Abstract
Microalgae are eukaryotic (e.g. green algae, diatoms) photosynthetic organisms capable of utilizing carbon dioxide and light for the synthesis of carbohydrates as energy compounds. They have been known since many years, but their large-scale cultivation has started a few decades ago. They have the potential to grow in open systems such as raceway ponds, circular ponds and lakes and also in controlled condition like closed photobioreactors. Microalgae are advantageous considering their higher productivity than terrestrial oilseed plants and ease of cultivation in wastewater and saline water. Microalgae do not compete with agricultural land for cultivation. They have dual role such as utilization of CO2 from atmosphere as well as remediation of wastewater by utilizing nutrients from wastewater to grow into biomass. Microalgae contain different types of major metabolites and high-value products such as proteins, lipids, carbohydrates, vitamins, pigments, antioxidants, minerals, etc. (Gupta et al. 2016; Mata et al. 2010; Rawat et al. 2011; Shriwastav et al. 2014; Francavilla et al. 2015). Their major metabolites are rich in essential amino acids and essential fatty acids, e.g. omega-3 fatty acids. Productivity of these major metabolites can be increased through mode of cultivation and nutrient limitation/stresses. Commonly, the lipids from microalgae are converted into biodiesel by the process of transesterification. After lipid extraction, a huge amount of residual biomass is left that is known as lipid-extracted algae (LEA). LEA still contains the high-value metabolites like proteins and carbohydrates in residual biomass (Ansari et al. 2015; Ju et al. 2012). Lipid-extracted algae can also serve as a good resource for biomethane, bioethanol and syngas production. In addition, protein fraction of LEA has promising potential as food and feed additive for animal and aquaculture. LEA biomass due to rich nitrogen content can also be employed as a fertilizer. Therefore, considering the rich chemical composition of microalgae, it can be considered as a good feedstock for the biorefinery.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Microalgae are eukaryotic (e.g. green algae, diatoms) photosynthetic organisms capable of utilizing carbon dioxide and light for the synthesis of carbohydrates as energy compounds. They have been known since many years, but their large-scale cultivation has started a few decades ago. They have the potential to grow in open systems such as raceway ponds, circular ponds and lakes and also in controlled condition like closed photobioreactors. Microalgae are advantageous considering their higher productivity than terrestrial oilseed plants and ease of cultivation in wastewater and saline water. Microalgae do not compete with agricultural land for cultivation. They have dual role such as utilization of CO2 from atmosphere as well as remediation of wastewater by utilizing nutrients from wastewater to grow into biomass. Microalgae contain different types of major metabolites and high-value products such as proteins, lipids, carbohydrates, vitamins, pigments, antioxidants, minerals, etc. (Gupta et al. 2016; Mata et al. 2010; Rawat et al. 2011; Shriwastav et al. 2014; Francavilla et al. 2015). Their major metabolites are rich in essential amino acids and essential fatty acids, e.g. omega-3 fatty acids. Productivity of these major metabolites can be increased through mode of cultivation and nutrient limitation/stresses. Commonly, the lipids from microalgae are converted into biodiesel by the process of transesterification. After lipid extraction, a huge amount of residual biomass is left that is known as lipid-extracted algae (LEA). LEA still contains the high-value metabolites like proteins and carbohydrates in residual biomass (Ansari et al. 2015; Ju et al. 2012). Lipid-extracted algae can also serve as a good resource for biomethane, bioethanol and syngas production. In addition, protein fraction of LEA has promising potential as food and feed additive for animal and aquaculture. LEA biomass due to rich nitrogen content can also be employed as a fertilizer. Therefore, considering the rich chemical composition of microalgae, it can be considered as a good feedstock for the biorefinery.
2 Biochemical Composition of Microalgae
Proteins, lipids, carbohydrates, pigments, vitamins and minerals comprise the biochemical constituents of microalgae. Among all, lipids, proteins and carbohydrates are the major constituents. The microalgal proteins (6–52%) are rich in essential amino acids, and their yield percentage depends upon the mode of cultivation and nutrient limitation. Microalgal lipids are very suitable for biodiesel production via fatty acid esterification to produce fatty acid methyl ester (FAME). The lipids are also a good source of essential unsaturated fatty acids such as alpha-linolenic acid (ALA, C18:3), eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6), so it has potential application to be used as a food and feed ingredient. Microalgae are also a good source of carbohydrates mainly in the form of starch, cellulose, sugar and other polysaccharides, and the biomass carbohydrate contents highly depend on types of species, cultivation condition and environmental factors. The overall microalgae carbohydrates have good digestibility.
2.1 Proteins
Microalgae abundantly are considered as a feedstock for biofuel production especially utilizing lipid, but besides lipid, they also contain many other valuable components. Proteins are the major primary metabolites in living organisms including the microalgae. Amino acids are the basic constituents of proteins which define the nutritional quality or value of protein on the basis of essential amino acid content, proportion and availability. Most of microalgal proteins are rich in essential amino acids. The proteins and amino acid profile of microalgae have been compared to different sources of food proteins and their proportion in which algal protein composition is nutritionally more favourable (Becker 2007). Recently, in different cases, microalgae proteins have recommended as a replacement protein source, due to their high nutrient quality and balanced content of essential amino acids (Romero Garcia et al. 2012). High protein yield directly depends upon cultivation condition and rich nitrogen source medium. In nitrogen limitation/starvation, fixed carbon produced by photosynthesis switches the metabolic pathway from protein to lipids or carbohydrates subsequently decreasing the protein yield (Singh et al. 2016).
2.2 Carbohydrates
Among the three major metabolites, carbohydrates are least rich in energy (15.7 kJ/g) (Wilhelm and Jakob 2011). The carbohydrates such as starch, cellulose and other polysaccharides are found in the form of storage products or the structural component of the cell wall. Microalgae cell lacks the lignin which makes them a good feedstock for food/feed ingredients since it requires no energy-intensive pretreatment. Although less in energy, microalgal carbohydrates have potential to become preferable feedstock for the production of biohydrogen, bioethanol, biobutanol and biomethane through integrating with biotechnological conversion technologies. The carbohydrate content in marine and freshwater microalgae varies significantly; microalgae P. cruentum and P. tricornutum contained 34.5 and 19.7% carbohydrates, respectively. While in fresh microalgae Scenedesmus sp., carbohydrate content was noted to be 23.3%. In nitrogen limitation, C. vulgaris accumulates 38.41%, Tetraselmis cordiformis accumulates 35%, Spirulina maxima accumulates 35% and Spirulina platensis accumulates 55–65% carbohydrates (Markou et al. 2012). It is also known that light energy is one of the most important energy sources for microalgae which affect the carbohydrate accumulation. High light intensity (200–400 μmol m−2 s−1.) resulted in high carbohydrates; Porphyridium sp. and Spirulina maxima were noted for threefold increase in carbohydrates upon enhanced light intensity (Markou et al. 2012).
2.3 Lipids
Lipids are one of the major primary metabolites of microalgae. The content of lipids varies between 15 and 60% on dry cell weight basis. Based on their polarity, microalgal lipids are generally classified into polar (structural) and non-polar or neutral (storage) lipids. Polar lipids are further subdivided into phospholipids and glycolipids. The function of the non-polar lipids, predominantly found in the form of TAG, is to store energy. These stored lipids are transesterified to produce biodiesel. Polar lipids form bilayer cell membrane and typically have high amount of PUFA; those have high potential for use in food/feed. Lipids in microalgae and their composition vary species to species such as some microalgae contain high amount of neutral lipid than others (Lv et al. 2010). Under starvation/nutrient limitation condition, microalga changes the metabolic pathway towards the storage of neutral lipids primarily in the form of TAG. For nutritional value of microalgae lipid, the controlled cultivation is very important that produces saturated and unsaturated fatty acids (PUFAs). Polyunsaturated fatty acids contained essential fatty acids such ALA, EPA and DHA which are used in feed and food for animals and humans.
2.4 Pigments
Microalgae colour is one of the most important characteristics which are determined by their pigments. These colour substances known as natural pigment have predominant role in the photosynthetic metabolism (D’Alessandro and Antoniosi Filho 2016). Apart from being photosynthetic components, they also have biological activities and act as antioxidants, anti-inflammatory agents, etc. Microalgae pigments are differentiated into three major classes: (a) carotenoids, (b) chlorophyll and (c) phycobiliproteins.
2.5 Carotenoids
These are the fat-soluble pigments, and their colour varies from brown, red, orange and yellow. The average carotenoid content in microalgae ranges in 0.1–0.2% which can go up to 14% on dry weight basis. Due to solubility in fat, they enter in blood circulation and get attached to different lipoprotein. The human body cannot synthesize these essential pigments, so it is important to supplement these in diets. Based on chemical structure, carotenoids are divided into two groups, carotenes including beta-carotene and lycopene and xanthophylls including astaxanthin, lutein and canthaxanthin. On the basis of involvement in photosynthesis, carotenoids are subdivided into primary and secondary carotenoids. In primary carotenoids, only those carotenoids are included which are directly involved in photosynthesis, e.g. beta-carotene and lutein. Both these carotenoids function in light-harvesting and photoprotective action. Secondary carotenoid, e.g. astaxanthin and canthaxanthin, is not involved in photosynthesis process. Haematococcus pluvialis is known as a prime source of natural astaxanthin. It can produce more astaxanthin under nutrient limitation and contains 0.2–3% astaxanthin on dry weight basis (Batista et al. 2013). Beta-carotene is orange-yellow in colour. It has a large demand as a natural pigment or nutritional supplementation, and it is also the precursor for vitamin A. Dunaliella salina is used at industrial scale to produce beta-carotene (14%) (Spolaore et al. 2006). Dunaliella salina is the first microalgae used for the commercial production of the high-value product (beta-carotene) from microalgae. The world market of carotenoid is growing by 2.3% annually; in 2010, it had the market of 1.2 billion USD which is expected to reach 1.4 billion USD by 2018 (BCC-Research, The Global Market for Carotenoids 2011).
2.6 Chlorophyll
It is green-coloured, fat-soluble pigment with porphyrin ring in its structure and is ubiquitously found in nature. These are responsible for photosynthesis by converting solar energy into chemical energy. Chlorophyll is tetrapyrrole in structure in which magnesium ion is centrally placed. On the basis of light absorption spectra of microalgae, chlorophyll has been grouped in many types, e.g. chlorophyll a, b, c, d and f. Chlorophyll a has a blue-green colour, chlorophyll b is a brilliant green, chlorophyll c is yellow green, chlorophyll d is a brilliant/forest green and chlorophyll f is emerald green. Most of the microalgae have chlorophyll a and c as the dominant chlorophylls in which chlorophyll a is the major light-harvesting complex and contains chlorophyll in the range of 0.5–1.0% on dry cell weight basis. The commercial application of chlorophyll is observed in food and feed industries, cosmetics and pharmaceuticals.
2.7 Phycobiliproteins
It is water-soluble pigments, made up of cell protein and reasonably easy to isolate and purify. Phycobiliprotein content varies from 2 to 8% on dry cell weight basis. It is water soluble, made up of protein and covalently bound with amino sulphur-containing amino acid—cysteine. Phycobiliprotein functions to accumulate light during photosynthesis. Phycobiliprotein, viz. phycocyanin and phycoerythrin, is commonly produced on commercial level from Spirulina spp. and Porphyridium spp., respectively. The phycobiliprotein is well known as the natural food colourant in pudding and as an antioxidant in immunology laboratories. Annual market for phycocyanin is around 5–10 million USD (Sekar and Chandramohan 2007).
3 Microalgae Cultivation
High-density cultivation of microalgae biomass for value-added product (VAP) extraction is still challenging mainly due to unavailability of water, land area requirement and inefficient illumination area, limitations of gas-liquid mass transfer, operational complications and contamination and production cost. Low density of microalgae biomass and small size of the microalgal cells add up more challenges to handle the culture for harvesting.
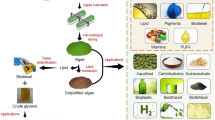
The commercial and cost-effective production of biofuels and other VAPs like food and feed ingredients from microalgae requires economic production of large quantity of algal biomass (Chisti 2007; Griffiths and Harrison 2009). Practically, suitable large-scale microalgae cultivation can be achieved via (i) open pond cultivation and (ii) closed photobioreactors (Carvalho et al. 2006). Figure 1 shows a generalized schematic representation of algae cultivation and biofuel production.
3.1 Open Ponds and Raceways
Open raceway ponds are open systems that are most widely used for outdoor microalgae cultivation using solar irradiations. The open ponds are generally constructed using concrete, and since their shape resembles with racetrack, these ponds are called as raceway ponds. The open raceway ponds are easy to construct, operate and maintain. The depth of the open raceway ponds varies from one region to another depending on the intensity of the available sunlight. The optimized depth can be in the range of 15–30 cm from the surface. Depth is set such that the shadowing effect of the microalgae culture can be avoided to enhance the biomass productivity. The microalgae culture in the pond requires continuous stirring for mixing and recirculation of both culture and the nutrients. The mixing helps to avoid the formation of concentration gradients, also provides homogenous illumination and overcomes the shadowing effect if caused. Generally, stirring is provided by the use of paddle wheels.
There are many advantages and disadvantages of open raceway pond over closed photobioreactor. The open raceway pond is directly affected by both biotic and abiotic factors. The main disadvantages are lower productivity than closed photobioreactor. It is mainly due to low atmospheric CO2 concentration and low gas-liquid mass transfer of CO2 from the atmosphere resulting in lower dissolved carbon in algal culture medium which remains insufficient to meet the needs of photosynthesis. To overcome this challenge, an external chemical source of carbon such as carbonates or direct injection of CO2 is done. Open raceway pond cultivation is also affected by water evaporation, fluctuations in temperature and variations in photoperiod. In addition, open raceway ponds, since being open to the environment, face contamination by other competing microalgal species making it a major challenge to maintain the monoalgal culture of a selected microalga. Therefore, extremophiles like Spirulina and Dunaliella salina are found to grow with lesser issues of contamination.
Apart from disadvantages, open raceway ponds have several advantages which include lower construction, operation and maintenance cost. Cleaning is less energy consuming than closed photobioreactors. These ponds can be constructed in deserts and nonarable lands. The net input energy is less than what is required for closed photobioreactor (Brennan and Owende 2010).
The cost of per kilogram of oil from algae grown in open raceway pond (7.64 USD) is cheaper than algae grown in closed photobioreactor (24.60 USD). The price of per kilogram algal biomass cultivated in open raceway pond (1.54 USD) is lesser than biomass obtained from photobioreactor (7.32 USD) (Rashid et al. 2014).
3.2 Photobioreactors
Basically, the photobioreactors allow monoalgal/axenic microalgae cultivation under controlled conditions to obtain high biomass for various food-, feed- and fuel-based applications. Several types of photobioreactors exist for cultivation of microalgae biomass. Biomass productivity, lipid content and lipid productivity of selected microalgae species in closed photobioreactors and the open ponds reported from various authors are summarized in Table 1. These include widely used tubular photobioreactors, plate reactors, bubble column reactors and not so frequently used semi-hollow spheres. Vertical-column photobioreactors are characterized by high mass transfer and good mixing with low shear stress. It has low energy consumption and can potentially be scaled up. It has reduced photoinhibition and photo-oxidation. In addition, it is advantageous for immobilization of microalgae and can be readily tempered (Ugwu et al. 2008). Flat panel photobioreactors on the other hand provide large illumination surface area and are noted for high biomass productivities (Ugwu et al. 2008). These are relatively cheap and easy to clean up and cause low oxygen build up. Tubular photobioreactor is considered for having large illumination area and is also suitable for outdoor cultivation. However, the vertical-column photobioreactors have limited/small illumination surface and are not considered worthy for scale up. Possible hydrodynamic stress is a challenge in flat-panel photobioreactors. Tubular photobioreactors suffer with the disadvantage of forming gradients of pH, dissolved oxygen and CO2 along the reactor tubes. The major limitation with all these reactor systems is that they are costly to set up and operate (Lam and Lee 2012).
4 Applications of Microalgae Biomass for Biofuels
Microalgal biomass is rich in lipids which are suitable to produce biodiesel by fatty acid methyl ester (FAME). The biodiesel production from microalgae lipid can be integrated with the other energy-producing processes that could make the biodiesel an economical and sustainable product. Apart from biodiesel, microalgae biomass can also be used to produce bioethanol by fermentation, biomethane by anaerobic digestion, biobutanol and syngas. Biofuel production capacities of various microalgal strains are summarized in Table 2.
4.1 Biodiesel
Microalgae are known as renewable feedstocks for biodiesel production due to ability to accumulate high amount of lipids. Among all major metabolites (lipids, proteins and carbohydrates) of microalgae, lipids have gained significant amount of interest to overcome fossil fuel crisis. The lipid content depends on species, and biomass condition such as lipids in lyophilized biomass of Chlorella pyrenoidosa (47%), dried biomass of Nannochloropsis oculata (26.8%), wet biomass of C. vulgaris ESP-31 (14–63%), algal cake of C. vulgaris ESP-31 (26.3%) and dried biomass of C. pyrenoidosa (56.3%) varies from species to species (Cao et al. 2013; Li et al. 2011; Tran et al. 2013). In normal cultivation condition, the capacity of lipid accumulation of various microalgae is low which hampers the biodiesel production cost. To surpass these challenges, many strategies have been developed such as cultivation in nutrient limitation/starvation, use of mixed culture, reactor design (open pond, closed photobioreactor, etc.) and supplementation of chemicals and hormones. Among all the lipid-enhancing strategies, the nutrient (nitrogen) limitation is widely used. Cultivation of Chlorococcum nivale and Scenedesmus deserticola in nitrogen starvation condition significantly enhanced lipid yield from 31.6 to 40.7% and 48 to 54%, respectively (Singh et al. 2016). In another study, Gao et al. (2013) found that cultivation of Chaetoceros muelleri under nitrogen limitation caused twofold increase in lipid yield (23–46%) and decrease in biomass productivity (19–12 mg L−1day−1). In chemical conversion of microalgal lipid to biodiesel via transesterification, lipid reacts with alcohol (e.g. methanol) in the existence of catalyst (e.g. acidic, alkaline or enzymes) and results in FAME and glycerol. There are two methods of transesterification, i.e. two-stage method in which biomass drying, lipid extraction and purification steps are involved, while in in situ transesterification (direct), lipid extraction and transesterification occur concomitantly. Johnson and Wen (2009) applied both methods of transesterification for S. limacinum biomass, and they obtained crude biodiesel (57%) and FAME (66.37%) by two-stage method and 66% of crude biodiesel and 63.46% of FAME by direct transesterification (Johnson and Wen 2009). Guldhe et al. (2016) achieved 90.87% of yield and 80.97% of biodiesel conversion from Scenedesmus obliquus by using whole cell lipase enzyme of Aspergillus niger as catalyst (Guldhe et al. 2016).
4.2 Biomethane
Lipid extraction for current liquid biofuel from microalgae leaves approximately 60–70% of residual biomass as byproduct. Anaerobic digestion of LEA biomass is used as a substrate for the production of methane and the release of nutrients such as soluble nitrogen, phosphorus, etc. Anaerobic digestion is a series of process in which microorganisms break down the biodegradable substance in the absence of oxygen. The four key steps involved in anaerobic digestion are hydrolysis, acidogenesis, acetogenesis and methanogenesis. In hydrolysis process, large or complex organic molecule (carbohydrate, proteins, lipids) is broken down in the small constituents (e.g. sugar, amino acids and fatty acids) by microorganism. In acidogenesis, microorganisms further break down the remaining complex molecules into ammonia, CO2 and H2S. In acetogenesis, acetoacetate, CO2 and H2 are formed. In methanogenesis, methanogenic bacteria utilized intermediate product of other steps and transform it into methane, CO2, and H2O. Among all four steps, hydrolysis is a rate-limiting step; the whole process depends on hydrolysis. LEA biomass used as a substrate and fermentative bacteria is used as an inoculum that converts carbohydrates and proteins into biomethane. Several factors are involved and influence biomethane production like upstream (cultivation, harvesting and lipid extraction) and downstream processing (biomass pretreatment, C/N ratio and inoculum). LEA biomass which has low C/N ratio is not suitable for biomethane production (Rashid et al. 2014). To overcome low C/N ratio, in many cases, rich carbon waste (e.g. biodiesel byproduct glycerol) is utilized to improve the biomethane production. Widely, C/N ratio of microalgal biomass varies from 4.16 to 7.82, and when this ratio is lesser than 20, it is unsuitable for microorganism. It has been observed that C/N ratio lower than 15 shows detrimental effect and produces ammonia nitrogen (Ehimen et al. 2013; Ward et al. 2014). Pretreatment is the vital step for methane production; pretreatment increases the surface area, makes the substrate more digestible and improves the fluidity in the reactor. Different types of treatment like mechanical, ultrasound, microwave, thermal, chemical treatment, biological and combined pretreatments are used; however, heat treatment is the most efficient and is widely used for biomethane production. Thermal pretreatment of microalgal biomass (at 50–250°C) enhances solubilization, sanitizes the feedstock and produces high yield of methane (Rodriguez et al. 2015). Thermal treatment on whole and LEA biomass of Nannochloropsis gaditana shows that methane production has been enhanced by 40 and 15% by whole and LEA biomass, respectively (Alzate et al. 2014). Thermal pretreatment (150–170°C) on whole N. salina increased the methane yield by 40% (0.31 L/gVS) (Bohutskyi et al. 2015). Hernandez et al. (2014) used supercritical CO2 extraction (SCCO2) techniques for lipid extraction from Tetraselmis sp. and found that LEA biomass has more potential (236 mL CH4/g VSadded) than whole algae. Lipid-extracting solvent (hexane, chloroform, etc.) system also affects the methane production (Choi et al. 2010; Yun et al. 2014).
4.3 Bioethanol
Bioethanol production from food crops (sugar cane and corn) can directly impact on food prices and deforestation. Second-generation feedstock for bioethanol production has a lot of challenges. Saccharification of lignocellulose is one of the major challenges because of resistance due to high content of lignin (Guo et al. 2013). In addition, these feedstocks are inexpensive than sugar, but lignocellulosic feedstock requires strong pretreatment prior to fermentation. Whole microalgae biomass as well as LEA biomass has potential to be used as an economical and sustainable feedstock for the production of bioethanol. Polysaccharide-rich microalgae biomass does not have lignin and therefore is easy and less resistant to conversion in fermentable sugar. Microalgal species like C. vulgaris and C. reinhardtii UTEX 90 stored starch as energy source; these species easily hydrolyze in glucose with chemical or enzymatic process (Brányiková et al. 2011; Choi et al. 2010). The combination of diluted acid and enzyme (cellulase) pretreatment method employed by Guo et al. (2013) for S. abundans PKUAC 12 biomass yielded 0.103 g of ethanol/g of dry weight algae. Among all pretreatment (chemical, enzymatical, combination of chemical and enzymatic, etc.) methods, dilute acid pretreatment is widely used. Chemical and enzymatic pretreatment was used for C. vulgaris with 51% carbohydrates, which resulted in 93.6% and 90.4% glucose yield, respectively (Ho et al. 2013). Hernández et al. (2015) compared the acid and enzymatic pretreatment of Chlorella sorokiniana and Nannochloropsis gaditana that caused monosaccharide yield of 128 and 129 mg/g DW, respectively. In case of Scenedesmus almeriensis under acid hydrolysis (for 60 min at 121°C), the yield of monosaccharides was 88 mg/g. Harun and Danquah (2011) used diluted acid (1% H2SO4 v/v at 140°C for 30 min) hydrolysis as pretreatment of Chlorococcum humicola, and 7.20 g/L bioethanol was obtained when15 g/L of microalgae were used for pretreatment. The cost of pretreatment can be minimized by using carbohydrate-rich microalgal species.
4.4 Biobutanol
Carbohydrates are one of the major primary metabolites of microalgae, and its contents depend on the type of species and mode of cultivation. In microalgae, most knocked primary metabolite is lipids for biodiesel production which leaves the defatted biomass after lipid extraction. Hence, whole and LEA biomass which contains carbohydrates can also be used to produce biobutanol. Butanol is one of the most plentiful biofuels produced by acetone-butanol-ethanol (ABE) fermentation process in which microorganism converts carbohydrate residues into acetone, butanol and ethanol via anaerobic process. Butanol is environmentally friendly, and it has potential to direct use in vehicles, and it is better than ethanol because of its greater energy content, better immiscible and lower volatility, corrodibility and hygroscopicity (Castro et al. 2015; Srirangan et al. 2012). In ABE fermentation process, the Clostridium species and Clostridium acetobutylicum are predominantly used for biobutanol production, and the ratio of the three products ( acetate, butanol and ethanol) in ABE fermentation is 3:6:1 (Cheng et al. 2015). Pretreatment is crucial to increase the surface area of microalgal biomass and to make it more susceptible for microorganisms for biobutanol production. Dilute acid hydrolysed microalgal biomass produced the lower ABE (2.74 g/L), while combination of acid and enzymatic hydrolysed biomass yielded highest ABE (9.74 g/L) (Kumar and Gayen 2011). Castro et al. (2015) optimized the acid hydrolysis of microalgal biomass, and they found that 1.0 M acid concentration at 80–90°C for 120 min is optimum to get the sugar yield of 166.1 g/kg of dry algae and 3.74 g/L butanol production. Cheng et al. (2015) used LEA biomass as a substrate and C. acetobutylicum as a model microorganism and achieved butanol yield of 0.13 g/g carbohydrates.
4.5 Syngas
Microalgae are a feedstock for renewable energy production, but most of their energy-forming processes are time consuming and energy intensive and required chemicals and enzymes for the process. Hence, it is very important to select an appropriate method that can make biofuel economically viable. Many conversion strategies have been utilized for biofuel production, and among all, the pyrolysis is a more explored technology in which microalgal biomass gets transformed into solid, liquid and gaseous products (Shie et al. 2010). Syngas, also known as synthetic gas, is a mixture of different gases such as CO2, CO and H2. The syngas is produced by gasification in which microalgal biomass undergoes the heat treatment and biomass breaks down and produces gases (synthetic natural gas and to create ammonia or methanol) as primary product and char tars as byproducts. Syngas production involves many reactions such as oxidation reaction, water gas reaction, methanation reaction, water-gas shift reaction, etc. The production of syngas also depends on microalgal biomass quality, instrument used for gasification and process parameters such as temperature and catalyst used for gasification (Raheem et al. 2015). In production of syngas, temperature is a vital parameter. Syngas yield increases from 28 to 57% when temperature is increased from 552°C to 952°C (Raheem et al. 2015). For production of syngas, Hiranoa et al. (1998) partially oxidized the Spirulina sp. (at 850–1000°C) to find out theoretical biomethanol production, and the findings showed that the microalgae biomass at 1000°C has optimum theoretical yield (0.64 g) of methanol per gram of biomass. Beneroso et al. (2013) carried out microwave-assisted pyrolysis to examine whole and extracted residues of Scenedesmus almeriensis at 400–800°C. The high yield of syngas (c.a. 94 vol%) was obtained at 800°C after pyrolysis of residues.
5 Process Constraints and Future Needs
Microalgae are the third-generation feedstock for biodiesel production. It has promising potential for biofuel production and also offers many other valuable products. Apart from CO2 sequestration, microalgae also carry out phytoremediation. The biggest process constraint is that high biomass is not achieved in microalgae cultivation. Low biomass production and single-product strategy are one of the bottlenecks in developing economical and sustainable microalgae industry. The requirement of huge volumes of water always remains a big challenge in microalgae cultivation. Economical and effective biomass harvesting technology is still in demand. The cost of biomass production remains high in closed photobioreactor, and open raceway ponds suffer from low biomass productivity and contamination issues. Multiproduct development strategy from microalgae biomass can make the microalgae biotechnology processes the viable and economical one. Integration of microalgae production with simultaneous wastewater treatment has the potential for sustainable biomass generation for biofuel and feed-/fertilizer-related products.
5.1 Factors Limiting Growth and Biomass Production
For large-scale commercial production of microalgae biomass, closed photobioreactor and open raceway systems are widely used. The choice of cultivation system depends on the final product; closed photobioreactors were always preferred for high-value product synthesis from microalgae. Both cultivation systems have their own advantages and disadvantages. Microalgae are photosynthetic organism, so light is one of the limiting factors for growth and biomass production. Light does not penetrate in the dense microalgae culture. In an open system, it is very hard to control and supply optimum light condition for optimum growth and biomass production. The other evaporation of water causes changes in ionic composition and pH of the medium. Seasonal variation also negatively affects photoperiod hours and biological clock of microalgae. Large-scale open system always has high-risk contamination. The unwanted microorganism such as protozoa, zooplankton and other undesirable microalgae species competes for nutrients. These unwanted microorganisms are known as grazer that grazes microalgae in 2–3 days. Zooplankton can reduce 90% of the microalgae cell density in 48 h, while Daphnia could bring massive change of over 99% in a few days (Rawat et al. 2013). In large-scale microalgae cultivation, mechanical failure in the system cannot be ignored. Therefore, the high biomass production remains one of the challenges in microalgae biotechnology.
5.2 Environmental Sustainability of Algal Biodiesel
Microalgae are the photosynthetic unicellular organism. It requires solar light and CO2 from environment to fix and grow into biomass. Biodiesel production from microalgae is eco-friendly because it releases low levels of NOx and SOx after combustion. Most importantly microalgal biodiesel is compatible with existing combustion engines without any further modifications (Rashid et al. 2014). Microalgae biodiesel also has similar fuel properties (density, viscosity, flash point, cold flow and heating value) like petrodiesel. Around the globe, climate change is one of the most debatable topics. In climate change, CO2 which is emitted by anthropogenic activities plays an important role. For production of one ton of microalgae biomass, microalgae consume 1.83 tons of CO2 (Chisti 2007). The microalgae cultivation could be integrated with industry such as cement factory to provide CO2 in proper utilization. It is very important to determine the carbon footprint. Carbon footprint of microalgal biodiesel is lower than the petroleum fuel. Microalgae water footprint (WF) is the water required for cultivation and media preparation. WF is predominantly based on evaporation rate, hydraulic retention time and photosynthesis rate. Evaporation rate highly depends upon local climate from 0.48 m3 m−2 year−1 to 2.28 m3 m−2 year−1 in arid regions (Usher et al. 2014). The average annual WF of microalgae biodiesel grown in open raceway pond and closed photobioreactor is 14–87 and 1–2 m3/GJ significantly lower than biodiesel produced from soybean (287 m3/GJ) (Usher et al. 2014). The carbon footprint is acceptable if it is lower than the petroleum fuel or equal on energy basis (Chisti 2013). Microalgae cultivation does not require freshwater; it can grow in domestic wastewater, municipal wastewater, industrial wastewater and marine water. Integration of wastewater treatment and microalgae cultivation can make biodiesel production a sustainable process (Gupta et al. 2016; Rawat et al. 2011; Shriwastav et al. 2014).
5.3 Economic Sustainability of Algal Biofuels
The price of microalgal biomass cultivated in open raceway pond and closed photobioreactor is $7.32 and $1.54, respectively, while the price of microalgae oil per kilogram grown on raceway and photobioreactor is $7.64 and $24.60, respectively. The cost of microalgal biodiesel is very high, and it must be reduced to make it commercially viable. The price of microalgae biodiesel per barrel is US$300–2600 in oil market (Rashid et al. 2014). It is also found in many studies that algal biofuel price is double of petrol fuel. According to Chisti et al., the price of microalgae oil without transport charge and taxes is $2.80 per litre. In current time, the price of crude oil is less than $60 per barrel. To replace 1% of annual US petroleum consumption, a huge amount (~31 million tons) of biomass with 40% oil (w/v) is required (Chisti 2013). To make microalgae economical and sustainable, low-cost microalgae cultivation, widely accepted harvesting process and green technology to extract high oil yield are required. Integration of microalgal biofuel technology to other technologies is very important to further reduce the overall biodiesel production cost. Integration like the use of treated wastewater for microalgal cultivation, use biomass for aquaculture feed and LEA for other applications will help to reduce the overall cost of microalgae products. The use of wet biomass directly to extract oil and transesterification for biodiesel production can also be one of the strategies. The use of residual biomass in aquaculture feed, piggery feed, poultry feed and animal feed could be alternative and novel idea. The high content of carbon in residual biomass can be used for biomethane, bioethanol, biobutanol and syngas production. It can also be used as a conventional fertilizer to enhance the crop productivity.
References
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2011) Microalgae as a sustainable energy source for biodiesel production: a review. Renew Sust Energ Rev 15(1):584–593
Alzate ME, Muñoz R, Rogalla F, Fdz-Polanco F, Pérez-Elvira SI (2014) Biochemical methane potential of microalgae biomass after lipid extraction. Chem Eng J 243:405–410
Ansari FA, Shriwastav A, Gupta SK, Rawat I, Guldhe A, Bux F (2015) Lipid extracted algae as a source for protein and reduced sugar: a step closer to the biorefinery. Bioresour Technol 179:559–564
Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A (2013) Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res 2(2):164–173
BCC-Research, 2011, The Global Market for Carotenoids; 2011
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25(2):207–210
Beneroso D, Bermudez JM, Arenillas A, Menendez JA (2013) Microwave pyrolysis of microalgae for high syngas production. Bioresour Technol 144:240–246
Bohutskyi P, Chow S, Ketter B, Betenbaugh MJ, Bouwer EJ (2015) Prospects for methane production and nutrient recycling from lipid extracted residues and whole Nannochloropsis salina using anaerobic digestion. Appl Energy 154:718–731
Brányiková I, Maršálková B, Doucha J, Brányik T, Bišová K, Zachleder V, Vítová M (2011) Microalgae—novel highly efficient starch producers. Biotechnol Bioeng 108(4):766–776
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14(2):557–577
Briassoulis D, Panagakis P, Chionidis M, Tzenos D, Lalos A, Tsinos C, Berberidis K, Jacobsen A (2010) An experimental helical-tubular photobioreactor for continuous production of Nannochloropsis sp. Bioresour Technol 101(17):6768–6777
Cao H, Zhang Z, Wu X, Miao X (2013) Direct biodiesel production from wet microalgae biomass of Chlorella pyrenoidosa through in situ transesterification. Biomed Res Int 2013:930686
Caporgno MP, Olkiewicz M, Pruvost J, Lepine O, Legrand J, Font J, Bengoa C (2016) A novel pre-treatment for the methane production from microalgae by using N-methylmorpholine-N-oxide (NMMO). Bioresour Technol 201:370–373
Carvalho AP, Meireles LA, Malcata FX (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Castro YA, Ellis JT, Miller CD, Sims RC (2015) Optimization of wastewater microalgae saccharification using dilute acid hydrolysis for acetone, butanol, and ethanol fermentation. Appl Energy 140:14–19
Chandra R, Venkata Mohan S (2011) Microalgal community and their growth conditions influence biohydrogen production during integration of dark-fermentation and photo-fermentation processes. Int J Hydrog Energy 36(19):12211–12219
Cheng HH, Whang LM, Chan KC, Chung MC, Wu SH, Liu CP, Tien SY, Chen SY, Chang JS, Lee WJ (2015) Biological butanol production from microalgae-based biodiesel residues by Clostridium acetobutylicum. Bioresour Technol 184:379–385
Chinnasamy S, Bhatnagar A, Hunt RW, Das KC (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol 101(9):3097–3105
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167(3):201–214
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100(2):833–838
Choi SP, Nguyen MT, Sim SJ (2010) Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour Technol 101(14):5330–5336
D’Alessandro EB, Antoniosi Filho NR (2016) Concepts and studies on lipid and pigments of microalgae: a review. Renew Sust Energ Rev 58:832–841
Dogaris I, Welch M, Meiser A, Walmsley L, Philippidis G (2015) A novel horizontal photobioreactor for high-density cultivation of microalgae. Bioresour Technol 198:316–324
Ehimen EA, Holm-Nielsen JB, Poulsen M, Boelsmand JE (2013) Influence of different pre-treatment routes on the anaerobic digestion of a filamentous algae. Renew Energy 50:476–480
Francavilla M, Kamaterou P, Intini S, Monteleone M, Zabaniotou A (2015) Cascading microalgae biorefinery: fast pyrolysis of Dunaliella tertiolecta lipid extracted-residue. Algal Res 11:184–193
Frumento D, Casazza AA, Al Arni S, Converti A (2013) Cultivation of Chlorella vulgaris in tubular photobioreactors: a lipid source for biodiesel production. Biochem Eng J 81:120–125
Gao Y, Yang M, Wang C (2013) Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol 147:484–491
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21(5):493–507
Guldhe A, Singh P, Kumari S, Rawat I, Permaul K, Bux F (2016) Biodiesel synthesis from microalgae using immobilized Aspergillus niger whole cell lipase biocatalyst. Renew Energy 85:1002–1010
Guo H, Daroch M, Liu L, Qiu G, Geng S, Wang G (2013) Biochemical features and bioethanol production of microalgae from coastal waters of Pearl River Delta. Bioresour Technol 127:422–428
Gupta SK, Ansari FA, Shriwastav A, Sahoo NK, Rawat I, Bux F (2016) Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio-fuels. J Clean Prod 115:255–264
Harun R, Danquah MK (2011) Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem 46(1):304–309
Hena S, Fatimah S, Tabassum S (2015) Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour Ind 10:1–14
Hernández D, Riaño B, Coca M, García-González MC (2015) Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem Eng J 262:939–945
Hernandez D, Solana M, Riano B, Garcia-Gonzalez MC, Bertucco A (2014) Biofuels from microalgae: lipid extraction and methane production from the residual biomass in a biorefinery approach. Bioresour Technol 170:370–378
Hiranoa A, Hon-Nami K, Kunito S, Hada M, Ogushi Y (1998) Temperature effect on continuous gasification of microalgal biomass: theoretical yield of methanol production and its energy balance. Catal Today 45:399–404
Ho SH, Huang SW, Chen CY, Hasunuma T, Kondo A, Chang JS (2013) Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol 135:191–198
Hu Z, Ma X, Li L, Wu J (2014) The catalytic pyrolysis of microalgae to produce syngas. Energy Convers Manag 85:545–550
Johnson MB, Wen Z (2009) Production of Biodiesel Fuel from the Microalga Schizochytrium limacinum by Direct Transesterification of Algal Biomass. Energy Fuel 23(10):5179–5183
Ju ZY, Deng D-F, Dominy W (2012) A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fishmeal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture 354-355:50–55
Kumar M, Gayen K (2011) Developments in biobutanol production: new insights. Appl Energy 88(6):1999–2012
Lam MK, Lee KT (2012) Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv 30(3):673–690
Lee OK, Oh YK, Lee EY (2015) Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour Technol 196:22–27
Li P, Miao X, Li R, Zhong J (2011) In situ biodiesel production from fast-growing and high oil content Chlorella pyrenoidosa in rice straw hydrolysate. J Biomed Biotechnol 2011:141207
Liu C-H, Chang C-Y, Liao Q, Zhu X, Liao C-F, Chang J-S (2013) Biohydrogen production by a novel integration of dark fermentation and mixotrophic microalgae cultivation. Int J Hydrog Energy 38(35):15807–15814
Lv X, Zou L, Sun B, Wang J, Sun M-Y (2010) Variations in lipid yields and compositions of marine microalgae during cell growth and respiration, and within intracellular structures. J Exp Mar Biol Ecol 391(1-2):73–83
Milano J, Ong HC, Masjuki HH, Chong WT, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sust Energ Rev 58:180–197
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 96(3):631–645
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14(1):217–232
Misra R, Guldhe A, Singh P, Rawat I, Bux F (2014) Electrochemical harvesting process for microalgae by using nonsacrificial carbon electrode: a sustainable approach for biodiesel production. Chem Eng J 255:327–333
Raheem A, Sivasangar S, Wan Azlina WAKG, Taufiq Yap YH, Danquah MK, Harun R (2015) Thermogravimetric study of Chlorella vulgaris for syngas production. Algal Res 12:52–59
Rashid N, Ur Rehman MS, Sadiq M, Mahmood T, Han J-I (2014) Current status, issues and developments in microalgae derived biodiesel production. Renew Sust Energ Rev 40:760–778
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2013) Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl Energy 103:444–467
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88(10):3411–3424
Rodriguez C, Alaswad A, Mooney J, Prescott T, Olabi AG (2015) Pre-treatment techniques used for anaerobic digestion of algae. Fuel Process Technol 138:765–779
Romero Garcia JM, Acien Fernandez FG, Fernandez Sevilla JM (2012) Development of a process for the production of L-amino-acids concentrates from microalgae by enzymatic hydrolysis. Bioresour Technol 112:164–170
Sekar S, Chandramohan M (2007) Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol 20(2):113–136
Shie JL, Tsou FJ, Lin KL, Chang CY (2010) Bioenergy and products from thermal pyrolysis of rice straw using plasma torch. Bioresour Technol 101(2):761–768
Shriwastav A, Gupta SK, Ansari FA, Rawat I, Bux F (2014) Adaptability of growth and nutrient uptake potential of Chlorella sorokiniana with variable nutrient loading. Bioresour Technol 174:60–66
Singh P, Guldhe A, Kumari S, Rawat I, Bux F (2015) Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem Eng J 94:22–29
Singh P, Kumari S, Guldhe A, Misra R, Rawat I, Bux F (2016) Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew Sust Energ Rev 55:1–16
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96
Srirangan K, Akawi L, Moo-Young M, Chou CP (2012) Towards sustainable production of clean energy carriers from biomass resources. Appl Energy 100:172–186
Tongprawhan W, Srinuanpan S, Cheirsilp B (2014) Biocapture of CO2 from biogas by oleaginous microalgae for improving methane content and simultaneously producing lipid. Bioresour Technol 170:90–99
Tran DT, Chen CL, Chang JS (2013) Effect of solvents and oil content on direct transesterification of wet oil-bearing microalgal biomass of Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized lipase as the biocatalyst. Bioresour Technol 135:213–221
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99(10):4021–4028
Usher PK, Ross AB, Camargo-Valero MA, Tomlin AS, Gale WF (2014) An overview of the potential environmental impacts of large-scale microalgae cultivation. Biofuels 5(3):331–349
Wang Y, Guo W, Cheng CL, Ho SH, Chang JS, Ren N (2016) Enhancing bio-butanol production from biomass of Chlorella vulgaris JSC-6 with sequential alkali pretreatment and acid hydrolysis. Bioresour Technol 200:557–564
Ward AJ, Lewis DM, Green FB (2014) Anaerobic digestion of algae biomass: a review. Algal Res 5:204–214
Ward A, Lewis D (2015) Pre-treatment options for halophytic microalgae and associated methane production. Bioresour Technol 177:410–413
Wilhelm C, Jakob T (2011) From photons to biomass and biofuels: evaluation of different strategies for the improvement of algal biotechnology based on comparative energy balances. Appl Microbiol Biotechnol 92(5):909–919
Yang Z, Guo R, Xu X, Fan X, Li X (2010) Enhanced hydrogen production from lipid-extracted microalgal biomass residues through pretreatment. Int J Hydrog Energy 35(18):9618–9623
Yang Z, Guo R, Xu X, Fan X, Luo S (2011) Fermentative hydrogen production from lipid-extracted microalgal biomass residues. Appl Energy 88(10):3468–3472
Yun Y-M, Cho S-K, Jung K-W, Kim M-S, Shin H-S, Kim D-H (2014) Inhibitory effect of chloroform on fermentative hydrogen and methane production from lipid-extracted microalgae. Int J Hydrog Energy 39(33):19256–19261
Zhao B, Ma J, Zhao Q, Laurens L, Jarvis E, Chen S, Frear C (2014) Efficient anaerobic digestion of whole microalgae and lipid-extracted microalgae residues for methane energy production. Bioresour Technol 161:423–430
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Ansari, F.A., Shekh, A.Y., Gupta, S.K., Bux, F. (2017). Microalgae for Biofuels: Applications, Process Constraints and Future Needs. In: Gupta, S., Malik, A., Bux, F. (eds) Algal Biofuels. Springer, Cham. https://doi.org/10.1007/978-3-319-51010-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-51010-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51009-5
Online ISBN: 978-3-319-51010-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)