Abstract
Hydrogen has been recognized as a promising alternative to traditional sources of energy, as it is renewable, readily available, environment friendly and does not produce harmful emissions, when burned. Biological hydrogen production has been carried out largely using microalgae and bacteria (dark fermentation). Microalgae can generate hydrogen either by biophotolysis of water or through photofermentation. Two enzymes, viz., hydrogenase and nitrogenase, perform critical tasks in biological hydrogen production processes. Hydrogenase enzyme has been observed in facultative anaerobic bacteria and green algae such as Scenedesmus obliquus, Chlorococcum littorale, Platymonas subcordiformis and Chlorella fusca. Conversely, microalgae such as Rhodopseudomonas capsulate, Rhodobacter sphaeroides and Rhodospirillum rubrum have been reported for photofermentative hydrogen production. Even though, microalgae have been successfully explored at a laboratory scale for biohydrogen production, low yield has been recognized as a limiting factor for its bulk production and commercialization. Current research is therefore engrossed more on overcoming the key challenges such as O2 sensitivity of hydrogenase enzyme, solar conversion efficiencies for CO2 fixation through genetic engineering and design of low-cost photobioreactors. This chapter primarily includes the various processes for biohydrogen generation using microalgae (photofermentation, direct biophotolysis and indirect biophotolysis), their advantages and limitations. Emphases have also been given on strategies to mitigate the present challenges.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Hydrogen Production
- Photosynthetic Bacterium
- Calvin Cycle
- Nicotinamide Adenine Dinucleotide Phosphate
- Oxygenic Photosynthesis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Hydrogen: A Future Energy Carrier
The global demand for energy is on an exponential rise over the years, while the fossil fuels reserves are diminishing at a faster pace (Nasr et al. 2014a). Additionally, fossil fuel combustion significantly affects the environment due to CO2 release (Nasr et al. 2013a). Accordingly, scientists are searching for exploring new and alternate energy sources that are sustainable and possibly could replace fossil fuels (Nasr et al. 2014b).
Hydrogen is emerging as a potential energy carrier of the future owing to its renewable nature, zero carbon dioxide emission during combustion (Nasr et al. 2013b), has more energy per unit weight and could be used in fuel cells (Nasr et al.2013c).
2 General Characteristics of Microalgae
Microalgae are primitive tiny plants found in aquatic habitats, however, lack various structures found in tracheophytes (vascular plants) such as leaves and roots (Amos 2004). The main cell composition of green algae includes nucleus, cell wall, chlorophyll and other pigments, pyrenoid, stigma and flagella. Cyanobacteria (blue-green algae) and green algae have the ability to carry out plant-type photosynthesis (Schnackenberg et al. 1996). Cyanobacteria are now categorized as prokaryotes due to their anatomical similarities to bacteria. Microalgae and Cyanobacteria perform oxygenic photosynthesis (Eq. 1), where water is split by sunlight into O2 and a strong reductant, typically ferredoxin, typically used to reduce CO2 to carbohydrates (sugars) (Tiwari and Pandey 2012).
(CH2O—represents carbohydrate general formula).
3 General Mechanisms of Hydrogen Production
Biohydrogen production is the biological conversion of water, sunlight and/or organic substrates into hydrogen by the action of nitrogenase or hydrogenase enzymes. Hydrogen, a by-product of nitrogen-fixation by the enzyme nitrogenase, is produced by reducing molecular nitrogen into ammonia. Hydrogenase is another key enzyme in biohydrogen production that catalyses the formation and decomposition of hydrogen (Tiwari and Pandey 2012).
Biologically, hydrogen can be produced through photobiological process (green algae, photosynthetic bacteria or cyanobacteria), or through dark fermentation (heterotrophic bacteria) (Hena et al. 2016). Microbial hydrogen production mainly involves three distinct mechanisms, based on the abundance of carbon and other energy sources (Nasr et al. 2015): (1) Dark fermentation, where the organic matter is converted to hydrogen, carbon dioxide and soluble metabolites (mainly volatile fatty acids) by a group of heterotrophic obligate or facultative anaerobic bacteria, in the absence of light (Nasr et al. 2013d), (2) Photofermentation, where organic acids (e.g. VFAs) are converted to hydrogen by the action of photosynthetic bacteria (e.g. Rhodobacter) in the presence of light and (3) Biophotolysis, where carbon dioxide and sunlight are used as energy sources for the dissociation of water into molecular hydrogen and oxygen by photoautotrophic organisms (cyanobacteria and green algae) (Gaffron and Rubin 1942).
Biological process of hydrogen generation has multiple benefits which include less energy demand and minimum capital investments (Nasr et al. 2014a). However, biological conversion efficiencies of substrates to hydrogen gas are influenced by various external factors including pH, temperature, substrate concentration, type of inoculum, etc. (Nasr et al. 2014b). The biohydrogen production efficiency of microalgae depends heavily on the species involved and their growth requirements.
4 Photoautotrophic Hydrogen Production
Photosystems (PS) are the complexes of pigments, such as chlorophylls, carotenoids and phycobiliproteins, in addition to several dozen proteins that are the functional units of photosynthesis (Singh et al. 2015). PS are able to capture photons by light harvesting pigments (also known as antenna) and then alter light (photon) into chemical energy via photosynthetic reaction centre (Miura et al. 1997). The initial form of chemical energy is further transformed as reductant to metabolic energy (reduced ferredoxin which then generates nicotinamide adenine dinucleotide phosphate (NADPH)) and a membrane potential proton-motive force which is then transformed into adenosine triphosphate (ATP) (Florin et al. 2001). ATP and NADPH are used to fix CO2 into glucose, further used along with nitrogen (as ammonia or nitrate), phosphorous (as phosphate) and other inorganic nutrients as the primary building material for all other algal cell components (carbohydrates, proteins, nucleic acids, fats, etc.) (Schnackenberg et al. 1996).
Eukaryotic microalgae are photoautotrophic (possess chlorophyll A and other pigments) organisms, and perform oxygenic photosynthesis using photosynthetic systems (PSII and PSI). Photosynthesis is a two-stage process (Fig. 1), which includes light reaction (the photo part) and Calvin cycle (the synthesis part) (Florin et al. 2001). During the light reaction of photosynthesis, the pigments in PSII (P680) absorb light (photons with wavelengths <680 nm), creating a powerful oxidant which can split water into protons (H+), electrons (e−) and O2 (Miura et al. 1997). A series of electron carriers and cytochrome complex transfer the released electrons to PSI (Schnackenberg et al. 1996). The photons (wavelength <700 nm) are absorbed by the pigments in PSI (P700) where NADPH is formed by reducing nicotinamide adenine dinucleotide phosphate (NADP+) by adding a pair of e− and H+. Additionally, the oxidized ferredoxin (Fdox) is reduced to reduced ferredoxin (Fdred), which is directed to the enzyme hydrogenase (Hase) for hydrogen liberation (Tiwari and Pandey 2012).
Schematic representation of photosynthesis and biophotolysis process (Amos et al., 2004)
Adenosine triphosphate (ATP) is also produced during light reactions by the addition of a phosphate group to ADP by chemiosmosis, through a process known as photophosphorylation (Bishop and Bishop 1987). The formation of ATP and NADPH thus marks the first step in the photosynthesis (conversion of light to chemical energy). Subsequently, the ATP and NADPH formed during light reactions are reduced with CO2 from the air (carbon fixation) via reductive pentose phosphate pathway or Calvin cycle for cell division (Weissman and Benemann 1977).
During Calvin cycle, the fixed carbon is reduced to carbohydrate by the addition of electrons (Miura et al. 1986). The excess reduced carbon thus formed is accumulated as carbohydrates (CH2O) and/or lipids inside the cells. The metabolic steps involved in Calvin cycle are termed as dark reactions, or light-independent reactions, as it does not require direct light (McCully and McKinlay 2016).
5 Hydrogenase-Dependent Hydrogen Production
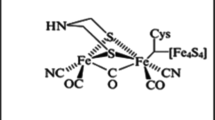
The hydrogen ions liberated during microalgal photosynthesis by splitting the water molecules to hydrogen ion and oxygen are transformed to hydrogen gas by the action of hydrogenase enzyme (Hena 2016). Hydrogenase oxidizes reduced ferredoxin to liberate molecular hydrogen in anaerobes, (Eq. 2, where the electron carrier “X” is assumed to be ferredoxin) (Winkler et al. 2002). Thus, addition of external iron source may be required for hydrogen production (Fig. 2). However, the oxygen-labile nature of hydrogenase is a bottleneck for sustainable hydrogen production using microalgae.
Hydrogenase enzyme has been found in green algae, Scenedesmus obliquus (Florin et al. 2001), in marine green algae Chlorococcum littorale (Schnackenberg et al. 1996), Playtmonas subcordiformis (Cao et al. 2001) and in Chlorella fusca (Winkler et al. 2002), though, hydrogenase activity was not reported from C. vulgaris and Dunaliella salina (Cao et al. 2001). Gaffron and Rubin (1942) have indicated that the electron donation (reducing) capacity of hydrogenase does not arise from water constantly, but may evolve intracellularly from starch like organic compounds. Greenbaum et al. (1995) have shown good conversion rate of light to hydrogen (10–20%), with light at 400–700 nm wavelength. Furthermore, they found that a mutant strain of Chlamydomonas could achieve CO2 fixation and hydrogen liberation using one photosystem only (photosystem II). Miura et al. (1986) proposed hydrogen production by a photo/dark cycle, and found that starch was reduced from CO2 during photosynthesis (in the presence of light), after which the starch was converted to hydrogen gas, organic acids and/or alcohols under anaerobic and dark conditions. They indicated that oxygen sensitivity of hydrogenase is overcome by green algae during anaerobic phase, and under light conditions, photosynthetic bacteria convert organic acids and alcohols to hydrogen gas. Asada and Kawamura (1986) have indicated that hydrogen gas could be produced by cyanobacteria through auto-fermentation in dark and anaerobic conditions, where the highest activity among the investigated cyanobacteria was witnessed for Spirulina species. Gaffron and Rubin (1942) found that Scenedesmus spp could produce hydrogen molecules under light conditions after exposure to dark and anaerobic conditions.
6 Nitrogenase-Dependent Hydrogen Production
In photosynthetic bacteria, nitrogenase enzyme plays a major role in catalysing hydrogen gas production (Kapdan and Kargi 2006). Nitrogen-fixation in prokaryotic organisms like cyanobacteria is catalysed by nitrogenase, whereas it is absent among eukaryotes, such as microalgae (Oldroyd and Dixon 2014). In photosynthetic bacteria, nitrogenase facilitates hydrogen production, though hydrogenases may involve both in hydrogen production and uptake under specific conditions. Nitrogenase catalysed hydrogen liberation arises as a side reaction during nitrogen-fixation process where photosynthetic bacteria, in the presence of light, could convert organic acids and other organic substrates into H2 and CO2 (Fig. 3). During nitrogen-fixation in cyanobacteria, molecular nitrogen is reduced to ammonia by the utilization of a reducing agent (ferredoxin) and ATP through an irreversible reaction (Flores et al. 2005).
Nitrogenase mediates reduction of proton in the absence of nitrogen gas.
In the presence of oxygen, ammonia and at high N/C ratio, nitrogenase activity is found inhibited, though hydrogen production can be resumed after ammonia depletion (Koku et al. 2003). Therefore, the process needs an atmosphere with limited ammonium and free oxygen availability (Yokoi et al. 1998). For example, in R. sphaeroides, a complete inhibition of hydrogen production was noticed at ammonia concentrations higher than 2 mM (Yokoi et al. 1998). Hydrogen production was also found to be limited in medium containing ammonia salts, whereas proteins (e.g. albumin, glutamate and yeast extract) enhanced hydrogen production when used as a nitrogen source (Oh et al. 2004). At higher nitrogen levels, the metabolism is directed more towards utilizing organic substrates for cell synthesis and growth than hydrogen production (Fascetti and Todini 1995). Removal efficiency of ammonia along with stimulation of hydrogen production could be enhanced by the supplementation of carbonate (Antal Lindblad 2005).
Localizing nitrogenase enzyme in the heterocysts of filamentous cyanobacteria is the most effective mechanism for depriving nitrogenase from oxygen and to provide it with energy ATP and reducing power (Llama et al. 1979). In filamentous cyanobacteria, vegetative cells perform oxygenic photosynthesis, whereas organic compounds are broken down to supply nitrogenase with reducing power. ATP is supplemented by PSI-dependent and anoxygenic photosynthesis within the heterocysts (Weissman and Benemann 1977). Additionally, previous studies on the enhancement of hydrogen production have indicated that the hydrogen-generation ability of cyanobacteria can be improved through nitrogen deprival (Weissman and Benemann 1977; Miyamoto et al. 1979)
7 Biophotolysis
Hydrogen gas is produced by microalgae under certain growth conditions, resulting in an overall net dissociation of water, known as “biophotolysis” (Miura 1995). Biophotolysis is a very interesting biological process, where water is converted to hydrogen and oxygen using solar energy. Microalgae have all the required genetic, enzymatic, metabolic and electron transport systems to convert water into hydrogen gas using light (Kapdan and Kargi 2006).
The biophotolysis process can be presented as:
Biophotolysis can be classified as “direct” and “indirect photolysis”. In direct biophotolysis, the reduced ferredoxin generated by the splitting of water during photosynthesis is directly used to reduce the hydrogen-producing hydrogenase or nitrogenase, without intermediate CO2 fixation (Miura 1995). However, if the hydrogen is generated from carbohydrates produced by microalgae during normal photosynthesis, then the process is known as “indirect biophotolysis” (Gaffron and Rubin 1942).
7.1 Direct Biophotolysis
In direct biophotolysis, the electrons generated by the absorption of light energy by PSII are transported to ferredoxin using light energy captured by PSI. The above process is catalysed by the enzyme hydrogenase (Benemann 1996) present in the stroma of algal chloroplast. Hydrogenase accepts electrons liberated from reduced ferredoxin and donates them to two protons to generate one H2 molecule. Equation (3) presents direct biophotolysis reaction (green algae and in vitro systems; potentially in cyanobacteria), whereas Eq. (4) demonstrates direct biophotolysis reaction with respiratory O2 uptake (green algae, possibly cyanobacteria) (Hena 2016).
Though direct biophotolysis is an attractive method for sustainable hydrogen production, in practice, the process is strongly limited by the powerful inhibition of hydrogenase activity by concomitantly released oxygen (Miura 1995). To combat the above challenge, alternate methods have been proposed such as spatial separation of hydrogen and oxygen, chloroplast immobilization, oxygen scavenging and gas purging.


7.2 Indirect Biophotolysis
Reduced carbon from the photosynthetic process is generally deposited as endogenous carbohydrates, in the form of starch in microalgae and glycogen in cyanobacteria (Dauvillée et al. 2006). These stored intracellular energy reserves such as carbohydrates may act as electron donors or reducing equivalents for hydrogenase and nitrogenase to function (Antal and Lindblad 2005). The energy from carbohydrates is released via fermentation in dark conditions, and the surplus reducing power may be shifted to protons (H+) by hydrogenase generating hydrogen (Gfeller and Gibbs 1984). Thus, indirect biophotolysis encompasses two stages, stage-1: photosynthesis for carbohydrate production, and stage-2: hydrogen production through dark fermentation of the stored carbohydrate. Through the above two-stage process, the release of oxygen and hydrogen can be spatially separated from each other (Benemann 1996). This separation makes the hydrogen purification process relatively simpler as CO2 can be easily separated from the gas (H2/CO2) mixture (Bélafi-Bakó et al. 2006).
Miura et al. (1997) studied hydrogen production using natural light/dark cycles via indirect biophotolysis. In their study, they have found that during photosynthesis, the CO2 is reduced to starch which is then fermented to hydrogen gas and organic acids under anaerobic and dark conditions. Another mechanism for indirect biophotolysis is by heterocystous cyanobacteria; filamentous species that could achieve water-splitting and CO2-fixing photosynthesis, as well as exclude O2 and reduce N2 (Prince and Kheshgi 2005). In heterocysts, localization of nitrogenase provides an oxygen free environment for cyanobacteria to fix nitrogen from air (Prince and Kheshgi 2005). Another approach of indirect biophotolysis is to carry out two reactions in separate stages, first O2 production (with CO2 fixation) followed by H2 production (with CO2 release) (Miura 1995).
8 Challenges and Technological Advancements in Biohydrogen Production from Microalgae
Although hydrogen production from microalgae is a much exploited area, there are few major drawbacks that limit its application at its commercial level. Some of these well-known challenges include (1) O2 sensitivity of hydrogenase enzyme, (2) non-dissipated proton gradient and state transitions, (3) small antenna size, (4) competition for photosynthetic reductant and (5) requirement of specific photobioreactor (Dubini and Ghiradi 2014). Nevertheless, the past decade has shown good progress in overcoming some of these shortcomings mainly through genetic engineering approaches. Several attempts have been made at pilot and industrial scale for the production of hydrogen by microalgae. However, most of these successful studies involved either genetically engineered microalgal strains or sulphur-deprived conditions (Dubini and Ghirardi 2014; Gimpel et al. 2015). Some of the recent advancements in this area are discussed in brief below.
Oxygen sequestration to mitigate the O2 sensitivity of hydrogenase enzymes is been investigated as an alternative approach for enhancing hydrogen biosynthesis in microalgae. Wu et al. (2011) have reported that introduction of leghaemoglobin (LbA) proteins (oxygen sequester protein from the root nodules of legumes) increased the hydrogen production in Chlamydomonas sp to fourfold compared to its wild type. With further modifications to the above strain, Wu et al. (2011) could increase the gene expression of HemH (ferrochelatase gene) and LbA (leghemoglobin gene) to 6.8 fold in the transgenic strain of Chlamydomonas. Similarly, two genetically modified Chlorella vulgaris strains (YSL01 and YSL16) with upregulated hydrogenase gene (HYDA) expression could liberate hydrogen through photosynthesis in the presence of oxygen (Hwang et al. 2014). Other alternative approaches were tested to remove O2 which included the establishment of new pathways in Chlamydomonas. It is known that pyruvate oxidase (PoX) enzyme is involved in the decarboxylation of pyruvate to CO2 and acetyl phosphate. The reaction is O2 dependent and was assumed that the intracellular O2 levels in Chlamydomonas could be reduced by introducing this gene (Dubini and Ghirardi 2014). The engineered algae strain could produce 2.5 fold higher hydrogen compared to its wild strain under very low light (30 μE m−2 s−1) and sulphur-deplete growth conditions (Gimpel et al. 2015).
Incomplete inactivation of O2 formation was also achieved in the transgenic strain of Chlorella sp. by knocking down the PSBO gene, a nuclear gene that encodes the plastid manganese-stabilizing protein of photosystem II (Lin et al. 2013). This was achieved by antisense RNA technology, i.e. by introducing a short interference RNA antisense-PSBO fragments to knock down the PSBO gene expression in the transgenic Chlorella sp. This was resulted in a tenfold improvement in hydrogen evolution in transgenic strain of Chlorella sp. (Lin et al. 2013).
Biohydrogen production by microalgae is also limited by the competition of hydrogenases enzymes for photosynthetic reductant from ferredoxin with other key enzymes viz., Ferredoxin-NADP++ reductase (FNR), Ferredoxin/thioredoxin reductase (FTR), nitrite reductase, sulphite reductase and glutamate synthase that are involved in major metabolic pathways (Dubini and Ghirardi 2014). To improve the electron flow and to reduce the competitions, engineering of FNR and the hydrogenase genes have all been exploited under in vitro conditions (Oey et al. 2016). Studies have also focused on engineering electron competitors genes such as RuBisCo, cyclic electron flow, starch degradation and respiration (Ruehle et al. 2008; Pinto et al. 2013) with a reportedly increase in hydrogen yield (Oey et al. 2016). Obtaining additional reducing power through genetic engineering is therefore expected to increase hydrogen yields in microalgal cells due to the reduced competitions by different enzymes. A study by Doebbe et al. (2007) has shown that the expression of HUP1 (hexose transporter gene) from Chlorella kessleri in C. reinhardtii has resulted the production of an improved strain that are able to use glucose as both carbon and electron source. They have also noticed a 1.5-fold improvement in hydrogen production rate in the modified strain. In another study, Pinto et al. (2013) have shown that expression of a genetically modified small sub-unit of Rubisco (RBCS-Y67A) in C. reinhardtii has resulted in the elimination of photosystem II activity in the modified strain leading to a 15-fold rise in hydrogen production rate under sulphur-deplete conditions (Dubini and Ghirardi 2014).
A truncated antenna mutant of Chlamydomonas sp. showed an eightfold increase in biohydrogen production under sulphur deprivation due to the increased light capture efficiency and decrease photo inhibition in the mutant strain (Kosourov et al. 2005). It is expected that the reduction of antenna size increases light harvesting efficiency of the microalgal cells as it enhances the light absorption and distribution leading to overall increase in photon conversion efficiency of the microalgal cells (Beckmann et al. 2009; Oey et al. 2013, 2016). A twofold increase in hydrogen production is also reported in C. reinhardtii by downregulation of three major proteins of light harvesting complex II (LHC II), i.e. LHC MB1, 2 and 3 with the use of RNAi constructs (Oey et al. 2013). Similarly, D1 mutant of Chlamydomonas sp. with non-functional photosystem II has exhibited a substantial improvement in hydrogen production rate in the transgenic strain (Scoma et al. 2012; Oey et al. 2016). It was also reported that the hydrogen production in microalgae can also be enhanced by modifying (locking) the electron transport chain and by lowering the cyclic electron transport thereby reducing the competition for electron with the hydrogenases (Kruse et al. 2005a; Tolleter et al. 2011; Oey et al. 2016).
Further, the use of latest gene editing technologies such as transcription activator-like effector nucleases (TALENs), zinc finger nucleases (ZFN) and CRISPER/Cas systems could offer specific and lasting gene editing (Cho et al. 2013; Sizova et al. 2013; Gao et al. 2015; Oey et al. 2016). In the recent past, immobilization of algal cells has shown excellent potential in biotechnological industry including biohydrogen production from microalgae. An immobilized wild type and tla1 sulphur-deprived mutant strain (truncated antenna) of C. reinhardtii on alginate films could produce higher hydrogen gas for a longer period (over 250 h) (Kosourov et al. (2011). More recently, several studies have also underlined the possibility of using microalgae to perform photoheterotrophic degradation of organic acid-rich dark fermentation effluent (Zhang et al. 2014).
9 Conclusions
Microalgae are regarded as a cheaper and viable source of biohydrogen production compared to other biomass based fuels. Biohydrogen production from microalgae can be achieved by various means such as biophotolysis and photofermentation. Though much progress has been made at the in vitro level, commercialization of this technology is far from real. This may require the integration of advanced engineering (next generation reactor configurations) and biotechnological approaches (genetic engineering). Though researchers were successful in integrating or modifying a specific challenge or character, scale up of the process may require inclusion of multiple characters into a single microalgal strain. Inclusion of all the required traits into a single microalgal strain for continuous biohydrogen production is still one of the biggest challenges that limit its commercialization. Advances in metabolic engineering may play a major role in development of sustainable substitutes for the long-term biohydrogen production such as simultaneous wastewater treatment and biohydrogen production using microalgae in the near future.
References
Amos W (2004) Updated cost analysis of photobiological hydrogen production from Chlamydomonas reinhardtii green algae, NREL/MP-560-35593. National Renewable Energy Laboratory, Golden
Antal T, Lindblad P (2005) Production of H2 by sulphur-deprived cells of the unicellular cyanobacteria Gloeocapsa alpicola and Synechocystis sp. PCC 6803 during dark incubation with methane or at various extracellular pH. J Appl Microbiol 98:114–120
Asada Y, Kawamura S (1986) Screening for cyanobacteria that evolve molecular hydrogen under dark and anaerobic conditions. J Ferment Bioeng 64:553–556
Bélafi-Bakó K, Búcsú D, Pientka Z, Bálint B, Herbel Z, Kovács K, Wessling M (2006) Integration of biohydrogen fermentation and gas separation processes to recover and enrich hydrogen. Int J Hydro Energy 31:1490–1495
Benemann J (1996) Hydrogen biotechnology: progress and prospects. Nat Biotechnol 14:1101–1103
Beckmann J, Lehr F, Finazzi G, Hankamer B, Posten C, Wobbe L, Kruse O (2009) Improvement of light to biomass conversion by de-regulation of light-harvesting protein translation in Chlamydomonas reinhardtii. J Biotechnol 142:70–77
Bishop M, Bishop C (1987) Photosynthesis and carbon dioxide fixation. J Chem Educ 64:302–305
Cao H, Zang L, Melis A (2001) Bioenergetic and metabolic processes for survival of sulfur deprived Dunaliella salina (Chlorophyta). J Appl Phycol 13:25–34
Cho SW, Kim S, Kim JM, Kim JS (2013) Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotech 31:230–232
Dauvillée V, Chochois M, Steup S, Haebel N, Eckermann G, Ritte J et al (2006) Plastidial phosphorylase is required for normal starch synthesis in Chlamydomonas reinhardtii. Plant J 48:274–285
Doebbe A, Rupprecht J, Beckmann J, Mussgnug JH, Hallmann A, Hankamer B, Kruse O (2007) Functional integration of the HUP1 hexose symporter gene into the genome of C. reinhardtii: impacts on biological H2 production. J Biotechnol 131:27–33
Dubini A, Ghirardi ML (2014) Engineering photosynthetic organisms for the production of biohydrogen. Photosynth Res 123:241–253
Fascetti E, Todini O (1995) Rhodobacter sphaeroids RV cultivation and hydrogen production in a one and two stage chemostat. Appl Microbiol Biotechnol 44:300–305
Flores E, Frias J, Rubio L, Herrero A (2005) Photosynthetic nitrate assimilation in cyanobacteria. Photosynth Res 83:117–133
Florin L, Tsokoglou A, Happe T (2001) A novel type of iron hydrogenase in the green alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain. J Biol Chem 276:6125–6132
Gaffron H, Rubin J (1942) Fermentative and photochemical production of hydrogen in algae. J Gen Physiol 26:219–240
Gao H, Wang Y, Fei X, Wright DA, Spalding MH (2015) Expression activation and functional analysis of HLA3, a putative inorganic carbon transporter in Chlamydomonas reinhardtii. Plant J. 82
Gfeller R, Gibbs M (1984) Fermentative metabolism of chlamydomonas reinhardtii. Plant Physiol 75:212–218
Gimpel JA, Henríquez V, Stephen PM (2015) In metabolic engineering of eukaryotic microalgae: potential and challenges come with great diversity front. Microbiology 6:1376
Greenbaum E, Lee J, Tevault C, Blankinship S, Mets L (1995) Correction CO2 fixation and photoevolution of H2 and O2 in a mutant of Chlamydomonas lacking photosystem I. Nature 376:438–441
Hena S (2016) Hydrogen Production by Microalgae, in Recent Advances in Microalgal. Biotechnology, 731 Gull Ave, Foster City, CA 94404, USA, OMICS Group eBooks 1–11
Hwang JH, Kim HC, Choi JA, Abou Shanab R, Dempsey BA, Regan JM et al (2014) Photoautotrophic hydrogen production by eukaryotic microalgae under aerobic conditions. Nat Commun 5:3234
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials: review. Enzyme Microb Technol 38:569–582
Koku H, Eroglu I, Gunduz U, Yucel M, Turker L (2003) Kinetics of biohydrogen production by the photosynthetic bacterium Rhodobacter spheroids. Int J Hydrog Energ 28:381–388
Kosourov S, Makarova V, Fedorov AS, Tsygankov A, Seibert M, Ghirardi ML (2005) The effect of sulfur re-addition on H2 photoproduction by sulfur-deprived green algae. Photosynth Res 85:295–305
Kosourov SN, Ml G, Seibert M (2011) A truncated antenna mutant of Chlamydomonas reinhardtii can produce more hydrogen than the parental strain. Int J Hydrog Energy 36(3):2044–2048
Kruse O, Rupprecht J, Bader KP, Thomas-Hall S, Schenk PM, Finazzi G, Hankamer B (2005a) Improved photobiological H2 production in engineered green algal cells. J Biol Chem 280:34170–34177
Lin HD, Liu BH, Kuo TT, Tsai HC, Feng TY, Huang CC et al (2013) Knockdown of PsbO leads to induction of HydA and production of photobiological H2 in the green alga Chlorella sp DT. Bioresour Technol 143:154–162
Llama M, Serra J, Rao K, Hall D (1979) Isolation and characterization of the hydrogenase activity from the non-heterocystous cyanobacterium Spirulina maxima. FEBS Lett 98:342–346
McCully, McKinlay J (2016) Disrupting Calvin cycle phosphoribulokinase activity in Rhodopseudomonas palustris increases the H2 yield and specific production rate proportionately. Int J Hydrog Energy 41:4143–4149
Miura Y (1995) Hydrogen production by biophotolysis based on microalgal photosynthesis. Process Biochem 30:1–7
Miura Y, Akano T, Fukatsu KH, Miyasaka K, Mizoguchi T et al (1997) Stably sustained hydrogen production by biophotolysis in natural day/night cycle. Energy Convers Manag 38:S533–S537
Miura Y, Ohta S, Mano M, Miyamoto K (1986) Isolation and characterization of a unicellular marine green alga exhibiting high activity in dark hydrogen production. Agric Biol Chem 50:2837–2844
Miyamoto I, Hallenbeck P, Benemann J (1979) Hydrogen production by the thermophilic alga Mastigocladus laminosus: effects of nitrogen, temperature, and inhibition of photosynthesis. Appl Environ Microbiol 28:440–446
Nasr M, Tawfik A, Ookawara S Suzuki M (2013a) Prediction of hydrogen production using artificial neural network, in Seventeenth International Water Technology Conference, IWTC17, stanbul, 5–7 Nov 2013
Nasr M, Tawfik A, Ookawara S, Suzuki M (2013b) Biological hydrogen production from starch wastewater using a novel up-flow anaerobic staged reactor. Bio. Resources 8:4951–4968
Nasr M, Tawfik A, Ookawara S, Suzuki M (2013c) Environmental and economic aspects of hydrogen and methane production from starch wastewater industry. J Water Environ Technol 11:463–475
Nasr M, Tawfik A, Ookawara S, Suzuki M (2013d) Hydrogen production from starch wastewater using anaerobic sludge immobilized on maghemite nanoparticle, in Seventeenth International Water Technology Conference, IWTC17, Istanbul, 5–7 Nov 2013
Nasr M, Tawfik A, Ookawara S, Suzuki M (2014a) Prediction of hydrogen production from starch wastewater using artificial neural networks. Int Water Technol J 1:36–44
Nasr M, Tawfik A, Ookawara S, Suzuki M, Kumari S, Bux F (2015) Continuous biohydrogen production from starch wastewater via sequential dark-photo fermentation with emphasize on maghemite nanoparticles. J Indus Eng Chem 21:500–506
Nasr M, Tawfik A, Suzuki M, Ookawara S (2014b) Mathematical modeling of bio-hydrogen production from starch wastewater via up-flow anaerobic staged reactor. Desalin Water Treat 52:1–9
Oey M, Ross IL, Stephen E, Steinbeck J, Wolf J, Radzun KA, Kügler J et al (2013) RNAi knock-down of LHCBM1, 2 and 3 increases photosynthetic H2 production efficiency of the green alga Chlamydomonas reinhardtii. PLoS One 8, e61375
Oey M, Sawyer AL, Ross IL, Hankamer B (2016a) Challenges and opportunities for hydrogen production from microalgae. Plant Biotechnol J 14:1487–1499
Oh Y, Scol E, Kim M, Park S (2004) Photoproduction of hydrogen from acetate by a chemoheterotrophic bacterium Rhodopseudomonas palustris P 4. Int J Hydrog Energy 29:1115–1121
Oldroyd G, Dixon R (2014) Biotechnological solutions to the nitrogen problem Current Opin. Biotechnology 26:19–24
Pinto TS, Malcata FX, Arrabac JD, Silva JM, Spreitzer RJ, Esquıvel MG (2013) Rubisco mutants of Chlamydomonas reinhardtii enhance photosynthetic hydrogen production. Appl Microbiol Biotechnol 97:5635–5643
Prince R, Kheshgi H (2005) The photobiological production of hydrogen: potential efficiency and effectiveness as a renewable fuel. Crit Rev Microbiol 31:19–31
Ruehle T, Hemschemeier A, Melis A, Happe T (2008) A novel screening protocol for the isolation of hydrogen producing Chlamydomonas reinhardtii strains. BMC Plant Biol 8:107
Schnackenberg J, Ikemoto H, Miyachi S (1996) Photosynthesis and hydrogen evolution under stress conditions in a CO2-tolerant marine green alga, Chlorococcum littorale. J Photochem Photobiol B Biol 34:59–62
Scoma A, Krawietz D, Faraloni C, Giannelli L, Happe T, Torzillo G (2012) Sustained H2 production in a Chlamydomonas reinhardtii D1 protein mutant. J Biotechnol 157:613–619
Singh N, Sonani R, Rastogi R, Madamwar D (2015) The phycobilisomes: an early requisite for efficient photosynthesis in cyanobacteria. EXCLI J:268–289
Sizova I, Greiner A, Awasthi M, Kateriya S, Hegemann P (2013) Nuclear gene targeting in Chlamydomonas using engineered zinc-finger nucleases. Plant J 73:873–882
Tiwari A, Pandey A (2012) Cyanobacterial hydrogen production–a step towards clean environment: review. Int J Hydrog Energy 37:139–150
Tolleter D, Ghysels B, Alric J, Petroutsos D, Tolstygina I, Krawietz D, Happe T et al (2011) Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 23:2619–2630
Weissman J, Benemann J (1977) Hydrogen production by nitrogen-starved cultures of Anabaena cylindrical. Appl Environ Microbiol 33:123–131
Winkler M, Heil B, Heil B, Happe T (2002) Isolation and molecular characterization of the [Fe]-hydrogenase from the unicellular green alga Chlorella fusca. Biochim Biophys Acta 1576:330–334
Wu S, Xu L, Huang R, Wang Q (2011) Improved biohydrogen production with an expression of codon-optimized hem H and lba genes in the chloroplast of Chlamydomonas reinhardtii. Bioresour Technol 102:2610–2616
Yokoi H, Mori S, Hirose J, Hayashi S, Takasaki Y (1998) H2 production from starch by mixed culture of Clostridium butyricum and Rhodobacter sp M-19. Biotechnol Lett 20:895–899
Zhang X, Rong J, Chen H, He C, Wang Q (2014) Current status and outlook in the application of microalgae in biodiesel production and environmental protection. Front Energy Res 2:1–15
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kumari, S., Nasr, M., Kumar, S. (2017). Technological Advances in Biohydrogen Production from Microalgae. In: Gupta, S., Malik, A., Bux, F. (eds) Algal Biofuels. Springer, Cham. https://doi.org/10.1007/978-3-319-51010-1_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-51010-1_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51009-5
Online ISBN: 978-3-319-51010-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)