Abstract
Myasthenia gravis is an autoimmune neuromuscular junction disorder. This uncommon disease is characterized by fluctuating muscle weakness that worsens with exertion and improves with rest. The initial presentation is consistent with involvement of the extrinsic ocular muscle. The myasthenia usually progresses to involve other bulbar muscles and limb musculature, resulting in generalized myasthenia gravis. Although the etiology of the disorder remains unknown, the role of circulating antibodies directed against the nicotinic acetylcholine receptor in its pathogenesis is well recognized. The disease is treatable; therefore, prompt diagnosis is crucial. Fortunately, significant progress has been made in our understanding of the disease, leading to new treatment modalities and a significant reduction in the related morbidity and mortality.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Myasthenia gravis (MG) is a disorder of the neuromuscular junction. Most cases of MG are autoimmune in origin although rarely there are cases of congenital genetic origin. The autoimmune disease is characterized by fluctuating muscle weakness which worsens with exertion and improves with rest. The disease usually involves the extraocular muscle initially and may progress to involve bulbar and limb musculature, resulting in generalized MG {1,2}. The disorder is of unknown etiology; however, the role of antibodies directed against the nicotinic acetylcholine receptor is well established in the pathogenesis. Since MG is eminently treatable, recognition of the signs and symptoms of MG is crucial. Recent progress in treatment options has led to a significant reduction in morbidity and mortality [1, 2].
Epidemiology
Acquired MG prevalence is approximately 20 per 100,000 in the US population. Gender and age both appear to influence the occurrence of MG. Below the age of 40 years, the female/male ratio is about 3:1. Between 40 and 50 years, it is roughly equal, but over the age of 50, MG occurs more commonly in men. Childhood MG is uncommon in Europe and North America, comprising 10 to 15% of cases. In Asian countries, up to 50% of patients have onset before 15 years, and these patients present mainly with purely ocular manifestations [4, 5].
Pathogenesis
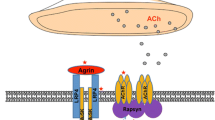
The nerve terminals innervating the neuromuscular junctions (NMJ) of skeletal muscles arise from the terminal arborization of α-motor neurons of the ventral horns of the spinal cord and brain stem. The NMJ itself consists of a synaptic cleft and a 20 nm thick space which contains acetylcholinesterase (AChE) along with other supporting proteins/proteoglycans. The NMJ postsynaptic membrane has deep folds with acetylcholine receptors (AChRs) tightly packed on the top of these folds.
When the nerve action potential reaches the synaptic bouton, depolarization opens voltage-gated calcium channels on the presynaptic membrane, triggering release of acetylcholine (ACh) into the synaptic cleft. The ACh diffuses into the synaptic cleft to reach postsynaptic membrane receptors where it triggers the end plate potential (EPP). ACh is then hydrolyzed by AChE within the synaptic cleft.
Muscle-specific receptor tyrosine kinase (MuSK), a postsynaptic transmembrane protein, forms part of the receptor for agrin, a protein present on synaptic basal lamina. Agrin/MuSK interaction triggers and maintains rapsyn-dependent clustering of AChR and other postsynaptic proteins [13]. Rapsyn, a peripheral membrane protein on the postsynaptic membrane, is necessary for the clustering of AChR. Mice lacking agrin or MuSK fail to form NMJs and die at birth due to profound muscle weakness [2, 24].
NMJ physiology influences susceptibility to MG muscle weakness. EPP generated in normal NMJ is several times larger than the threshold needed to generate the postsynaptic action potential. This neuromuscular transmission “safety factor” is reduced in MG patients. Reduction in number or activity of the AChR molecules at the NMJ decreases the EPP. The EPP may be adequate at rest to generate an action potential, but when the quantal release of ACh is reduced after repetitive activity, the EPP may fall below the threshold needed to trigger the action potential [22]. This results in blocking of muscle fiber contraction and muscle weakness. If the EPP at rest is consistently below the action potential threshold, persistent weakness occurs.
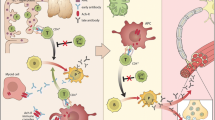
Effector Mechanisms of Anti-AChR Antibodies (Anti-AChR Abs)
Anti-AChR Abs affect NMT by at least three mechanisms [2]: (i) complement binding and activation at the NMJ, (ii) antigenic modulation (accelerated AChR endocytosis of molecules cross-linked by antibodies), (iii) and functional AChR block—preventing normal ACh from attaching and acting on the AChR.
Role of CD4+ T Cells in MG
Pathogenic anti-AChR Abs are high-affinity IgGs, and their synthesis requires activated CD4+ T cells to interact with and stimulate B cells. Thymectomy is believed to benefit patients with MG by removal of these AChR-specific CD4+ T cells [20]. Treatment with anti-CD4+ antibodies has also been shown to have a positive therapeutic impact. AIDS patients with reduction in CD4+ T cells notice myasthenic symptom improvement.
Role of CD4+ T-Cell Subtypes and Cytokines in MG and EAMG (Experimental Autoimmune MG)
CD4+ T cells are classified into two main subtypes: Th1 and Th2 cells. Th1 cells secrete pro-inflammatory cytokines, such as IL-2, IFN-γ, and TNF-α, which are important in cell-mediated immune responses. Th2 cells secrete anti-inflammatory cytokines, like IL-4, IL-6, and IL-10, which are important inducers of humoral immune responses. IL-4 further stimulates differentiation of Th3 cells that secrete TGF-β, which is involved in immunosuppressive mechanisms [17].
MG patients have abundant anti-AChR Th1 cells in the blood that recognize many AChR epitopes and are capable of inducing B cells to produce high-affinity anti-AChR antibodies. Th1 cells are indispensible in the development of EAMG as proven in animal models. Therapies against Th1 cytokines (TNF-α and IFN-γ) have been proven in animal models to improve EAMG symptoms [22, 23].
Anti-AChR Th2 cells have a complex role in EAMG pathogenesis. They can be protective, but their cytokines IL-5, IL-6, and IL-10 may also facilitate EAMG development [2]. CD4+ T cells that express CD25 marker and transcription factor Foxp3 are called “Tregs” and are important in maintaining self-tolerance. Tregs in MG patients may be functionally impaired and have been shown to increase after thymectomy with concomitant symptom improvement. Natural killer (NK) and natural killer T (NKT) cells also have important roles in MG and EAMG. Natural killer T (NKT) cells with Tregs help in regulating anti-AChR response. Mouse models have shown inhibition of EAMG development after stimulation of NKT cells [23]. IL-18, secreted by antigen-presenting cells (APCs), stimulates NK cells to produce IFN-γ, which permits and enhances Th1 cells to induce EAMG. IL-18-deficient mice are resistant to EAMG, and pharmacologic block of IL-18 suppresses EAMG. MG patients have been shown to have increased serum level of IL-18, which tends to decrease with clinical improvement [15].
Other Autoantigens in MG
Seronegative MG patients are those patients who have clinical MG but do not demonstrate anti-AChR antibodies in blood. Some of these patients have anti-MuSK antibodies (up to 40% of this subgroup). Other ethnic groups or locations (e.g., Chinese and Norwegians) have lower frequencies of anti-MuSK antibodies in seronegative MG patients. MG patients with anti-MuSK antibodies do not have anti-AChR Abs, except as reported in a group of Japanese patients [16].
Agrin/MuSK signaling pathway maintains the structural and functional integrity of the postsynaptic NMJ apparatus in the adult muscle cell. Anti-MuSK antibodies affect the agrin-dependent AChR cluster maintenance at the NMJ, leading to reduced AChR numbers. Complement-mediated damage may also be responsible for decreasing the AChR numbers at the NMJ when targeted by anti-MuSK Abs. Some human muscle cell culture studies have shown cell cycle arrest, downregulation of AChR subunit with rapsyn, and other muscle protein expression, on exposure to sera from anti-MuSK-positive MG patients [2]. Other antimuscle cell protein antibodies (e.g., antititin and antiryanodine receptor antibodies) are also postulated to have pathogenic roles in autoimmune MG.
Immunological Test
The most commonly used immunological test for the diagnosis of MG measures the serum concentrations of anti-AChR antibodies and is highly specific for myasthenia gravis [46]. False positives are rare and may occur with low titers in LEMS (5%), motor neuron disease (3–5%), and polymyositis (<1%).
The sensitivity of this test is approximately 85% for gMG and 50% for oMG [47, 48]. Anti-AChR antibody concentrations cannot be used to predict the severity of disease in individual patients since the concentration of the antibodies does not correlate with the clinical picture. Seronegativity may occur with immunosuppression or if the test is done too early in the disease [49, 50]. As indicated above, striated muscle antibodies against muscle cytoplasmic proteins (titin, myosin, actin, and ryanodine receptors) are detected mainly in patients with thymomatous MG and also in some thymoma patients without MG [24, 51]. The presence of these antibodies in early-onset MG raises the suspicion of a thymoma. Titin antibodies and other striated muscle antibodies are also found in up to 50% of patients with late-onset and nonthymomatous MG and are less helpful as predictors of thymoma in patients over 50 years [51]. Anti-KCNA4 antibodies might be a useful marker to identify patients with thymoma but can be also seen in myocarditis/myositis [52]. Patients with gMG who are anti-AChR antibody negative should be tested for anti-MuSK antibodies which are found in approximately 40% of patients in this group. As noted, low-affinity anti-AChR antibodies binding to clustered AChRs have been found in 66% of sera from patients with seronegative gMG [53]. Whether low-affinity antibodies are present in oMG remains to be determined, but this cell-based assay might eventually provide a more sensitive diagnostic test in this subgroup. Chest CT or MRI is done in all patients with confirmed MG to exclude the presence of a thymoma. Iodinated contrast agents should be used with caution because they might exacerbate myasthenic weakness [54, 55]. MG often coexists with thyroid disease, so baseline testing of thyroid function should be obtained at the time of diagnosis.
Clinical Feature
The cardinal feature of MG is fluctuating weakness that is fatigable, worsening with repetitive activities and improving with rest. Weakness is worsened by exposure to heat, infection, and stress [3]. The fluctuating nature of weakness distinguishes MG from other disorders which present with weakness. Typically, the weakness involves specific skeletal muscle groups. The distribution of the weakness is generally ocular, bulbar, proximal extremities, and neck, and in a few patients, it involves the respiratory muscles. In patients with MG, the weakness is mild in 26%, moderate in 36%, and severe in 39%, associated with dysphagia, depressed cough, and reduced vital capacity [27]. Extraocular muscle (EOM) weakness is by far the most common initial symptom of MG, occurring in approximately 85% of patients. Generalized progression will develop in 50% of these patients within 2 years [27]. Early MG usually presents with fluctuating ptosis and diplopia. Diplopia can be elicited by having the patient look laterally for 20–30 s resulting in eye muscle fatigue. The ptosis can be unilateral or bilateral, and sustained up-gaze for 30 or more seconds will usually induce it. The ptosis can be severe enough to totally occlude vision. The most commonly involved EOM is the medial rectus. But, on clinical examination, usually more than one extraocular muscle is weak with pupillary sparing. The weakness does not follow any pattern of specific nerve or muscle involvement, distinguishing it from other disorders such as vertical gaze paresis, distinct cranial nerve palsy, or internuclear ophthalmoplegia (INO).
Bulbar muscle involvement during the course of MG can be seen in approximately 60% of patients. It may present as fatigable chewing, particularly on chewing solid food with jaw closure more involved than jaw opening [38, 39]. Painless dysphagia and dysarthria may be the initial presentation in approximately 15% of patients [39]. The lack of ocular involvement in these patients may result in misdiagnosis as motor neuron disease or primary myopathy. Weakness involving respiratory muscles is rarely the presenting feature of MG, but respiratory insufficiency certainly may occur later as the disease progresses [35]. Respiratory muscle weakness can lead to myasthenic crisis which can be life threatening, requiring mechanical ventilation. It can be precipitated by infections and certain medications such as aminoglycosides, telithromycin, neuromuscular blocking agents, magnesium sulfate, beta-blockers, and fluoroquinolone antibiotics.
Involvement of the limbs in MG produces predominantly proximal muscle weakness. The upper extremities tend to be more often affected than the lower extremities. Occasionally predominant distal muscle weakness occurs [40]. Facial muscles are frequently involved and can make the patient appear expressionless. Neck extensor and flexor muscles are commonly affected. The weight of the head may overcome the extensors, producing a “dropped head syndrome.” Although it has become evident that the natural course of MG with adequate treatment is general improvement in 57% and remission in 13% after the first 2 years, severe weakness can be accompanied by high mortality. Only 20% of patients remain unchanged, and mortality from the disease is 5–9%. Only 4% of the patients who survive the first 2 years become worse. Of those who will develop generalized myasthenia, virtually, all do so by 2–3 years [3].
Clinical Classification
The Myasthenia Gravis Foundation of America (MGFA) clinical classification divides MG into five main classes and several subclasses [26]. It is designed to identify subgroups of patients with MG who share distinct clinical features or severity of disease that may indicate different prognoses or responses to therapy. It should not be used to measure outcome and is as follows:
Class I MG is characterized by the following:
-
1.
Any ocular muscle weakness.
-
2.
May have weakness of eye closure.
-
3.
All other muscle strengths are normal.
Class II MG is characterized by the following:
-
1.
Mild weakness affecting muscles other than ocular muscles
-
2.
May also have ocular muscle weakness of any severity
Class IIa MG is characterized by the following:
-
1.
Predominantly affecting limb muscles, axial muscles, or both
-
2.
May also have lesser involvement of oropharyngeal muscles
Class IIb MG is characterized by the following:
-
1.
Predominantly affecting oropharyngeal muscles, respiratory muscles, or both
-
2.
May also have lesser or equal involvement of limb muscles, axial muscles, or both
Class III MG is characterized by the following:
-
1.
Moderate weakness affecting muscles other than ocular muscles
-
2.
May also have ocular muscle weakness of any severity
Class IIIa MG is characterized by the following:
-
1.
Predominantly affecting limb muscles, axial muscles, or both
-
2.
May also have lesser involvement of oropharyngeal muscles
Class IIIb MG is characterized by the following:
-
1.
Predominantly affecting oropharyngeal muscles, respiratory muscles, or both
-
2.
May also have lesser or equal involvement of limb muscles, axial muscles, or both
Class IV MG is characterized by the following:
-
1.
Severe weakness affecting muscles other than ocular muscles
-
2.
May also have ocular muscle weakness of any severity
Class IVa MG is characterized by the following:
-
1.
Predominantly affecting limb muscles, axial muscles, or both
-
2.
May also have lesser involvement of oropharyngeal muscles
Class IVb MG is characterized by the following:
-
1.
Predominantly affecting oropharyngeal muscles, respiratory muscles, or both
-
2.
May also have lesser or equal involvement of limb muscles, axial muscles, or both
Class V MG is characterized by the following:
Diagnosis
Serological Testing
MG is a condition which fulfills all the major criteria for a disorder mediated by autoantibodies against the acetylcholine receptor (AChR-Ab) or against a receptor-associated protein, muscle-specific tyrosine kinase (MuSK-Ab).
Patients with positive AChR-Ab or MuSK-Ab assays have seropositive myasthenia gravis (SPMG). Demonstration of these antibodies is possible in approximately 90% of patients with generalized MG and provides the laboratory confirmation of the disease [1, 2]. In those patients with purely ocular MG, the sensitivity of AChR-Ab testing is considerably lower, detectable in about half of patients. There are rare cases of ocular myasthenia that are MuSK-Ab positive, but most large case series of ocular myasthenia gravis have not found patients who are MuSK-Ab positive.
Acetylcholine receptor antibodies
Immunologic assay to detect the presence of circulating AChR-Ab is the first step in the laboratory confirmation of MG. There are three AChR-Ab assays: binding, blocking, and modulating. Most authors use the term AChR-Ab as synonymous with the binding antibodies, and these are what are referenced in most studies that report the diagnostic sensitivity of these tests in MG for the reasons discussed below. These antibodies are polyclonal and are present in approximately 85% of patients with generalized disease. Essentially all patients (98 to 100%) with myasthenia gravis and thymoma are seropositive for these antibodies [7, 8]. The negative predictive value of thymoma in the absence of acetylcholine antibodies (binding) is high at 99.7% [8].
The assay for the binding antibody is the most sensitive. One study found these antibodies in 93, 88, and 71% of individuals with moderate to severe generalized myasthenia gravis, mild generalized myasthenia, and ocular myasthenia, respectively [9]. Others have found binding AChR-Ab in 80 to 90% of those with generalized disease [2, 10, 11] and in 40 to 55% of those with ocular myasthenia. Binding AChR antibodies are measured by standard radioimmunoassay and are highly specific for MG. There are virtually no false-positive results in healthy or disease-matched populations [12–14]. There are rare false positives in low titers in Lambert-Eaton myasthenic syndrome (5%), motor neuron disease (3–5%), and polymyositis (<1%) [9, 14, 15]. They are also rarely seen in some disorders that are not usually confused with myasthenia: primary biliary cholangitis, systemic lupus erythematosus, thymoma without myasthenia, and in first-degree relatives of patients with myasthenia gravis [16, 17].
Blocking AChR-Ab are present in about half of patients with generalized disease. They are present in fewer than 1% of patients with negative binding antibodies, but they have no significant false positives.
Assays for modulating AChR-Ab increase the sensitivity by ≤5% when added to the binding studies [11], and false-positive results are more of a problem [17].
Binding antibody studies are sufficient in most circumstances. The blocking and modulating antibody assays add relatively little to the diagnostic sensitivity [14]. However, the demonstration of blocking antibodies may be helpful if a possible false-positive binding antibody result is suspected.
AChR-Ab titers correlated poorly with disease severity between patients. A low-titer or even antibody-negative patient may have much more severe clinical disease than a patient with high titers. However, in an individual patient, the titers tend to fall with successful immunotherapy, and they parallel clinical improvement.
Ideally, serologic testing for AChR-Ab should be performed prior to initiating immunomodulating therapy for myasthenia gravis, as such therapy can sometimes lead to apparent seronegativity [11]. In one cohort of 143 seropositive patients, 9% became seronegative after treatment when retested in clinical remission. In addition, repeat serologic testing 6–12 months after initial testing has been reported to detect positive seroconversion in approximately 15% of patients with myasthenia gravis who were initially seronegative [11, 18].
MuSK antibodies
Antibodies to the muscle-specific receptor tyrosine kinase (MuSK) are present in 38–50% of those with generalized myasthenia gravis who are AChR-Ab negative [11, 19–25]. MuSK is a receptor tyrosine kinase that mediates agrin-dependent AChR clustering and neuromuscular junction formation during development. MuSK antibody-positive MG may have a different cause and pathologic mechanism than AChR-Ab-positive disease [19, 26].
MuSK antibodies are generally not present in those with well-established ocular MG, but they have been detected in a few cases [27, 28]. Although nearly half of patients with AChR-Ab-negative myasthenia gravis will have MuSK antibodies, those with AChR-Ab-positive myasthenia do not have antibodies to MuSK in most studies to date [19–24]. However, one group found that 11% of patients with AChR-Ab-positive myasthenia did have antibodies to MuSK as well [29]. MuSK antibodies appear to be much less common in some AChR-Ab-negative myasthenia populations, being found in only 1 of 27 Taiwanese patients [30] and 0 of 17 Scandinavian patients [31].
One consistent finding is that patients with AChR-Ab-negative MG and MuSK antibodies have a much lower frequency of thymic pathology than patients with AChR-Ab-positive MG [32–35]. Thymic hyperplasia is frequent in AChR-Ab-positive myasthenia, but this pathology is much less frequent in the MuSK-Ab-positive group.
In the appropriate clinical setting (i.e., a patient with the typical clinical features of myasthenia gravis (see “Clinical features” below) who is AChR-Ab negative), MuSK antibody testing can clarify the diagnosis and perhaps direct treatment [20]. However, the initial management of clinically apparent MG should be the same for patients with or without AChR antibodies. This would change only if future studies find additional therapeutic differences related to MuSK antibody status.
Seronegative myasthenia
The term seronegative MG, also called antibody-negative MG, refers to the 6–12% of patients with myasthenia who have negative standard assays for both AChR antibodies and MuSK antibodies. The term was previously used only for those who were AChR antibody negative, regardless of MuSK antibody status.
Patients with seronegative MG are more likely to have purely ocular disease than those who are seropositive. There is also a trend for those with generalized seronegative MG to have a better outcome after treatment [25].
Seronegative MG is an autoimmune disorder with most of the same features as seropositive myasthenia gravis [18, 25]. The electrophysiologic findings are identical. Patients with seronegative MG respond in a similar fashion to pyridostigmine, plasma exchange, glucocorticoids, and immunosuppressive therapies, as well as thymectomy.
Newer diagnostic antibody assays may further reduce the percentage of patients that are considered seronegative. As an example, approximately 50% of patients with seronegative MG have low-affinity AChR antibodies (also called clustered AChR antibodies) when tested by a specialized cell-based immunofluorescence assay. Other studies have demonstrated antibodies against LRP4, an agrin receptor required for agrin-induced activation of MuSK and AChR clustering and neuromuscular junction formation. These antibodies have been found in 2–50% of patients with seronegative MG. These assays are not commercially available and are not yet in widespread clinical use.
Electrophysiological Tests
The two principal electrophysiologic tests for the diagnosis of MG are repetitive nerve stimulation (RNS) study and single-fiber electromyography (SFEMG). RNS tests neuromuscular transmission. It is performed by stimulating the nerve supramaximally at 2–3 Hz. A 10% decrement between the first and the fifth evoked muscle action potential is consistent with a diagnosis of MG. In the absence of the decrement, exercise can be used to induce exhaustion of muscles and document decrement. The test is abnormal in approximately 75% of patients with gMG and 50% of patients with oMG [44, 45].
SFEMG is the most sensitive diagnostic test for MG. It is done by using a special needle electrode that allows identification of action potentials from individual muscle fibers. It allows simultaneous recording of the action potentials of two muscle fibers innervated by the same motor axon. The variability in time of the second action potential relative to the first is called “jitter.” In MG, the jitter will increase because the safety factor of transmission at the neuromuscular junction is reduced. SFEMG reveals abnormal jitter in 95–99% of patients with MG if appropriate muscles are examined [44, 45]. Although highly sensitive, increased jitter is not specific for primary NMJ disease. It may be abnormal in motor neuron disease, polymyositis, peripheral neuropathy, Lambert-Eaton myasthenic syndrome (LEMS), and other neuromuscular disorders. However, it is specific for a disorder of neuromuscular transmission when no other abnormalities are seen on standard needle EMG examination [42].
Management
Management of MG should be individualized according to patient characteristics and the severity of the disease. There are two approaches for management of MG based on the pathophysiology of the disease. The first is by increasing the amount of ACh that is available to bind with the postsynaptic receptor using an acetylcholinesterase inhibitor agent, and the second is by using immunosuppressive medications that decrease the binding of acetylcholine receptors by antibodies.
There are four basic therapies used to treat MG:
-
1.
Symptomatic treatment with acetylcholinesterase inhibitors
-
2.
Rapid short-term immunomodulating treatment with plasma exchange (PE) and intravenous immunoglobulin (IVIg)
-
3.
Chronic long-term immunomodulating treatment with glucocorticoids and other immunosuppressive drugs
-
4.
Surgical treatment
Acetylcholinesterase Inhibitors
Acetylcholinesterase inhibitors are the first-line treatment in patients with MG. Response to treatment varies from marked improvement in some patients to little or no improvement in others. Acetylcholinesterase inhibitors are used as a symptomatic therapy and act by increasing the amount of available acetylcholine at the NMJ [46]. They do not alter disease progression or outcome. Pyridostigmine is the most commonly used drug. It has a rapid onset of action within 15 to 30 min, reaching peak activity in about 2 h. The effect lasts for about 3–4 h. The initial oral dose is 15–30 mg every 4–6 h and is titrated upwards depending on the patient’s response. Adverse side effects of pyridostigmine are mostly due to the cholinergic properties of the drug such as abdominal cramping, diarrhea, increased salivation and bronchial secretions, nausea, sweating, and bradycardia. Nicotinic side effects are also frequent and include muscle fasciculation and cramping. High doses of pyridostigmine exceeding 450 mg daily, administered to patients with renal failure, have been reported to cause worsening of muscle weakness [47].
Short-Term Immunomodulating Therapies
Plasma exchange (PE) and intravenous immunoglobulin (IVIg) have rapid onset of action with improvement within days, but this is a transient effect. They are used in certain situations such as myasthenic crisis and preoperatively before thymectomy or other surgical procedures. They can be used intermittently to maintain remission in patients with MG who are not well controlled despite the use of chronic immunomodulating drugs.
Plasma Exchange (PE)
PE improves strength in most patients with MG by directly removing AChR from the circulation [48]. Typically one exchange is done every other day for a total of four to six times. Adverse effects of PE include hypotension, paresthesias, infections, thrombotic complications related to venous access, and bleeding tendencies due to decreased coagulation factors [50].
Intravenous Immunoglobulin Therapy (IVIg)
IVIg are preparations of immunoglobulins isolated from pooled human plasma by ethanol cryoprecipitation. IVIg is usually administered for 5 days at a dose of 0.4 g/kg/day. Different doses and schedules involving fewer infusions at higher doses are also used. The mechanism of action of IVIg is complex. Therapeutic mechanisms include inhibition of cytokines, competition with autoantibodies, and inhibition of complement deposition. Interference with the binding of Fc receptor on macrophages, Ig receptor on B cells, and interference with antigen recognition by sensitized T cells are other mechanisms [50]. More specific techniques to remove pathogenic anti-AChR antibodies utilizing immunoadsorption have been developed recently and offer a more targeted approach to MG treatment. Clinical trials showed significant reduction of blocking antibodies with concomitant clinical improvement in patients treated with immunoadsorption techniques [41].
IVIg is considered to be relatively safe, but rare cases of severe complications such as thrombosis, renal insufficiency, volume overload, and hemolytic anemia are reported [42].
Compared to plasma exchange, IVIg is similar in terms of efficacy and complication rates [43]. However, plasma exchange (PE) has considerable cost advantages over IVIg with a cost-benefit ratio of 2:1 for treatment of myasthenia gravis [44].
Long-Term Immunotherapies
The goal of immune-directed therapy of MG is to induce a remission or near remission of the disease.
Corticosteroids
Corticosteroids were the first and most commonly used immunosuppressant medications in MG. Prednisone is generally used when symptoms of MG are not adequately controlled by cholinesterase inhibitors alone. Good response can be achieved with initial high doses which are then tapered to the lowest dose to maintain the response. Temporary exacerbation can occur after starting high doses of prednisone within the first 7–10 days and can last for several days [35, 36]. In mild cases, cholinesterase inhibitors are usually used to manage this worsening. In cases of severe exacerbation, PE or IVIg can be given before or with corticosteroid therapy to prevent or reduce the severity of corticosteroid-induced weakness and to induce a more rapid response. Oral prednisone might be more effective than anticholinesterase drugs in oMG and should therefore at least be considered in all patients with oMG [37, 38].
Nonsteroidal Immunosuppressive Agents
Azathioprine, a purine analog, reduces nucleic acid synthesis, thereby interfering with T- and B-cell proliferation. It has been utilized as an immunosuppressant agent in MG since the 1970s and is effective in 70–90% of patients with MG [45]. It usually takes up to 15 months to detect clinical response. When used in combination with prednisone, it might be more effective and better tolerated than prednisone alone [49]. Adverse side effects include hepatotoxicity and leukopenia [50]. The patients being considered for treatment with azathioprine should be screened for thiopurine methyltransferase (TPMT) deficiency either by plasma levels or genetic testing. Those people who have low levels of TPMT are at higher risk of adverse effects from azathioprine and should not receive the drug.
Mycophenolate mofetil selectively blocks purine synthesis, thereby suppressing both T-cell and B-cell proliferation. Widely used in the treatment of MG, its efficacy in MG was actually suggested by a few nonrandomized clinical trials [31, 32].
The standard dose used in MG is 1000 mg twice daily, but doses up to 3000 mg daily can be used. Higher doses are associated with myelosuppression, and complete blood counts should be monitored at least once monthly. The drug is contraindicated in pregnancy and should be used with caution in renal diseases, GI diseases, bone marrow suppression, and elderly patients [33].
Cyclophosphamide administered intravenously and orally is an effective treatment for MG [34]. More than half of the patients become asymptomatic within 1 year of treatment. Undesirable side effects include hair loss, nausea, vomiting, anorexia, and skin discoloration, which limit its use to the management of patients who do not respond to other immunosuppressive treatments [2].
Cyclosporine blocks the synthesis of IL-2 cytokine receptors and other proteins critical to the function of CD4+ T cells. Cyclosporine is used mainly in patients who do not tolerate or respond to azathioprine. Large retrospective studies have supported its use as a steroid-sparing agent [45].
Tacrolimus has been used successfully to treat MG at low doses. It has the theoretical advantage of less nephrotoxicity than cyclosporine. However, there are more controlled trial data supporting the use of cyclosporine. Like other immunosuppressive agents, tacrolimus also has the potential for severe side effects [2].
MG patients resistant to therapy have been successfully treated with cyclophosphamide in combination with bone marrow transplant or with rituximab, a monoclonal antibody against the B-cell surface marker CD20 [26].
Etanercept, a soluble and a recombinant tumor necrosis factor (TNF) receptor blocker, has also been shown to have steroid-sparing effects in studies on small groups of patients [2, 27].
Surgical Management
Thymectomy Surgical treatment is strongly recommended for patients with thymoma. The clinical efficacy of thymectomy for patients with autoimmune MG without thymoma has been questioned because the evidence supporting its use has not been demonstrated in randomized controlled trials. However, many case reports and series suggest that thymectomy is also of benefit in generalized autoimmune MG, especially when performed in younger patients. The benefit of thymectomy evolves over several years. Thymectomy is advised as soon as the patient’s degree of weakness is sufficiently controlled to permit surgery. Patients undergoing surgery are usually pretreated with low-dose glucocorticoids and IVIg or PE. Thymectomy may not be a viable therapeutic approach for anti-MuSK antibody-positive patients because their thymus glands lack the germinal centers and infiltrates of lymphocytes that characterize thymi in patients who have anti-AChR antibodies. This supports a different pathologic mechanism in anti-MuSK-Ab-positive and anti-AChR-Ab-positive MG [78, 79]. Most experts still consider thymectomy to be a therapeutic option in anti-AChR-Ab-positive generalized MG with disease onset before the age of 50 years [2].
Prognosis
Given current treatment, which combines cholinesterase inhibitors, immunosuppressive drugs, PE, IVIg, immunosuppressive therapy, and supportive care in an intensive care unit (ICU) setting (when appropriate), most patients with MG have a near-normal life span. Mortality is now 3–4%, with principal risk factors being age older than 40 years, short history of progressive disease, and thymoma. Prior to modern therapies, the mortality from MG was as high as 30–40%. Fortunately, in most cases the term “gravis” is no longer applicable to most patients.
Morbidity results from intermittent impairment of muscle strength, which may cause aspiration, increased incidence of pneumonia, falls, and even respiratory failure if not treated [14]. In addition, the medications used to control the disease may produce adverse effects.
Today, the only terribly feared condition arises when the weakness involves the respiratory muscles. Weakness might become so severe as to require ventilatory assistance. Those patients are said to be in myasthenic crisis.
The disease frequently presents (40%) with only ocular symptoms. However, the EOMs are almost always involved within the first year. Of patients who show only ocular involvement at the onset of MG, only 16% still have exclusively ocular disease at the end of 2 years.
In patients with generalized weakness, the nadir of maximal weakness usually is reached within the first 3 years of the disease. As a result, half of the disease-related mortality also occurs during this period. Those who survive the first 3 years of disease usually achieve a steady state or improve. Worsening of disease is uncommon after 3 years.
Thymectomy results in complete remission of the disease in a number of patients. However, the prognosis is highly variable.
A retrospective study of 38 patients with MG indicated that the disease, particularly late-onset MG, is associated with a high risk for cancers outside of the thymus, whether or not the patient also has thymoma [16]. Extrathymic neoplasms occurred in 12 of the study patients. All of these tumors were solid and heterogeneous to their organ of origin. Some of the tumors were diagnosed before and some after the patients were diagnosed with MG.
Altogether the tumors represented nine different types of neoplasm, as follows:
-
Two each of squamous cell carcinoma of the mouth, invasive bladder cancer, and prostate adenocarcinoma
-
One each of basal cell skin cancer; lung, gastric, breast, and colon adenocarcinoma; and renal cell cancer
The only statistically significant variable among the patients was age, with the extrathymic tumors being found only in patients over 50 years. None of the patients with these neoplasms had thyroid disease or an autoimmune disease other than MG.
Congenital Myasthenic Syndromes
Congenital myasthenic syndromes (CMS) are characterized by fatigable weakness of skeletal muscle (e.g., ocular, bulbar, limb muscles) with onset at or shortly after birth or in early childhood. Rarely symptoms may not manifest until later in childhood. Cardiac and smooth muscles are not involved. Severity and course of disease are highly variable, ranging from minor symptoms to progressive disabling weakness. In some subtypes of CMS, myasthenic symptoms may be mild, but sudden severe exacerbations of weakness or even sudden episodes of respiratory insufficiency may be precipitated by fever, infections, or stress. Major findings of the neonatal onset subtype include feeding difficulties; poor suck and cry; choking spells; eyelid ptosis; and facial, bulbar, and generalized weakness. In addition arthrogryposis multiplex congenita may be present, and respiratory insufficiency with sudden apnea and cyanosis may occur. Later childhood onset subtypes show abnormal muscle fatigability with difficulty in activities such as running or climbing stairs; motor milestones may be delayed; fluctuating eyelid ptosis and fixed or fluctuating extraocular muscle weakness are common presentations.
Diagnosis/Testing
The diagnosis of CMS is based on clinical findings, a decremental EMG response of the compound muscle action potential (CMAP) on low-frequency (2–3 Hz) stimulation, absence of anti-acetylcholine receptor (AChR) and anti-MuSK antibodies in the serum, and lack of improvement of clinical symptoms with immunosuppressive therapy. Mutations in one of multiple genes encoding proteins expressed at the NMJ are currently known to be associated with subtypes of CMS, including the genes encoding different subunits of the acetylcholine receptor:
-
CHRNE (εAChR subunit)
-
CHRNA1 (αAChR subunit)
-
CHRNB1 (βAChR subunit)
-
CHRND (δAChR subunit)
-
AGRN encoding agrin
-
CHAT encoding choline O-acetyltransferase
-
COLQ encoding acetylcholinesterase collagenic tail peptide
-
DOK7 encoding protein Dok-7
-
GFPT1 encoding glucosamine-fructose-6-phosphate aminotransferase 1
-
MUSK encoding muscle, skeletal receptor tyrosine protein kinase
-
RAPSN encoding rapsyn (43-kd receptor-associated protein of the synapse)
-
SCN4A encoding the sodium channel protein type 4 subunit alpha
Management
Treatment of manifestations: Most individuals with CMS benefit from acetylcholinesterase (AChE) inhibitors and/or the potassium channel blocker 3,4-diaminopyridine (3,4-DAP); however, caution must be used in giving 3,4-DAP to young children and individuals with fast-channel CMS (FCCMS). Individuals with COLQ and DOK7 mutations usually do not respond to long-term treatment with AChE inhibitors. Some individuals with slow-channel CMS (SCCMS) are treated with quinidine, which has some major side effects and may be detrimental in individuals with acetylcholine receptor (AChR) deficiency. Fluoxetine is reported to be beneficial for SCCMS. Ephedrine and albuterol have been beneficial in a few individuals, especially as a therapeutic option for those with DOK7 or COLQ mutations.
Prevention of primary manifestations: Prophylactic anticholinesterase therapy to prevent sudden respiratory insufficiency or apneic attacks provoked by fever or infections in those with mutations in CHAT or RAPSN. Parents of infants are advised to use apnea monitors and be trained in CPR.
Agents/circumstances to avoid: Drugs known to affect neuromuscular transmission and exacerbate symptoms of myasthenia gravis (e.g., ciprofloxacin, chloroquine, procaine, lithium, phenytoin, beta-blockers, procainamide, quinidine).
Evaluation of relatives at risk: If the disease-causing mutations in the family are known, molecular genetic testing can be used to clarify the genetic status of at-risk asymptomatic family members, especially newborns or young children, who could benefit from early treatment to prevent sudden respiratory failure.
Genetic Counseling
Congenital myasthenic syndromes are inherited in an autosomal recessive or, less frequently, autosomal dominant manner.
In autosomal recessive CMS (AR-CMS), the parents of an affected child are obligate heterozygotes and therefore carry one mutant allele. Heterozygotes (carriers) are asymptomatic. At conception, each sibling of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffected and not a carrier.
In autosomal dominant CMS (AD-CMS), some individuals have an affected parent, while others have a de novo mutation. The proportion of cases caused by de novo mutations is unknown. Each child of an individual with AD-CMS has a 50% chance of inheriting the mutation.
Prenatal testing for pregnancies at increased risk is possible through laboratories offering either testing for the gene of interest or custom testing.
The Future and Myasthenia
Complement inhibition is an attractive therapeutic approach for MG because it is effective in RODENT EAMG (e.g., ref. 24).
Moreover, anti-C5 inhibitors show short-term safety and are effective in a variety of human disorders, including myocardial infarction [10], coronary artery bypass graft surgery [11], and lung transplantation [12]. Thus, therapeutic approaches based on inhibition of complement activation will likely be tried for MG in the future.
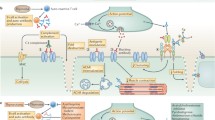
However, the ultimate goal for MG treatment is to eradicate the rogue anti-AChR autoimmune response specifically and reestablish tolerance to the AChR without affecting the other functions of the immune system or causing other adverse effects. Such targeted immunosuppressive approaches are still far from clinical use. However, their success in EAMG suggests that approaches for specific modulation of the autoimmune anti-AChR response may become part of MG patient care in the next decade. We will summarize here the different approaches that have proven successful for the prevention and treatment of EAMG induced by immunization with AChR. We will also analyze the possible technical and biological limitations to their application for the treatment of human MG.
Approaches that have proven successful in rodent EAMG include the following: (a) administration of AChR or parts of its sequence in a manner known to induce tolerance; (b) depletion of AChR-specific B cells or T cells; and (c) interference with formation of the complex between MHC class II molecules, epitope peptide, T-cell receptor, and CD4 molecule.
Antigen presentation under special circumstances may lead to antigen-specific tolerance in adult animals rather than activated CD4+ T cells. Earlier studies showed that in rats, presentation of AChR epitopes by unsuitable APCs (fixed B cells that had been incubated with AChR under conditions favoring AChR uptake and processing) caused unresponsiveness of the AChR-specific CD4+ T cells to further stimulation with AChR [13]. More recently, several studies have demonstrated that DCs, especially after treatment with TGF-β, IFN-γ, or IL-10, when injected into rats with developing or ongoing EAMG, suppressed or ameliorated the myasthenic symptoms [14–16]. The effect was correlated with a reduced production of anti-AChR Abs without a reduced proliferative response of T cells to the AChR. Approaches based on the use of tolerance-inducing APCs, which should present all AChR epitopes and therefore influence all AChR-specific T cells, might be useful for the treatment of MG. Should pulsing of the APCs with human AChR be needed, biosynthetic human AChR subunits could be used as antigens.
Mucosal or subcutaneous administration of AChR or synthetic or biosynthetic AChR peptides to rodents—approaches known to induce antigen-specific tolerance in adult animals—prevented or delayed EAMG development [19]. Depending on the dose of the antigen administered, anergy/deletion of antigen-specific T cells (at high doses) and/or expansion of cells producing immunosuppressive cytokines (TGF-β, IL-4, IL-10) (at low doses) are major mechanisms in mucosal tolerance induction. The use of mucosal toleration procedures in human MG, however, is problematic because those procedures can be a double-edged sword [20]; they reduce AChR-specific CD4+ T-cell responses but may also stimulate AChR-specific B cells to produce Abs, thereby worsening the disease. Also, a large amount of human AChRs would be required, which may be difficult to obtain.
Conjugates of a toxin with AChR or synthetic AChR sequences, when administered to animals with EAMG, eliminated B cells producing anti-AChR Abs [21]. This is probably because the AChR moiety of the conjugate docks onto the membrane-bound Abs of AChR-specific B cells, which can then be killed by the toxic domain. This approach has two caveats. First, the toxin may damage other cells. Second, anti-AChR CD4+ T cells can recruit new B cells to synthesize more anti-AChR Abs.
AChR-specific CD4+ T cells can be specifically eliminated in vitro by APCs genetically engineered to express relevant portions of the AChR, Fas ligand (to eliminate the activated AChR-specific T cells with which they interact), and a portion of Fas-associated death domain, which prevents self-destruction by the Fas ligand [22]. It is not known yet whether this strategy can be safely used to modulate EAMG in vivo.
Activation of CD4+ T cells requires interaction and stable binding of several proteins on the surfaces of the CD4+ T cell and of the APC. In experimental systems, interfering with formation of this complex usually reduced the activity of autoimmune CD4+ T cells. This may be obtained by administering or inducing Abs that recognize the binding site for the antigen of the T-cell receptor (known as T-cell vaccination) [23]. T-cell vaccination is already used in clinical trials for the treatment of multiple sclerosis, rheumatoid arthritis, and psoriasis [24]. It is effective in EAMG, and it is a promising future strategy for the treatment of MG [24]. The mechanisms of action of T-cell vaccination are complex, and they likely include the induction of modulatory CD4+ and CD8+ T cells [24]. Another approach used synthetic peptide analogs of an epitope recognized by autoimmune CD4+ T cells that bind the MHC class II molecules but cannot stimulate the specific CD4+ cells. These are known as altered peptide ligands (APLs). APLs compete with peptide epitopes derived from the autoantigen, thereby turning off the autoimmune response. APLs might also stimulate modulatory anti-inflammatory CD4+ T cells or anergize the pathogenic CD4+ T cells [25]. The rich epitope repertoire of anti-AChR CD4+ T cells in MG patients reduces the therapeutic potential of approaches that interfere with activation of specific CD4+ T cells; targeting only a few epitopes may not significantly reduce the anti-AChR response. Moreover, these treatments are likely to produce only transient improvement that ceases when administration of the anti-T-cell Ab is discontinued.
MG and EAMG have offered unique opportunities to investigate the molecular mechanisms of an Ab-mediated autoimmune disease. Many factors have contributed to making MG the best understood human autoimmune disease. These include the simplicity of the pathogenic mechanism in MG, where NMJ failure explains all symptoms; the deeper understanding of the structure and the function of the NMJ and its molecular components, most notably, the AChR; and the increasing understanding of the mechanisms that modulate immune responses and maintain tolerance. Hopefully increasing knowledge of the immunobiology of MG will form a foundation for designing new and specific therapeutic approaches aimed at curbing the rogue autoimmune response and reestablishing immunological tolerance without interfering with the other immune functions.
If this expectation is fulfilled, MG, which has been a benchmark to understanding autoimmunity in humans, will become a reference point for the design of specific immunosuppressive treatments of other autoimmune Ab-mediated diseases.
References
Robertson N. Enumerating neurology. Brain. 2000;123(4):663–4.
Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Investig. 2006;116(11):2843–54.
Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141–9.
Zhang X, Yang M, Xu J, et al. Clinical and serological study of myasthenia gravis in hu bei province, China. J Neurol Neurosurg Psychiatry. 2007;78(4):386–90.
Kurukumbi M, Weir RL, Kalyanam J, Nasim M, Jayam-Trouth A. Rare association of thymoma, myasthenia gravis and sarcoidosis: a case report. J Med Case Reports. 2008;2:245.
Marsteller HB. The first American case of myasthenia gravis. Arch Neurol. 1988;45(2):185–7.
Wilks, Sir Samuel, Bart. In: Roll Munk’s, editor. Reprinted by RCPs, 1955, P.86.
Willis T. Pathologiae cerebri et nervosi generis specimen. Oxford, UK: Ja Allestry; 1667.
Jolly F. Ueber myasthenia gravis pseudoparalytica, vol. 32. Berlin: Klin Wochenschr; 1895.
Hughes T. The early history of myasthenia gravis. Neuromuscul Disord. 2005;15(12):878–86.
Walker MB. Case showing the effect of prostigmin on myasthenia gravis. J R Soc Med. 1935;28:759–61.
Pascuzzi RM. The history of myasthenia gravis. Neurol Clin. 1994;12(2):231–42.
Nastuk WL, Strauss AJL, Osserman KE. Search for a neuromuscular blocking agent in the blood of patients with myasthenia gravis. Am J Med. 1959;26(3):394–409.
Simpson JA. Myasthenia gravis, a new hypothesis. Scott Med. 1960;5:419–36.
Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180(4088):871–2.
Sathasivam S. Steroids and immunosuppressant drugs in myasthenia gravis. Nat Clin Pract Neurol. 2008;4(6):317–27.
Gilhus NE, Owe JF, Hoff JM, Romi F, Skele GO, Aarli JA. Myasthenia gravis: a review of available treatment approaches. Autoimmune Diseases. 2011;10:1–6.
Leite MI, Waters P, Vincent A. Diagnostic use of autoantibodies in myasthenia gravis. Autoimmunity. 2010;43(5–6):371–9.
Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475–90.
Vernino S, Lennon VA. Autoantibody profiles and neurological correlations of thymoma. Clin Cancer Res. 2004;10(21):7270–5.
Leite MI, Scröbel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. 2005;57(3):444–8.
Vincent A, McConville J, Farrugia ME, Newsom-Davis J. Seronegative myasthenia gravis. Semin Neurol. 2004;24(1):125–33.
Morgenthaler TI, Brown LR, Colby TV, Harper CM, Coles DT. Thymoma. Mayo Clin Proc. 1993;68(11):1110–23.
Romi F, Skeie GO, Gilhus NE, Aarli JA. Striational antibodies in myasthenia gravis: reactivity and possible clinical significance. Arch Neurol. 2005;62(3):442–6.
Romi F, Skeie GO, Aarli JA, Gilhus NE. The severity of myasthenia gravis correlates with the serum concentration of titin and ryanodine receptor antibodies. Arch Neurol. 2000;57(11):1596–600.
Jaretzki A, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Ann Thorac Surg. 2000;70(1):327–34.
Hughes BW, Kusner LL, Kaminski HJ. Molecular architecture of the neuromuscular junction. Muscle Nerve. 2006;33(4):445–61.
Glass DJ, Bowen DC, Stitt TN, et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85(4):513–23.
Morgutti M, Conti-Tronconi BM, Sghirlanzoni A, Clementi F. Cellular immune response to acetylcholine receptor in myasthenia gravis: II. Thymectomy and corticosteroids. Neurology. 1979;29(5):734–8.
Weiner HL. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14.
Christadoss P, Goluszko E. Treatment of experimental autoimmune myasthenia gravis with recombinant human tumor necrosis factor receptor Fc protein. J Neuroimmunol. 2002;122(1–2):186–90.
Feferman T, Maiti PK, Berrih-Aknin S, et al. Overexpression of IFN-induced protein 10 and its receptor CXCR3 in myasthenia gravis. J Immunol. 2005;174(9):5324–31.
Shi FD, Wang HB, Li H, et al. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nat Immunol. 2000;1(3):245–51.
Jander S, Stoll G. Increased serum levels of the interferon-γ-inducing cytokine interleukin-18 in myasthenia gravis. Neurology. 2002;59(2):287–9.
Keesey JC. Clinical evaluation and management of myasthenia gravis. Muscle Nerve. 2004;29(4):484–505.
Vincent A, Leite MI. Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis. Curr Opin Neurol. 2005;18(5):519–25.
Grob D, Arsura L, Brunner NG, Namba T. The course of myasthenia gravis and therapies affecting outcome. Ann N Y Acad Sci. 1987;505:472–99.
Pal S, Sanyal D. Jaw muscle weakness: a differential indicator of neuromuscular weakness-preliminary observations. Muscle Nerve. 2011;43(6):807–11.
Grob D. Course and management of myasthenia gravis. JAMA. 1953;153(6):529–32.
Werner P, Kiechl S, Löscher W, Poewe W, Willeit J. Distal myasthenia gravis—frequency and clinical course in a large prospective series. Acta Neurol Scand. 2003;108(3):209–10.
Pascuzzi RM. The edrophonium test. Semin Neurol. 2003;23(1):83–8.
Meriggioli MN, Sanders DB. Advances in the diagnosis of neuromuscular junction disorders. Am J Phys Med Rehabil. 2005;84(8):627–38.
Sethi KD, Rivner MH, Swift TR. Ice pack test for myasthenia gravis. Neurology. 1987;37(8):1383–5.
Sanders DB, Howard JF, Johns TR. Single-fiber electromyography in myasthenia gravis. Neurology. 1979;29(1):68–76.
Oh SJ, Kim DE, Kuruoglu R, Bradley RJ, Dwyer D. Diagnostic sensitivity of the laboratory tests in myasthenia gravis. Muscle Nerve. 1992;15(6):720–4.
Lindstrom JM, Seybold ME, Lennon VA. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26(11):1054–9.
Lennon VA. Serologic profile of myasthenia gravis and distinction from the Lambert-Eaton myasthenic syndrome. Neurology. 1997;48(4):S23–7.
Vincent A, Newsom-Davis J. Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: results in 153 validated cases and 2967 diagnostic assays. J Neurol Neurosurg Psychiatry. 1985;48(12):1246–52.
Mittag TW, Caroscio J. False-positive immunoassay for acetylcholine-receptor antibody in amyotrophic lateral sclerosis. N Engl J Med. 1980;302(15):868.
Koon HC, Lachance DH, Harper CM, Lennon VA. Frequency of seronegativity in adult-acquired generalized myasthenia gravis. Muscle Nerve. 2007;36(5):651–8.
Cikes N, Momoi MY, Williams CL, et al. Striational autoantibodies: quantitative detection by enzyme immunoassay in myasthenia gravis, thymoma, and recipients of D-penicillamine or allogeneic bone marrow. Mayo Clin Proc. 1988;63(5):474–81.
Suzuki S, Satoh T, Yasuoka H, et al. Novel autoantibodies to a voltage-gated potassium channel KV1.4 in a severe form of myasthenia gravis. J Neuroimmunol. 2005;170(1–2):141–9.
Leite MI, Jacob S, Viegas S, et al. IgG1 antibodies to acetylcholine receptors in “seronegative” myasthenia gravis. Brain. 2008;131(7):1940–52.
Chagnac Y, Hadani M, Goldhammer Y. Myasthenic crisis after intravenous administration of iodinated contrast agent. Neurology. 1985;35(8):1219–20.
Eliashiv S, Wirguin I, Brenner T, Argov Z. Aggravation of human and experimental myasthenia gravis by contrast media. Neurology. 1990;40(10):1623–5.
Drachman DB. Medical progress: myasthenia gravis. N Engl J Med. 1994;330(25):1797–810.
Bosch EP, Subbiah B, Ross MA. Cholinergic crisis after conventional doses of anticholinesterase medications in chronic renal failure. Muscle Nerve. 1991;14(10):1036–7.
Batocchi AP, Evoli A, Schino CD, Tonali P. Therapeutic apheresis in myasthenia gravis. Ther Apher. 2000;4(4):275–9.
Gold R, Schneider-Gold C. Current and future standards in treatment of myasthenia gravis. Neurotherapeutics. 2008;5(4):535–41.
Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291(5503):484–6.
Psaridi-Linardaki L, Trakas N, Mamalaki A, Tzartos SJ. Specific immunoadsorption of the autoantibodies from myasthenic patients using the extracellular domain of the human muscle acetylcholine receptor α-subunit. Development of an antigen-specific therapeutic strategy. J Neuroimmunol. 2005;159(1–2):183–91.
Brannagan TH, Nagle KJ, Lange DJ, Rowland LP. Complications of intravenous immune globulin treatment in neurologic disease. Neurology. 1996;47(3):674–7.
Barth D, Nabavi Nouri M, Ng E, Nwe P, Bril V. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology. 2011;76(23):2017–23.
Robinson J, Eccher M, Bengier A, Liberman J. Costs and charges for plasma exchange (PLEX) versus intravenous immunoglobulin (IVIg) in the treatment of neuromuscular disease. Neurology. 2012;78:PD6.008.
Pascuzzi RM, Branch Coslett H, Johns TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol. 1984;15(3):291–8.
Evoli A, Batocchi AP, Palmisani MT, Lo Monaco M, Tonali P. Long-term results of corticosteroid therapy in patients with myasthenia gravis. Eur Neurol. 1992;32(1):37–43.
Kupersmith MJ, Moster M, Bhiiiyan S, Warren F, Weinberg H. Beneficial effects of corticosteroids on ocular myasthenia gravis. Arch Neurol. 1996;53(8):802–4.
Bhanushali MJ, Wuu J, Benatar M. Treatment of ocular symptoms in myasthenia gravis. Neurology. 2008;71(17):1335–41.
Palace J, Newsom-Davis J, Lecky B. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Neurology. 1998;50(6):1778–83.
Kissel JT, Levy RJ, Mendell JR, Griggs RC. Azathioprine toxicity in neuromuscular disease. Neurology. 1986;36(1):35–9.
Chaudhry V, Cornblath DR, Griffin JW, O’Brien R, Drachman DB. Mycophenolate mofetil: a safe and promising immunosuppressant in neuromuscular diseases. Neurology. 2001;56(1):94–6.
Ciafaloni E, Massey JM, Tucker-Lipscomb B, Sanders DB. Mycophenolate mofetil for myasthenia gravis: an open-label pilot study. Neurology. 2001;56(1):97–9.
Meriggioli MN, Ciafaloni E, Al-Hayk KA, et al. Mycophenolate mofetil for myasthenia gravis: an analysis of efficacy, safety, and tolerability. Neurology. 2003;61(10):1438–40.
Spring PJ, Spies JM. Myasthenia gravis: options and timing of immunomodulatory treatment. BioDrugs. 2001;15(3):173–83.
Tindall RSA, Phillips JT, Rollins JA, Wells L, Hall K. A clinical therapeutic trial of cyclosporine in Myasthenia gravis. Ann N Y Acad Sci. 1993;681:539–51.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Richeh, W., Engand, J.D., Paddison, R.M. (2017). Myasthenia Gravis: Clinical Features, Immunology, and Therapies. In: Minagar, A., Alexander, J. (eds) Inflammatory Disorders of the Nervous System. Current Clinical Neurology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-51220-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-51220-4_11
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-51218-1
Online ISBN: 978-3-319-51220-4
eBook Packages: MedicineMedicine (R0)