Abstract
-
Moderate-to-severe sleep-disordered breathing (SDB) is common in the general population, particularly between ages 50 and 70.
-
Obstructive sleep apnea (OSA) is a major public health concern presenting a high associated risk for hypertension, cardiovascular disease, type II diabetes, and motor vehicle accidents. The dentist should always evaluate for the presence of risk factors in patients with any signs of SDB.
-
PSG is the gold standard for the diagnosis of OSA and sleep disorders.
-
The CPAP device is still considered the most effective therapeutic approach for the management of SDB in both adults and children; however, their side effects and low adherence make clinicians look for alternative treatment options.
-
Oral appliances (OA), especially mandibular advancement devices (MAD), have become viable option for patients with mild-to-moderate OSA or with primary snoring.
-
The sleep physician should be the one providing an accurate diagnosis and prescribe OA when indicated. The dentist is considered a key part of the multidisciplinary management team for OSA.
-
When using OA, then dentist should use custom-made ones allowing for the titration of the device and should monitor closely the resolution of OSA symptoms and possible side effects.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
-
Moderate-to-severe sleep-disordered breathing (SDB) is common in the general population, particularly between ages 50 and 70.
-
Obstructive sleep apnea (OSA) is a major public health concern presenting a high associated risk for hypertension, cardiovascular disease, type II diabetes, and motor vehicle accidents. The dentist should always evaluate for the presence of risk factors in patients with any signs of SDB.
-
PSG is the gold standard for the diagnosis of OSA and sleep disorders.
-
The CPAP device is still considered the most effective therapeutic approach for the management of SDB in both adults and children; however, their side effects and low adherence make clinicians look for alternative treatment options.
-
Oral appliances (OA), especially mandibular advancement devices (MAD), have become viable option for patients with mild-to-moderate OSA or with primary snoring.
-
The sleep physician should be the one providing an accurate diagnosis and prescribe OA when indicated. The dentist is considered a key part of the multidisciplinary management team for OSA.
-
When using OA, then dentist should use custom-made ones allowing for the titration of the device and should monitor closely the resolution of OSA symptoms and possible side effects.
1 Introduction and Diagnostic Subtypes
Sleep-related breathing disorders in adults are a heterogeneous group of disorders characterized by different abnormalities of respiration during sleep. The term sleep-disordered breathing (SDB) has been used interchangeably with the term obstructive sleep apnea (OSA), which has been now supplanted by the term obstructive sleep apnea–hypopnea (OSAH) syndrome. However, in strict terms, we should use the term SDB to encompass the entire group of respiratory abnormalities occurring during sleep, not only just the obstructive sleep events. Taking these terms in consideration, the ICSD-3 classifies the sleep-relating breathing disorders as in Table 13.1 below.
Although classically the spectrum of sleep-disordered breathing ranged from primary snoring to severe sleep apnea (Fig. 13.1) and that can be even considered the better approach for the dentist to understand these types of disorders, it is important to point out this aspect in order to keep a balance between the clinical presentation and the rigor of the nomenclature in the sleep medicine field. The term obstructive sleep apnea–hypopnea (OSAH) syndrome should only be applied when making reference to the spectrum of disorders in the family of obstructive respiratory events, including OSA among others.
Firstly, it is important to differentiate the terms OSA and central sleep apnea (CSA) since it will have a definite impact in the treatment that we can provide to our patients. Further, it will also define the role of the dentist or the orofacial pain/oral medicine practitioner on the management of these conditions. Nevertheless, OSA is the most common and serious medical condition and is characterized by recurrent cessation or substantial reduction in breathing during sleep. OSA is characterized by repeated cessation of breathing during sleeping, due mostly to complete or partial oropharyngeal obstruction; therefore, it is a serious, potentially life-threatening condition. OSA is usually found in severe snorers, in which there are periods of decreased breathing or a complete cessation of breathing during sleep due to an obstruction of airflow. The differences in the definition of OSA, CSA, and a combination of these two (referred as “mixed apnea”) are displayed in Table 13.2.
The term “upper airway resistance syndrome” (UARS) was coined by Guilleminault et al. In 1993 UARS is usually defined as the presence of daytime sleepiness associated to a sleep-disordered breathing and microarousals related to respiratory effort (RERA) but without sufficient apneas/hypopneas episodes for meeting the criteria for OSA. The diagnosis is based on both the association of clinical symptoms and polysomnographic findings. UARS patients usually complain of snoring associated with daytime sleepiness.
2 Clinical Presentation
Several types of events can occur during sleep-disordered breathing (SDB) and a detailed description of them can be found in Table 13.3.
The clinical spectrum of SDB conditions tends to progress with aging as seen on Fig. 13.1.
Firstly, the primary snoring condition is primarily a “social” problem where no excessive daytime sleepiness is present, and the sleep study is within normal observations. Primary snoring can be further categorized as intermittent, mild chronic, and heavy chronic as it increases in severity.
Secondly, as for UARS, snoring and excessive daytime sleepiness are present though sleep study is found normal.
Lastly, obstructive sleep apnea (OSA) is the most common condition with three severity stages (Table 13.4) according to the apnea–hypopnea index (AHI), which is the number of apnea and hypopnea events per hour of sleep. This condition is usually associated with:
-
Profound snoring, gasping, snoring, and cessation of breathing during sleep
-
Excessive daytime sleepiness
-
An abnormal sleep study
-
Multiple systemic effects (life-threatening potential)
More clinical differences between OSA and UARS can be found in Table 13.5, according to polysomnography (PSG) and presence of other signs, symptoms, and medical conditions.
3 Epidemiology and Etiology (Risk Factors)
Primary Snoring:
-
Occurs in all age groups, but increases with age (40–60% of adults over 40 report snoring).
-
In children, it is usually related to the presence of enlarged tonsils or adenoids.
-
More prevalent in males, particularly in Hispanic, Asian, and African American.
-
Three times more common in obese people.
Obstructive Sleep Apnea (OSA): Overall prevalence is 9% in females and 24% in males
-
Symptomatic OSA in 2% of females and 4% of males.
-
Have signs of potential sleep-disordered breathing (SDB) since RDI >5.
-
Moderate-to-severe SDB is frequent in the general population, affecting 17% of males and 9% of females between ages 50 and 70.
OSA Risk Factors
Several risk factors have been described of being responsible for the development and progression of OSA. These can be categorized as modifiable and non-modifiable OSA risk factors and are presented in Fig. 13.2.
4 Pathophysiology and Mechanisms
The critical abnormality in OSA is the repetitive complete or partial collapse of the upper airway during sleep. Because of the relationship between form and function, upper airway anatomy must be taken into consideration in OSA pathophysiology. The upper airway has a collapsible segment that extends from the hard palate to the vocal cords. Thus, the size and the shape of the upper airway will determine the probability of suffering OSA. When there is a restriction in the size of the bony compartment, and excess of soft tissue around the airway or a combination of the two, there will be an excess of extraluminal tissue pressure, producing subsequently a reduction in the caliber and thus affecting negatively the degree of patency (Fig. 13.3).
Certain skeletal conditions as retrognathia; retro-positioning of the maxilla, mandibular, and hypoplasia; or an inferiorly positioned hyoid bone reduce the volume of the bony compartment. In addition, deposition of fat tissue around the upper airway as seen in obesity, macroglossia, adenotonsillar enlargement, thickening of the lateral pharyngeal walls, enlargement of the soft palate, and edema/inflammation are among the soft tissue factors that can favor the airway collapse.
However, the activity of the pharyngeal dilatator muscles and the central control of the ventilation are also key factors in the pathophysiology of OSA. The impairment of mechanoreceptor sensitivity, of the upper airway neuromuscular reflexes, and of the strength and endurance of pharyngeal dilatator muscles can produce a decrease in the pharyngeal dilator muscle activity. The dysregulation of the upper airway neuromuscular reflexes can be a consequence from a neuro-sensorial injury due to inflammation and trauma on the upper airway caused by snoring.
4.1 Systemic Effects of OSA
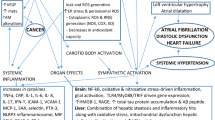
Obstructive sleep apnea (OSA) is a potential life-threatening condition since apnea may trigger a cascade of primary events and physiological consequences involving dysfunctions in respiratory, cardiovascular, and central nervous systems (Figs. 13.4 and 13.5). Hence, these OSA events may lead to increased heart rate and blood pressure (hypertension), cardiovascular disease, type 2 diabetes, neurocognitive impairment, and increased risk to suffer motor vehicle accidents. The risk of heart failure is increased by 140%, the risk of stroke by 60%, and the risk of coronary heart disease by 30% in OSA patients.
Some community studies showed evidence that OSA is a predisposing factor for cardiovascular mortality, independently of traditional cardiovascular risk factors. The increase in morbidity and mortality is likely to involve intermediate pathways that include dyslipidemia, glucose intolerance, and hypertension along with central obesity which defines the so-called metabolic syndrome. This is the reason why OSA is thought to contribute to the development of type 2 diabetes. Out of the two phenotypic components of OSA, hypoxemia is the one more closely associated with glucose intolerance and cardiovascular disease, whereas sleep microarousals are more closely associated with incident hypertension.
The neurocognitive deficits associated with OSA include a decrease in vigilance, memory, and executive function and can be a result of the effects of sleep loss in the prefrontal cortex. Although daytime sleepiness is easily recovered after OSA treatment, it seems that permanent injury to the brain’s cognitive centers is secondary to the chronic intermittent hypoxia. Hence, incomplete recovery of those initial neurocognitive deficits does occur even after an appropriate OSA treatment. It is also important to note that in children, OSA-driven neurocognitive effects can be related to cognitive–behavioral problems, attention-deficit/hyperactivity disorder (ADHD) and subsequently impaired academic performance.
5 Diagnosis and Diagnostic Criteria
The diagnosis of OSA and SDB is made based on the recognition of typical clinical signs and symptoms, the clinical examination, and the evaluation of sleep studies. Although overnight full PSG is the gold standard for the diagnosis of OSA and sleep disorders according to the American Academy of sleep medicine, the use of home sleep monitors along with a comprehensive clinical evaluation may be used as an alternative to PSG for patients who have a high pretest probability for OSA.
The suspicion of having OSA is based on the presence of clinical signs and symptoms. Those can be divided in daytime (e.g., excessive daytime sleepiness, restless sleep, morning headaches, neurocognitive impairment, depression) and nighttime symptoms (e.g., snoring, choking or gasping, GERD).
The use of different questionnaires can be of help, and they are used mainly as a screening tool to detect the presence of excessive daytime sleepiness, which is a main finding in patients with OSA. The Epworth Sleepiness Scale (ESS) is one example of a quantifiable subjective measure of sleepiness. In this scale, the individual is asked to rate on a scale of 0–3 (0, no chance; 3, high likelihood) the chance of dozing in a series of eight situations. This score has a modest correlation with physiological measures of sleep but has a better correlation with the respiratory disturbance index in patients with obstructive sleep apnea.
In addition, a set of four basic questions represented by the acronym STOP can also be used. A positive response to two or more questions represents an increased risk for sleep apnea. The expanded version, the STOP–BANG questionnaire, has been demonstrated to highly predict sleep apnea’s presence (Table 13.6). This questionnaire can have four more questions represented by the acronym BANG. If the score is eight, the probability for severe sleep apnea is nearly 82%.
A comprehensive clinical examination also provides the clinician with the anatomical risk factors that may lead to the diagnosis of OSA. The Table 13.7 below displays a summary of the most common physical findings and signs and symptoms in OSA patients.
Imaging plays a role in the anatomic assessment of the airway and adjacent structures. Although imaging techniques for the head and neck are not regularly used to diagnose OSA, the use of certain techniques can help to (1) visualize the airway, (2) detect anatomic abnormalities, and (3) ultimately predict the risk for upper airway obstruction that may contribute to the presence of a SBD. Thus, cephalometric analysis, MRI, acoustic reflections, and computed tomography scans are used as part of the patient comprehensive exam. Cone beam computed tomography (CBCT) scans are becoming more and more popular in the dental field, and since these techniques include the teeth, jaws, spine, cranial base, and facial soft tissues, they provide an excellent opportunity to evaluate the functional and developmental relationships between these structures. Furthermore they allow us to visualize and calculate the airway dimension.
Sleep studies are the basic diagnostic tools to provide the definite diagnosis of SDB and OSA, where a complete collection of physiological data from the wake/sleep stages is required for interpretation by a trained sleep medicine specialist.
The American Academy of Sleep Medicine (AASM) defined four levels of sleep studies from which an objective-based assessment is made. These four levels are differentiated per the number of physiological signals recorded as well as if the sleep study is attended or not by a sleep technologist.
Type I sleep study or polysomnography (PSG) is an overnight sleep study performed in a sleep center and monitored by a sleep technologist in a nearby control room with the record and registration of at least seven physiological measures. The PSG is considered to be the “gold standard” in sleep medicine relative to objective-based sleep studies. In Table 13.8 a description of the different types of sleep studies and monitors, with comments about the clinical application of each one, is showed.
6 Rationale for Treatment
The treatment of snoring and OSA will depend on the severity of the disease (particularly in OSA), and this will determine the treatment options and sequence of care.
7 Treatment Options, Goals, and Sequencing of Care
This section will focus on OSA and primary snoring conditions. These are the only conditions that can be managed by an experienced dentist in collaboration with a sleep medicine specialist. Ideally, an effective treatment approach for OSA is to reverse excessive daytime somnolence and fatigue, reduce the risks associated with this condition, minimize the impact of cardiovascular effects, and improve quality of life. In many cases the participation of other healthcare professionals such as the ENT, pneumologists, cardiologists, and maxillofacial surgeons, among others, is mandatory. In fact, the ideal treatment plan is to always establish different patient’s needs depending on the severity of the apnea and other health issues related to the patient’s condition. Below is a description of cognitive–behavioral, medical devices, pharmacological, and surgical options for OSA management:
-
1.
Cognitive–behavioral: We should never underestimate the importance of patient education, especially those items addressed to have an impact on risk factors’ modification. Weight loss, changes on sleep position, smoking cessation, alcohol and sedative drug intake avoidance, and sleep hygiene measures are included among the most common approaches.
-
2.
Medical devices: The most successful medical modalities for the management of OSA are the use of positive airway pressure (PAP) devices and oral appliances:
-
2.1.
PAP devices: The most successful medical treatment for OSA is the use of positive airway pressure devices, which maintain upper airway patency during sleep simply by providing a pneumatic splint. PAP devices can be delivered by continuous (CPAP), bi-level (BPAP), or auto-titrating (APAP) modalities. CPAP supplies a flow of positive air pressure adjusted to the level needed to keep the airway open, delivered through a facial device. It reduces AHI, blood pressure, and cardiac arrhythmias and improves oxygen saturation levels, sleep efficiency, self-reported sleep, and well-being. The main disadvantage is that CPAP devices are sometimes difficult to use, thus affecting compliance. Despite CPAP being highly efficacious in preventing upper airway collapse, patients’ acceptance, tolerance, and adherence are often low, thereby reducing effectiveness. Several risk factors and comorbid conditions seem to be associated with decreased compliance, especially depression. PAP therapy is considered the first line of treatment for those patients suffering from moderate-to-severe OSA. The success rate with CPAP therapy is considered around 95% of cases.
-
2.2.
Oral appliances: In those cases where patient is intolerant to CPAP, when the level of compliance to CPAP therapy is low, and in those cases with primary snoring or mild-to-moderate OSA, the use of oral appliances (OAs) should be considered. There are two main types of oral appliances: tongue retaining devices and mandibular repositioning devices (a.k.a. mandibular advancement devices). It is important to clarify that their level of efficacy is always lower than the CPAP therapy.
-
2.1.
While mandibular advancement devices (MADs) increase the anteroposterior dimensions of the oropharynx and velopharynx by repositioning and maintaining the lower jaw in a forward position during sleep, tongue retaining devices (TRDs) provide a forward movement of the tongue producing more favorable changes in the retroglossal region. Anyway the positive effect is attributed to changes in airway configuration. Due to comfort and compliance issues, MADs are usually the first choice unless the patient is edentulous where tongue retaining devices seem to be the first option. There are numerous types of MAD available whose differences rely on material of fabrication, design, advancement mechanisms, size, and thickness.
The level of efficacy of OA for the management of OSA is estimated in 76% in cases of mild OSA (5–15 events/hour sleep), 61%i in moderate cases (15–30 events/hour), and 40% in severe cases (more than 30 events/hour). Different studies have shown that therapy with OA is usually more successful in younger patients and female gender and patients with small neck, lower body mass index (BMI), and retrognathic mandible and positional OSA cases.
The complications and side effects with the use of OA are frequent but usually minor and temporary and include increase or decrease in salivation, tooth movement, tooth soreness, masticatory muscles or TMJ pain, worsening in OSA (around 13% of patients), bite discomfort, and occlusal changes (posterior open bite). There are relative contraindications for the use of OA. These are usually severe or active periodontal disease, active TMD pathology, inadequate dentition (less than six teeth remaining), central sleep apnea, growing children, morbid obesity, unmotivated patients, severe hypoxemia, severe OSA, and concomitant severe cardiovascular pathology.
The use of OA in the treatment of OSA in mild-to moderate cases as well as severe cases intolerant to CPAP therapy is recommended as the current practice parameter. OA also can be used as a part of combination therapy with CPAP and/or upper airway surgery. The flowchart and decision tree for the management of snoring or OSA with a CPAP or MAS are shown in Fig. 13.6 for a better understanding.
-
3.
Pharmacological: A large range of pharmacological approaches have been explored over the years, but their effectiveness to treat OSA has been proved minimal, and research is undergoing to find better options. Nasal corticosteroids can reduce AHI index and can help with allergic nasal congestion and vasomotor rhinitis associated with CPAP. Only modafinil (200–400 mg/day) or armodafinil (150–250 mg/day) has shown mild-to-moderate positive effects in specific OSA cases.
-
4.
Surgical: This is only indicated in severe OSA clinical scenarios when patients have an anatomic obstruction. There are different surgical techniques for treating OSA, whose aim is to relieve the obstruction by removing or bypassing it or increasing airway size. The selection of what modality to use depends on patient’s anatomy and physiology. The selection process only comes after a full evaluation by an ENT or a maxillofacial surgeon specialized in OSA management. The most common surgical procedures for OSA treatment are tracheostomy, tonsillectomy/adenoidectomy (for children mainly), septoplasty/turbinate reduction, uvulopalatopharyngoplasty (UPPP), tongue base reduction, hyoid suspension, genioglossus advancement, maxillomandibular advancement, or a combination of these techniques.
Treatment algorithm for the management of primary snoring and OSA with OA (Modified from Pliska and Almeida [34]. Permission to modify and reproduce was granted)
Suggested Reading
Kryger M, Roth T, Dement W. Principles and practice of sleep medicine. 3rd ed. Philadelphia: W B Saunders Co; 2000.
Ramar K, Dort LC, Katz SG, Lettieri CL, Harrod CG, Thomas SM, Chervn RD. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015: an American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2015;11(7):773–827.
Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnea. Lancet. 2014;383(9918):736–47.
Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–76.
Satela MJ. International classification of sleep disorders-third edition highlights and modifications. Chest. 2014;146(5):1387–94.
Rules for scoring respiratory events in sleep: update of the 2007. AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597–619.
Thomas RJ. Multimodality therapy for sleep apnea syndromes. J Clin Sleep Med. 2012;8(5):565–7.
Gharibeh T, Mehra R. Obstructive sleep apnea syndrome: natural history, diagnosis, and emerging treatment options. Nat Sci Sleep. 2010;2:233–55.
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43.
Gold AR, Dipalo F, Gold MS, O’Hearn D. The symptoms and signs of upper airway resistance syndrome. A link to the functional somatic syndromes. Chest. 2003;123:87–95.
Bao G, Guilleminalult C. Upper airway resistance syndrome-one decade later. Curr Opin Pulm Med. 2004;10:461–7.
Palombini L, Lopes M, Tufik S, Guilleminault C, Bittencourt LR. Upper airway resistance syndrome: still not recognized and not treated. Sleep Sci. 2011;4(2):72–8.
Scherr SC, Dort LC, Almeida F, et al. Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring. A report of the American Academy of Dental Sleep Medicine. J Dent Sleep Med. 2014;1(1):39–50.
del Campo MF, Ruiz Albi T, Zamarrón SC. Upper airway resistance syndrome – a twenty-five years experience. In: Sleep disorders, Dr. Chris Idzikowski, editor. 2012. ISBN: 978-953-51-0293-9.
Sutherland K, Vanderveken OM, Tsuda H, Marklund M. Oral appliance treatment for obstructive sleep apnea. A review. J Clin Sleep Med. 2014;10(2):215–27.
Pham LV, Schwartz AR. The pathogenesis of obstructive sleep apnea. J Thorac Dis. 2015;7(8):1358–72.
Iber C, Ancoli-Israel S, Chesson A, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester: American Academy of Sleep Medicine; 2007.
Hoekema A, Stegenga B, De Bont L. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: a systematic review. Crit Rev Oral Biol Med. 2004;15(3):137–55.
Lim J, et al. Oral appliances for obstructive sleep apnea. Cochrane Database Syst Rev. 2003;(4);CD004435.
Bailey D, Attanasio R. Sleep disorders: dentistry’s role. Dent Cl N Am. 2001;45(4).
Clark G. Mandibular advancement devices and sleep disordered breathing. Sleep Med Rev. 1998;2(3):163–74.
Schmidt-Nowara W, Lowe A, Wiegand L, et al. Oral appliances for the treatment of snoring and obstructive sleep apnea: a review. Sleep. 1995;18(6):501–10.
Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances. Sleep. 1995; 18(6):511–513.
Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61.
Marklund M, Sahlin C, Stenlund H, et al. Mandibular advancement device in patients with obstructive sleep apnea. Long- term effects on apnea and sleep. Chest. 2001;120:162–9.
Ferguson K, Ono T, Lowe A, et al. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109:1269–75.
Clark G, Blumenfeld I, Yoffe N, et al. A crossover study comparing the efficacy of continuous positive airway pressure with anterior mandibular positioning devices on patients with obstructive sleep apnea. Chest. 1996;109:1477–83.
Walker-Engstrom M, Tegelberg Å, Wilhelmsson B, Ringqvist I. 4-Year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea. A randomized study. Chest. 2002;121:739–46.
Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep. 1999;22(2):237–40.
Fritsch K, et al. Side effects of mandibular advancement devices for sleep apnea treatment. Am J Respir Care Med. 2001;164(5):813–8.
Robertson CJ. Dental and skeletal changes associated with long-term mandibular advancement. Sleep. 2001;24(5):531–7.
Bailey DR, Atannasio R. Screening and comprehensive evaluation for sleep related breathing disorders. In sleep medicine and dentistry. Dent Clin N Am. 2012;56:331–42.
Atannasio R, Bailey DR. Sleep assessment studies. In: Atannasio R, Bailey DR, editors. Dental management of sleep disorders. Iowa: Blackwell Publishing; 2010.
Pliska BT, Almeida F. Effectiveness and outcome of oral appliance therapy. Dent Clin N Am. 2012;56(2):433–44.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Romero, A.G., Ferreira, J.N.A.R. (2017). Obstructive Sleep Apnea and Snoring. In: Ferreira, J., Fricton, J., Rhodus, N. (eds) Orofacial Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-51508-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-51508-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51507-6
Online ISBN: 978-3-319-51508-3
eBook Packages: MedicineMedicine (R0)