Abstract
Pyrometallurgical studies conducted for Mn extraction using Turkish Mn-ore reserves were reviewed. Turkish Mn-ores are low-grade ones having ~30% Mn. The most important Mn ore reserves in Turkey are in the Denizli-Tavas region, where more than 2 million tons of proven reserves are reported. Mining rights to the Denizli-Tavas manganese belongs to Eregli Iron and Steel Works Co. (ERDEMIR). The ore is usually charged to the blast furnaces of ERDEMIR during hot metal production. So far, nearly 20 Master’s thesis studies have been conducted for the analysis of manganese extraction in Turkey. In the scope of the present work, only pyrometallurgical and related activities (i.e. ore beneficiation, calcination, etc.) relevent to the treatment of a specific ore were examined.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Manganese is an important element since it increases the strength, toughness and hardenability of ferrous products, especially steel. It is also used as deoxidizer and desulphurizer in steelmaking. Most of the manganese used in the iron and steel industry are in the form of ferromanganese and silicomanganese . Turkey has some low-grade manganese ores having ~30% Mn. However, there is no ferromanganese/silicomanganese production plant in Turkey. Therefore, the demand of the Turkish iron and steel industry for manganese ferroalloys has been met by imports. The most important Mn-ore reserve is present in the Western Anatolia region, namely Denizli-Tavas. More than 2 million tons of proven reserves are reported in that area. In the last two decades, pyrometallurgical and hydrometallurgical studies were performed to treat this ore and similar reserves in Turkey. In the scope of this work, only pyrometallurgical and pyrometallurgical related (i.e. ore beneficiation, calcination, etc.) activities were reviewed. In this paper, important results obtained from these studies were summarized and outlined to create a question mark in the reader’s mind whether industrial production of manganese ferroalloys from those ores is possible together with the method that could be used. The results presented should hopefully give alternative ideas related to the use of these valuable deposits in iron and steelmaking. Mining rights for the Denizli-Tavas manganese ore belongs to Eregli Iron and Steel Works Co. (ERDEMIR). The ore is usually charged to the blast furnaces of ERDEMIR during hot metal production. Therefore, various alternative uses should be considered and applied such as a mini blast furnace (and mini basic oxygen furnace) process for high/medium carbon ferromanganese production in the steelmaking company and the use of the ore in steelmaking converters as a manganese source. The first alternative obviously decreases the amount of ferromanganese imports, due to production from domestic sources. Selection of a blast furnace method is related to both high electricity prices in Turkey and relatively low ore grades. The second alternative is rather challenging and could be a topic of another paper.
Literature Review
So far, nearly 20 Master’s thesis studies have been conducted for the analysis of manganese extraction in Turkey. In the scope of present work, only pyrometallurgical and pyrometallurgical related (i.e. ore beneficiation, calcination, etc.) activities were examined. Among these, the ones dealing with treatment of a specific ore were reviewed. In other words, literature about hydrometallurgical studies and studies including treatment of manganese containing chemicals were outside of the authors’ interest. Thirteen studies have been performed for pyrometallurgical manganese extraction using Turkish manganese ore reserves. Eleven of them are related to the treatment of the biggest manganese ore reserves in Turkey, which are present in the Western Anatolia region, namely Denizli-Tavas. This is a carbonate type ore body having 30–31% Mn. Chemical composition of the Denizli-Tavas manganese ore is tabulated in Table 1. In this region, more than 2 million tons of proven reserves are reported. Only two studies are related to treatment of other reserves present in Central and Eastern Anatolia, namely Karaman and Erzincan, respectively. Those are very small ore reserves that are not suitable for industrial scale treatment.
Pyrometallurgical studies conducted for the treatment of manganese ores of Turkey may be grouped into three categories: (1) Ferromanganese /Silicomanganese production, (2) Carbothermic reduction, (3) Pretreatment operations for increasing Mn content of the ore.
Six studies have been performed in the first category. The first pyrometallurgical study was conducted by Celik and Bilgen [1]. According to their study, Denizli-Tavas manganese ore was smelted using electrical and induction furnaces. The ore was mixed with charcoal and limestone, and smelted at 1440 °C for two hours. It was reported that an iron-manganese alloy with 67% Mn was obtained with 60% manganese recovery.
Emeksiz [2] investigated the production of high carbon ferromanganese from Denizli-Tavas manganese ore. The ore was first calcined and then smelted in graphite crucibles in an induction furnace at 1600 °C. Four small graphite crucibles inside a large graphite crucible were placed in the furnace. The smaller graphite crucibles had outer diameters of 60 mm, inner diameters of 50 mm and depths of 73 mm. The charges for smelting were contained in the smaller graphite crucibles. Smelting time, charge basicity (i.e. CaO/SiO2), coke to ore weight ratio and CaF2 addition were the main experimental variables and the effect of each variable on manganese recovery were studied. Two hours smelting time together with coke to ore ratio less than 0.2 were reported to yield optimum results. The main problem encountered was the poor metal-slag separation. The reason of this problem was considered to be inadequate metal depth in the crucibles. This result was more pronounced when excess coke was added to the charge such that coke to ore weight ratio was greater than 0.3.
Imer [3] studied the production of high carbon ferromanganese from the same ore with the same experimental variables. First smelting experiments were conducted in an Al2O3 crucible contained in a graphite crucible in an induction furnace. The Al2O3 crucible was found to have interacted with the liquid slag that formed during the experiment and the liquid slag was found to have leaked through the Al2O3 crucible and to have collected at the bottom of the outer graphite crucible. A similar experiment conducted in an electronically controlled muffle furnace led to the same problems mentioned above. Similar difficulties arose in an experiment conducted with an MgO crucible. As a result of these findings, smelting experiments were decided to be conducted in graphite crucibles. With cylindrical graphite crucibles the leakage problem was overcome, but the metal-slag separation was not good as in the case of study performed by Emeksiz [2]. A conical shaped graphite crucible shown in Fig. 1 was considered to be helpful in obtaining good metal-slag separation. It was found that metal-slag separation was satisfactory in the experiment conducted with such a crucible design. Imer [3] performed a series of experiments with these crucibles using an electronically controlled muffle furnace. A schematic drawing of the alloy-slag system obtained at the end of the experiments is presented in Fig. 1.
According to Imer’s findings [3], the manganese concentration and the weight of the metal increased with time in the experiments conducted for 1, 2 and 4 h. The manganese content of the metal increased with increasing basicity in the experiments conducted with CaO/SiO2 ratios of 1, 1.5 and 2.0. CaF2 addition was found to help obtain good metal-slag separation using additions of 5 and 10% of the weight of the (ore + coke + lime + scrap iron) mixture. The manganese content of the ferromanganese produced was very close to standard ferromanganese [4]. These aforementioned two studies showed that this ore could be used for the production of ferromanganese. However, some pretreatment processes were advised to upgrade the manganese content of the ore. The study conducted by Akarca [5] had this target and it could be counted in the third category previously indicated. He studied the possibility of concentration of Denizli-Tavas region manganese ore by magnetic concentration and gravity separation methods. It was observed that a concentrate containing 39.46% Mn can be produced by magnetic separation with 89.07% manganese recovery and a concentrate of 45.34% Mn with 62.37% manganese recovery can be produced by gravity separation. Since Denizli-Tavas manganese ore is a carbonate type, calcination is, of course, a simple way to increase manganese content. In that respect, Keceli [6] studied the mineralogy of the ore. He reported that the manganese carbonate (rhodochrosite) and manganese hydroxide (manganite) were found to be most abundant, 21 and 16% of the ore by weight, respectively (Table 2). According to experiments conducted, it was proposed to carry out calcination at 900 °C, and the calcination time (with a good stirring) should be a minimum of 1 h, and the particle size of ore should be about 1 mm. With those conditions satisfied, the manganese content of the ore could be increased to above 35%.
Ari [7] conducted a set of experiments using a laboratory scale arc furnace to determine the feasibility of ferromanganese and silicomanganese production using Denizli-Tavas manganese ore. He found that the ore is suitable for the production of high carbon ferromanganese and silicomanganese. When a charge containing 13.69% fixed carbon and 7.69% CaO was smelted, an alloy with 85.08% Mn and 1.28% Si was obtained with 82.50% manganese recovery. The smelting period was indicated as 30 min. In relation to silicomanganese production, a charge containing 15.60% fixed carbon and SiO2 sand/ore wt ratio of 0.28 yielded a silicomanganese alloy having 69.7% Mn and 18.2% Si. The smelting period was noted as half an hour and manganese recovery was reported as 70.7%. He also included an economic analysis showing that industrial scale ferromanganese production from Denizli-Tavas manganese ore is feasible.
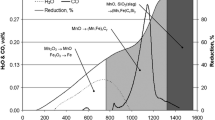
Keskinkilic [8] investigated silicomanganese production from Denizli-Tavas manganese ore using electronically controlled muffle furnace. Experiments were conducted in the same design of graphite crucibles as used in the previous study [3]. Results of the experiments performed with SiO2 sand addition indicated that the charge having basicity of 0.58 and smelted at 1700 °C for a 1.5 h smelting period was the optimum conditions for silicomanganese smelting. Variations of manganese and silicon recoveries with charge basicity are shown in Fig. 2. Both manganese and silicon recoveries were found to increase as charge basicity increased from 0.39 to 0.58. Further increase in charge basicity gave rise to a decrease in both manganese and silicon recoveries. Maximum manganese and silicon recoveries were obtained at the charge basicity value of 0.58. At this point, manganese and silicon recoveries were found as 64 and 29%, respectively. Both manganese and silicon recoveries were expected to be higher. These relatively small recoveries may be attributed to the large mass losses in the experiments, which were also reported in the work conducted by Ari [7]. Such losses were most probably related to increased vapor pressure of manganese at increased temperature. Regardless of the losses encountered in the laboratory scale set up, it was finally concluded that production of silicomanganese [9] from Denizli-Tavas manganese ore is possible.
In the literature, the most recent study in relation to manganese ferroalloy production from a domestic ore was conducted by Cardakli [10]. He used a manganese ore sample (ore grade was about 42%) taken from a small reserve present in Erzincan. Experiments conducted using the same smelting system revealed that a high carbon ferromanganese alloy having manganese content more than 75% could be produced using Erzincan manganese ore. Manganese recovery during the smelting experiments was reported as about 60–80%.
The remaining five studies present in the literature were related to carbothermic reduction of manganese oxides using Turkish manganese ores. Aytis [11] investigated the reduction mechanism of manganese oxides. With a series of experiments using Denizli-Tavas manganese ore, he first outlined the well-known reduction sequence of manganese oxides starting from MnO2 until MnO. Then, he conducted a number of experiments between 1100 and 1350 °C under argon atmosphere using graphite as the reducing agent. It was indicated that the reduction was found to occur in two stages: (1) The first stage includes the rapid reduction of higher oxides of manganese and iron to MnO and FeO. (2) The second stage is slower during which MnO and FeO are reduced to mixed carbide of iron and manganese. Kalfaoglu [12] studied the solid state reduction behavior of Denizli-Tavas manganese ore to produce ferromanganese , by using pure carbon in the form of graphite. Weight loss data was collected with respect to time as the reactions took place in the furnace. Experiments were done at 1100, 1150, 1200, 1250, and 1300 °C, and a constant amount of carbon and calcined manganese ore were used for each experiment. Reactions were conducted under argon atmosphere. The weight loss was converted to percent reduction, R%, using the formula given below.
Mass of oxygen removed was calculated from the mass of CO removed and which was assumed to be equal to the weight loss. Mass of removable oxygen was taken as the oxygen present in the reducible oxides in the system. Iron oxides (assumed as Fe2O3 in stoichiometric calculations) and manganese oxides (assumed as Mn2O3 in stoichiometric calculations) were considered to be the reducible oxides. Therefore, all other oxides were assumed to be non-reducible in the calculation of R%. It was found that approximately 60% reduction of the manganese ore took place at and below 1200 °C. At and above 1250 °C, it was reported that this reduction efficiency increased to approximately 90%. It was concluded from his study that it is possible to produce high carbon ferromanganese alloy from the Denizli-Tavas manganese ore by solid state reduction with carbon. According to the weight loss data and Energy Dispersive Analysis of X-Rays (EDAX), the overall mechanism was observed to take place in three stages: (1) Reduction of higher oxides to lower oxides. (2) Nucleation of a metal phase. (3) Growth of the metal phase together with the reduction of MnO to Mn [12].
Akil [13] aimed to find the optimum conditions to produce a charge material for ferromanganese production or steelmaking with high content of iron and manganese carbides from Denizli-Tavas manganese ore by carbothermic reduction. The experiments conducted using a horizontal tube furnace under argon atmosphere revealed that solid state reduction performed at 1250 °C for 4 h with a charge containing 100% stoichiometric active carbon and 5% CaO yielded about 84% reduction of the calcined ore. This percentage reduction value was again calculated from Eq. (1). Stoichiometric active carbon was defined as the necessary carbon for the chemical reactions shown as (2) and (3). It was indicated that the ore product obtained this way had higher manganese content (~53%), so it could be used for ferromanganese production or as an additive in the production of high carbon steels.
Cil [14] studied the effect of mechanical activation on the extent of solid state reduction of manganese oxides. Some of the Denizli-Tavas ore samples were subjected to planetary milling for different milling times and the reduction degree of those samples were compared with the ones reduced without mechanical activation. It was found that the extent of reduction for non-activated manganese ore at 1100 and 1200 °C for 1 h were reported as 34.29 and 55.11%, respectively. On the other hand, the reduction degrees of the activated (60 min) manganese ore at the same reduction conditions were found as 41.70 and 70.43%, respectively. Therefore, it was concluded that mechanical activation was beneficial to increase the extent of reduction of manganese oxides.
Aranci [15] investigated the effect of carbonized tea plant wastes on the production of ferromanganese using the carbothermic method from ferrous manganese oxide ore subjected to mechanical activation. The manganese ore obtained from Karaman was mixed with Elazig-Keban region magnetite ore and the carbonization product obtained from tea wastes from the Karadeniz CAYKUR plants. The whole mixture was subjected to mechanical activation for 10–120 h. The product was pelletized and then subjected to reduction at 1000, 1100, 1200, 1300 and 1400 °C for various periods ranging from 30 to 120 min. It was concluded that the mechanical activation process influenced the degree of reduction positively. It was also indicated that carbonized tea plant’s wastes could be possibly used in the carbothermic reduction.
Conclusions
According to laboratory-scale pyrometallurgical studies using ore from Turkish Mn reserves, the following main conclusions were drawn:
-
Denizli-Tavas manganese ore (or other similar ore bodies in Turkey) is suitable for ferromanganese production. However, some pretreatment operations are advised to increase the ore grade. Mechanical activation improves recovery.
-
Smelting of silicomanganese from Denizli-Tavas manganese ore (or other similar ore bodies in Turkey) is possible.
-
Solid-state carbothermic reduction studies revealed that a product of higher manganese content was obtained. Therefore, it could be used for ferromanganese production or as an additive in the production of high carbon steels.
References
O. Celik, N. Bilgen, Denizli-Tavas Mangan Cevherlerinin Pirometalurjik Yöntemle Değerlendirilmesi, Report, General Directorate of Mineral Research and Exploration, MTA, Report No. 502 (1990)
F. Emeksiz, in Smelting of High Carbon Ferromanganese from Denizli-Tavas Manganese Ore. MS Thesis, Middle East Technical University (1991)
S.T. Imer, in Smelting of High Carbon Ferromanganese from Denizli-Tavas Manganese Ore. MS Thesis, Middle East Technical University (1997)
American Society for Testing and Materials (ASTM), “Annual Book of ASTM Standards,” Volume 01.02, “Ferrous Castings; Ferroalloys,” 59–61 (1993)
S.S. Akarca, Beneficiation of Denizli-Tavas Region Manganese Ores. MS Thesis, Middle East Technical University (2000)
H. Keceli, in Calcination of Denizli Tavas Manganese Ore. MS Thesis, Middle East Technical University (1997)
M.E. Ari, in Tavas Manganez Cevherlerinden Laboratuvar Tipi Ark Fırınında Ferromanganez/Ferrosilikomanganez Üretim Koşullarının Belirlenmesi. MS Thesis, Istanbul Technical University (1996)
E. Keskinkilic, in Smelting of Silicomanganese from Denizli-Tavas Manganese Ore. MS Thesis, Middle East Technical University (2001)
American Society for Testing Materials (ASTM), Annual book of ASTM standards, Vol. 01.02, “Ferrous Castings; Ferroalloys,” 256–257 (1993)
I.S. Cardakli, in Production of High Carbon Ferromanganese from a Manganese Ore Located in Erzincan. MS Thesis, Middle East Technical University (2010)
C. Aytis, in Production of Ferromanganese by Carbothermic Reduction. MS Thesis, Yildiz Technical University (1997)
F. Kalfaoglu, in Solid State Carbothermic Reduction of Denizli-Tavas Manganese Ore. MS Thesis, Middle East Technical University (1997)
C. Akil, in Optimization of Conditions to Produce Manganese and Iron Carbides from Denizli-Tavas Manganese Ore by Solid State Reduction. MS Thesis, Middle East Technical University (2007)
G. Cil, in Effect of Activation on Ferromanganese Production from Denizli-Tavas Manganese Ore by Carbothermal Reduction. MS Thesis, Sakarya University (2013)
E. Aranci, in Effect of Carbonized Tea Plant Wastes to Production of Ferromanganese by Carbothermal Method from Ferrous Manganese Oxide Ores Subjected to Mechanical Activation. MS Thesis, Firat University (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Keskinkilic, E. (2017). Pyrometallurgical Studies for Manganese Extraction Using Turkish Ore Reserves. In: Hwang, JY., et al. 8th International Symposium on High-Temperature Metallurgical Processing. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51340-9_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-51340-9_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51339-3
Online ISBN: 978-3-319-51340-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)