Abstract
This chapter discusses the clinical manifestations, pathogenesis, and treatment of Guillain-Barre syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP), including recent advances in immunopathogenesis as well as the treatments in pipeline. GBS and CIDP are part of the spectrum of autoimmune-mediated neuropathy. The target antigen remains elusive in CIDP and acute inflammatory demyelinating polyneuropathy, the most common variant of GBS in North America. Autoimmunity against different gangliosides is involved in the pathogenesis of GBS variants, i.e., acute motor axonal neuropathy, Miller Fisher syndrome, and acute motor and sensory axonal neuropathy, as well as their sub-variants. As GBS is a monophasic disease, immunomodulatory treatment, currently consisting of intravenous immunoglobulin infusions or plasma exchange, is required only during the acute phase, unless there are relapses. On the other hand, maintenance treatment with the aforementioned treatment modalities, as well as corticosteroids or other immunosuppressants, is frequently needed in CIDP patients. Further advances in the understanding of immunopathogenesis of these diseases will likely result in development of new treatments involving targeted immunotherapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Guillain-Barre syndrome
- GBS
- Chronic inflammatory demyelinating polyneuropathy
- CIDP
- Acute inflammatory demyelinating polyneuropathy
- AIDP
- Acute motor axonal neuropathy
- AMAN
- Miller Fisher syndrome
- MFS
- Ganglioside
- GM1
- GD1a
- GQ1b
- GT1a
- Campylobacter jejuni
- Neurofascin 155
- Contactin
- Caspr 1
- Intravenous immunoglobulin
- IVIG
- Plasma exchange
- PLEX
Guillain-Barre Syndrome (GBS)
GBS represents a spectrum of polyneuropathies, which arise from immune-mediated attack on different myelin or axonal antigens of peripheral and/or cranial nerves. GBS is the most common cause of flaccid paralysis worldwide after the elimination of poliomyelitis [1]. GBS encompasses a spectrum of diseases (i.e., subtypes) with varied clinical manifestations, reflective of the target antigen of autoimmune attack (myelin vs. axon) as well as the location of immunopathology within the peripheral nervous system (nerve roots, plexi, distal nerves, cranial nerves). Besides the autoimmune etiology, the GBS subtypes share the acute to subacute onset and albuminocytological dissociation in the CSF.
Subtypes of GBS have been defined based on the clinical manifestations, neurophysiological features, and presence of different antibodies to neural glycolipid components. Acute inflammatory demyelinating polyneuropathy (AIDP) constitutes the typical primarily demyelinating form of the disease. AIDP is the most common subtype of GBS in Europe and North America and is typically characterized by acute onset of flaccid, hypo-, or areflexic paralysis [1, 2]. The clinical course consists of progressive weakness within hours to days and maximum weakness and disability within 4 weeks. Muscle weakness (including proximal limb and respiratory) usually dominates the clinical presentation. However, sensory symptoms, usually a distal paresthesia, very often allow distinguishing AIDP from some of its mimics such as myasthenia gravis and botulism. Dysautonomia is prevalent in AIDP and is one of its life-threatening manifestations. A less common, atypical presentation, which is encountered in 8% of the patients, is paraparesis without arm weakness. [3] Patients with paraparetic GBS, however, usually have sensory symptoms and areflexia, as well as abnormal conduction studies in the upper extremities [3]. Acute motor axonal neuropathy (AMAN) is the second most common form of GBS in North America and Europe, accounting for 6–78% of the cases, and the most common in China and Bangladesh [4]. AMAN patients have a purely motor picture (positive sensory symptoms in only 10% of patients). In contrast to AIDP, dysautonomia and cranial nerve involvement are rare, and deep tendon reflexes are often normal to brisk in AMAN [4]. AMAN is also associated with a more rapid progression early in the course, with earlier peak than AIDP (11.5 vs. 18 days) [5]. Acute motor and sensory axonal neuropathy is the third GBS subtype which has sensory involvement (in contrast to AMAN) and is characterized by less favorable recovery because of axonal degeneration. Miller Fisher syndrome (MFS), the fourth major subtype of GBS, accounts for 5–12% of the GBS cases [6]. MFS typically presents with a triad of ophthalmoparesis, ataxia, and areflexia, and the patients generally do not develop significant weakness or respiratory impairment and have a good prognosis. MFS by itself has different clinical subtypes: acute ataxic neuropathy (without ophthalmoplegia), acute ophthalmoparesis (without ataxia), and a variant with CNS symptoms such as hypersomnolence (Bickerstaff’s encephalitis) [1]. Yet another less common, local subtypes of MFS include pharyngeal-cervical-brachial variant, which is characterized by rapidly progressive weakness of oropharyngeal, cervical, and upper extremity muscles accompanied by areflexia of the upper extremities [7].
Examination of cerebrospinal fluid (CSF) demonstrates albuminocytological dissociation in all the variants of GBS. Another useful diagnostic test is nerve conduction study and abnormal nerve conduction study, which demonstrates segmental demyelination in AIDP and axonal neuropathy in AMAN, AMSAN, and MFS and its variants [8]. It should be noted that conduction block, which is characteristic for AMAN, is secondary to functional blockage of axonal salutatory conduction and not secondary to segmental demyelination, leading to the recommendation that at least two sets of nerve conduction studies over time to differentiate AIDP from AMAN [9].
Pathology
AIDP is characterized by lymphocytic (mainly T cell) and macrophage infiltration and associated segmental demyelination, which affect nerve roots, plexi, and proximal portions of the nerves, which are more myelinated [10, 11]. Complement activation has been suggested to play an early role, as deposition of complement activation marker C3d and terminal complement complex C5b-9 on the surface of Schwann cells and myelin degeneration were shown to precede macrophage infiltration in patients who succumbed in early stage of AIDP [12].
On the other hand, postmortem findings in AMAN subtype may show Wallerian degeneration of the motor axons; presence of macrophages within the periaxonal space, which surround or displace the axons; and intact myelin sheath [13]. Some of the AMAN patients with fatal paralysis have had minimal axonal degeneration in the postmortem study consistent with functional impairment of axonal electrical conduction in these cases [13]. Axonal degeneration of the motor and sensory nerves is the hallmark of the neuropathology in AMSAN [13]. Because of the benign clinical course of MFS, the pathological studies are limited. Although segmental demyelination is reported in a patient with MFS [14], the patient more likely had AIDP and associated ophthalmoplegia.
Immunopathogenesis
About two thirds of GBS cases occur after a respiratory or gastrointestinal infection, and the pathogen can be identified in about half of these cases [15]. Some of the more common preceding infections include C. jejuni cytomegalovirus, Epstein-Barr virus, Mycoplasma pneumonia, Hemophilus influenza, influenza A, and hepatitis E virus [2]. The best explanation for the association of GBS and aforementioned infections is molecular mimicry between the components of pathogens and axonal or myelin structures. C. jejuni is the most common antecedent infection in GBS, ranging from 26 to 65% of the cases depending on the geographic location [4]. Patients with AMAN after C. jejuni infection have high titers of antibodies to GM1 and GD1a, which is the result of cross-reactivity between lipo-oligosaccharides from the bacterial wall of C. jejuni and respective gangliosides of the motor nerve axons [16, 17]. On the other hand, lipo-oligosaccharides that mimic the carbohydrate moiety of peripheral nerve gangliosides are expressed in only a subset of C. jejuni strains, Penner D: 19 serogroup, as it is different from other serotypes in containing genes for enzymes involved in synthesis of sialic acids which result in molecular mimicry with gangliosides GM1, GD1a ND GD1B [1]. As a result, GBS is a relatively rare outcome of these infections: e.g., only one out of 5000 C. pylori gastroenteritis results in GBS [18]. Whether C. jejuni infection is a cause of AIDP is a matter of controversy. A previous study showed that only 5 of 22 (23%) of patients with GBS post C. jejuni infection had AIDP, but when they were followed by repeated nerve conduction studies, all of those who had prolonged motor distal latencies normalized in less than 2 weeks suggestive for impaired axonal conductivity (seen in AMAN) rather than segmental demyelination seen in AIDP, which is associated with more slowing of the nerve conduction study in the same time period during remyelination [19]. A neuropathy characterized by severe axonal degeneration and seropositivity for IgG or IgM GM1 antibodies has also been reported in patients who received ganglioside injections for chronic pain [20]. IgG antibodies against GQ1b and GD1a are detected in more than 90% of patients with MFS [21–23], as well as patients with AIDP who have ophthalmoplegia. As about half of patients with pharyngeal-cervical-brachial variants are seropositive for IgG anti-GT1a antibodies which cross-reacts with GQ1b, it is considered to be in the broad spectrum of MFS [7].
Differences in anatomical expression of gangliosides explain the diverse phenotypic manifestations of GBS variants. GM1 is suggested to be expressed more in the motor than sensory nerve roots, therefore providing possible explanation for motor involvement of AMAN [23]. On the other hand, GM1/GD1a is also present in the sensory nerves [24]. The predominant or pure motor involvement could be the result of specificity of autoantibodies for epitopes of these gangliosides that are only present in the motor axons. Furthermore, nodes of Ranvier of the distal, intramuscular portion of the motor axons are suggested to be particularly susceptible to complement activation by antibodies to GD1a [25]. The blood-nerve barrier is more permeable in the unmyelinated distal branches of the motor nerves and the nerve roots, making these parts of the peripheral nerves more vulnerable to circulating factors such as autoantibodies and complement [26, 27]. Ophthalmoplegia and areflexia in MFS which is associated with antibodies directed to GQ1b are explained by high expression of GQ1b in the oculomotor nerves and muscle spindles [23].
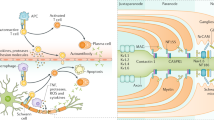
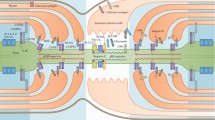
The autoantigen involved in AIDP is so far unknown, and most of the AIDP patients are not seropositive for antiganglioside antibodies. Some of the putative antigens include proteins which are expressed at the nodes of Ranvier (neurofascin 186, gliomedin, sodium channels, ankyrin, and spectrin) and at the paranode (neurofascin 155, contactin/Caspr 1, and connexins Cx31.3, Cx3232) [23].
A recently identified molecular target is moesin in patients with CMV infection as antibodies against moesin were present in most of AIDP cases after CMV but not with other GBS patients or other neurological disease controls [28]. Moesin is expressed in the microvilli of the Schwann cells and has been proposed to have a critical role in myelination [29].
There is also evidence for involvement of T cells in the pathogenesis of GBS, based on: (1) T cell infiltration is present in experimental allergic neuritis (EAN) which is considered as an animal model of GBS. (2) There is increased frequency of Th1 and Th17 levels in the blood and of T cell-related cytokines (IFN gamma, IL-17, and IL-22) in the cerebrospinal fluid of GBS patients [30–32]. (3) Reduced number and abnormal function of CD4+Foxp3+ (Treg) cells, which have a critical role in immune homeostasis, have been demonstrated in the blood of GBS patients [32, 33].
Animal Models
Experimental allergic neuritis (EAN) has been considered as an animal model for human GBS. EAN is usually (but not always) a monophasic illness, which is induced by vaccination of rats, mice, rabbits, and guinea pigs with peripheral nerve homogenate or different myelin proteins such as P0, PMP 22, and P2 [34–37]. It presents with weakness and ataxia after a period of about 2 weeks after the vaccination. Perivascular T cell infiltration is noted 2–3 days before the onset of demyelination and paralysis [36, 37]. T cell infiltration results in activation of monocytes to tissue macrophages, which subsequently strip myelin and cause axonal injury by secreting cytokines such as tumor necrosis factor alpha. B cells also play a role in the pathogenesis of EAN, and autoantibodies against the myelin play a synergistic role in causing demyelination, after the blood-nerve barrier has become more permeable because of T cell activation and subsequent infiltration of macrophages [38]. Although the target antigen in EAN remains to be elusive, neurofascin 186 and gliomedin, which are involved in clustering of voltage-gated Na channels at the nodes of Ranvier, have been suggested as potential antigenic targets [39, 40]. In the EAN model induced by vaccination with peripheral myelin in rat, antibodies to neurofascin and gliomedin cause dismantling of nodal organization and Na channel clusters, therefore leading to conduction block prior to onset of demyelination [39, 40].
B cell immunity, particularly autoantibodies to gangliosides, appears to have a primary role in the pathogenesis of GBS variants. Immunization of Japanese white rabbits with a bovine brain ganglioside mixture or isolated GM1 results in an AMAN phenotype: acute monophasic flaccid paralysis, seropositivity for anti-GM1 antibodies, axonal degeneration, IgG deposits at the nodes of Ranvier and lymphocytic infiltration in the periaxonal space, and lack of segmental demyelination [41, 42]. On the other hand, GQ1b and GD1a antibodies cause conduction block at the motor nerve terminals in a mouse model [25].
Treatment of GBS
Treatment of GBS consists of supportive treatment as well as immunotherapy in more severe cases. Supportive care is better provided in an intensive care unit in the progressive phase of the disease.
Supportive Treatment
-
1.
Respiratory care
Respiratory failure is one of the most serious short-term complications of GBS. About 25% of patients with GBS who are unable to walk and 30–50% of patients who are admitted to ICU undergo intubation and mechanical ventilation [43]. The need for mechanical ventilation should be anticipated in GBS when there is rapidly progressive course as manifested by time to peak disability less than 7 days, time from the onset of symptoms to hospitalization less than 7 days, and presence of more than 30% reduction of vital capacity, NIF, and PEF during the course of hospitalization [44, 45]. It is essential to anticipate the need for mechanical ventilation (MV) and proceed with elective intubation in selected patients. It is therefore recommended to assess FVC every 2–4 h during the day and every 4–6 h at night in a patient with declining respiratory function. A vital capacity of less than 20 mL/kg, maximal inspiratory pressure less than 30 cm H2O, maximal expiratory pressure less than 40 cm H2O, and a reduction of more than 30% in vital capacity, maximal inspiratory pressure, or maximal expiratory pressure anticipate need for oncoming respiratory failure [44]. Elective intubation and MV are recommended in patients with significant respiratory distress, fatigue, sweating, tachycardia, active aspiration, FVC < 15 mL/kg, hypercarbia (PaCO2 48 mm Hg), and hypoxemia (PaO2 on room air <56 mm Hg) [1, 46].
-
2.
Dysautonomia
Autonomic dysfunction in GBS is more common in the acute stage of the disease, can involve sympathetic or parasympathetic systems, and is a major cause of mortality [2]. In a study on pediatric GBS patients, hypertension and tachycardia occurred in 70 and 77% of the patients, respectively, and they were more likely with increasing motor weakness [47]. In another study on 156 GBS patients, tachycardia, hypertension, and hypotension were noted in 38, 69, and 11% of the patients, respectively [48]. Less common manifestations include transient ECG changes such as ST segment elevation and diffusely inverted T waves secondary to coronary vasospasm [49]. Careful assessment for fluctuations in blood pressure and pulse rate and appropriate treatment which may involve symptomatic treatment and even insertion of a pacemaker are therefore important aspects of the GBS care, especially during the ICU care, but also during the recovery period [1].
Gastrointestinal dysfunction was noted in 45% of a large cohort of GBS patients [48], while adynamic ileus was reported in 15% of GBS patients admitted to the ICU in another study [50]; however, the authors speculated that some of the cases could have been due to other factors such as abdominal surgery, immobility, and use of medications such as opioids.
About a quarter of GBS patients (39% of AIDP and 19% of the AMAN cases) had urinary symptoms, including urinary retention in about 10% of the cases [51, 52]. Urinary dysfunction in GBS is proposed to be caused by either hypo- or hyperactivity of lumbosacral nerves [52]. Besides incontinence and urinary retention which will require the use of a catheter, patients may develop underactive detrusor, overactive detrusor, and, to a lesser extent, hyperactive sphincter. Urinary symptoms may be persistent and affect the quality of life in the patients who have recovered from the acute phase, i.e., urinary frequency and urgency were present in one third and nocturia in half of the patients who recovered from GBS patients when these patients were followed for 6 years [53].
Immunomodulatory Treatments
GBS was associated with mortality in 10% of patients and severe residual neurological deficit in 20 of cases before the introduction of immunotherapy [54]. As detailed below, immunomodulatory treatments directed at removal (plasma exchange (PLEX)) or modulation of immunoglobulins and probably T cell responses (intravenous immunoglobulins (IVIG)) have been proven to be effective in GBS. In contrast to many other autoimmune neurological diseases, steroids have not shown to hasten recovery nor affect the long-term outcome [55], and their use is not recommended in GBS, neither alone nor combined with PLEX or IVIG [1, 2].
-
1.
Plasma Exchange (PLEX)
The immunomodulatory action of PLEX is through the removal of autoantibodies and complement components. It is usually administered at five plasma volume exchanges (50 ml/kg each) usually every other day, over a period of up to 2 weeks [56, 57]. PLEX is more effective if done early in the course of the illness, preferentially the first week after the onset of symptoms [58]. However, larger exchanges of 1.5 plasma volumes have also been used. Hughes et al. reviewed four clinical studies involving 585 severely affected GBS patients and concluded that there is significant improvement and less disability in the treated patients after 4 weeks and 1 year after of randomization [56–60]. The treated patients also had a higher chance of full strength recovery (odds ratio 1.24, confidence interval 1.07–1.45), as well as lower disability and higher likelihood for full recovery in 1 year [59]. In milder GBS patients who did not lose the ability to ambulate, patients who received two sessions of PLEX over 3 days had shorter onset of motor recovery (4 vs. 8 days) and better improvement after 1 month compared to those who did not receive PLEX [57]. On the other hand, in GBS patients who could not stand unaided, there was a higher likelihood of regaining full motor strength in 1 year after four sessions of PLEX (x1.5 plasma volume each) than after two sessions (64% vs. 48%) [57]. Six exchanges were similar in efficacy to four in the severe GBS cases in the latter study.
-
2.
Intravenous Immunoglobulin (IVIG)
IVIG has become the preferred treatment for GBS because of the availability and convenience of use [1]. The therapeutic effect of IVIG in GBS may arise from blocking pathogenic autoantibodies and antibody-mediated complement activation [8]. On the other hand, IVIG has shown to result in reduced number of Th1 and Th17 and expansion of the population of Treg cells in GBS patients [31, 32]. IVIG, when started within 2 weeks of onset of weakness, has been shown to be effective in AIDP patients with more severe disease manifested as inability to walk 10 m unaided (GBS disability scale score ≥3) [59]. IVIG treatment has been demonstrated to be as effective as PLEX if given within 2 weeks in patients who lose the ability to walk [61, 62]. The dosage of IVIG used in the GBS clinical trials has been 2 g/kg divided over 5 days [59]. The same dose can be divided over 2–4 days in selected cases, although a study suggested more posttreatment relapses in children who received the dose in 2 days [63]. It has been suggested that some patients may have a better response with a higher dose than 2 g/kg total or a second course of treatment, for the following reasons: (1) about 10% of the IVIG-treated GBS patients have a relapse, which usually responds to further treatment with IVIG [64], and (2) a subgroup of GBS have poor initial response and slower recovery, which has been correlated with lower levels of serum immunoglobulin concentrations due to different pharmacokinetics [65]. The latter subgroup may benefit from a higher dose or a second course of treatment [65].
Although the optimal immunomodulatory treatment for AMAN is still unclear, PLEX has been suggested to be more efficient and cost-effective than IVIG [2, 66]. The prognosis of MFS is generally good without treatment. Although the recovery started earlier in the MFS patients who received IVIG, the final outcome was not changed by the use of PLEX or IVIG in a study [67].
-
3.
Oncoming Treatments
Considering the role of anti-ganglioside antibodies and complement activation in the pathogenesis of GBS variants, modulation of complement activation through monoclonal antibodies and synthetic serine protease inhibitors is emerging as a new treatment for GBS [8]. Eculizumab is a humanized monoclonal antibody, which binds plasma C5 and blocks its cleavage to C5b, therefore preventing the formation of membrane attack complex [68]. Eculizumab prevented the occurrence of anti-GQ1b-mediated neuropathy in a murine model [69]. Nafamostat, a synthetic serine protease inhibitor which is used as a short-acting anticoagulant during hemodialysis, has been shown to ameliorate the phenotype of anti-GM1 antibody-mediated neuropathy in a rabbit model due to its anticomplement activity [70].
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
The term CIDP refers to a chronic form of an acquired inflammatory polyneuropathy that is clinically differentiated from AIDP by its time course. CIDP encompasses a spectrum of phenotypic variants with common features of chronicity, demyelination evident on the nerve conduction studies, and albuminocytological dissociation in the CSF.
Clinical Manifestations
Classical CIDP is characterized by symmetrical proximal and distal muscle weakness, sensory loss, and hyporeflexia or areflexia, with either a relapsing or progressive course [71]. Proximal weakness and upper extremity involvement are common in classical CIDP, which is in contrast to most other types of polyneuropathy which are generally characterized by a more distal pattern of involvement [72]. Sensory changes may include numbness, paresthesias, and difficulty with proprioception and balance. Neuropathic pain is a rather infrequent feature in CIDP [73], but rarely pain is the presenting feature [74]. Respiratory compromise and dysautonomia are uncommon in CIDP (in contrast to GBS) and occur in less than 10% of patients [75]. Facial, ocular, and oropharyngeal involvement is infrequent as well and is estimated to occur in about 15% of patients [76]. CIDP is differentiated from GBS by its time course: the time to nadir in CIDP is more than 8 weeks (it is usually <2 weeks and maximally 4 weeks in GBS) [2]. In two thirds of those affected, the disease has a progressive course, with the remainder experiencing relapses.
CIDP Variants
Only 50% of patients with CIDP present with classic features described above [77]. Other variants of CIDP include sensory-predominant, motor-predominant, ataxic, chronic inflammatory sensory polyradiculopathy (CISP), and multifocal acquired demyelinating sensory and motor (MADSAM) neuropathy.
Five to thirty-five percent of CIDP patients present with sensory symptoms in their lower extremities [78]. Despite this purely sensory presentation from the clinical standpoint, motor nerve conduction abnormalities consistent with demyelination can be found in many of these patients, and a pure sensory variant of CIDP has only been reported rarely [79, 80]. On the other hand, many of the patients with purely sensory variant will develop motor involvement years later [81]. Sensory CIDP may mimic sensory ganglionopathy if the sensory action potentials are absent and motor conduction studies are entirely normal. In these instances, nerve biopsy may be required for the diagnosis [79, 82]. A rare (~5%) predominantly sensory ataxic form of CIDP (chronic immune sensory polyradiculopathy (CISP)) is a distinct clinical entity that involves large fibers of the dorsal roots rather than distal sensory nerves [83, 84]. In these cases peripheral nerve conduction studies may be unrevealing, and somatosensory conduction potentials may need to confirm demyelination of the sensory nerve roots [85]. The motor-predominant variant of CIDP presents with relatively symmetric proximal and distal muscle weakness, demyelination on the nerve conduction study, and minimal or absent sensory involvement, which occurs in about 7–10% of patients with CIDP, more commonly in young adults <20 years of age [78, 86, 87]. The main differential diagnosis for motor variant of CIDP is multifocal motor neuropathy. Multifocal acquired demyelinating sensory and motor (MADSAM, aka Lewis-Sumner syndrome) neuropathy is a focal variant which occurs in about 6–15% of CIDP patients [78]. MADSAM presents with an asymmetrical muscle weakness and sensory changes, usually starting in one or both upper extremities. Later in its clinical course, MADSAM may become more diffuse and involve both lower extremities as well.
It is differentiated from axonal mononeuritis multiplex by the presence of segmental demyelination in the nerve conduction study, involving both motor and sensory nerves.
Pathology
Postmortem studies as well as MRI and ultrasonography have demonstrated involvement of nerve roots, plexi, and proximal nerve trunks, as well as focal involvement of more distal portion of peripheral nerves in CIDP patients [88, 89]. The classic histopathological findings include demyelination, remyelination (thick myelin sheath and onion bulb formation), endoneurial edema, and presence of inflammatory infiltrates (CD4, CD8 lymphocytes) in the perineurium and endoneurium [73]. Macrophages intercalate between the layers of Schwann cell membranes, including outer mesaxon, extending their elongated processes into the myelin lamellae and breaking them down [90]. Due to the focal distribution of lesions, up to 20% of biopsies may show no inflammatory changes. Only 10–50% of nerve biopsies show inflammatory cell infiltrates, due to the focal nature of the disease [90]; on the other hand, 20–40% only show features of axonal degeneration [73, 91, 92].
Immunopathogenesis
CIDP is an autoimmune disease as proven by its response to immunomodulatory treatments, presence of inflammatory infiltrates in the peripheral nerves, and development of a chronic relapsing EAN in animal models, similar to CIDP from the pathological and electrophysiological standpoint [93, 94].
Immunopathogenesis of CIDP is complex and involves both cellular and humoral arms of the immune system, affecting peripheral myelin. Breakdown in the blood-nerve-barrier (BNB), which protects the microenvironment of the nerve from exogenous proteins such as potentially pathogenic immunoglobulins, plays a key role in the pathogenesis of CIDP. Abnormal permeability of BNB can be detected via contrast enhancement seen in the MRI of the inflamed nerve trunks and plexi of patients with CIDP [95, 96].
Similar to AIDP, the target antigen remains unknown in CIDP, but unlike GBS, CIDP is characteristically not preceded by an antecedent infection. Although about a third of cases were preceded by an infection in a previous study [97], other studies have challenged that data by finding that the antecedent infections were present in only 10% of patients with CIDP, which does not differ from the prevalence of in the general population [98]. On the other hand, the onset has not been consistently linked to any one specific antecedent infection, with the exception of rare association of CIDP and HIV infection [99, 100]. CIDP has been rarely reported in association with malignant melanoma, which is explained by presence of shared antigens, such as myelin-associated glycoprotein and different gangliosides, between melanocytes and Schwann cells, as they both are derived from neuroectodermal origin [101–104].
Cellular Immunity
Aberrant T cell activation plays an important role in the pathogenesis of CIDP as suggested by several lines of evidence: (i) sural nerve biopsies of CIDP patients frequently demonstrate endoneurial infiltration by CD4+, CD8+ T cells, and macrophages [105]; (ii) changes in T cell subsets, function, and interleukin profiles have been reported in the blood and CSF of patients with CIDP [106]; and (iii) gamma delta T cells, which are capable of recognizing nonprotein antigens such as gangliosides, were observed in 14 of 20 CIDP nerve biopsy specimens [107].
It is yet unclear whether the initial activation of T cells occurs in lymphoid organs or within the peripheral nerve. Upon the activation of peripheral CD4+ T cells, they release multiple inflammatory cytokines (interleukin (IL)-2, interferon-γ (IFNγ), and IL-17 as well as the chemokines (interferon gamma-induced protein (IP)-10 and macrophage inflammatory protein 3 β (MIP3β) and stimulate the increase in the expression of the endothelial adhesion molecules (VCAM, ICAM, ELAM) that mediate the adherence and transmigration of T cells through BNB and into the nerve compartment. When in the endoneurium, T cells release pro-inflammatory cytokines and metalloproteinases (MMP), further breaking down the BNB. Both MMP-2 and MMP-9 were found to be upregulated in nerves of CIDP patients [108]. As T cells transmigrate BNB, they become locally activated due to the upregulation of MHC II and co-stimulatory molecules B7-1 and B7-2 by infiltrating macrophages as well as Schwann cells. An antigen-driven, major histocompatibility complex class I-restricted CD8+ T cell-mediated immune attack has also been suggested to play a role in the pathogenesis of CIDP [109, 110]. An oligoclonal or polyclonal repertoire of CD8+ T cells is found in peripheral nerves of patients with CIDP which correlates with the same expansion in their blood [109]. On the other hand, IVIG corrects this prominent oligoclonal repertoire of CD8+ T cells [110].
Another important checkpoint that controls the extent of inflammatory reaction and autoimmunity is Treg cells. In patients with CIDP, Treg cells are reduced in number and have been found to be less functional than in healthy controls [111, 112]. The B7-1/B7-2 CD28/CTLA4 signaling pathways are important in the lymphocyte activation and homeostasis of Treg cells, with CD28 signaling promoting and CTLA4 signaling downregulate T cell activation [36, 113]. The importance of the aforementioned pathways in the pathogenesis of CIDP is demonstrated by occurrence of a spontaneous autoimmune neuropathy in B7-2 knockout nonobese diabetic mice (see below).
Endoneurial macrophages and Schwann cells may function as antigen-presenting cells particularly in regard with nonprotein antigens, as indicated by overexpression of MHC-like molecules CD1a and CD1b in these cells in the nerve biopsies of CIDP patients [114, 115]. Moreover, Schwann cells may participate as accessory cells in T cell activation as they express CD58 molecule (LFA-3) [115]. Macrophages recruited into the site of inflammation represent one of the dominant effector cells in CIDP [116]. They form clusters around the endoneurial vessels and participate in antigen presentation, in the release of pro-inflammatory cytokines, and at the end stage in stripping away the damaged myelin and phagocytizing it.
Humoral Immunity
Different lines of evidence suggest that humoral immunity has an important role in the pathogenesis of CIDP. Firstly, sural nerve biopsies of some patients with CIDP have shown complement and immunoglobulin deposition on the surface of Schwann cells and compact myelin [117, 118]; secondly, serum proteins from CIDP patients bind to the segments of healthy nerves, which results in demyelination and conduction blocks, when injected interneurally [119]; thirdly, the efficacy of plasma exchange in the treatment of CIDP implicates the important role of humoral factors in its pathogenesis.
It is therefore plausible that after the BNB is first damaged by the action of T cells and macrophages detailed above, autoantibodies mediate demyelination by complement fixation and by directing macrophages to the antigenic targets via Fc receptors, leading to opsonization and phagocytosis.
Although the target antigen in CIDP remains elusive, antibodies to a number of myelin and axonal antigens such as glycolipids GM1, LM1, and LM1-containing ganglioside complex, beta tubulin, galactocerebroside, chondroitin sulfate, and proteins P0, P2, and P0-related glycoprotein have been reported in sera from CIDP patients [120, 121]. On the other hand, these antibodies have not been detected in most patients with CIDP, and only antibodies against PO were shown to be pathogenic in vivo with passive transfer or intraneural injection [122]. The presence of these autoantibodies may represent an epiphenomenon of the ongoing inflammation rather than denote causality.
Proteins in the non-compact myelin in the nodal, paranodal, and juxtanodal regions have an important role for the maintenance of structural integrity of the nodes of Ranvier and therefore saltatory conduction. As the search for a target antigen among major compact myelin proteins has been so far unsuccessful, the attention has shifted toward non-compact myelin proteins such as gliomedin, neurofascin, contactin, and Caspr 1 [40, 123, 124]. The complex of contactin/Caspr/neurofascin-155 has a critical function in the integrity of paranodal junctions [125]. In a study by Deveaux et al., 30% of patients with CIDP had IgG antibodies that bound to the nodes of Ranvier and paranodes of the rodent nerves, and the binding was specific to gliomedin, neurofascin 186, and contactin [123]. Another study showed that 13 of 533 Japanese patients with CIDP had an IgG4 antibody to contactin 1; seropositivity was associated with sensory ataxia and poor responsiveness to IVIG treatment [126]. In another study and using the same group of patients, antibodies to neurofascin-155 were identified in 7% of the patients [127]; those who were seropositive were more likely to have sensory ataxia (42%), tremors (13%), and demyelinating CNS lesions (8%) and also were poorly responsive to IVIG [127]. Poor response to IVIG in patients positive to neurofascin-155 and contactin 1 has been suggested to be due to the fact that antibodies are of IgG4 type, which do not result in complement fixation and have low affinity to Fc receptors, two postulated immunomodulatory mechanisms of IVIG [127].
Antibodies to contactin/Caspr/neurofascin-155 complex are pathogenic as serum of anti-contactin-positive CIDP patients prevents adhesive interaction between contactin. Caspr and neurofascin-155 therefore alter the structure of paranodal junctions in myelinated neuronal culture [125].
Animal Models
Immunization of rabbits with a high dose of bovine myelin results in a relapsing or progressive form of EAN [93]. Chronic EAN has been created in the Lewis rats by immunization with myelin after treatment with low-dose cyclosporine A (CsA), which is explained by inhibition of T cell apoptosis and therefore perpetuation of inflammatory response by low-dose CsA [128]. Higher doses of CsA actually resulted in attenuation of the disease severity, attributed to suppression of overall T cell responses, which leads to prevention of the occurrence of EAN [128].
Spontaneous autoimmune polyneuropathy (SAP) in nonobese diabetic (NOD) mice is another model of inflammatory neuropathy 36. The NOD mouse strain is a model of type 1 diabetes, but it also has the propensity to develop other autoimmune diseases. When B7-2 was knocked out in these mice, they did not develop insulitis and diabetes, but on the other hand, all female and one third of male mice developed a chronic demyelinating neuropathy beginning at 20 weeks of age with pathological (heavy infiltration by CD4+, CD8+ T cells and dendritic cells in peripheral nerves and dorsal root ganglia) and electrophysiological (demyelination, conduction blocking) characteristics of CIDP 129. There was overexpression of B7-1 by the antigen presenting cells in that model. The disease was reproduced by treatment of NOD mice with antibody against B7-2, and by transfer of CD4+ T cells but not by sera from SAP animals [129]. Interferon gamma secreting Th1 cells that are reactive against certain episodes of myelin protein zero (P0) are shown to have a critical role in SAP in B7-2 deficient mouse model [130].
Treatment
CIDP is considered a treatable form of autoimmune neuropathy, and therefore a variety of immunomodulatory and immunosuppressive agents have been studied for its treatment.
Several controlled and retrospective studies as well as a few randomized trials have confirmed the efficacy of current first-line treatments: corticosteroids, IVIG, and PLEX [131–133]. Approximately, 50–70% of patients with CIDP respond to one of these treatments, with another 50% of the remainder responding to one of the other therapies [78, 134].
Corticosteroids
Steroids are the oldest treatment used for CIDP. The mechanism of action of steroids is multimodal and includes decrease in circulating lymphocytes, inflammatory cytokines, macrophage activation, and lymphocyte transmigration. A 3-month, randomized, placebo-controlled trial showed the efficacy of high-dose prednisone (120 mg) on alternate days in 28 CIDP patients [135]. A clinical response to steroid treatment occurs between 2 weeks and several months with an average of about 8 weeks [91, 121]. Although oral steroids are effective, daily dosing is commonly poorly tolerated due to multiple side effects (osteoporosis, weight gain, glycemic control, stomach irritation). As a result, pulse treatments with intravenous methylprednisolone or oral dexamethasone have been investigated as an alternative approach. When the efficacy of dexamethasone 40 mg daily for 4 days a month was compared to prednisolone at 60 mg in a double-blind, randomized, controlled trial, remission occurred in about 40% of patients at both arms at 12 months [136]. The median time to remission was however shorter in the dexamethasone (20 weeks) versus prednisone group (39 weeks). Another retrospective study evaluated intravenous methylprednisolone, loading dose of 1 g/day for 3–5 days followed 1 g/week for 4–8 weeks, and then a slow taper over a period of 2 months to 2 years [137]. There was favorable response as assessed by remission rate and improved disability score, in 13 out of 16 patients at 6-month follow-up, and IV methylprednisolone regimen was equal in efficacy to IVIG and oral prednisolone arms in that study. There were fewer steroid-related side effects in the IV methylprednisolone than the prednisone arm.
Intravenous Immunoglobulins (IVIG)
IVIG has been used as a preferred treatment for CIDP for almost two decades.
Axonal loss, as demonstrated by muscle atrophy clinically or low or absent motor potentials on EMG, is an important predictor of lack of response to IVIG [138].
The mechanism of action of IVIG in CIDP is multimodal and includes blocking or decreased production of pathogenic antibodies and decreased complement deposition [139]. IVIG also modulates cellular immune system and decreases the concentration of adhesion molecules and cytokine secretion by the endothelial cells [139]. Wong et al. showed significantly reduced ratio of sialylated/agalactosylated IgG-Fc in CIDP patients, and decrease in that ratio was associated with more severe disease [140]. Treatment with IVIG resulted in increased levels of sialylated IgG-Fc which correlated with clinical improvement [140]. The effect of IVIG on the T cell profile and Treg cells is described above [32].
IVIG is administered at 2 g/kg divided over 3–5 days and followed by maintenance infusions of 0.5–1 g/kg every 2–4 weeks. The frequency and dose of the maintenance therapy are adjusted based on the clinical response of the patient. IVIG is overall well tolerated by most patients. Infusion reactions include chills, rash, nausea, headache, and myalgias. These can be prevented or improved by premedicating patients with acetaminophen and diphenhydramine and slowing the infusion rate [141]. Other serious but not common side effects include renal failure (typically in patient with underlying renal insufficiency), congestive heart failure (in patients with pre-existing heart disease), anaphylactic reactions (more common in IgA-deficient patients), and thromboembolic events such as deep venous thrombosis and ischemic stroke. Other rare side effects include aseptic meningitis, neutropenia, and uveitis [141]. The efficacy of IVIG was proven in the CIDP Efficacy (ICE) trial, which is thus far the largest and longest (up to 48 weeks) randomized, double-blind, placebo-controlled, crossover trial in this disease. The trial used a loading dose of 2 g/kg administered over 2 to 5 days, followed by maintenance infusions of 1 g/kg administered every 3 weeks for 6 months, and demonstrated improvement in adjusted INCAT disability score and grip strength and lower rate of relapse compared to the placebo arm [142].
Subcutaneous IG (SCIg) is being investigated as an alternative to IVIG in those patients who cannot tolerate IVIG infusions. These have been used for two decades for other autoimmune disorders and require more frequent administration but at lower doses. Recent randomized trials showed efficacy of SCIg in improving the muscle strength in CIDP patients who were previously responsive to IVIG [143, 144].
Two IVIG formulations (Gammagard 5% IVIG and Kiovig 10% IVIG) were compared for their efficacy and side effect profile in a study, which demonstrated similar efficacy and side effect profile [145]. No randomized trials of IVIG versus SCIg have thus far been conducted. The effectiveness of IVIG versus pulsed IV methylprednisolone (500 mg IV daily for 4 days, followed by a monthly administration for 6 months) was compared in a randomized controlled trial, which showed that IVIG was less frequently discontinued because of inefficacy or side effects at 6 months (87.5% vs. 47.6%, respectively); however, the relapse rate after discontinuation was higher in the IVIG group, while in the patients who remained in the methylprednisolone group, no patients relapsed at 6 months of treatment [146].
Plasma Exchange (PLEX)
PLEX has been demonstrated to be effective in CIDP in multiple studies, including the two short-term randomized placebo-controlled trials [147, 148]. In the study by Hahn et al., PLEX was effective in 80% of the patients as indicated by improvement in grip strength, clinical disability grade, and the mean neurologic disability score, as well as summated motor potential amplitudes and conduction velocities [148]. Of those patients who responded to the plasma exchange, most improved within 4 weeks of receiving therapy with no significant difference in responsiveness between those with progressive and relapsing disease, i.e., five of seven patients with progressive course and seven of eight patients with relapsing course improved in that study. Despite good response initially, after discontinuation of therapy, about two thirds of patients will experience deterioration within several weeks [133, 148]. There are no specific guidelines for the use of PLEX in CIDP beyond 4 weeks; clinical response, timing, and degree of deterioration should be used to guide decision-making regarding frequency of subsequent PLEX sessions. Usually, a maintenance therapy with one PLEX session at least every 8 weeks may be needed, sometimes in addition to other immunomodulatory medications [121].
Plasma exchange administration requires a central catheter placement and about three to five sessions per treatment. Adverse effects include bleeding, infection at the site of the catheter, hypotension, anemia, and hypocalcemia due to citrate toxicity [133]. Pre-existing coagulation abnormalities, thrombocytopenia, and hemodynamic instability warrant the use of another treatment modality.
Other Treatments
A large number of immunosuppressants (azathioprine, cyclophosphamide, methotrexate, cyclosporine A, mycophenolate mofetil, rituximab) and immunomodulatory drugs (alpha and beta interferon) have been tried for CIDP. Although some of the aforementioned medications are commonly used in CIDP patients as steroid-sparing drugs, none have been shown to be effective in CIDP in randomized, controlled trials [149]. When azathioprine was added to a regimen of alternate-day steroid treatment, the outcome was not different [150]; on the other hand, azathioprine has been used in the treatment of CIDP patients who also had diabetes in small case series [151, 152]. A double-blinded randomized study did not show efficacy of a weekly dose of oral methotrexate in patients in CIDP who were also on IVIG and prednisone [153]. Interferon B1a was shown not to be effective in a cohort of ten patients with treatment-resistant CIDP in a randomized, double-blind, placebo-controlled study [154]. High-dose cyclophosphamide (200 mg/kg over 4 days) infusion was reported to be effective in a cohort of four CIDP patients who had failed other treatments, with remissions that could last more than 3 years [155]. Cyclosporine has been reported to be effective to sustain remission in a child with CIDP and to reduce the required dose of prednisolone in another [156]. In a retrospective study on eight CIDP patients, neuropathy disability score improved in all eight, and in six of eight, the concomitant medications could be stopped or dose reduced by >50% [157]. On the other hand, another study on 21 CIDP patients suggested efficacy of mycophenolate in only one third of patients [158]. Autologous hematopoietic stem cell transplantation (AHSCT) has been successfully used for treatment-resistant CIDP [159]. In a prospective study, 11 patients with therapy-refractory CIDP underwent AHSCT with a median follow-up time of 28 months. Eight had a drug-free remission at their last follow-up [159].
Other treatment modalities are being investigated, including agents affecting B cells, T cells, transmigration molecules, and signal transduction pathways.
Rituximab, which is a monoclonal antibody against CD20 and acts by depleting the precursors of antibody-producing B cells, was used in 13 patients with refractory CIDP, eight of whom had concurrent hematological disease (B cell lymphoma, Waldenstrom macroglobulinemia, and IgM monoclonal gammopathy of unknown significance) [160]. Nine of 13 (7 of 8 with hematological disease) showed improved in that study, with median duration of 2 months from rituximab infusion to a response and mean duration of response of 1 year. In another study, rituximab was used in four patients with anti-CNTN1/NF155-positive, IVIG-resistant, CIDP patients [161]. The autoantibody titer diminished in all the patients and three of the four improved clinically.
Alemtuzumab is a monoclonal antibody directed against the CD52, therefore resulting in lymphocytic depletion via apoptosis. In a cohort of seven patients with treatment-resistant CIDP who underwent treatment with alemtuzumab, two had remissions and another two needed a lower dose of IVIG [162]. Fingolimod, a sphingosine-1-phosphate receptor modulator approved for relapsing-remitting multiple sclerosis, is currently under investigation for the treatment of CIDP in a randomized, double-blind, placebo-controlled trial.
Supportive Therapies
Physical therapy and supportive equipment such as canes, walking sticks, walkers, braces, and ankle-foot orthotics may be helpful in assisting CIDP patients in walking and other activities of daily living. Physical therapy may help maintain range of motion and prevent joint contractures. Neuropathic pain, anxiety, depression, and fatigue may need to be treated with symptomatic medications. Exercise can be helpful in combatting fatigue and encouraging endurance.
References
Yuki N, Hartung HP. Guillain-Barre syndrome. N Engl J Med. 2012;366:2294–304.
van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469–82.
van den Berg B, Fokke C, Drenthen J, van Doorn PA, Jacobs BC. Paraparetic Guillain-Barre syndrome. Neurology. 2014;82:1984–9.
Kuwabara S, Yuki N. Axonal Guillain-Barre syndrome: concepts and controversies. Lancet Neurol. 2013;12:1180–8.
Hiraga A, Kuwabara S, Ogawara K, et al. Patterns and serial changes in electrodiagnostic abnormalities of axonal Guillain-Barre syndrome. Neurology. 2005;64:856–60.
Aranyi Z, Kovacs T, Sipos I, Bereczki D. Miller Fisher syndrome: brief overview and update with a focus on electrophysiological findings. Eur J Neurol. 2012;19:15–20. e11–13
Wakerley BR, Yuki N. Pharyngeal-cervical-brachial variant of Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 2014;85:339–44.
Wakerley BR, Yuki N. Guillain-Barre syndrome. Expert Rev Neurother. 2015;15:847–9.
Uncini A, Manzoli C, Notturno F, Capasso M. Pitfalls in electrodiagnosis of Guillain-Barre syndrome subtypes. J Neurol Neurosurg Psychiatry. 2010;81:1157–63.
Asbury AK, Arnason BG, Adams RD. The inflammatory lesion in idiopathic polyneuritis. Its role in pathogenesis. Medicine. 1969;48:173–215.
Prineas JW. Pathology of the Guillain-Barre syndrome. Ann Neurol. 1981;9(Suppl):6–19.
Hafer-Macko CE, Sheikh KA, Li CY, et al. Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann Neurol. 1996;39:625–35.
Griffin JW, Li CY, Ho TW, et al. Guillain-Barre syndrome in northern China. The spectrum of neuropathological changes in clinically defined cases. Brain (A Journal of Neurology). 1995;118(Pt 3):577–95.
Phillips MS, Stewart S, Anderson JR. Neuropathological findings in Miller Fisher syndrome. J Neurol Neurosurg Psychiatry. 1984;47:492–5.
Jacobs BC, Rothbarth PH, van der Meche FG, et al. The spectrum of antecedent infections in Guillain-Barre syndrome: a case-control study. Neurology. 1998;51:1110–5.
Yuki N, Taki T, Inagaki F, et al. A bacterium lipopolysaccharide that elicits Guillain-Barre syndrome has a GM1 ganglioside-like structure. J Exp Med. 1993;178:1771–5.
Koga M, Takahashi M, Masuda M, Hirata K, Yuki N. Campylobacter gene polymorphism as a determinant of clinical features of Guillain-Barre syndrome. Neurology. 2005;65:1376–81.
Tam CC, Rodrigues LC, Petersen I, Islam A, Hayward A, O'Brien SJ. Incidence of Guillain-Barre syndrome among patients with Campylobacter infection: a general practice research database study. J Infect Dis. 2006;194:95–7.
Kuwabara S, Ogawara K, Misawa S, et al. Does Campylobacter jejuni infection elicit "demyelinating" Guillain-Barre syndrome? Neurology. 2004;63:529–33.
Illa I, Ortiz N, Gallard E, Juarez C, Grau JM, Dalakas MC. Acute axonal Guillain-Barre syndrome with IgG antibodies against motor axons following parenteral gangliosides. Ann Neurol. 1995;38:218–24.
Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain (A Journal of Neurology). 2002;125:2591–625.
Kusunoki S, Kaida K. Antibodies against ganglioside complexes in Guillain-Barre syndrome and related disorders. J Neurochem. 2011;116:828–32.
Dalakas MC. Pathogenesis of immune-mediated neuropathies. Biochim Biophys Acta. 1852;2015:658–66.
Gong Y, Tagawa Y, Lunn MP, et al. Localization of major gangliosides in the PNS: implications for immune neuropathies. Brain (A Journal of Neurology). 2002;125:2491–506.
McGonigal R, Rowan EG, Greenshields KN, et al. Anti-GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain (A Journal of Neurology). 2010;133:1944–60.
Burkel WE. The histological fine structure of perineurium. Anat Rec. 1967;158:177–89.
Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol. 1990;5:265–311.
Sawai S, Satoh M, Mori M, et al. Moesin is a possible target molecule for cytomegalovirus-related Guillain-Barre syndrome. Neurology. 2014;83:113–7.
Gatto CL, Walker BJ, Lambert S. Local ERM activation and dynamic growth cones at Schwann cell tips implicated in efficient formation of nodes of Ranvier. J Cell Biol. 2003;162:489–98.
Li S, Yu M, Li H, Zhang H, Jiang Y. IL-17 and IL-22 in cerebrospinal fluid and plasma are elevated in Guillain-Barre syndrome. Mediators Inflamm. 2012;2012:260473.
Li S, Jin T, Zhang HL, et al. Circulating Th17, Th22, and Th1 cells are elevated in the Guillain-Barre syndrome and downregulated by IVIg treatments. Mediators Inflamm. 2014;2014:740947.
Maddur MS, Rabin M, Hegde P, et al. Intravenous immunoglobulin exerts reciprocal regulation of Th1/Th17 cells and regulatory T cells in Guillain-Barre syndrome patients. Immunol Res. 2014;60:320–9.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87.
Waksman BH, Adams RD. Allergic neuritis: an experimental disease of rabbits induced by the injection of peripheral nervous tissue and adjuvants. J Exp Med. 1955;102:213–36.
Rostami A, Gregorian SK, Brown MJ, Pleasure DE. Induction of severe experimental autoimmune neuritis with a synthetic peptide corresponding to the 53-78 amino acid sequence of the myelin P2 protein. J Neuroimmunol. 1990;30:145–51.
Soliven B. Animal models of autoimmune neuropathy. ILAR (Journal/National Research Council, Institute of Laboratory Animal Resources). 2014;54:282–90.
Astrom KE, Webster HD, Arnason BG. The initial lesion in experimental allergic neuritis. A phase and electron microscopic study. J Exp Med. 1968;128:469–95.
Taylor JM, Pollard JD. Dominance of autoreactive T cell-mediated delayed-type hypersensitivity or antibody-mediated demyelination results in distinct forms of experimental autoimmune neuritis in the Lewis rat. J Neuropathol Exp Neurol. 2001;60:637–46.
Lonigro A, Devaux JJ. Disruption of neurofascin and gliomedin at nodes of Ranvier precedes demyelination in experimental allergic neuritis. Brain (A Journal of neurology). 2009;132:260–73.
Devaux JJ. Antibodies to gliomedin cause peripheral demyelinating neuropathy and the dismantling of the nodes of Ranvier. Am J Pathol. 2012;181:1402–13.
Yuki N, Yamada M, Koga M, et al. Animal model of axonal Guillain-Barre syndrome induced by sensitization with GM1 ganglioside. Ann Neurol. 2001;49:712–20.
Susuki K, Yuki N, Schafer DP, et al. Dysfunction of nodes of Ranvier: a mechanism for anti-ganglioside antibody-mediated neuropathies. Exp Neurol. 2012;233:534–42.
van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol. 2008;7:939–50.
Lawn ND, Fletcher DD, Henderson RD, Wolter TD, Wijdicks EF. Anticipating mechanical ventilation in Guillain-Barre syndrome. Arch Neurol. 2001;58:893–8.
Sharshar T, Chevret S, Bourdain F, Raphael JC. French Cooperative Group on Plasma Exchange in Guillain-Barre S. Early predictors of mechanical ventilation in Guillain-Barre syndrome. Crit Care Med. 2003;31:278–83.
Ropper AH, Kehne SM. Guillain-Barre syndrome: management of respiratory failure. Neurology. 1985;35:1662–5.
Dimario Jr FJ, Edwards C. Autonomic dysfunction in childhood Guillain-Barre syndrome. J Child Neurol. 2012;27:581–6.
Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barre syndrome: a long-term follow-up study. Neurology. 2010;75:1439–47.
Hiraga A, Nagumo K, Suzuki K, Sakakibara Y, Kojima S. [A patient with Guillain-Barre syndrome and recurrent episodes of ST elevation and left ventricular hypokinesis in the anterior wall]. No to shinkei =. Brain Nerve. 2003;55:517–20.
Burns TM, Lawn ND, Low PA, Camilleri M, Wijdicks EF. Adynamic ileus in severe Guillain-Barre syndrome. Muscle Nerve. 2001;24:963–5.
Sakakibara R, Hattori T, Kuwabara S, Yamanishi T, Yasuda K. Micturitional disturbance in patients with Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 1997;63:649–53.
Sakakibara R, Uchiyama T, Kuwabara S, et al. Prevalence and mechanism of bladder dysfunction in Guillain-Barre Syndrome. NeurourolUrodyn. 2009;28:432–7.
Amatya B, Khan F, Whishaw M, Pallant JF. Guillain-Barre syndrome: prevalence and long-term factors impacting bladder function in an Australian community cohort. J Clin Neurol. 2013;9:144–50.
Winer JB, Hughes RA, Osmond C. A prospective study of acute idiopathic neuropathy. I. Clinical features and their prognostic value. J Neurol Neurosurg Psychiatry. 1988;51:605–12.
Hughes RA, van Doorn PA. Corticosteroids for Guillain-Barre syndrome. Cochrane Database Syst Rev. 2012;8:CD001446.
Efficiency of plasma exchange in Guillain-Barre syndrome: role of replacement fluids. French Cooperative Group on Plasma Exchange in Guillain-Barre syndrome. Ann Neurol. 1987;22:753–61.
Appropriate number of plasma exchanges in Guillain-Barre syndrome. The French Cooperative Group on Plasma Exchange in Guillain-Barre Syndrome. Ann Neurol. 1997;41:298–306.
Plasmapheresis and acute Guillain-Barre syndrome. The Guillain-Barre syndrome Study Group. Neurology. 1985;35:1096–1104.
Hughes RA, Swan AV, Raphael JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barre syndrome: a systematic review. Brain (A Journal of Neurology). 2007;130:2245–57.
Greenwood RJ, Newsom-Davis J, Hughes RA, et al. Controlled trial of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet. 1984;1:877–9.
van der Meche FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barre syndrome. Dutch Guillain-Barre Study Group. N Engl J Med. 1992;326:1123–9.
Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barre syndrome. Plasma Exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group. Lancet. 1997;349:225–30.
Korinthenberg R, Schessl J, Kirschner J, Monting JS. Intravenously administered immunoglobulin in the treatment of childhood Guillain-Barre syndrome: a randomized trial. Pediatrics. 2005;116:8–14.
Kleyweg RP, van der Meche FG. Treatment related fluctuations in Guillain-Barre syndrome after high-dose immunoglobulins or plasma-exchange. J Neurol Neurosurg Psychiatry. 1991;54:957–60.
Kuitwaard K, de Gelder J, Tio-Gillen AP, et al. Pharmacokinetics of intravenous immunoglobulin and outcome in Guillain-Barre syndrome. Ann Neurol. 2009;66:597–603.
Dada MA, Kaplan AA. Plasmapheresis treatment in Guillain-Barre syndrome: potential benefit over IVIg in patients with axonal involvement. Ther Apher Dial (Official Peer-Reviewed Journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy). 2004;8:409–12.
Mori M, Kuwabara S, Fukutake T, Hattori T. Intravenous immunoglobulin therapy for Miller Fisher syndrome. Neurology. 2007;68:1144–6.
Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350:552–9.
Halstead SK, Zitman FM, Humphreys PD, et al. Eculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine model. Brain (A Journal of Neurology). 2008;131:1197–208.
Phongsisay V, Susuki K, Matsuno K, et al. Complement inhibitor prevents disruption of sodium channel clusters in a rabbit model of Guillain-Barre syndrome. J Neuroimmunol. 2008;205:101–4.
Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society – first revision. Eur J Neurol. 2010;17:356–63.
Gorson KC, Katz J. Chronic inflammatory demyelinating polyneuropathy. Neurol Clin. 2013;31:511–32.
Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle Nerve. 2001;24:311–24.
Boukhris S, Magy L, Khalil M, Sindou P, Vallat JM. Pain as the presenting symptom of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). J Neurol Sci. 2007;254:33–8.
Henderson RD, Sandroni P, Wijdicks EF. Chronic inflammatory demyelinating polyneuropathy and respiratory failure. J Neurol. 2005;252:1235–7.
Dyck PJ, Lais AC, Ohta M, Bastron JA, Okazaki H, Groover RV. Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc. 1975;50:621–37.
Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. 2015;86:973–85.
Viala K, Maisonobe T, Stojkovic T, et al. A current view of the diagnosis, clinical variants, response to treatment and prognosis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010;15:50–6.
Oh SJ, Joy JL, Kuruoglu R. "Chronic sensory demyelinating neuropathy": chronic inflammatory demyelinating polyneuropathy presenting as a pure sensory neuropathy. J Neurol Neurosurg Psychiatry. 1992;55:677–80.
Rajabally YA, Wong SL. Chronic inflammatory pure sensory polyradiculoneuropathy: a rare CIDP variant with unusual electrophysiology. J Clin Neuromuscul Dis. 2012;13:149–52.
van Dijk GW, Notermans NC, Franssen H, Wokke JH. Development of weakness in patients with chronic inflammatory demyelinating polyneuropathy and only sensory symptoms at presentation: a long-term follow-up study. J Neurol. 1999;246:1134–9.
Simmons Z, Tivakaran S. Acquired demyelinating polyneuropathy presenting as a pure clinical sensory syndrome. Muscle Nerve. 1996;19:1174–6.
Yato M, Ohkoshi N, Sato A, Shoji S, Kusunoki S. Ataxic form of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Eur J Neurol. 2000;7:227–30.
Sinnreich M, Klein CJ, Daube JR, Engelstad J, Spinner RJ, Dyck PJ. Chronic immune sensory polyradiculopathy: a possibly treatable sensory ataxia. Neurology. 2004;63:1662–9.
Yiannikas C, Vucic S. Utility of somatosensory evoked potentials in chronic acquired demyelinating neuropathy. Muscle Nerve. 2008;38:1447–54.
Sabatelli M, Madia F, Mignogna T, Lippi G, Quaranta L, Tonali P. Pure motor chronic inflammatory demyelinating polyneuropathy. J Neurol. 2001;248:772–7.
Hattori N, Misu K, Koike H, et al. Age of onset influences clinical features of chronic inflammatory demyelinating polyneuropathy. J Neurol Sci. 2001;184:57–63.
Pitarokoili K, Schlamann M, Kerasnoudis A, Gold R, Yoon MS. Comparison of clinical, electrophysiological, sonographic and MRI features in CIDP. J Neurol Sci. 2015;357:198–203.
Matsuda M, Ikeda S, Sakurai S, Nezu A, Yanagisawa N, Inuzuka T. Hypertrophic neuritis due to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): a postmortem pathological study. Muscle Nerve. 1996;19:163–9.
Vital C, Vital A, Lagueny A, et al. Chronic inflammatory demyelinating polyneuropathy: immunopathological and ultrastructural study of peripheral nerve biopsy in 42 cases. Ultrastruct Pathol. 2000;24:363–9.
Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy. Clinical characteristics, course, and recommendations for diagnostic criteria. Arch Neurol. 1989;46:878–84.
Gorson KC, Allam G, Ropper AH. Chronic inflammatory demyelinating polyneuropathy: clinical features and response to treatment in 67 consecutive patients with and without a monoclonal gammopathy. Neurology. 1997;48:321–8.
Harvey GK, Pollard JD, Schindhelm K, Antony J. Chronic experimental allergic neuritis. An electrophysiological and histological study in the rabbit. J Neurol Sci. 1987;81:215–25.
Adam AM, Atkinson PF, Hall SM, Hughes RA, Taylor WA. Chronic experimental allergic neuritis in Lewis rats. Neuropathol Appl Neurobiol. 1989;15:249–64.
Crino PB, Grossman RI, Rostami A. Magnetic resonance imaging of the cauda equina in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 1993;33:311–3.
Morgan GW, Barohn RJ, Bazan 3rd C, King RB, Klucznik RP. Nerve root enhancement with MRI in inflammatory demyelinating polyradiculoneuropathy. Neurology. 1993;43:618–20.
McCombe PA, Pollard JD, McLeod JG. Chronic inflammatory demyelinating polyradiculoneuropathy. A clinical and electrophysiological study of 92 cases. Brain (A Journal of Neurology). 1987;110(Pt 6):1617–30.
Chio A, Cocito D, Bottacchi E, et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. J Neurol Neurosurg Psychiatry. 2007;78:1349–53.
Gibbels E, Diederich N. Human immunodeficiency virus (HIV)-related chronic relapsing inflammatory demyelinating polyneuropathy with multifocal unusual onion bulbs in sural nerve biopsy. A clinicomorphological study with qualitative and quantitative light and electron microscopy. Acta Neuropathol. 1988;75:529–34.
Chimowitz MI, Audet AM, Hallet A, Kelly Jr JJ. HIV-associated CIDP. Muscle Nerve. 1989;12:695–6.
Bird SJ, Brown MJ, Shy ME, Scherer SS. Chronic inflammatory demyelinating polyneuropathy associated with malignant melanoma. Neurology. 1996;46:822–4.
Weiss MD, Luciano CA, Semino-Mora C, Dalakas MC, Quarles RH. Molecular mimicry in chronic inflammatory demyelinating polyneuropathy and melanoma. Neurology. 1998;51:1738–41.
Rousseau A, Salachas F, Baccard M, Delattre JY, Sanson M. Chronic inflammatory polyneuropathy revealing malignant melanoma. J Neurooncol. 2005;71:335–6.
Noronha AB, Harper JR, Ilyas AA, Reisfeld RA, Quarles RH. Myelin-associated glycoprotein shares an antigenic determinant with a glycoprotein of human melanoma cells. J Neurochem. 1986;47:1558–65.
Schmidt B, Toyka KV, Kiefer R, Full J, Hartung HP, Pollard J. Inflammatory infiltrates in sural nerve biopsies in Guillain-Barre syndrome and chronic inflammatory demyelinating neuropathy. Muscle Nerve. 1996;19:474–87.
Chi LJ, Xu WH, Zhang ZW, Huang HT, Zhang LM, Zhou J. Distribution of Th17 cells and Th1 cells in peripheral blood and cerebrospinal fluid in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010;15:345–56.
Winer J, Hughes S, Cooper J, Ben-Smith A, Savage C. gamma delta T cells infiltrating sensory nerve biopsies from patients with inflammatory neuropathy. J Neurol. 2002;249:616–21.
Renaud S, Erne B, Fuhr P, et al. Matrix metalloproteinases-9 and -2 in secondary vasculitic neuropathies. Acta Neuropathol. 2003;105:37–42.
Schneider-Hohendorf T, Schwab N, Uceyler N, Gobel K, Sommer C, Wiendl H. CD8+ T-cell immunity in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2012;78:402–8.
Mausberg AK, Dorok M, Stettner M, et al. Recovery of the T-cell repertoire in CIDP by IV immunoglobulins. Neurology. 2013;80:296–303.
Chi LJ, Wang HB, Wang WZ. Impairment of circulating CD4+CD25+ regulatory T cells in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2008;13:54–63.
Sanvito L, Makowska A, Gregson N, Nemni R, Hughes RA. Circulating subsets and CD4(+)CD25(+) regulatory T cell function in chronic inflammatory demyelinating polyradiculoneuropathy. Autoimmunity. 2009;42:667–77.
Ledbetter JA, Imboden JB, Schieven GL, et al. CD28 ligation in T-cell activation: evidence for two signal transduction pathways. Blood. 1990;75:1531–9.
Khalili-Shirazi A, Gregson NA, Londei M, Summers L, Hughes RA. The distribution of CD1 molecules in inflammatory neuropathy. J Neurol Sci. 1998;158:154–63.
Van Rhijn I, Van den Berg LH, Bosboom WM, Otten HG, Logtenberg T. Expression of accessory molecules for T-cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain (A Journal of Neurology). 2000;123(Pt 10):2020–9.
Sommer C, Koch S, Lammens M, Gabreels-Festen A, Stoll G, Toyka KV. Macrophage clustering as a diagnostic marker in sural nerve biopsies of patients with CIDP. Neurology. 2005;65:1924–9.
Dalakas MC, Engel WK. Immunoglobulin and complement deposits in nerves of patients with chronic relapsing polyneuropathy. Arch Neurol. 1980;37:637–40.
Hays AP, Lee SS, Latov N. Immune reactive C3d on the surface of myelin sheaths in neuropathy. J Neuroimmunol. 1988;18:231–44.
Yan WX, Taylor J, Andrias-Kauba S, Pollard JD. Passive transfer of demyelination by serum or IgG from chronic inflammatory demyelinating polyneuropathy patients. Ann Neurol. 2000;47:765–75.
Koller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343–56.
Dalakas MC, Medscape. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011;7:507–17.
Yan WX, Archelos JJ, Hartung HP, Pollard JD. P0 protein is a target antigen in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 2001;50:286–92.
Devaux JJ, Odaka M, Yuki N. Nodal proteins are target antigens in Guillain-Barre syndrome. J Peripher Nerv Syst. 2012;17:62–71.
Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2013;73:370–80.
Labasque M, Hivert B, Nogales-Gadea G, Querol L, Illa I, Faivre-Sarrailh C. Specific contactin N-glycans are implicated in neurofascin binding and autoimmune targeting in peripheral neuropathies. J Biol Chem. 2014;289:7907–18.
Miura Y, Devaux JJ, Fukami Y, et al. Contactin 1 IgG4 associates to chronic inflammatory demyelinating polyneuropathy with sensory ataxia. Brain (A Journal of Neurology). 2015;138:1484–91.
Devaux JJ, Miura Y, Fukami Y, et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology. 2016;86:800–7.
McCombe PA, van der Kreek SA, Pender MP. The effects of prophylactic cyclosporin A on experimental allergic neuritis (EAN) in the Lewis rat. Induction of relapsing EAN using low dose cyclosporin A. J Neuroimmunol. 1990;28:131–40.
Salomon B, Rhee L, Bour-Jordan H, et al. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med. 2001;194:677–84.
Kim HJ, Jung CG, Jensen MA, Dukala D, Soliven B. Targeting of myelin protein zero in a spontaneous autoimmune polyneuropathy. J Immunol. 2008;181:8753–60.
Hughes RA, Mehndiratta MM. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2012;8:CD002062.
Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013;12:CD001797.
Mehndiratta MM, Hughes RA. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2012;9:CD003906.
Cocito D, Paolasso I, Antonini G, et al. A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2010;17:289–94.
Dyck PJ, O'Brien PC, Oviatt KF, et al. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann Neurol. 1982;11:136–41.
van Schaik IN, Eftimov F, van Doorn PA, et al. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:245–53.
Lopate G, Pestronk A, Al-Lozi M. Treatment of chronic inflammatory demyelinating polyneuropathy with high-dose intermittent intravenous methylprednisolone. Arch Neurol. 2005;62:249–54.
Iijima M, Yamamoto M, Hirayama M, et al. Clinical and electrophysiologic correlates of IVIg responsiveness in CIDP. Neurology. 2005;64:1471–5.
Buttmann M, Kaveri S, Hartung HP. Polyclonal immunoglobulin G for autoimmune demyelinating nervous system disorders. Trends Pharmacol Sci. 2013;34:445–57.
Wong AH, Fukami Y, Sudo M, Kokubun N, Hamada S, Yuki N. Sialylated IgG-Fc: a novel biomarker of chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2016;87:275–9.
Brannagan 3rd TH. Intravenous gamma globulin (IVIg) for treatment of CIDP and related immune-mediated neuropathies. Neurology. 2002;59:S33–40.
Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7:136–44.
Markvardsen LH, Debost JC, Harbo T, et al. Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2013;20:836–42.
Markvardsen LH, Harbo T, Sindrup SH, et al. Subcutaneous immunoglobulin preserves muscle strength in chronic inflammatory demyelinating polyneuropathy. Eur J Neurol. 2014;21:1465–70.
Kuitwaard K, van den Berg LH, Vermeulen M, et al. Randomised controlled trial comparing two different intravenous immunoglobulins in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry. 2010;81:1374–9.
Nobile-Orazio E, Cocito D, Jann S, et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol. 2012;11:493–502.
Dyck PJ, Daube J, O'Brien P, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med. 1986;314:461–5.
Hahn AF, Bolton CF, Pillay N, et al. Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double-blind, sham-controlled, cross-over study. Brain (A Journal of Neurology). 1996;119(Pt 4):1055–66.
Hughes RA, Swan AV, van Doorn PA. Cytotoxic drugs and interferons for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2004:CD003280.
Dyck PJ, O'Brien P, Swanson C, Low P, Daube J. Combined azathioprine and prednisone in chronic inflammatory-demyelinating polyneuropathy. Neurology. 1985;35:1173–6.
Stewart JD, McKelvey R, Durcan L, Carpenter S, Karpati G. Chronic inflammatory demyelinating polyneuropathy (CIDP) in diabetics. J Neurol Sci. 1996;142:59–64.
Haq RU, Pendlebury WW, Fries TJ, Tandan R. Chronic inflammatory demyelinating polyradiculoneuropathy in diabetic patients. Muscle Nerve. 2003;27:465–70.
Group RMCT. Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial): a pilot, multicentre study. Lancet Neurol. 2009;8:158–64.
Hadden RD, Sharrack B, Bensa S, Soudain SE, Hughes RA. Randomized trial of interferon beta-1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1999;53:57–61.
Brannagan 3rd TH, Pradhan A, Heiman-Patterson T, et al. High-dose cyclophosphamide without stem-cell rescue for refractory CIDP. Neurology. 2002;58:1856–8.
Visudtibhan A, Chiemchanya S, Visudhiphan P. Cyclosporine in chronic inflammatory demyelinating polyradiculoneuropathy. Pediatr Neurol. 2005;33:368–72.
Bedi G, Brown A, Tong T, Sharma KR. Chronic inflammatory demyelinating polyneuropathy responsive to mycophenolate mofetil therapy. J Neurol Neurosurg Psychiatry. 2010;81:634–6.
Gorson KC, Amato AA, Ropper AH. Efficacy of mycophenolate mofetil in patients with chronic immune demyelinating polyneuropathy. Neurology. 2004;63:715–7.
Press R, Askmark H, Svenningsson A, et al. Autologous haematopoietic stem cell transplantation: a viable treatment option for CIDP. J Neurol Neurosurg Psychiatry. 2014;85:618–24.
Benedetti L, Briani C, Franciotta D, et al. Rituximab in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a report of 13 cases and review of the literature. J Neurol Neurosurg Psychiatry. 2011;82:306–8.
Querol L, Rojas-Garcia R, Diaz-Manera J, et al. Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol Neuroimmunol Neuroinflamm. 2015;2:e149.
Marsh EA, Hirst CL, Llewelyn JG, et al. Alemtuzumab in the treatment of IVIG-dependent chronic inflammatory demyelinating polyneuropathy. J Neurol. 2010;257:913–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Grebenciucova, E., Rezania, K. (2017). Immunopathogenesis and Treatment of Guillain-Barre Syndrome and Chronic Inflammatory Demyelinating Polyneuropathy. In: Minagar, A., Alexander, J. (eds) Inflammatory Disorders of the Nervous System. Current Clinical Neurology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-51220-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-51220-4_10
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-51218-1
Online ISBN: 978-3-319-51220-4
eBook Packages: MedicineMedicine (R0)