Abstract
The discovery of NdFeB permanent magnets helps to perform technologies with reduced weight and gives them access to miniaturization. The industrial process of production of the Nd-Fe alloy by electrolysis used to manufacture of NdFeB magnets consists in reducing Nd3+ ions dissolved in a LiF-NdF3-Nd2O3 salt on iron cathode at 1050 °C. This route was chosen since the electroplating of neodymium at low temperature molten chloride leads to low recovery yields. This is usually attributed to the comproportionation reaction between the electrodeposited metal and its chloride salt (NdCl3) leading to the formation of NdCl2. In this work, the neodymium electrochemical behavior is reviewed in order to understand its reduction mechanism. Meal addition to LiCl-KCl-NdCl3 melt is usually used to simulated comproportionation reaction in solution. Neodymium deposition yields are studied. Stability of Nd2+ in two different solvent (LiCl-KCl and LiCl) is also discussed through electrodeposition tests on the gram scale.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Rare earth elements (REE) take an important place in a economical point of view and are considered today as critical raw materials with high supply risk. They are used in various consumer materials and products, military equipment of high technology, wind turbines (permanent magnets), cars (Ni-MH batteries), computers (hard-disk drives), etc. The discovery in the 1980s of NdFeB permanent magnets helps to perform technologies with reduced weight and give them access to miniaturization. The rare earths metals used in NdFeB alloys are either produced by metallothermy or by electrolysis in molten salts.
The industrial process for Nd-Fe alloy production by electrolysis used in the manufacture of NdFeB magnets consists in reducing Nd3+ ions dissolved in a LiF-NdF3-Nd2O3 salt bath on an iron reactive cathode at 1050 °C. The high temperature is selected to obtain a liquid alloy Nd-Fe (80-20%) at the iron cathode surface. This route is chosen since the electroplating of neodymium in low temperature molten chloride leads to low recovery yields [1,2,3]. This is usually attributed to the comproportionation reaction between the electrodeposited Nd(0) metal and its chloride salt (NdCl3) leading to the formation of NdCl2:
This work investigates the neodymium electrochemical behavior on inert cathode in different molten chloride salts using transient electrochemical techniques. The comproportionation reaction is highlighted after electrowining tests on the gram scale or simulated by metal addition in molten salt containing NdCl3.
Experimental
Electrochemical Cell
All the experiments were carried out in a controlled purified argon atmosphere glove-box containing less than 10 ppm of water and oxygen. Chloride salts: LiCl and KCl (Sigma Aldrich: 99.0%) are stored in a drying oven at 150 °C under air and atmospheric pressure during several days. NdCl3 (Sigma Aldrich: 99.99%) is used as received and kept in the glove box under Ar atmosphere.
Electrochemical Measurements
Salt chloride mixture was fused in an alumina crucible placed in a quartz cell inside a furnace. The cell was kept under Ar (99.999%).
Electrochemical behavior studies were performed with cyclic voltammetry (CV) and square wave voltammetry (SWV). The quasi reference electrodes (RE) for the LiCl-KCl and LiCl systems were W (Ø: 1.0 mm) and Pt (Ø: 1.0 mm) rod respectively. A W wire (Ø: 0.5 mm) was used as working electrode (WE) and a Mo spiral as counter electrode (CE).
Electrodeposition Tests
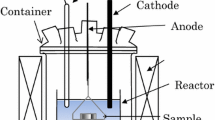
Electrowining tests of neodymium were carried out in LiCl-KCl eutectic salt on an inert Mo cathode (Ø: 5 mm, h: 10–20 mm) (Fig. 1). The eutectic salt containing NdCl3 was electrolyzed under constant current at 450 °C. The anodic reaction: oxidation of chloride was into chlorine gas on glassy carbon anode. In this type of cell, the reaction between chlorine gas and the metal deposited at the cathode is the primary source of Faraday yield drop, this assembly used is effective in limiting this problem by a high argon flush (10 L/h) [4].
Results and Discussions
Electrochemical Measurements
Cyclic voltammetry and square-wave voltammetry (SWV) were used to study neodymium reduction mechanism. Cyclic voltammetry curves in two molten chloride salts, i.e. LiCl-KCl (450 °C) and LiCl (650 °C) containing NdCl3 at 1 wt% are presented in Fig. 2. In both case, the reduction of Nd(III) to neodymium metal on the inert cathode occurs in a two-step process. The first step (peaks A and A′) is attributed to Nd(III)/Nd(II) reduction and the second step (peaks B and B′) to Nd(II)/Nd(0) transition leading to metal deposition. The anodic peak observed on the reverse scan corresponding to the second step is specific to a anodic striping (metal dissolution).
The shape difference between A and A′ can be attributed to the chloro-acidity of LiCl and LiCl-KCl salts which plays an important role on the stability of neodymium complexes and thus on its reduction potential [5]. According to Fig. 2, Nd(III) chloro-complex seems to be more stable in LiCl-KCl eutectics than in LiCl salt. In LiCl-KCl, Nd3+/Nd2+ signal is not clearly defined since the potential difference between the Nd3+/Nd2+ and Nd2+/Nd0 signals is very low (ΔE < 100 mV) whereas the difference in LiCl pure salt is larger than 300 mV.
This two-step neodymium reduction study is completed by square wave voltammetry analysis. Figure 3 shows square wave voltammograms of the LiCl-KCl-NdCl3 (1 wt%) at 450 °C and LiCl-NdCl3 (1 wt%) at 650 °C systems on an inert W working electrode. Two reduction peaks are clearly observed corresponding to the two-step reduction of neodymium:
These results are consistent with the ones obtained from cyclic voltammograms. Each peak can be modeled with a Gaussian shape curve [6]. The mathematical analyses of the peaks allow calculating the number of exchanged electron by measuring half peak width (W1/2) according to equation:
The value of number of exchanged electron is close to 1 for the first peak (A and A′). If the curve-fitting of the first signal is not questionable, the results obtained on the second peak are neither consistent nor reproducible in particular for LiCl salt (Table 1). Moreover, the ratio between the differential current density of the two peaks should be equal to the ratio of exchanged electrons, i.e. 2 if Nd3+/Nd2+ and Nd2+/Nd0 transitions are considered. The ratio found is rather 4 in LiCl-KCl and 8 in LiCl (Table 1). The comproportionation reaction between the electrodeposited Nd(0) metal and its chloride salt (NdCl3) could have an influence on those calculations leading to incorrect number of electrons.
Lithium underpotential deposition takes place when the concentration of neodymium increases in the salt phase or at low scan rate in cyclic voltammetry. This characteristic alloy was also observed for several lanthanides elements such as lanthanum and cerium. This is identified on Fig. 4 at:
-
Low Nd3+ concentration (1 wt%) at low scan rate (<10 mV/s) (Fig. 4a)
-
High Nd3+ concentration (~3 wt%) at any scan rate.
Electrowining Test
Electrodeposition tests of neodymium metal is realized by electrowining under constant current density (cathodic current density: −0.250 to −0.450 A/cm2) in molten LiCl-KCl-NdCl3 (5 wt%) at 450 °C. Faradic yield is defined by the ratio of the mass of neodymium metal (determined by ICP-OES) to the theoretical mass calculated according to the Faraday’s law with a current efficiency of 100%:
where Q is the number of coulomb (C), M the molecular weight of neodymium (g mol−1), z the number of electron and F the Faraday constant (C mol−1).
The results show a low metal recovery with low faradic yields (lower than 50%). Novoselova and Smolenski [7] and Kvam et al. [8] attributed the faradic losses to the comproportionation reaction . In the tests carried out in this work, the metal is deposited as a fine divided powder unlike many rare earths metals (lanthanum and cerium) are deposited in the same experimental conditions (dendritic structure).
Stability of Nd(II) After Addition of Nd Metal
After several electrolysis, the open circuit potential (EOCP) of the tungsten WE is shifted to the Nd(III)/Nd(II) redox potential (i.e. −3.3 V vs. Cl2/Cl−) whereas in LiCl-KCl-NdCl3 without Nd metal, the EOCP is rather −1.5 V versus Cl2/Cl−. The coexistence of Nd(III) and Nd(0) in the electrolyte leads to a spontaneous reaction:
According to the Nernst equation, the presence of Nd(II) and Nd(III) in the electrolyte sets the EOCP at the neodymium redox couple:
where E° is the standard potential of Nd(III)/Nd(II) couple (V vs. Cl2/Cl−) and R the ideal gas constant (J K−1 mol−1).
Moreover Nd(II) oxidation is clearly observed when the voltammogram is started in the anodic direction (grey curve on Fig. 5) with a positive residual current (I > 50 mA/cm2) corresponding to the oxidation of Nd(II) to Nd(III). The Nd(III)/Nd(II) reduction signal tends to disappear on the cyclic voltammogram (black curve on Fig. 5). All these observations fit with the formation of Nd(II) when Nd(0) is in contact with Nd(III) as already observed by Yamana et al. [9] and Hayashi et al. [10].
This experiment has been repeated by adding a Nd metallic ingot (placed in a boron nitride basket) into LiCl-KCl-NdCl3 solution. Similar changes of the open circuit potential is observed (Fig. 6), it went slowly (in 50 h probably because the metal surface was partially oxidized) from tungsten equilibrium potential in LiCl-KCl-NdCl3 (EOCP: −1.5 V vs. Cl2/Cl−) to the Nd(III)/Nd(II) system (EOCP: −3.3 V vs. Cl2/Cl−) which means that equilibrium of comproportionation reaction is shifted to the production of Nd2+ (Eq. 6).
EOCP stays for several weeks at the Nd(III)/Nd(II) potential showing that Nd2+ is stable in reductive conditions (presence of an excess of Nd metal in the crucible). Surprisingly, Nd(III) signal doesn’t completely disappear because an equilibrium is established between the potential of the Nd(III)/Nd(II) and Nd(II)/Nd(0) couple and no more Nd(III) can be reduced.
Square wave voltammetry is carried out from EOCP towards cathodic and anodic senses (Fig. 7). Oxidation of Nd(II) is clearly identified in the anodic one (exchange of 1 electron, Table 2):
On the square wave voltammogram obtained from EOCP to the cathodic direction, a first signal that could be attributed to the reduction of residual Nd(III) appears, followed by a reduction peak characteristic of metal deposition.
The calculation of number of electrons on the second reduction peak is close to a two electrons transfer corresponding to:
Conclusion
The electrochemical behaviour of neodymium is carried out in LiCl-KCl and LiCl. Neodymium (III) reduction mechanism into metal occurs in two steps through the formation of divalent Nd(II) compound. The reduction potential of Nd(III) into Nd(II) is strongly dependent on the solvent composition (chloro-acidity). Lithium underpotential deposition is observed at high concentration of Nd(III) in LiCl-KCl melt. Nd metal electrowining tests shows that both recovery and faradic yields are low and metal is obtained as a fine powder contrary to other rare earth elements deposits that exhibit dendritic morphology. The low deposition efficiency is usually attributed to the chemical reaction between Nd(III) and Nd(0) leading to the formation of Nd(II). The formation of Nd(II) in LiCl-KCl is observed after several successive electrolysis of LiCl-KCl-NdCl3 solution or by contacting Nd metal ingot with Nd(III) containing salt. The Nd(II) is stable in LiCl-KCl at 450 °C for several weeks as long as Nd metal is present in excess in the solution (reductive condition).
References
H. Vogel, B. Friedrich, Development and Research Trends of the Neodymium Electrolysis—A Literature Review (2015)
B.M.F. Chambers, Electrolytic Production of Neodymium Metal From a Molten Chloride Electrolyte (1991)
C. Pernel, J. Serp, M. Ougier, R. Malmbeck, E. Commission, Electrochemical investigations of La, Nd and Am in molten chloride salts in view of Am/Ln partitioning. Eur. Comm. Karlsruhe, 1–7 (2004)
M. Gibilaro, L. Massot, P. Chamelot, L. Cassayre, P. Taxil, Electrochemical extraction of europium from molten fluoride media. Electrochim. Acta 55(1), 281–287 (2009)
K. Fukasawa, Systematic Study on the Thermodynamic Stability of Lanthanides and Actinides in Molten Alkali and Alkaline Earth Chlorides (2012)
L. Ramaley, M.S. Krause, Theory of square wave voltammetry. Anal. Chem. 41(11), 1362–1365 (1969)
A. Novoselova, V. Smolenski, Electrochemical behavior of neodymium compounds in molten chlorides, Electrochim. Acta, vol 87, (Jan 2013), pp. 657–662
K.R. Kvam, D. Bratland, H.A. Øye, The solubility of neodymium in the systems NdCl3-LiCl and NdCl3-LiCl-KCl. J. Mol. Liq. 83(1–3), 111–118 (1999)
H. Yamana, B.G. Park, O. Shirai, T. Fujii, A. Uehara, H. Moriyama, Electrochemically produced divalent neodymium in chloride melt. J. Alloys Compd. 408–412, 66–70 (2006)
H. Hayashi, M. Akabori, T. Ogawa, K. Minato, Spectrophotometric study of Nd2+ ions in LiCl-KCl eutectic melt. Zeitschrift fur Naturforsch. Sect. A J. Phys. Sci. 59(10), 705–710 (2004)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Diaz, L., Chamelot, P., Gibilaro, M., Massot, L., Serp, J. (2017). Electrochemical Behavior of Neodymium in Molten Chloride Salts. In: Kim, H., Alam, S., Neelameggham, N., Oosterhof, H., Ouchi, T., Guan, X. (eds) Rare Metal Technology 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51085-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-51085-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51084-2
Online ISBN: 978-3-319-51085-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)