Abstract
The aim of this study is processing of domestic molybdenum concentrate to produce technical grade molybdenum trioxide may be the starting material for the production of various molybdenum products. In order to determine the optimum parameters for the total oxidizing roasting process of molybdenite concentrate to produce MoO3 and purify the MoO3 product by removing the copper content; first, MoS2 was roasted by controlling reaction temperature and duration in a chamber type furnace. The highest Mo concentration rate was obtained as 56.6 wt% of Mo at 650 °C for the roasting duration of 45 min. Secondly, roasted product was leached with H2SO4 to remove copper. Leaching conditions were optimized by investigating the effects of different H2SO4 concentrations and S/L ratios. The minimum copper content was obtained as 0.13 wt% at leach residue. The raw materials and the products were characterized by using AAS (atomic absorption spectrometry) and XRD (X-Ray Diffraction) techniques.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Generally molybdenum metal is produced from its high grade sulphide concentrate through oxidizing roasting of molybdenite (MoS2) in order to obtain molybdenum trioxide (MoO3). Then purification of MoO3 and hydrogen reduction of MoO3 follows.
Molybdenum is widely used for the production of ferromolybdenum, which is required for the production of alloyed steel. Almost 80% of Mo, produced from molybdenum trioxide, is used for steel making in industries.
Usually, concentrates are roasted to obtain low copper and lead levels to produce a calcine that is essentially MoO3 which contains low sulphur. Such calcines can be used directly in steel making, because liquid iron will reduce MoO3 to metal in high yield.
Technical-grade MoO3 is produced by roasting MoS2 in air atmosphere in a multiple-hearth furnace. The roasted MoO3 product usually has <0.1% sulphur content. Technical-grade MoO3 typically contains 85–90 wt% MoO3, the balance is silica with some Fe2O3 and Al2O3.
In some cases, additional hydrometallurgical processing is needed to produce technical-grade MoO3. In steel making, using molybdenum trioxide with high copper content has some harmful effects on the mechanical properties of the alloyed steel produced. Therefore, the up-gradation of molybdenum trioxide is required in steel industry. The up-graded molybdenum trioxide must have less than 0.5 wt% Cu content for ferromolybdenum production [1,2,3,4].
Experimental Procedure
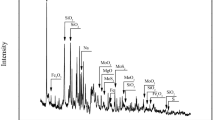
The raw material which was used in total oxidizing roasting experiments, is local molybdenite concentrate which has 0.40 wt% Cu and 0.05 wt% Mo content. The XRD pattern of MoS2 concentrate is shown in Fig. 1.
MoS2 concentrate was analyzed by using chemical analysis and AAS. Chemical analysis of MoS2 concentrate is given in Table 1.
Present study was conducted in two main stages: First, MoS2 was roasted by controlling parameters such as reaction temperature and duration in a chamber type furnace. To make a homogeneous roasting , alumina boats which have large surface area, were used. The samples were put in the alumina boats as thin layers. Samples were roasted at 600, 625, 650 °C with 15, 30, 45, 60, 90 and 120 min.
In the second experimental sets, roasted products were leached by H2SO4 to remove copper. Leaching conditions were optimized by investigating the effects of different H2SO4 concentrations and S/L ratio. Leaching experiments were done with Merck quality 95–98% H2SO4 with the duration of 15 min, at room temperature and 400 rpm mixing rate. All the samples were 10 g. In order to investigate the effects of different H2SO4 concentrations and S/L ratios, 10 g of samples were leached with 0.2, 0.4, 0.6 M acid concentrations with 1/5, 1/2 and 1/1 solid-liquid ratios.
Results and Discussion
In the first experimental set, effect of roasting temperature and duration on molybdenum trioxide recovery were investigated. In Fig. 2, the change of sulphur content at the concentrate depending on the roasting temperature and duration, is given.
As it is seen from Fig. 2, the sulphur content of the concentrates decreases rapidly till 40 min of roasting . It can be said that roasting at 650 °C with the duration of 45 min has given the closest results to the standard technical-grade MoO3 . As the roasting temperature and duration decrease, the sulphur amount increases.
The chemical analysis of the samples which were roasted at different temperatures and durations, is given in Table 2.
The optimum Mo concentration rate was obtained as 56.58 wt% at 650 °C for the roasting duration of 45 min.
In the second experimental sets, roasted product was leached by H2SO4 to remove copper. Leaching conditions were optimized by investigating the effects of different H2SO4 concentrations and S/L ratio.
The metal concentrations in H2SO4 solutions are given in Table 3.
The concentration values were put in Formula (1) in order to calculate the metal recovery from H2SO4 solutions.
In Fig. 3, the effects of different H2SO4 concentrations and S/L ratios on Cu recovery from the solution are given.
As it is seen from Fig. 4, almost 96% of Cu was removed from calcine by using 0.6 M H2SO4 solution at 1/5 solid-liquid ratio [5, 6].
Figure 4 presents the XRD patterns of leached samples with 0.6 M H2SO4 solution.
Figure 4 shows that, the main phases of leaching residue are MoO3. There are not seen any copper compounds rather the roasted samples. Because the majority of the copper was solution treated.
Conclusions
Based on the results of present study of roasting molybdenite concentrate at different temperatures and durations, leaching of roasted samples with different H2SO4 concentrations at different solid-liquid ratios, following conclusions can be given.
-
(1)
Optimum Mo recovery was determined as 56.58% Mo and 1.05% S with the roasting temperature of 650 °C and duration of 45 min.
-
(2)
Leaching with 0.6 M H2SO4 solution at 1/5 solid-liquid ratio ended up with 95.92% Cu recovery from the H2SO4 solution. These parameters gave the best results as 0.13% Cu content in the leaching residue.
References
S.C. Grund, K. Hanusch, H.J. Breuning, H.U. Wolf, Ullmann’s Encyclopedia of Industrial Chemistry (ABD, Wiley-VCH Verlag GmbH & Co. KGaA.O, Weinheim, NY, 2006)
C.K. Gupta, Extractive Metallurgy of Molybdenum (CRC Press, USA, 1992)
Url-1. http://www.imoa.info
F. Habashi, in Handbook of Extractive Metallurgy, Volume III: Precious Metals, Refractory Metals, Scattered Metals, Radioactive Metals, Rare Earth Metals, (Wiley-VCH, Weinheim, 1997), pp. 1361–1402
J. Lee, B. Kim, M.K. Jha, J. Jeong, Leaching of impurities for the up-gradation of molybdenum oxide and cementation of copper by scrap iron. Int. J. Miner. Process. 88, 7–12 (2008)
J.R. Kumar, J.Y. Lee, H.S. Jeon, J.S. Kim, Up-gradation of MoO3 and separation of copper, iron, zinc from roasted molybdenum ore by a leaching process. Braz. J. Chem. Eng. 30, 391–397 (2013)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Benzeşik, K., Sonmez, S., Yücel, O. (2017). Evaluation of Molybdenum Concentrates. In: Hwang, JY., et al. 8th International Symposium on High-Temperature Metallurgical Processing. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51340-9_43
Download citation
DOI: https://doi.org/10.1007/978-3-319-51340-9_43
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51339-3
Online ISBN: 978-3-319-51340-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)