Abstract
Classically, the pupil light reflex pathway is considered to be a simple reflex arc consisting of the retinal ganglion cells, intercalated neurons in the midbrain, the oculomotor nerve, and short ciliary nerves. However, there are some specialties in the structure of the afferent pupillary pathway that should be taken into account when interpreting pupillary disorders and that can help in the topodiagnosis of the lesion. Moreover, studies in patients with lesions of the retrogeniculate pathway showed that the pupillary pathway is more complex than previously assumed and the retrogeniculate visual pathway and the visual cortex are also involved in the pupillary light reaction. Clear anatomic evidence is still lacking but pupillographic measurements in patients with various disorders of the visual pathway support the existence of two pupillomotor channels that drive the pupil light reaction – the subcortical (more primitive, luminance channel associated with the intrinsically photosensitive retinal ganglion cells) and the suprageniculate (responds to shifts in structured stimuli, is driven by the rods and cones, and receives input from the visual cortex and extrastriate areas). The chapter summarizes possible pupillary findings in patients with homonymous hemianopia.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Pupil
- Pupil light reflex
- Relative afferent pupillary defect
- Pupil perimetry
- Chromatic pupillography
- Swinging flashlight test
- Hemihypokinesia
- Hemiakinesia
- Intrinsically photosensitive retinal ganglion cells
- Melanopsin
7.1 Introduction
The neural pathway of the pupillary light reflex as first described by Wernicke [1, 2] in 1880s consists of four neurons (Fig. 7.1). Afferent fibers of the retinal ganglion cells travel in the optic nerve and undergo hemidecussation at the chiasm before entering the optic tract. In the posterior third of the optic tract, the pupillomotor fibers separate from the sensory fibers, branch medial via the brachium of the superior colliculus to the lateral geniculate nucleus, and synapse in the ipsilateral pretectal nucleus in the dorsal midbrain. Intercalated neurons from each pretectal nucleus then project to both Edinger-Westphal nuclei and parasympathetic fibers from the Edinger-Westphal nuclei innervate the iris pupillary sphincter muscle. According to this model, the suprageniculate visual pathway should have no influence on the pupillary light reflex. However, studies in patients with lesions of the retrogeniculate pathway showed that the pupillary pathway is more complex than previously assumed and the retrogeniculate visual pathway and the visual cortex are also involved in the pupillary light reaction.
The human pupillary pathway as first described by Wernicke consists of four neurons (excluding photoreceptors and bipolar cells in the retina): retinal ganglion cells (1), intercalated neurons in the midbrain (2), oculomotor nerve (3), and short ciliary nerves (4). The simplicity of this model can be no longer accepted (From Wilhelm [2], with permission)
Homonymous hemianopia means vision loss on the same side of the visual field in both eyes and is indicative of a lesion involving the visual pathway posterior to the chiasm. Patients with a visual field defect should always have their pupils examined and this applies even more so in the case of homonymous visual field defects. This chapter should summarize possible pupillary findings in patients with homonymous hemianopia.
7.2 Examination of Pupils
Examination of the pupils offers objective evaluation of visual function as well as of the vegetative pathways to the eye. Essential information is gathered within a short time. This makes pupillary inspection a valuable part of routine ophthalmological, neurological, and general medical examinations. Due to the proximity of pupillary pathways to various anatomic structures, pupillary dysfunction can be caused by a variety of disorders, some of which may be life threatening. Due to differences in the course of pupillomotor and sensory fibers, pupillary tests can help in the localization of a visual pathway lesion. The ophthalmologist plays a key role in detecting pupillary disorders and in directing further investigations. Therefore, one should have a good knowledge of the diagnostic significance of pupillary function and dysfunction.
There are several ways of how to examine the pupil light reaction. Some methods are based on the asymmetry in the afferent visual pathway, another on the examination of the visual field by means of measuring the pupil light reaction to focal light stimuli or on stimulation methods that are similar to multifocal electroretinography. Recently developed chromatic pupillography can identify pupil light response mediated by the rods, cones, or the intrinsically photosensitive retinal ganglion cells containing melanopsin.
7.2.1 Relative Afferent Pupillary Defect and Swinging Flashlight Test
The most frequently evaluated pupillary parameter in clinical practice is the relative afferent pupillary defect (RAPD). It is typically related to lesions within the anterior visual pathway and is almost always present in unilateral or asymmetric bilateral diseases of the optic nerve, chiasm, or the optic tract. It can be diagnosed by means of the swinging flashlight test and is characterized by diminished pupillary constriction on direct illumination with a normal consensual response to illumination of the contralateral eye.
Swinging flashlight test can be performed as follows: In a darkened room ask the patient to fixate an object in a few meters’ distance. Shine with the ophthalmoscope in an angle of 45° from below and from the distance of 20–40 cm into the eyes. Move the light quickly from one eye to the other and observe the direct pupil light reaction of both pupils. Both pupils should be illuminated for the same time (ca. 2 s) and the switch between both eyes should be repeated at least five times. If a relative afferent pupillary defect is present on one side, then at the illumination of this eye both pupils will either enlarge without any previous contraction or this contraction will be smaller and shorter. RAPD can be quantified by means of neutral density filters and expressed in log units: A filter is placed between light source and the “good eye”. If there is still a RAPD defect visible, a filter with higher density is chosen until the difference in pupillary constriction between both eyes disappears or even the RAPD switches side. The density of the filter necessary to compensate the side difference is a measure for the RAPD.
7.2.2 Pupil Perimetry
Pupil perimetry or campimetry is an objective visual field test that measures pupil light reaction (PLR) to focal light stimuli projected onto the retina. Light stimuli are presented at various locations in the visual field, similar as in standard perimetry. However, as the threshold for the pupil light response is higher than the differential light threshold in conventional perimetry, stimuli in pupil perimetry have to be brighter or larger. Brighter stimuli increase straylight, and larger stimuli reduce spatial resolution of pupil perimetry. This is the major problem of all systems applied in pupil perimetry. To overcome this, M-sequence techniques known from multifocal electroretinography have been applied but not yet tested against conventional pupil perimetry.
Visual field defects in pupil perimetry can be recognized by a reduced or absent pupil light reaction within these areas. Studies dealing with clinical applications of pupil perimetry have shown that most diseases affecting the retina and the visual pathway caused pupil field scotomata which match the defects found in standard perimetry (Figs. 7.2, 7.3, and 7.4) [3,4,5].
(Top) Visual field in a patient with sphenoid wing meningioma causing a lower altitudinal defect in the left eye. (Bottom) Pupil field of the left eye as detected by means of pupil perimetry. The column represents the mean value of pupil light response amplitude in millimeters at each tested location in the visual field. Corresponding pupil field defect in the lower hemifield can be recognized by a reduced pupil light reaction in this area
(Left) Schematic drawing of advanced concentric visual field loss in a patient with retinitis pigmentosa as detected by kinetic perimetry (Goldmann stimulus V4). (Right) Corresponding pupil field with pupil light reaction present only within the preserved visual field (From Skorkovská et al. [4], with permission)
Pupil perimetry can be performed either by means of a special pupillographic device or by a modified standard perimeter. However, most of these devices serve for research purposes and only a few machines are available commercially. In our laboratory, the pupillographic device consists of a computer, a 19-inch CRT screen for the stimulus presentation, and a third monitor for continuous monitoring of fixation by observation (Fig. 7.5). Stimuli are displayed on the computer screen at a distance of 20 cm from the subject’s eye. A small red spot is presented for fixation. Blinds around the device prevent stray light from the room disturbing the measurement. The pupil reaction is recorded by means of an infrared-sensitive video camera. The pupil edges can be determined by the contrast of the dark fundus and a very light iris infrared reflex. During the test the examiner can observe the quality of fixation, the stimulus sequence, as well as the continuous pupillographic curve. For the stimuli, white light is usually used and different stimulus intensities can be tested with a constant background luminance of 2.7 cd/m2. The stimulus is usually presented for 200 ms every 2000 ms.
In contrast to standard visual perimetry, pupil perimetry represents a method for objective visual field examination. It can be very useful particularly in patients suspected of stimulation [6] or in patients who do not manage standard perimetry well enough.
7.2.3 Chromatic Pupillography
Recently it was found that not only the rods and cones, but also other retinal elements – retinal ganglion cells containing melanopsin (ipRGCs) – are intrinsically photosensitive and capable of phototransduction [7,8,9,10]. Unlike rods and cones they do not or only marginally contribute to image formation. They serve more as a detector of the surrounding light intensity and are involved in the management of circadian rhythm. In addition to that, axons of the ipRGCs are connected with the pretectal area and can drive pupil light reaction, particularly at high intensities of light (100 cd/m2). This explains why people who lost sight because of a photoreceptor disease still may have normal pupil light reaction and circadian rhythm [11, 12].
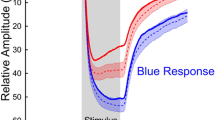
Rods and cones are located in the outer retina, ipRGCs in the inner retinal layer. Each type of photoreceptors has its different wavelength sensitivity. The peak sensitivity of the ipRGCs is in the blue spectrum around 480 nm. By registering the pupil light reaction to light stimuli of different color and intensity, it is possible to separately test the function of different population of retinal photoreceptors, and like this evaluate and monitor the function of outer retina (rods and cones) and inner retina (ipRGC). This method is called chromatic pupillography and appears as a highly sensitive method for objective examination of neuroretinal function that might become a useful complement to electrophysiological tests, at this moment more for research purposes or clinical trials (Figs. 7.6 and 7.7) [13].
The relative pupil light response amplitude to red and blue light stimulus in healthy subjects. With blue light, the relative amplitude is significantly greater and the time to maximal pupil constriction significantly longer compared to red light for all tested time points (indicated by the vertical lines A–D). Blue light evokes the “sustained” pupil contraction (driven by ipRGCs), while the red light rather the “transient” contraction (driven by rods and cones) (From Skorkovská et al. [13], with permission)
7.3 RAPD in Optic Tract Lesions
Optic tract lesions are characterized by homonymous visual field defects, asymmetric bilateral optic disc atrophy (more pronounced contralateral to the lesion), and contralateral RAPD (Fig. 7.8). The closer the lesion is located to the chiasm the more incongruent are the visual field defects. Visual acuity is usually not affected. The suggested causes for this contralateral RAPD in an optic tract lesion are a greater nasal photoreceptor density, a ratio of crossed to uncrossed fibers in the chiasm of 53:47, and a temporal visual field 61–71% larger than the nasal field [14]. A tract lesion disrupts fibers from the contralateral nasal retina and the ipsilateral temporal retina, thus disproportionally diminishing input from the contralateral eye and producing a corresponding RAPD. However, the magnitude of RAPD in patients with an optic tract lesion can range from 0.3 logE to 1.0 logE and this can, probably, be completely explained neither by the rather small asymmetry of crossed to uncrossed fibers nor the difference between temporal and nasal hemifield [15].
Schematic representation of different findings according to the course of the pupil light reflex pathway (OT optic tract, M midbrain, N3 oculomotor nerve, OR optic radiation). Lesions of the optic tract result in homonymous hemianopia with contralateral relative afferent pupillary defect (RAPD). Lesions of the brachium of the superior colliculus cause contralateral RAPD but no visual field defect. In suprageniculate lesions with sufficient distance from lateral geniculate body homonymous hemianopia without RAPD develops
Patients with an optic tract lesion represent a unique model for studies of the hemifield organization of the afferent pupillomotor system. A complete tract lesion enables the comparison of the pupil light reaction from temporal and nasal retina without the disturbing influence of stray light because only the intact retinal half can participate in the pupil light reaction. Because of stray light such an estimation of the nerve fiber distribution in the pupillary pathway is not precisely possible in a healthy eye with both retinal halves functioning. By means of pupillography it could be shown that in case of separate light stimulation of either of the retinal halves in optic tract lesions, the pupil light reaction was always greater in the preserved temporal visual field ipsilateral to the site of the tract lesion, compared to the functional contralateral nasal visual field. So, RAPD in optic tract lesions probably reflects the difference in light sensitivity of the intact temporal and nasal visual field [16].
7.4 RAPD Without Visual Field Loss
Prior to the termination of retinal ganglion cell axons in LGN, the pupillomotor fibers branch off and travel via the brachium of the superior colliculus to the ipsilateral pretectal nucleus, where they synapse with the next neuron of the pupillomotor pathway. This small region between the optic tract and pretectal area is called pretectal afferent pupillary pathway and is located inside the dorsal midbrain in the brachium of the superior colliculus. A pathology in this area will cause a contralateral RAPD without any visual impairment – that means no decrease in visual acuity, no visual field loss and no optic atrophy (Fig. 7.8). If the lesion was located more proximally (e.g., in optic tract), a visual field defect would be present and on the other hand, if the lesion was more distally (e.g., in Edinger-Westphal nucleus), an anisocoria would be observed.
There are several reports [17,18,19] in the literature dating back to 1920s that describe patients with a unilateral RAPD without any visual impairment. Most of the patients had a pathology in the dorsal midbrain and all authors considered the cause lesion of the pretectal afferent pupillary pathway in dorsal midbrain. Recently, it was shown by means of pupil perimetry that the pupil field in these patients looked exactly like the visual field in an optic tract lesion [20]. So, the RAPD without visual loss is simply a variant of the RAPD in an optic tract lesion, in which the site of the lesion is moved towards dorsal midbrain and leaves the visual function intact.
7.5 RAPD in Suprageniculate Lesions with Homonymous Visual Field Defect
Detection of a RAPD in acute homonymous hemianopias has been commonly used in differentiating infrageniculate from suprageniculate lesions, since neither optic atrophy nor a RAPD should occur in acquired affections of the optic radiation or the visual cortex. However, there are exceptions.
For instance, RAPD has been described in patients with congenital occipital hemianopia [21]. The suggested mechanism was transsynaptic optic tract atrophy after intrauterine or perinatal damage to the suprageniculate visual pathway, which presumably affected also the afferent pupillary fibers to the pretectal area of the midbrain. This explanation sounds plausible and in accordance with what was written above.
Further, there are numerous studies, reporting disturbances of the PLR in patients with acquired HVFDs due to lesions not involving the optic tract, that are no more compatible with the traditional model of the pupillary pathway: either the presence of pupillary “hemiakinesia” or “hemihypokinesia” in the blind part of the visual field [3,4,5, 22,23,24,25,26,27] or RAPD contralateral to the brain lesion, as a response to full-field light stimulation [28, 29]. Results of these studies provide evidence that the pupil light reaction is not a pure subcortical pathway.
Further progress in understanding the underlying anatomic pupillary pathway could be achieved thanks to advances in neuroimaging. Modern methods of analysis enable us to define any lesion very precisely. Like this, clinically relevant RAPD, as a response to full-field light stimulation, could be limited to suprageniculate lesions that were found closer than 10 mm to the LGN or involving it, but sparing the optic tract. In lesions located more than 18 mm from the LGN, RAPD did not occur [29]. It was concluded that RAPD was probably not caused by a lesion of the visual pathway itself, but by a lesion of the intercalated neurons between the visual pathway and the pupillomotor centers in the pretectal area of the midbrain, comparable to the lesions that cause RAPD without visual field loss. Further, using a new strategy of lesion analysis by combining subtraction techniques with the stereotaxic probabilistic cytoarchitectonic map it was found that a region in the early course of the optic radiation in the temporal white matter, close to the LGN, seems to be associated with the presence of RAPD. This finding is consistent with the hypothesis that the connection between visual pathway and pretectal area in the dorsal midbrain is probably closely related to the LGN and its involvement in suprageniculate homonymous hemianopias can lead to RAPD. So, there seems to be more input from suprageniculate neurons and the occipital cortex but the exact anatomy of this connection is still unclear. It may be that the critical area in the early course of the optic radiation near LGN is the site of integration of cortical signals in relation to the PLR into the pupillomotor pathway. Another explanation could be that some afferent pupillomotor fibers of infrageniculate origin bypass the LGN and then travel through this critical area to the mesencephalon.
In summary, the classical view of the pupillary pathway in postchiasmal lesions of the visual pathway is basically true. Infra- and suprageniculate lesions can still be distinguished by the presence of RAPD. However, it must be kept in mind that RAPD can develop also in lesions in the surroundings of the pretectal area. And the situation is even more complicated in case of pupillary hemihypokinesia that is to be discussed.
7.6 Pupillary Hemihypokinesia
According to the classic idea of the pupillary pathway, infrageniculate lesions should present with a hypokinesia, suprageniculate lesions should not. However, many studies [3,4,5, 22,23,24,25,26,27] in patients with retrogeniculate damage and homonymous visual field defects have provided evidence for impairment of pupil responses to small localized stimuli registered by pupillography. Early clinical reports dating back to 1940s were later reproduced by other groups using modern pupillometric techniques in patients well documented by magnetic resonance imaging or computed tomography, and currently there is no doubt that the retrogeniculate visual pathway or even visual cortex is involved in the pupillary light reaction. In patients with retrogeniculate damage the so-called pupillary hemihypokinesia can be observed which differs from RAPD.
Pupillary hemihypokinesia (or akinesia) means a reduced or absent pupil light reaction to perimetric stimuli in the blind part of the visual field and was observed in all kinds of postchiasmal lesions (Fig. 7.9). The first pupillometric measurements in patients with suprageniculate lesions have been performed already by Harms in 1949 [22] and have challenged the Wernicke’s description of the pupil light reflex. Harms found reduced pupil light reaction in war veterans with occipital lobe injuries. At that time, his results were called into question and the findings ascribed to the transsynaptic degeneration or to an overlooked pregeniculate damage. Harm’s findings were eventually many times reproduced, later also with the help of modern pupillographic equipment and sophisticated imaging methods. Still, even today we can only speculate about the underlying cause of this phenomenon.
(Top) Visual field in a patient with superior left homonymous quadrantanopia due to an ischemia. (Bottom) Pupil field of the same patient showing a reduced or absent pupil light reaction in the affected portion of the visual field (From Skorkovská et al. [4], with permission)
The findings, for example, can be explained by the view, that in pre- and retrogeniculate lesions different components of the light response may be involved to a different extent. The steady-state component of the pupillary light response regulates the resting pupil diameter depending on the ambient light level; it is characterized by a large spatial summation and a wide dynamic range. This component is represented basically by the subcortical pupillary pathway. The transient component of the pupil light response is responsible for the constriction of the pupil in response to brisk light stimuli. In the presence of this component, the steady-state signal is largely discarded. The transient component reflects merely novel changes in luminance contrast; it is characterized by a “limited spatial summation, band-pass temporal response characteristics, and high contrast gain” [30, 31]. It is obvious that the stimulus characteristics of pupil perimetry predominantly address this transient component. There is strong evidence that – after cortical processing of specific stimulus characteristics – projections from the extrastriate visual cortex contribute considerably to the transient pupil response component.
Indeed, pupillographic measurements with specific stimuli (isoluminant pattern stimuli, chromatic stimuli or moving stimuli) in patients with a retrogeniculate lesion indicate the possible existence of two separate pupillomotor channels: the PLR in the blind hemifield was reduced but not absent. However, all the other specific, “higher” pupil responses to stimulus attributes, like stimulus color, structure, or motion, were completely lost. On the other hand, studies in patients with Parinaud syndrome [32] demonstrated that there was a small, residual PLR and preserved reactions to pattern and color stimuli as well as preserved pupillary sleepiness-related oscillations. Again, the existence of a cortical input to the pupillary pathway was suggested, since the retinal afferent input to the pretectal nuclei had been apparently damaged.
Hence, it is considered that two or more distinct channels could serve the PLR: a more primitive “luminance channel,” which connects the retina directly with the pretectal area and responds to diffuse light, and “pattern channel,” which is mediated suprageniculately and responds to shifts in structured stimuli, like isoluminant grating, motion, and isoluminant color stimuli. The PLR is primarily mediated by the luminance channel and to a smaller extent by the “weaker,” suprageniculate pattern channel (Fig. 7.10). It seems that the intrinsically photosensitive retinal ganglion cells operate merely on the subcortical level, while the cortical pathway may rely more on ganglion cells that carry predominantly cone inputs. Additionally, it needs to be considered that a pupillary constriction could also be evoked by temporarily canceling the inhibition of the Edinger-Westphal nucleus by the central sympathetic inhibiting system. This might provide a second pathway for pupillary constriction.
Schematic drawing of the current view of the pupillary light reflex pathway. Afferent pupillomotor fibers travel in the optic nerve and undergo hemidecussation at the chiasm before entering the optic tract. In the posterior third of the optic tract, the pupillomotor fibers branch medial via the brachium of the superior colliculus to the lateral geniculate nucleus (LGN) and synapse in the ipsilateral pretectal nucleus (PN) in the dorsal midbrain. Intercalated neurons from each pretectal nucleus then project to both Edinger-Westphal nuclei. Parasympathetic fibers from the Edinger-Westphal nuclei (NEW) travel with the oculomotor nerve to the ciliary ganglion (CG) and via the short ciliary nerves (SCN) innervate the iris pupillary sphincter muscle. However, there seems to be more input from suprageniculate neurons and the visual cortex (CX), although the exact anatomy of this connection is still unclear. It may be that stimuli with different attributes are processed at a different level – subcortically or by suprageniculate neurons and the visual cortex. The proposed site of integration of cortical signals to the pupillary response should be located in the early course of the optic radiation near the LGN (From Papageorgiou et al. [29], with permission)
Conclusion
Pupillary findings in patients with pregeniculate lesions of the visual pathway are consistent with the subcortical course of the pupil light reflex arc. However, the evidence of pupillary hemihypokinesia in patients with homonymous visual field defects due to retrogeniculate lesions of the visual pathway supports the hypothesis that the afferent pupillary system is not purely a subcortical reflex arc but consists of two pathways: one of these via intrinsically photosensitive retinal ganglion cells (ipRGCs) directly reaching the dorsal midbrain, the other running through the normal RGCs via the visual cortex; although the exact anatomy of this pathway is still unclear. The subcortical pathway accounts for changes in pupil diameter to stimuli of high intensity, whereas the cortical part responds particularly to higher stimulus attributes like color, structure, or motion. Future research will certainly provide further understanding of the problem.
References
Wernicke C. Über hemianopische Pupillenreaktion. Fortschr Med. 1883;1:9–53. (Article in German).
Wilhelm H. Pupille und retrogenikuläre Sehbahn. Ophthalmologe. 1996;93:319–24. (Article in German).
Schmid R, Lüdtke H, Wilhelm B, Wilhelm H. Pupil campimetry in patients with visual field loss. Eur J Neurol. 2005;12(8):602–8.
Skorkovská K, Wilhelm H, Lüdtke H, Wilhelm B. How sensitive is pupil campimetry in hemifield loss? Graefes Arch Clin Exp Ophthalmol. 2009;247(7):947–53.
Kardon RH. Pupil perimetry. Curr Opin Ophthalmol. 1992;3(5):565–70.
Skorkovská K, Lüdtke H, Wilhelm H, Wilhelm B. Pupil campimetry in patients with retinitis pigmentosa and functional visual field loss. Graefes Arch Clin Exp Ophthalmol. 2009;247(6):847–53.
Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600–5.
Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–70.
Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81.
Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin knockout mice. Science. 2003;299(5604):245–7.
Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007;27(3):195–204. Review.
Wilhelm BJ. [The eye of the inner clock—pupil research in a new light.] Das Auge der Inneren Uhr – Pupillenforschung in neuem Licht. Klin Monbl Augenheilkd. 2010;227(11):840–4. (Article in German).
Skorkovská K, Maeda F, Kelbsch C, Peters T, Wilhelm B, Wilhelm H. Pupillary response to chromatic stimuli. Cesk Slov Neurol N. 2014;77/110(3):334–8.
Kupfer C, Chumbley L, Downer J. Quantitative histology of optic nerve, optic tract and lateral geniculate nucleus of man. J Anat. 1967;101(Pt 3):393–401.
Schmid R, Wilhelm B, Wilhelm H. Naso-temporal asymmetry and contraction anisocoria in the pupillomotor system. Graefes Arch Clin Exp Ophthalmol. 2000;238(2):123–8.
Kardon RH, Kawasaki A, Miller NR. Origin of the relative afferent pupillary defect in optic tract lesions. Ophthalmology. 2006;113(8):1345–53.
Johnson RE, Bell RA. Relative afferent pupillary defect in a lesion of the pretectal afferent pupillary pathway. Can J Ophthalmol. 1987;22(5):282–4.
Forman S, Behrens MM, Odel JG, Spector RT, Hilal S. Relative afferent pupillary defect with normal visual function. Arch Ophthalmol. 1990;108(8):1074–5.
King JT, Galetta SL, Flamm ES. Relative afferent pupillary defect with normal vision in a glial brainstem tumor. Neurology. 1991;41(6):945–6.
Papageorgiou E, Wermund T, Wilhelm H. Pupil perimetry demonstrates hemifield pupillary hypokinesia in a patient with a pretectal lesion causing a relative afferent pupil defect but no visual field loss. J Neuroophthalmol. 2009;29(1):33–6.
Tychsen L, Hoyt WF. Relative afferent pupillary defect in congenital occipital hemianopia. Am J Ophthalmol. 1985;100(2):345–6.
Harms H. Grundlagen, Methodik und Bedeutung der Pupillenperimetrie für die Physiologie und Pathologie des Sehorgans. Albrecht Von Graefes Arch Ophthalmol. 1949;149:1–68.
Harms H. Hemianopische Pupillenstarre. Klin Monbl Augenheilkd. 1951;118:133–47. (Article in German).
Bresky R, Charles S. Pupil motor perimetry. Am J Ophthalmol. 1969;68(1):108–12.
Cibis GW, Campos EC, Aulhorn E. Pupillary hemiakinesia in suprageniculate lesions. Arch Ophthalmol. 1975;93:1322–7.
Alexandridis E, Krastel H, Reuther R. Pupillenreflexstörungen bei Läsionen der oberen Sehbahn. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1979;209(3):199–208. (Article in German).
Hellner KA, Jensen W, Mueller-Jensen A. [Videoprocessing pupillographic perimetry in hemianopsia] Fernsehbildanalytische pupillographische Perimetrie bei Hemianopsie. Klin Monbl Augenheilkd. 1978;172(5):731–5. (Article in German).
Wilhelm H, Wilhelm B, Petersen D, Schmidt U, Schiefer U. Relative afferent pupillary defects in patients with geniculate and retrogeniculate lesions. Neuro Ophthalmol. 1996;16(4):219–24.
Papageorgiou E, Ticini LF, Hardiess G, Schaeffel F, Wiethoelter H, Mallot HA, et al. The pupillary light reflex pathway: cytoarchitectonic probabilistic maps in hemianopic patients. Neurology. 2008;70(12):956–63.
Barbur JL, Keenleyside MS, Thompson WD. Investigations of central visual processing by means of pupillometry. In: Kulikowski JJ, Dickinson CM, Murray TJ, editors. Seeing contour and colour. Oxford: Pergamon Press; 1987. p. 431–51.
Barbur JL. Learning from the pupil – studies of basic mechanisms and clinical applications. In: Chalupa LM, Werner JS, editors. The visual neurosciences. Cambridge: MIT Press; 2004. p. 641–56.
Wilhelm BJ, Wilhelm H, Moo S, Barbur JL. Pupil response components: studies in patients with Parinaud’s syndrome. Brain. 2002;125(Pt 10):2296–307.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Skorkovská, K., Wilhelm, B., Wilhelm, H. (2017). Pupillary Disorders in Homonymous Visual Field Defects. In: Skorkovská, K. (eds) Homonymous Visual Field Defects. Springer, Cham. https://doi.org/10.1007/978-3-319-52284-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-52284-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52282-1
Online ISBN: 978-3-319-52284-5
eBook Packages: MedicineMedicine (R0)