production of organic manure, conversion of biomass to energy, etc. The biogas produced by the process also has many applications such as electricity production using fuel cell, cooking fuel, fuel in many industries, etc. Despite much advancement, the biomethanation still have many areas to improve such as slow rate. The main bottleneck which requires improvements are biogas productivity, high capital investment, modular design, and operating cost.
References
Abdelgadir A, Chen X, Liu J, Xie X, Zhang J, Zhang K, Wang H, Liu N (2014) Characteristics, process parameters, and inner components of anaerobic bioreactors. Biomed Res Int 2014. doi:10.1155/2014/841573
Abouelenien F, Fujiwara W, Namba Y, Kosseva M, Nishio N, Nakashimada Y (2010) Improved methane fermentation of chicken manure via ammonia removal by biogas recycle. Bioresour Technol 101:6368–6373. doi:10.1016/j.biortech.2010.03.071
Ahring BK (2003) Biomethanation II. Springer, Berlin
Archer DB, Harris JE (1986) Methanogenic bacteria and methane production in various habitats. Soc Appl Bacteriol Symp Ser 13:185–223
Bal AS, Dhagat NN (2001) Upflow anaerobic sludge blanket reactor – a review. Indian J Environ Health 43:1–82
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Chandrasekhar K, Venkata Mohan S (2012) Bio-electrochemical remediation of real field petroleum sludge as an electron donor with simultaneous power generation facilitates biotransformation of pah: effect of substrate concentration. Bioresour Technol 110:517–525. doi:10.1016/j.biortech.2012.01.128
Chandrasekhar K, Venkata Mohan S (2014a) Bio-electrohydrolysis as a pretreatment strategy to catabolize complex food waste in closed circuitry: function of electron flux to enhance acidogenic biohydrogen production. Int J Hydrog Energy 39:11411–11422. doi:10.1016/j.ijhydene.2014.05.035
Chandrasekhar K, Venkata Mohan S (2014b) Induced catabolic bio-electrohydrolysis of complex food waste by regulating external resistance for enhancing acidogenic biohydrogen production. Bioresour Technol 165:372–382. doi:10.1016/j.biortech.2014.02.073
Chandrasekhar K, Lee YJ, Lee DW (2015a) Biohydrogen production: strategies to improve process efficiency through microbial routes. Int J Mol Sci 16:8266–8293. doi:10.3390/ijms16048266
Chandrasekhar K, Amulya K, Venkata Mohan S (2015b) Solid phase bio-electrofermentation of food waste to harvest value-added products associated with waste remediation. Waste Manag 45:57–65. doi:10.1016/j.wasman.2015.06.001
Chattanathan SA, Adhikari S, McVey M, Fasina O (2014) Hydrogen production from biogas reforming and the effect of H2S on CH4 conversion. Int J Hydrog Energy 39:19905–19911. doi:10.1016/j.ijhydene.2014.09.162
Choong YY, Norli I, Abdullah AZ, Yhaya MF (2016) Impacts of trace element supplementation on the performance of anaerobic digestion process: a critical review. Bioresour Technol 209:369–379. doi:10.1016/j.biortech.2016.03.028
Conklin A, Stensel HD, Ferguson J (2006) Growth kinetics and competition between Methanosarcina and Methanosaeta in mesophilic anaerobic digestion. Water Environ Res Res Publ Water Environ Fed 78:486–496
Díaz I, Fdz-Polanco M (2012) Robustness of the microaerobic removal of hydrogen sulfide from biogas. Water Sci Technol 65:1368–1374. doi:10.2166/wst.2012.013
Diekert G, Wohlfarth G (1994) Metabolism of homoacetogens. Antonie Van Leeuwenhoek 66:209–221. doi:10.1007/BF00871640
Ferry JG (2012) Methanogenesis: ecology, physiology, biochemistry & genetics. Springer Science & Business Media
Ge X, Xu F, Li Y (2016) Solid-state anaerobic digestion of lignocellulosic biomass: recent progress and perspectives. Bioresour Technol 205:239–249. doi:10.1016/j.biortech.2016.01.050
Gerardi MH (2003) The microbiology of anaerobic digesters, Wastewater microbiology series. Wiley-Interscience, Hoboken, NJ
Gorris LG, van der Drift C (1994) Cofactor contents of methanogenic bacteria reviewed. BioFactors 4:139–145
Griffin ME, McMahon KD, Mackie RI, Raskin L (1998) Methanogenic population dynamics during start-up of anaerobic digesters treating municipal solid waste and biosolids. Biotechnol Bioeng 57:342–355. doi:10.1002/(SICI)1097-0290(19980205)57:3
Gujer W, Zehnder AJB (1983) Conversion processes in anaerobic digestion. Water Sci Technol 15:127–167
Hungate RE (1967) Hydrogen as an intermediate in the rumen fermentation. Arch Für Mikrobiol 59:158–164. doi:10.1007/BF00406327
Jain S, Jain S, Wolf IT, Lee J, Tong YW (2015) A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew Sust Energ Rev 52:142–154. doi:10.1016/j.rser.2015.07.091
Kang JW, Jeong CM, Kim NJ, Kim MI, Chang HN (2010) On-site removal of H2S from biogas produced by food waste using an aerobic sludge biofilter for steam reforming processing. Biotechnol Bioprocess Eng 15:505–511. doi:10.1007/s12257-009-0134-8
Kimari GW, Jiang W, Zhang K (2015) Biogas production from high-solid organic biowastes. In: Gas biofuels from waste biomass: principles and advances, pp 87–117
Kiran Kumar A, Venkateswar Reddy M, Chandrasekhar K, Srikanth S, Venkata Mohan S (2012) Endocrine disruptive estrogens role in electron transfer: bio-electrochemical remediation with microbial mediated electrogenesis. Bioresour Technol 104:547–556. doi:10.1016/j.biortech.2011.10.037
Kotelnikova S, Pedersen K (1997) Evidence for methanogenic Archaea and homoacetogenic bacteria in deep granitic rock aquifers. FEMS Microbiol Rev 20:339–349. doi:10.1111/j.1574-6976.1997.tb00319.x
Kumar P, Pant DC, Mehariya S, Sharma R, Kansal A, Kalia VC (2014) Ecobiotechnological strategy to enhance efficiency of bioconversion of wastes into hydrogen and methane. Indian J Microbiol 54(3):262–267. doi:10.1007/s12088-014-0467-7
Kumar P, Sharma R, Ray S, Mehariya S, Patel SKS, Lee JK, Kalia VC (2015) Dark fermentative bioconversion of glycerol to hydrogen by Bacillus thuringiensis. Bioresour Technol 182:383–388. doi:10.1016/j.biortech.2015.01.138
Kumar P, Ray S, Kalia VC (2016) Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour Technol 200:413–419. doi:10.1016/j.biortech.2015.10.045
Lettinga G (1995) Anaerobic digestion and wastewater treatment systems. Antonie Van Leeuwenhoek 67:3–28
Li W-Z, Qian Y, Chang C-C, Ju M (2015) Anaerobic process. Water Environ Res 87:1075–1094. doi:10.2175/106143015X14338845155381
Narihiro T, Sekiguchi Y (2007) Microbial communities in anaerobic digestion processes for waste and wastewater treatment: a microbiological update. Curr Opin Biotechnol 18:273–278. doi:10.1016/j.copbio.2007.04.003
Nguyen D, Gadhamshetty V, Nitayavardhana S, Khanal SK (2015) Automatic process control in anaerobic digestion technology: a critical review. Bioresour Technol 193:513–522. doi:10.1016/j.biortech.2015.06.080
Noike T, Endo G, Chang JE, Yaguchi J, Matsumoto J (1985) Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol Bioeng 27:1482–1489. doi:10.1002/bit.260271013
Novaes RF (1986) Microbiology of anaerobic digestion. Water Sci Technol 18:1–14
O’Flaherty V, Collins G, Mahony T (2006) The microbiology and biochemistry of anaerobic bioreactors with relevance to domestic sewage treatment. Rev Environ Sci Biotechnol 5:39–55. doi:10.1007/s11157-005-5478-8
Peu P, Picard S, Diara A, Girault R, Béline F, Bridoux G, Dabert P (2012) Prediction of hydrogen sulphide production during anaerobic digestion of organic substrates. Bioresour Technol 121:419–424. doi:10.1016/j.biortech.2012.06.112
Prasad RD, Prasad RD (2012) Empirical study on factors affecting biogas production. Int Sch Res Not 2012:e136959. doi:10.5402/2012/136959
Romero-Güiza MS, Vila J, Mata-Alvarez J, Chimenos JM, Astals S (2016) The role of additives on anaerobic digestion: a review. Renew Sust Energ Rev 58:1486–1499. doi:10.1016/j.rser.2015.12.094
Sambo AS, Garba B, Danshehu BG (1995) Effect of some operating parameters on biogas production rate. Renew Energy, World Renew Energy Cong Clim Change, Energy Environ 6:343–344. doi:10.1016/0960-1481(95)00027-H
Schmidt T, Nelles M, Scholwin F, Pröter J (2014) Trace element supplementation in the biogas production from wheat stillage – optimization of metal dosing. Bioresour Technol 168:80–85. doi:10.1016/j.biortech.2014.02.124
Sebola R, Tesfagiorgis H, Muzenda E (2014) Production of biogas through anaerobic digestion of various waste: review. In: Proceedings of International Conference on Chemical, Integrated Waste Management and Environmental Engineering (ICCIWEE’2014). Johannesburg, pp 196–201
Skiadas IV, Gavala HN, Schmidt JE, Ahring BK (2003) Anaerobic granular sludge and biofilm reactors. Adv Biochem Eng Biotechnol 82:35–67
Sowers KR (2014) Methanogenesis. In: Reference module in biomedical research
Stamatelatou K, Antonopoulou G, Michailides P (2014) Biomethane and biohydrogen production via anaerobic digestion/fermentation. In: Waldron KW (ed) Advances in biorefineries: biomass and waste supply chain exploitation, pp. 476–524. doi: 10.1533/9780857097385.2.476
Strik DPBTB, Domnanovich AM, Holubar P (2006) A pH-based control of ammonia in biogas during anaerobic digestion of artificial pig manure and maize silage. Process Biochem 41:1235–1238. doi:10.1016/j.procbio.2005.12.008
Tijani H, Abdullah N, Yuzir A (2015) Integration of microalgae biomass in biomethanation systems. Renew Sust Energ Rev 52:1610–1622. doi:10.1016/j.rser.2015.07.179
Venkata Mohan S, Chandrasekhar K (2011a) Self-induced bio-potential and graphite electron accepting conditions enhances petroleum sludge degradation in bio-electrochemical system with simultaneous power generation. Bioresour Technol 102:9532–9541. doi:10.1016/j.biortech.2011.07.038
Venkata Mohan S, Chandrasekhar K (2011b) Solid phase microbial fuel cell (smfc) for harnessing bioelectricity from composite food waste fermentation: influence of electrode assembly and buffering capacity. Bioresour Technol 102:7077–7085. doi:10.1016/j.biortech.2011.04.039
Venkata Mohan S, Devi MP, Venkateswar Reddy M, Chandrasekhar K, Juwarkar A, Sarma PN (2011) Bioremediation of petroleum sludge under anaerobic microenvironment: influence of biostimulation and bioaugmentation. Environ Eng Manag J 10:1609–1616. doi:10.1007/3-540-45838-7_2
Venkata Mohan S, Chandrasekhar K, Chiranjeevi P, Babu PS (2013) Chapter 10 – biohydrogen production from wastewater. In Larroche AP-SCCH (ed) Biohydrogen. Elsevier, Amsterdam, pp 223–257. ISBN: 978-0-444-59555-3 doi: 10.1016/B978-0-444-59555-3.00010-6
Wang X, Lu X, Li F, Yang G (2014) Effects of temperature and Carbon-Nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: focusing on ammonia inhibition. PLoS One 9:e97265. doi:10.1371/journal.pone.0097265
Zinder SH (1990) Conversion of acetic acid to methane by thermophiles. FEMS Microbiol Lett 75:125–137. doi:10.1111/j.1574-6968.1990.tb04090.x
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Patel, V., Pandit, S., Chandrasekhar, K. (2017). Basics of Methanogenesis in Anaerobic Digester. In: Kalia, V. (eds) Microbial Applications Vol.2. Springer, Cham. https://doi.org/10.1007/978-3-319-52669-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-52669-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52668-3
Online ISBN: 978-3-319-52669-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)