Abstract
The earliest studies on the firing properties of hippocampal neurons revealed coding of both spatial and non-spatial dimensions of experience. Since then, distinct lines of investigation have elaborated these findings to provide compelling evidence that the hippocampal neurons represent the events we remember within spatial as well as temporal frameworks. This characterization suggests that neural networks in the hippocampus underlie a “memory space” that organizes the features of memory dependent on hippocampal function.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
A comprehensive understanding of the hippocampus requires identifying the nature of information encoded by its information processing elements combined with interpretation of the overall network representations that underlie cognitive and memory functions. Here I will attempt an overview of our knowledge about information processing by hippocampal neurons and networks. This will not be a comprehensive review—there have been several recent collections that survey the firing properties of hippocampal neurons in behaving animals and humans (Hartley et al. 2013; Mizumori 2007; Derdikman and Knierim 2014). Rather, here I will provide examples of the broad variety of hippocampal coding properties and attempt a synthesis of what these findings tell us about single neuron and network coding mechanisms that underlie memory representations.
Ancient History: The Early Studies on Firing Patterns of Hippocampal Neurons in Behaving Animals
In the early 1970s, several investigators adopted newly developed methods using single sharp electrodes or bundles of small-diameter flexible wires to record the activity of principal neurons in the hippocampus. Their studies pre-dated the advent of digitized recordings and computerized data analysis, and so depended on human observation to correlate auditory artifacts of neuronal spiking with ongoing behavior or simple automated averaging of spiking over time to compute firing rates time-locked to specific stimuli. These papers identified both spatial and non-spatial correlates of hippocampal neural activity that we still struggle to reconcile today.
The first of these publications was a short communication by O’Keefe and Dostrovsky (1971) that described the firing properties of neurons recorded from the dorsal hippocampus in rats using sharp electrodes as they moved through or were positioned within an open field environment. They focused on the activity patterns of eight hippocampal neurons that fired solely or maximally when a rat was in a particular part of the open field. The activity of most of these cells was also dependent on specific sensory stimuli (e.g. a tactile or visual stimulus) and the direction of orientation within the environment. The more extensive follow-up study by O’Keefe (1976) described many more hippocampal cells whose activity was dependent on spatial location and emphasized a distinction between “place cells” that fired when the rat occupied or ran past a particular location and “misplace cells” whose spatially specific activity depended on exploratory sniffing, usually when the rat did not find an expected object at the location. So, while these firing patterns were immediately interpreted as supporting the idea that hippocampal neurons map space, the data were equally clear that hippocampal neuronal firing patterns also encoded specific stimuli, behaviors, and cognitive states.
Quite independently, and around the same time, James Olds and his colleagues recorded from single neurons using fine wire electrodes positioned in various brain areas. They established an approach to identifying “learning centers” in the rat brain defined as areas where neurons developed short-latency responses time locked to stimuli (tones) as animals were classically conditioned to expect food delivery following the tones (Olds et al. 1972). Using this paradigm they identified neuronal responses to the conditioned tone observed throughout the hippocampus (Segal and Olds 1972). In these studies no effort was employed to control or determine the location of the animal within the small conditioning chamber. However, typically the neurons did not respond to the tones or reward delivery during a preliminary pseudo-conditioning session, suggesting that the stimulus-driven responses depended specifically on the learned association and not solely other aspects of sensory experience, behavior, or location.
In 1973 James Ranck published an extensive analysis of hippocampal neuron firing patterns observed in rats performing a variety of behaviors in an open field, including eating, drinking, grooming, being held, bar pressing, and sleeping. He observed correlations between neural activity and ongoing behavior in almost all hippocampal neurons, and reported that no two principal cells had the identical behavioral correlate. Four main types emerged from his analysis: “approach-consummate cells” that fired during the approach to and consumption of food, “approach-consummate mismatch cells” that fired similarly during approach and also during exploration of a missing water bottle (like O’Keefe’s misplace cells), “appetitive cells” that fired during orienting movements and approach but not consummatory behavior, and “motion-punctuate cells” that fired at the end of orienting movements or change in direction of movement. No effort was made to control for spatial location in this study, and Ranck acknowledged that, “perhaps spatial characteristics are the entire basis of firing in these cells” (Ranck 1973). However, the distinctions between the different behavioral correlates of these cell types seems unlikely explained purely by differences in where the behaviors occurred.
Finally, Theodore Berger, Richard Thompson, and their colleagues recorded multi-units and single neurons in the hippocampus of rabbits undergoing tone-cued classical eye-blink conditioning (Berger et al. 1976, 1983). They reported the emergence of tone-evoked conditioned responses of hippocampal neurons that paralleled both success in learning across trials and the time course of the conditioned eye-blink within trials. In these studies position within space was strictly controlled in that the animals were immobilized within a restraining device throughout learning. Thus the learning and behavioral correlates of conditioned eye-blinks cannot be attributed to spatial coding.
In many ways these early observations already provided insights into the broad scope of information that is encoded by hippocampal neural activity patterns that are evident in current studies. Place is a major determinant of the firing patterns of hippocampal neurons in animals that freely move through the environment. This property of hippocampal neurons was recognized in the awarding of the 2015 Nobel Prize to O’Keefe, who discovered the spatial firing patterns of hippocampal neurons. However, differences in spatial location do not account fully for firing patterns of many neurons, such as the misplace/mismatch neurons of O’Keefe and Ranck suggestive of additional correlates of cognitive and memory function. In addition, the coding of specific sensory stimuli was implicated in O’Keefe’s original study and more systematically in Olds and colleagues’ observations on conditioned neural responses. And, just as Ranck’s observations are strongly suggestive that specific actions (e.g., approach behavior) seem to play some role, the findings of conditioned eye-blink related responses by Berger & Thompson strongly indicate that learned actions are encoded by hippocampal neurons in immobilized animals where location cannot explain the neural firing patterns.
Subsequent work on hippocampal neuron firing patterns in behaving animals and humans has expanded in four main directions. First, many studies have explored the spatial firing properties of hippocampal neurons, identifying cues that control, as well as other factors that modulate, spatial firing patterns. Second, many other studies have explored how learning of non-spatial information or actions is encoded by hippocampal neurons, along with or independent of spatial information. Third, recent evidence has indicated that hippocampal neurons encode time much like they encode space, suggesting a parallel dimension for mapping experiences. Fourth, another new direction involves explorations of how hippocampal neuronal ensembles integrate representations of multiple related experiences into networks of memories (also called “schemas”). These directions will be examined in turn. As you read this review, note that, while the coding of position in space has received the greatest attention in this literature, there is considerable evidence that position coding is often subordinate to other abstract features (the “context”) of a behavioral task, and the finding of robust temporal coding indicates that space may be only one of the dimensions employed by hippocampal networks to organize memories.
Spatial Coding by Hippocampal Neurons
As can been deduced from the early observations, a mixture of spatial and non-spatial parameters influences hippocampal neural activity. In particular, it is clear that non-spatial events must be considered because of the findings on classical eyelid conditioning that show coding of learned behavior when space is held constant. Thus, when animals are freely moving in space, it might be that overt or subtle distinctions in ongoing perception, behavior, or cognition are confounded with, and drive the observation of position correlates of hippocampal neurons.
This issue was addressed by Olton et al. (1978) who identified clear place fields of hippocampal neurons in rats performing a task where they traversed the arms of an 8-arm radial maze and were required to remember visited arms. Despite the behavioral sequence being identical on all maze arms, many hippocampal neurons fired as the animal ran through particular locations on only one or a few of the arms, thus distinguishing the spatial correlate on some arms from the absence of activity during matched behavior on all arms. Another way the issue was addressed employed a clever behavioral paradigm created by Muller and colleagues (1987) that involved recording from hippocampal neurons as rats foraged for small bits of food dropped within an open field. The aim of this approach was to control for potential behavioral influences by testing whether a position correlate would emerge in a situation where foraging behavior is constant over all locations in the environment, thereby experimentally “subtracting” its influence. The results were striking: many hippocampal neurons had clear-cut place fields during random foraging in an open field. The observation of strong position coding when behavior is constant, involving either continuous foraging throughout a two-dimensional open field or identical movement sequences through linear tracks or mazes, have been replicated many times.
Variants of these linear maze and open field paradigms have been employed to characterize the sensory cues that determine position coding by hippocampal neurons. These findings can be summarized as follows. Nearly all of our information on hippocampal neuronal firing patterns comes from data on CA1 and CA3 pyramidal cells in the dorsal hippocampus of rats and to some degree in mice, monkeys, and humans (see Muller 1996; Eichenbaum et al. 1999, for more detailed reviews). As the animal explores or merely traverses a large environment, one can readily correlate dramatic increases in a cell’s firing rate when the rat arrives at a particular location, called the “place field”, and these cells are called “place cells”. From a baseline of less than 1 spikes/s, the firing rate can exceed 100 Hz, although during some passes through the place field the cell may not fire at all. Typically a large fraction of cells, perhaps 40–75%, have place fields in any environment, although the low baseline firing rates may let many cells without place fields go undetected. Place fields vary in size from quite small to half the size of an environment and are dispersed throughout the environment, although they may be concentrated at areas of particular salience such as where rewards occur (e.g., Hollup et al. 2001; McKenzie et al. 2013). In most of the environments used to date, most hippocampal cells have only one or two place fields, although in large environments they can have many place fields (Rich et al. 2014).
Sensory Cues That Govern the Spatial Firing Patterns of Place Cells
Many studies have focused on identifying the environmental cues that drive spatially specific activity. O’Keefe (1979) defined place cells as neurons whose activity is not dependent on any particular stimulus, but rather reflects the presence and topography of multiple environmental cues. Several studies have shown that a variety of visual and nonvisual cues can determine the location of place fields (e.g., Hill and Best 1981; Muller et al. 1987; Save et al. 2000; Gener et al. 2013; but see Cressant et al. 1997). O’Keefe and Conway (1978) performed the first study where multiple spatial cues were provided and then manipulated to determine which cues controlled spatial representations, and found that some cells were controlled by only one or two of the cues and others by any subset of the cues. More recent studies indicate that place cells are driven by relatively few relatively proximal cues. O’Keefe and Burgess (1996) showed that the shape and locus of most place fields within a simple rectangular chamber are determined by the dimensions of, and spatial relations between, only a few nearby walls of the environment (see also Hetherington and Shapiro 1997). Several other studies have shown that place cells can encode subsets of the spatial cues and that these representations are independent of the spatial representations of other cells in the same environment. Shapiro, Tanila, and colleagues (Shapiro et al. 1997; Tanila et al. 1997a, b, c) and Knierim (2002) examined the responses of hippocampal cells to systematic manipulations of a large set of spatial cues, including both distant cues outside a maze and proximal cues on the floors of maze arms. Different place cells encoded individual proximal and distant stimuli, combinations of proximal or distant stimuli, or relations between proximal and distant cues. The place fields of some cells were fully controlled by as little as a single cue within a very complex environment, and most cells were controlled by different subsets of the controlled cues. More recently Leutgeb et al. (2005) examined firing patterns of hippocampal neurons as rats explored multiple small environments (boxes) within multiple large environments (rooms) and reported that whether or not place cells fire and the locations of place fields depend on distant (“global”) cues that lie outside of the small environment, whereas the firing rate, but not location of place fields depends on proximal cues (called “rate coding”). However, when distant cues are minimized, place fields can be entirely determined by local cues (Young et al. 1994; Hetherington and Shapiro 1997).
Not Necessarily Location Per Se: Length and Distance
Place fields do not necessarily represent specific locations but rather can reflect continuous spatial dimensions of length and distance. O’Keefe and Burgess (1996) recorded from rats as they foraged in rectangular chambers whose walls varied in length. They found that place fields stretch along a wall of an environment that is elongated, indicating that when environmental cues are continuously variable, place cells represent spatial dimensions continuously. Gothard et al. (1996a, b) found that when a particularly salient cue or enclosure within an open field is moved repeatedly and randomly, the spatial firing patterns of some cells become tied to that cue. When rats were trained to shuttle between a mobile starting box and a goal location defined by landmarks in an open field, some cells fired relative to the static environmental cues, whereas others fired relative to a landmark-defined goal site, or in relation to the start box. When rats were trained to shuttle between a movable start-end box and goal site on a linear track, the anchor of the spatial representation of many cells switched between these two cues, depending on which was closer. Under these conditions the majority of the activated hippocampal cells did not exhibit location-specific activity that was associated with fixed environmental cues. Instead, their activity could be characterized as “spatial” only to the extent that they fired at specific distances from a particular stimulus or goal. Distance coding has also been observed in rats running on a treadmill where external spatial cues signaling motion are absent (Kraus et al. 2013) and in a task where spatial cues are variable and distance provides salient information about location (Ravassard et al. 2013; Aghajan et al. 2015).
Place Cells Encode Both the Similarities and Differences Between Environments That Share Spatial Features
Several studies have shown that place cells are not linked together to form a cohesive map of the environment. Tanila et al. (1997b) found that ensembles of simultaneously recorded place cells changed their firing patterns independently associated with distinct subsets of the cues, indicating that the spatial representation was not cohesive but instead coded for spatial cues that were common to and distinct in multiple environments. In several cases where two cells had overlapping place fields associated with one configuration of the cues, each cell responded differently when the same cues were rearranged. This finding shows that each cell was controlled by a different subset of the cues at the same time, and that their differential encodings are not due to shifts between two different spatial “reference frames” used by all cells at different times (Gothard et al. 1996b). Skaggs and McNaughton (1998) confirmed this finding by recording from a large number of place cells simultaneously in rats foraging randomly in two identical enclosures, between which they could move freely. Each hippocampal ensemble contained cells that had similar place fields and others that had distinct spatial firing patterns between the two enclosures. In this situation, some cells encoded the physical cues, whereas the activity of others at the same time reflected the knowledge that the two environments were distinct.
Spatial Representations Are Context Dependent
One view of place cells is that they compose a representation of the context in which specific events occur. What constitutes a “context”, as opposed to a set of individual cues is not clear, and whether its domain includes spatial and temporal, as well as other aspects of the situation in which events occur is also not clear. The data suggests that all aspects of the background context in which specific events occur and when places are occupied can dramatically affect hippocampal neural activity. For example, the spatial firing patterns, and the extent to which firing is dependent on spatial orientation, are dramatically different when a rat forages randomly or produces repeated paths as it traverses the identical environment (Markus et al. 1995). Similarly, when different starting points in a radial maze determined the locations of goals, the firing patterns of place cells changed dramatically (Smith and Mizumori 2006). Notably, some places cells fire similarly in the two situational contexts whereas others change dramatically—showing that the hippocampus represents both the commonalities and differences in the two context-defined situations.
Seemingly subtle changes in environmental cues can also produce dramatic changes in the spatial firing patterns of hippocampal neurons. For example, changes in the background color or background odor of an environment can dramatically change the spatial firing patterns of individual hippocampal neurons (Anderson and Jeffrey 2003). Notably, again some cells do not change for each contextual shift, whereas others do. What cues and the extent of situational change that causes changes in firing patterns is not clear, but several studies have examined the dynamics of firing pattern changes when cues are gradually altered. When the shape of an environment is gradually altered (Wills et al. 2005), or critical cues are gradually changed (Rotenberg and Muller 1997), most place cells do not alter their firing patterns initially, but at some level of change, dramatically alter their firing patterns. This sudden switch of firing patterns when a threshold of cue alteration is passed suggests an attractor state dynamic (not unlike that of many other brain areas) in which the contextual representation switches from pattern completion to pattern separation. Area CA3 demonstrates a particularly sharp discrimination gradient in making this switch (Leutgeb et al. 2004; Lee et al. 2004). It appears that hippocampal cell assemblies can rapidly switch between spatial representations as animals perform different tasks within the same environment (Fenton et al. 1998; Jackson and Redish 2007).
Spatial firing patterns can also dramatically change when the affective association of a constant spatial environment is altered. Several studies have reported major alternations in hippocampal spatial representations of previously neutral environments when a rat is shocked in the environment, thus altering the meaning of the environment to evoke fear (Moita et al. 2004; Wang et al. 2012) or vice versa (Wang et al. 2015).
Several other recent studies have focused on changes in context defined by the behavioral demands of a task. In several of these studies, rats alternate routes that involve left and right turns through a T-maze where they traverse a part of the maze that is common to both routes. In this and similar tasks, many hippocampal neurons have distinct firing patterns, even when the rat traverses the common maze area depending on whether the rat is performing a left-turn or right-turn trial (Wood et al. 2000; Frank et al. 2000; Ferbinteanu and Shapiro 2003; Ainge et al. 2007; Bower et al. 2005; Lee et al. 2006; Griffin et al. 2007; reviewed in Shapiro et al. 2006). Importantly, some cells fire similarly as the rat performs both routes, indicating the hippocampus represents both the distinct paths and the common elements among them. Furthermore, the distinct firing patterns of place cells predict success in the alternation task (Robitsek et al. 2013). Also, the same pattern of findings occurs when the choice of different goals is guided by motivational context (hunger or thirst), indicating that the distinctions in firing patterns are not due to the accumulated movements (i.e., path integration) prior to the overlapping segment of the maze, but rather to the cognitive state associated with different routes through the maze (Kennedy and Shapiro 2009). A recent extension on these findings showed that, when the alternation task is separated into distinct sample and choice phases, most hippocampal neurons have different spatial firing patterns in the distinct trial phases, and within that, some cells also differentiate the two routes within each phase (Griffin et al. 2007). These data are consistent with other findings discussed above showing that different cognitive states within a single overall behavioral task are represented distinctly and linked by representations of their common features by hippocampal neurons.
Finally, new findings suggest that the ventral hippocampus, not examined in the studies described above, may represent large scale space that constitutes a meaningful spatial “context”. Kjelstrup et al. (2008) compared the sizes of place fields in the dorsal and ventral hippocampus and found that place fields become larger as one records along the dorsal to ventral portions of the hippocampus. More recently, Komorowski et al. (2013) also recorded along this axis as rats performed a task where they were required to employ their current spatial context (one of two chambers) to remember which of two objects contained a reward, and found that ventral hippocampal neurons had large place fields, many of which filled most of all of one of the contexts. However, these fields never bridged between contexts in animals successfully performing the task, suggesting that ventral hippocampal networks code for representations of spatial and meaningful contexts.
Where the Rat “Thinks” It Is
Notably, the spatial activity patterns of place cells may be more determined by where the rat may “think” it is rather than being explicitly driven by spatial cues. This possibility is consistent with the observation that the spatial firing patterns of place cells can persist even when all of the spatial cues are removed or the room is darkened (O’Keefe and Speakman 1987; Muller and Kubie 1987; Quirk et al. 1990), although the selectivity of spatial firing may be degraded in the dark (Markus et al. 1994). Also, the findings discussed above showing that place cells form categorical representations even in circumstances of ambiguous spatial cues (Skaggs and McNaughton 1998) or continuously changing spatial cues (Leutgeb et al. 2004), indicates that the animal’s perspective on where it is can dominate over the actual spatial cues. Also, when a rat is first introduced into a new environment, place cells may continue firing associated with the cues of a former highly experienced environment, and then suddenly “re-map” after successive exposures (Bostock et al. 1991; see also Sharp et al. 1990). In a direct test of whether the animal’s conception of its location can govern place cell activity, O’Keefe and Speakman (1987) tested rats in a task where they had to remember where removed spatial cues had been. They found that errors in their choice behavior predicted shifts of their hippocampal place fields, suggesting that these codings were determined by the orientation of the maze remembered by the rat, thus providing a compelling link between hippocampal spatial coding and spatial memory but also showing that place cells reflect an internal representation of space rather than a representation that depends on external cues.
Direction of Movement Influences Place Cells When Movements Through Space Are Meaningfully Directional
According to O’Keefe (1979) true place cells fire whenever an animal is in the place field, regardless of its orientation or ongoing behavior. However, the only situation where large numbers of true place cells are observed is when animals forage by random walk through an environment, where behavior is held constant and the meaning of movement directions is homogeneous. However, in contrast to this open field foraging, in virtually any situation where movement directions are meaningfully different, distinct movement directions influence spatially specific activity. For example, in the radial maze task where animals regularly perform runs outward on each maze arm to obtain a reward, and then return to the central platform to initiate the next choice, outward and inward arm movements reflect meaningfully distinct behavioral episodes that occur repetitively. Correspondingly, hippocampal neurons reflect the relevant “directional structure” imposed by this protocol, and almost all place cells fire only during outward or inward journeys (McNaughton et al. 1983), and directionality is also observed when animals perform the same task in an open field, indicating that directionality is not due to the constraints of location by walls of the arms on a radial maze (Weiner et al. 1989). Similarly, place cells are activated selectively during distinct approach and return episodes and from variable goal and start locations in open fields and linear tracks. Furthermore, Muller et al. (1994) showed that the same place cells that are non-directional during random foraging are highly directional in a radial maze. Most impressively, Markus et al. (1995) directly compared the directionality of place cells under different task demands, and found that place cells that were non-directional when rats foraged randomly in an open field, were directional when they systematically visited a small number of reward locations. Taken together, these findings emphasize that place cells exhibit movement-related firing patterns whenever particular movements are associated with meaningfully different events. Also, directionality of place fields is obtained only following experience in directional movements (Navratilova et al. 2012).
Conclusions About Spatial Coding in Hippocampal Neurons
The phenomenon of place cells in freely moving animals is highly robust and observed both in situations where the hippocampus is necessary for memory performance (e.g., the radial maze) and where it is not (foraging for food in an open field). A broad variety of individual spatial and non-spatial cues and cognitive states can drive or strongly influence place cells, so they do not provide a simple cohesive map of coordinate locations within a space defined by geometric relations among spatial cues as O’Keefe (1979) originally envisioned. On the other hand, perhaps the most straightforward explanation of place cells is that they reflect where an animal “thinks” it is in space as well as where it “thinks” it is going. This view is consistent with the notion that the hippocampal representation of space is “cognitive” as opposed to stimulus driven. A critical remaining question is whether the function of this cognitive map of space is dedicated to navigation, as some have suggested (McNaughton et al. 1996, 2006; Moser et al. 2008; Hartley et al. 2013) or whether the purpose of the map is to represent where events occur in spatial context, as has been suggested by recent studies on humans and animals (Eichenbaum et al. 2007; Davachi 2006; Diana et al. 2007). Much of the evidence that place cells are components of a dedicated spatial mapping system rest on the observation that hippocampal cells (and other cells in neighboring regions) can encode spatial parameters (location, head direction, borders, distance traveled; Hartley et al. 2013), but these findings may well just reflect the relevant dimensions of specific experiences that are dominated by spatial dimensions and lack non-spatial stimuli and behavioral demands. Deciding between these views rests instead on the extent to which hippocampal neurons encode specific stimuli, behavioral actions, and non-spatial cognitive events that fall outside the domain of spatial navigation and instead are consistent with a spatial framework for memories.
Representation of Stimuli, Behavioral Actions, and Cognitive States Independent of, or Along With Position
The Berger & Thompson studies described above indicate that hippocampal neurons can have clear learning and behavioral correlates in animals entirely restrained within a specific location. However, it may well be that space still plays a role even in this highly controlled task, because the same behavior related firing pattern may depend upon the location where conditioning occurs, as does the behavior in this kind of classical conditioning (Penick and Solomon 1991). To address this possibility, many studies employed learning and memory tasks where explicitly distinct sensory or behavioral events occur in multiple positions in an environment, with the aim of distinguishing the extent to which firing patterns are dependent on the nature of the event, on where it occurs, or both. These studies have revealed that hippocampal neuronal firing patterns distinguish both the different events and the positions and spatial contexts where they occur.
Sensory Driven Responses
Many studies in rodents, monkeys, and humans have described hippocampal neuronal activity associated with a very broad range of non-spatial stimuli and behavioral events. In rodents, many studies have observed robust activation of hippocampal neurons associated with visual, tactile, olfactory, and auditory cues in several learning and memory paradigms (reviewed in Eichenbaum et al. 1999; Eichenbaum 2004). These findings join with many other reports of robust activation of hippocampal neurons associated with combinations of specific stimuli, match/non-match stimulus comparisons, and the locations of these events in animals performing discrimination and recognition memory tasks (Eichenbaum et al. 1987; Wood et al. 1999; Wiebe and Staubli 1999; Deadwyler et al. 1995; Otto and Eichenbaum 1992; Hampson et al. 1993; Wible et al. 1986). The extent to which non-spatial and spatial cues are represented depends on the context of behavioral demands. For example, in the same environment with the same olfactory cues, hippocampal neurons strongly encode location when rewards are associated with the location of the cue, but fire associated with the odors when the odor identity is associated with reward (Muzzio et al. 2009). Similarly, Lee and Kim (2010) reported that hippocampal neuronal activity shifted from spatially determined to stimulus determined as learning about the stimuli developed. In addition, hippocampal neurons signal learned behavioral actions. Lenck-Santini et al. (2008) described hippocampal neurons that fire during learned “jump” avoidance responses, reminiscent of Ranck’s (1973) pioneering descriptions of a variety of behavioral correlates of hippocampal neurons in rats and the findings on conditioned eye-blink related responses described by Berger et al. (1976), a finding extended in recent studies on classical eye-blink conditioning (Hattori et al. 2015; McEchron and Disterhoft 1997).
Consistent with these findings in rodents, a large fraction of hippocampal neurons in head-fixed monkeys fire robustly associated with learned associations between specific visual stimuli and eye-movement responses (Wirth et al. 2003). Similarly, a large fraction of hippocampal neurons in monkeys respond to visual stimuli modulated by their familiarity in the naturalistic recognition task described above (Jutras and Buffalo 2010). Furthermore, multiple studies have reported that hippocampal neurons in humans also respond to visual stimuli and their responses are modulated by familiarity in recognition tasks (Fried et al. 1997) and distinguish the stimuli that are recalled from those forgotten (Rutishauser et al. 2008). Hippocampal neuronal responses also predict memory for learned verbal paired associates (Cameron et al. 2007). Human hippocampal neurons exhibit sparse and distributed coding of individual remembered stimuli (Wixted et al. 2014) and rapidly develop as humans learn associations between objects and locations (Ison et al. 2015), and many hippocampal neurons generalize across closely related stimuli (Quiroga et al. 2005; Krieman et al. 2000a) and fire while the subject is imagining a cued stimulus (Krieman et al. 2000b). These studies provide strong evidence that many hippocampal neurons fire associated with specific stimuli and actions when space is held constant (e.g. eye-blink conditioning) and are driven by conditioned stimuli when the animal is immobile (Olds et al. 1972; the studies in monkeys and humans).
Conjoint Sensory-Behavioral and Spatial Responses
Several other studies have shown that hippocampal neurons conjoin sensory-behavioral events and positions where they occur. The most striking of these studies also involve tracking learning about sensory stimuli and related conditioned behavioral responses. These studies show that hippocampal neuronal activation that occurs during the exploration of specific objects is embedded within the spatial firing patterns (place fields) of those neurons. For example, following tone-cued fear conditioning, hippocampal neurons come to be driven by the conditioned tone stimulus when the animal is within the place field of that neuron (Moita et al. 2003; Wang et al. 2012). Also, in rats performing a variant of the novel object exploration task, hippocampal neurons fired associated with specific objects and their familiarity embedded within the spatial firing patterns (place fields) of these neurons (Manns and Eichenbaum 2009). In rats performing a context-guided object-reward association task, hippocampal neurons fire when animals sample specific objects within particular locations and spatial contexts. In this experiment, the spatial specificity of responses occurred early and the object related activity paralleled learning to respond to different objects in only one context (Komorowski et al. 2009). Similarly, after training on somatosensory or auditory discrimination tasks, hippocampal neurons encode tactile and auditory cues along with the locations where they were experienced and rewarded (Itskov et al. 2011, 2012; Vinnik et al. 2012). This combination of studies clarifies that position-related firing precedes the adoption of stimulus or action specificity and suggests that the hippocampal network constitutes a spatial framework onto which memories of stimuli are incorporated. This conclusion is consistent with a large literature that positions the hippocampus as convergence site for streams of information processing about objects and space (reviewed in Davachi 2006; Eichenbaum et al. 2007), and suggests the mechanism for coding objects and events in space is conjunctive object and place coding by single hippocampal neurons.
Conclusions About Non-spatial Coding in Hippocampal Neurons
There is considerable evidence that a broad range of specific significant stimuli can drive hippocampal neuronal activity and that hippocampal neurons fire associated with specific learned behaviors. At the same time, however, whenever these sensory and behavioral events occur in multiple locations, these activity patterns differ across locations. Thus, sensory-behavioral responses of hippocampal neurons are embedded within a spatial framework of hippocampal representation.
Time as an Additional Framework for Encoding Memories
There is considerable recent evidence that the hippocampus is involved in representing the flow of events in time, in parallel to its representation of the organization of events in space (Eichenbaum 2013, 2014), and indeed it has been suggested that bridging between successive events to link them in time may be a fundamental function of hippocampal circuitry (Rawlins 1985; Levy 1989; Wallenstein et al. 1998; Howard et al. 2014). Consistent with this idea, hippocampal lesions impair memory for the order of sequences of events (Fortin et al. 2002; Kesner et al. 2002) and ensemble activity patterns of CA1 neurons gradually change while rats sample sequences of odors, and this signal of continuously evolving temporal context predicted success in remembering the odor sequence (Manns et al. 2007). These findings, and more discussed below, suggest that temporal coding by the hippocampus is not merely representing the passage of time, but supports representation of the order of events in experiences, which can be used to guide subsequent behavior.
Several studies have now identified hippocampal principal neurons that fire at a particular moments in time of a temporally structured event, composing temporal maps of specific experiences. Across these studies, the location of the animal is held constant or firing patterns associated with elapsed time are distinguished from those associated with spatial and behavioral variables, and the firing patterns of these cells are dependent on the critical temporal parameters that characterize the task. Because these properties parallel those of place cells in coding locations in spatially structured experiences, we called these neurons “time cells” (MacDonald et al. 2011), even though these neurons are the same cells that exhibit spatial firing specificity in other circumstances.
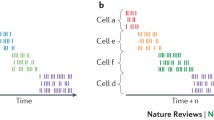
Time cells have now been observed in several experiments. Pastalkova et al. (2008) recorded from single CA1 neurons as rats performed a spatial T-maze task where alternating left-turns and right-turns, and trials were separated by a fixed period of wheel running. They were the first to report that hippocampal neurons fire reliably at specific moments during wheel running and the entire period of each wheel run was filled by a sequence of brief neuronal activations. Importantly, the firing sequences differed between trials in which the rat subsequently turned left or right—even though the rat was largely in the same location (that is, in the running wheel) and performing the same behavior (that is, running)—but they were consistent between left-turn trials and consistent between right-turn trials, suggesting that a sequence was linked to the content of the trial. Subsequently, Kraus et al. (2013) also observed time cells in rats running in place on a treadmill in between trials on a T-maze, and showed that these cells are influenced independently and conjunctively by elapsed time and distance traveled on the treadmill (Fig. 1).
Hippocampal neurons encode the flow of time while a rat runs in place. (a) Spatial alternation task in which on each trial the rat runs in place on a treadmill for 15 s. (b) Raster display, histogram, and heat plot of the time related firing pattern of four hippocampal neurons that fire at different moments during treadmill running. (c) Normalized firing rates of 23 hippocampal neurons over the course of treadmill running period. Adapted from Kraus et al. (2013)
Another study (Gill et al. 2011) examined activity patterns of CA1 neurons in rats performing a place-reversal task. In the first half of each daily session, trials began at any of three arms of a plus-maze and the rats had to go to the remaining arm to obtain a reward; in the second half of the session, another arm became the ‘reward arm’ and trials started from any of the other three arms. In between trials, rats were placed on a small platform outside the maze for several seconds. During the course of training, time-specific firing patterns emerged during the inter-trial periods, and the firing sequences differed between the two sessions. The rats could move freely during the delay, but cells that had reliable place fields were excluded from the analysis, indicating that the measured activity patterns encoded time rather than place.
In another study MacDonald et al. (2011) examined whether CA1 neurons also fired at specific moments in a non-spatial task where rats learned to associate each of two visually distinct objects with one of two cups of scented sand (Fig. 2a). On each trial, rats approached and sampled one of the two objects and, after a fixed delay, were exposed to one of the two odor cups. If the odor matched the object, the rat had to dig in the sand to retrieve a buried reward. During the delay period, individual neurons fired at successive moments that fill out the entire period, and firing patterns differed depending on which object the rats had to remember and were consistent between trials in which the same object had to be remembered. Extensive general linear model (GLM) analysis was used to distinguish activity patterns associated with the animal’s location, speed and head direction during the delay period from the time elapsed. Although these spatial and behavioral parameters contributed to the activity patterns of many of the recorded cells, the analysis also revealed a contribution of time that was independent of these variables. Furthermore, the firing patterns of many of these neurons changed (i.e. they ‘re-timed’) when the delay was increased. This happened even though the behavior and locations of the animal during the initial period did not change, indicating that the firing patterns of these cells reflected the passage of time rather than variations in behavior or place. Importantly, the cells firing later in the delay period were active for longer durations (i.e. had larger “time-fields”; also see Kraus et al. 2013, Fig. 1; MacDonald et al. 2013). This pattern suggests a scalar coding of time, which parallels a hallmark property of time judgments in humans and animals (Howard and Eichenbaum 2013). Each of these studies provided evidence for the existence of an evolving temporal signal that takes the form of a succession of briefly firing neurons.
Context-guided object memory task. (a) Rats enter either of two spatial contexts (A and B) in which they are presented with the same two object stimuli (X and Y) in either of the two positions shown. In Context A, object X contains a reward whereas in Context B, object Y contains a reward. (b) Dendrogram illustrating the relationships between representations of each type of event (x-axis) as linked (y-axis) by specific task dimensions (right). At the top of this schema, events that occur in different contexts are widely separated in representational space, indicated by anti-correlation between events that occur in different contexts, putting context as the highest superordinate dimension. Within each context-based network, events are then separated by positions within a context, i.e., positions are subordinate to contexts. Next, within positions, events are separated by different reward associations, i.e., reward association is subordinate to position. Finally, closest together in this schema are events that involve different objects that have the same reward association in the same location and context, i.e., object identity is subordinate to reward values. Adapted from McKenzie et al. (2014)
Further evidence supporting the existence of temporal signals that are independent of place or distance has come from recent studies showing time cells in head-fixed animals in which the animal’s location and behavior were kept constant and movement was eliminated. For example, in one study (MacDonald et al. 2013) rats performed an odor-cued delayed matching to sample task in which each trial began with the presentation of one of multiple sample odors for 1 s. Following a fixed delay, a test odor was presented. In order to receive a reward, the animal had to respond only to the test odor that matched the sample on that trial. We found that approximately 30 % of hippocampal cells encoded specific moments during the delay. Another study in head-fixed animals used two-photon calcium imaging to investigate the evolution of firing patterns among large ensembles of hippocampal neurons as mice underwent classical conditioning (Modi et al. 2014). On each trial, mice heard a brief tone that was followed, after a temporal gap, by an air-puff to the eye. During acquisition of the conditioned eye-blink response, CA1 cells developed time-locked firing sequences throughout the trial, including during the temporal gap.
Conclusions About Temporal Coding by Hippocampal Neurons
Time cells have been observed in a range of behavioral conditions, including during delay periods in maze tasks in which rats alternate goals (Gill et al. 2011; Pastalkova et al. 2008; Kraus et al. 2013), bridging temporal gaps between associated non-spatial cues (MacDonald et al. 2011), during the delay period in a in non-spatial matching to sample task (MacDonald et al. 2013), and throughout trials in trace eyelid conditioning (Modi et al. 2014). Importantly, in some of these studies, the animal is immobilized and thus space plays no role in ongoing behavior or memory (MacDonald et al. 2013; Modi et al. 2014; Naya and Suzuki 2011). The findings of these studies establish a broad scope of temporally structured episodes in which the hippocampus encodes the temporal organization of specific experiences. Furthermore, some of the studies in animals have closely linked the emergence of time cell sequences to the encoding of specific memories and to subsequent memory accuracy (Gill et al. 2011; Modi et al. 2014; MacDonald et al. 2013), thus indicating a causal role of time cell firing patterns to memory performance. Also, the representation of temporally ordered sequences of events by the hippocampus extends to monkeys and humans. In monkeys, hippocampal neuronal activity signals elapsed time in a memory delay between associated objects (Naya and Suzuki 2011). In humans, hippocampal neurons fire in sequence associated with learning (Paz et al. 2010) and memory (Gelbard-Sagiv et al. 2008) of the flow of events experienced in movie clips.
Combined Spatial and Temporal Coding
In addition, many studies have reported that ensembles of simultaneously recorded place cells that fire in sequential locations as animals traverse a path through a maze, subsequently also ‘replay’ the corresponding sequence of firings during ‘off-line’ periods, including sleep and quiet wakefulness when the animal is not moving through those locations (Carr et al. 2011; Karlsson & Frank 2009). Thus, spatial coding observed as rats actively run through a maze is recapitulated in temporal coded firing sequences when the rat is not moving. Disruption of these replay events impairs subsequent memory of the path (Jadhav et al. 2012). Moreover, field potentials associated with replays of sequences associated with alternative choice paths in a maze predict acquisition of learned performance (Singer et al. 2013). In addition, replay can be observed in sequential firing patterns associated with place-cell sequences that are about to occur as a rat takes a novel path in an open field (Pfeiffer and Foster 2013), and these replays converge on the target goal location (Pfeiffer and Foster 2015). The findings on replay strongly indicate a temporal coding of spatial representations relevant to memory.
The significance of prominent temporal representation as an aspect of non-spatial coding in the hippocampus is high in two ways. First, as introduced by Tulving (1983) episodic memories are defined by a temporal organization that embodies the temporal organization of events in personal experiences. We know that the hippocampus is critical to episodic memory and to memory for the temporal order of events, even when space is not relevant. Now the existence of time cells provides a mechanism by which the hippocampus organizes memories for events in time. Second, the existence of time cells offers a parallel temporal organizing mechanism to the spatial organizing mechanism offered by place cells. Therefore, the hippocampus could support representations of episodes by mapping objects and events within a framework of space and time, conferring upon those memories connections that reflect the spatial and temporal associations between distinct but related events embodied within a mapping by place and time cells (Eichenbaum 2013, 2014).
Linking Related Experiences into Memory Networks
McClelland et al. (1995) suggested that a key function of the hippocampus is to integrate new memories with the existing organization of related knowledge. Experimental evidence supporting this idea came from studies showing that rats integrate related memories and this capacity depends on the hippocampus (Dusek and Eichenbaum 1997, 1998; Bunsey and Eichenbaum 1996). More recently, Tse et al. (2007) showed that when rats learn to find specific food flavors in particular places in an open field, they develop an organized representation of the spatial relations among the objects in a particular environment and rely on the hippocampus for rapid assimilation of new flavor-place associations within the relational representation. Relating these findings to place cells, McKenzie et al. (2013) reported that hippocampal neurons encode multiple reward locations and rapidly assimilate and reorganize the overall network representation to incorporate new reward locations (see also Dupret et al. 2010).
In a more ambitious study, McKenzie et al. (2014) characterized hippocampal neural activity in a task where rats learned multiple context-dependent object-reward associations (Fig. 2a). Analyses of single neuron firing patterns revealed considerable variation in the types of non-spatial and spatial information encoded in hippocampal neural activity patterns, showing that hippocampal neuronal activity in complex tasks is “high-dimensional” in the sense that hippocampal neurons exhibit considerable mixed selectivity to multiple relevant non-spatial and spatial dimensions that are salient in a large range of memory tasks. In an effort to understand how these dimensions are organized in hippocampal networks, McKenzie et al. characterized the neural ensemble representations using a Representational Similarity Analysis (RSA) that compared population vectors accumulated during each type of event defined as a particular object in a specific position associated with reward or non-reward value within one of two spatial contexts. The RSA generated correlation coefficients that characterized the similarity of ensemble firing patterns among all pairs of event types. Then a hierarchical clustering analysis was used to determine the pairs of events that were most similar, then iteratively, the combined pairs of events that were most similar, and so on (Fig. 2b). This analysis revealed a hierarchy of relations among events: Events that involved the different objects of the same value were lowest in the hierarchy and embedded within specific positions. Next, events that involved different values were embedded within positions. Next, events at each position within a context were embedded within each context. Finally, representations of events across contexts were anti-correlated. Thus, hippocampal ensemble coding represented the identity of the objects, their reward assignments, the positions within a context in which they were experienced, and the context in which they occurred and networked these representations to form a systematic “map” of relations between the different types of memories.
Furthermore, after initial learning of one set of object associations, new object associations were rapidly assimilated into the relational structure that was established by initial learning. In addition, within the overall representation, items that had in common their reward associations in particular positions had strongly similar representations, even when they were never experienced together. These results suggest that, at the time of learning, new information is encoded within extant networks that stored related information, consistent with the view that new information is assimilated within networks of related memory traces to form hippocampal networks of related experiences (Eichenbaum 2004; McKenzie and Eichenbaum 2011). Similarities in hippocampal coding between familiar and novel conditions likely reflects the integration of related memories, arguably a primary purpose of memory systems in schema development and memory consolidation (McClelland et al. 1995; Tse et al. 2007). This overlapping code at the time of learning builds relational representations that can support transitive associations between separately learned experiences via of their common associations with a behaviorally relevant context (Dusek and Eichenbaum 1997; Bunsey and Eichenbaum 1996; Zeithamova et al. 2012).
The notion of relational representations that link memories in space can be readily extended to the linking of memories that are characterized by their flow in time. Thus, in studies described above where rats traverse different but overlapping routes through a T-maze, a typical finding is that some neurons represent the distinct memories that correspond to specific routes, even when rats traverse the overlapping segment of the maze, whereas other neurons fire similarly in the common segment thus providing a link between the distinct memories (Wood et al. 2000). Indeed, even in situations where animals traverse similarly structured routes in different mazes, whereas most neurons fire at distinct places in each maze, some fire similarly at positions that are functionally equivalent in the different mazes (Singer et al. 2010) or in different locations in the same maze (McKenzie et al. 2013). Thus, hippocampal networks create schemas that link spatial-temporal memories in situations where different routes have common features. Thus, the mechanism for interleaving of memories may be hippocampal neurons that encode overlapping features of multiple memories.
Conclusions: The Hippocampus as a Memory Space
The above review on hippocampal neuronal firing patterns allows me to address the following key questions: (1) What is the function of strong position coding by hippocampal neurons? And, (2) how are the various non-spatial and temporal coding properties of hippocampal neurons integrated with spatial coding?
It is remarkable that, after 40 years of research following the pioneering discoveries about hippocampal neurons in the 1970s, we have yet to reach a consensus on the nature of the hippocampal code. The early observations on hippocampal neurons in behaving animals revealed both behavioral and spatial firing properties. Each is quite apparent when the other is tightly controlled. Thus, in the studies following the early work, when behavior was held constant over locations, cells that exhibit spatial coding (place cells) are prevalent. Conversely, when space is held constant by immobilization, behavioral and temporal correlates of hippocampal activity are readily apparent in a variety of learning paradigms. Importantly, in a broad variety of testing paradigms when space, time, and sensory and behavioral events are salient, hippocampal neurons encode and integrate all of these dimensions of experience. The hundreds of studies on hippocampal neurons over these years has confirmed and extended these fundamental features of information coding by hippocampal neurons and networks. It is not too simplistic to conclude that the hippocampal network reflects all the salient events in attended experience, just as it should as indicated by its core function in memory. But how should we conceive the organization of information that supports this mirror of experience?
These properties support the notion that the hippocampus creates a “memory space” that binds in memory the elements of experiences and links memories via their common elements (Eichenbaum et al. 1999). By rapidly forming associations among any subset of its inputs, and between its inputs and reactivated relational memories, the hippocampus plays a critical role in the generation, recombination, and flexible use of information of all kinds. The representational schemes that underlie the memory space include representations of events as the relations among objects within the context in which they occur, representations of episodes as the flow of events across time, and representations that interleave events and episodes into relational networks, supporting the ability to draw novel inferences from memory (Eichenbaum 2004). This interpretation applies equally well to spatial and non-spatial domains of memory (Eichenbaum and Cohen 2014).
Considering the original definition of cognitive maps might provide progress towards a clarification of hippocampal function. According to Tolman (1948), a cognitive map is a form of mental organization of cognition, a tool for systematic organization of information across multiple domains of life. O’Keefe and Nadel (1978) interpreted the notion of a cognitive map narrowly to refer to a mental mapping of physical space and argued that the hippocampus performs spatial computations and represents geographical maps of the real world. The principals of cognitive mappings, however, can very well apply to episodic memories by viewing events as items organized in a spatial-temporal context (Butterly et al. 2012; Eichenbaum and Cohen 2014; Tavares et al. 2015). The memory space hypothesis takes the view that hippocampal networks map our location and movements within a broad range of life-spaces, supporting our ability to navigate spatial, temporal, and associational dimensions of personal experience (Eichenbaum et al. 1999; Eichenbaum 2004; see also Buzsaki and Moser 2013; Milivojevic and Doeller 2013).
References
Aghajan ZM, Acharya L, Moore JJ, Cushman JD, Vuong C, Mehta MR (2015) Impaired spatial selectivity and intact phase precession in two-dimensional virtual reality. Nat Neurosci 18:121–128
Ainge JA, Tamosiunaite M, Woergoetter F, Dudchencko PA (2007) Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J Neurosci 27:9769–9779
Anderson MI, Jeffery KJ (2003) Heterogeneous modulation of place cell firing by changes in context. J Neurosci 23:8827–8835
Berger TW, Alger BE, Thompson RF (1976) Neuronal substrates of classical conditioning in the hippocampus. Science 192:483–485
Berger TW, Rinaldi PC, Weisz DJ, Thompson RF (1983) Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J Neurophsiol 50:1197–1219
Bostock E, Muller RU, Kubie JL (1991) Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1:193–206
Bower MR, Euston DR, McNaughton BL (2005) Sequential-context dependent hippocampal activity is not necessary to learn sequences with repeated elements. J Neurosci 15:1313–1323
Bunsey M, Eichenbaum H (1996) Conservation of hippocampal memory function in rats and humans. Nature 379:255–257
Butterly DA, Petroccione MA, Smith DM (2012) Hippocampal context processing is critical for interference free recall of odor memories in rats. Hippocampus 22:906–913
Buzsáki G, Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci 16:130–138
Cameron KA, Yashar S, Wilson CL, Fried I (2007) Human hippocampal neurons predict how well word pairs will be remembered. Neuron 30:289–298
Carr MF, Jadhav SP, Frank LM (2011) Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci 14:147–153
Cressant A, Muller RU, Poucet B (1997) Failure of centrally placed objects to control the firing fields of hippocampal place cells. J Neurosci 17:2531–2542
Davachi L (2006) Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16:693–700
Deadwyler SA, Bunn T, Hampson RE (1995) Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J Neurosci 16:354–372
Derdikman D, Knierim JJ (eds) (2014) Space, time and memory in the hippocampal formation. Springer, Vienna
Diana RA, Yonelinas AP, Ranganath C (2007) Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci 11:379–386
Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J (2010) The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci 13:995–1002
Dusek JA, Eichenbaum H (1997) The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci U S A 94:7109–7114
Dusek JA, Eichenbaum H (1998) The hippocampus and transverse patterning guided by olfactory cues. Behav Neurosci 112:762–771
Eichenbaum H (2004) Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44:109–120
Eichenbaum H (2013) Memory on time. Trends Cogn Sci 17:81–88
Eichenbaum H (2014) Time cells in the hippocampus: a new dimension for mapping memories. Nat Rev Neurosci 15:732–744
Eichenbaum H, Cohen NJ (2014) Can we reconcile the declarative memory and spatial navigation views of hippocampal function? Neuron 83:764–770
Eichenbaum H, Kuperstein M, Fagan A, Nagode J (1987) Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor discrimination task. J Neurosci 7:716–732
Eichenbaum H, Dudchencko P, Wood E, Shapiro M, Tanila H (1999) The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23:209–226
Eichenbaum H, Yonelinas AR, Ranganath C (2007) The medial temporal lobe and recognition memory. Ann Rev Neurosci 30:123–152
Fenton AA, Wsierska M, Kaminsky Y, Bures J (1998) Both here and there: simultaneous expression of autonomous spatial memories in rats. Proc Natl Acad Sci U S A 95:11493–11498
Ferbinteanu J, Shapiro ML (2003) Prospective and retrospective memory coding in the hippocampus. Neuron 40:1227–1239
Fortin NJ et al (2002) Critical role of the hippocampus in memory for sequences of events. Nat Neurosci 5:458–462
Frank LM, Brown EN, Wilson M (2000) Trajectory encoding in the hippocampus and entorhinal cortex. Neuron 27:169–178
Fried I, MacDonald KA, Wilson CL (1997) Single neurons activity in human hippocampus and amygdala during recognition of faces and objects. Neuron 18:753–765
Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I (2008) Internally generated reactivation of single neurons in human hippocampus during free recall. Science 322:96–101
Gener T, Perez-Mendez L, Sanchez-Vives MV (2013) Tactile modulation of hippocampal place fields. Hippocampus 23:1453–1462
Gill PR, Mizumori SJ, Smith DM (2011) Hippocampal episode fields develop with learning. Hippocampus 21:1240–1249
Gothard KM, Skaggs WE, Moore KM, McNaughton BL (1996a) Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci 16:823–835
Gothard KM, Skaggs WE, McNaughton BL (1996b) Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J Neurosci 16:8027–8040
Griffin AL, Eichenbaum H, Hasselmo ME (2007) Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci 27:2416–2423
Hampson RE, Heyser CJ, Deadwyler SA (1993) Hippocampal cell firing correlates of delayed-match-to-sample performance in the rat. Behav Neurosci 107:715–739
Hartley T, Lever C, Burgess N, O’Keefe J (2013) Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc B Biol Sci 369(1635):20150510
Hattori S, Chen L, Weiss C, Disterhoft JF (2015) Robust hippocampal responsivity during retrieval of consolidated associative memory. Hippocampus 25:655–669
Hetherington PA, Shapiro ML (1997) Hippocampal place fields are altered by the removal of single visual cues in a distance-dependent manner. Behav Neurosci 111:20–34
Hill AJ, Best PJ (1981) Effects of deafness and blindness on the spatial correlates of hippocampal unit activity in the rat. Exp Neurol 74:204–217
Hollup SA, Molden S, Donnett JG, Moser M-B, Moser EI (2001) Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J Neurosci 21:1635–1644
Howard MW, Eichenbaum H (2013) The hippocampus, time, and memory across scales. J Exp Psychol Gen 142:1211–1230
Howard MW, MacDonald CJ, Tiganj Z, Shankar KH, Du Q, Hasselmo ME, Eichenbaum H (2014) A unified mathematical framework for coding time, space, and sequences in the hippocampal region. J Neurosci 34:4692–4707
Itskov PM, Vinnik E, Diamond ME (2011) Hippocampal representation of touch-guided behavior in rats: persistent and independent traces of stimulus and reward location. PLoS One 6(1):e16462. doi:10.1371/journal.pone.0016462
Ison MJ, Quian Quiroga R, Fried I (2015) Rapid encoding of new memories by individual neurons in the human brain. Neuron 87:220–230
Itskov PM, Vinnik E, Honey C, Schnupp J, Diamond ME (2012) Sound sensitivity of neurons in rat hippocampus during performance of a sound-guided task. J Neurophysiol 107:1822–1834
Jackson J, Redish AD (2007) Network dynamics of hippocampal cell-assemblies resemble multiple spatial maps within single tasks. Hippocampus 17:1209–1229
Jadhav SP, Kemere C, German PW, Frank LM (2012) Awake hippocampal sharp-wave ripples support spatial memory. Science 336(6087):1454–1458
Jutras MJ, Buffalo EA (2010) Recognition memory signals in the macaque hippocampus. Proc Natl Acad Sci U S A 107:401–406
Karlsson MP, Frank LM (2009) Awake replay of remote experiences in the hippocampus. Nat Neurosci 12:913–918
Kennedy PJ, Shapiro ML (2009) Contextual memory retrieval: motivational states activate distinct hippocampal representations. Proc Natl Acad Sci U S A 106:10805–10810
Kesner RP et al (2002) The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci 116:286–290
Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB (2008) Finite scale of spatial representation in the hippocampus. Science 321:140–143
Komorowski RW, Manns JR, Eichenbaum H (2009) Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens. J Neurosci 29:9918–9929
Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, Eichenbaum H (2013) Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci 33:8079–8087
Kraus BJ, Robinson RJ II, White JA, Eichenbaum H, Hasselmo ME (2013) Hippocampal ‘time cells’: time versus path integration. Neuron 78:1090–1101
Kreiman G, Koch C, Fried I (2000a) Category-specific visual responses of single neurons in the human medial temporal lobe. Nat Neurosci 3:946–953
Kreiman G, Koch C, Fried I (2000b) Imagery neurons in the human brain. Nature 408:357–361
Lee I, Kim J (2010) The shift from a response strategy to object-in-place strategy during learning is accompanied by a matching shift in neural firing correlates in the hippocampus. Learn Mem 17:381–393
Lee I, Yoganarasimha D, Rao G, Knierim JJ (2004) Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature 430:456–459
Lee I, Griffin AL, Zilli EA, Eichenbaum HM (2006) Gradual translocation of spatial correlates of neuronal firing in the hippocampus toward prospective reward locations. Neuron 51:539–650
Lenck-Santini PP, Fenton AA, Muller RU (2008) Discharge properties of hippocampal neurons during performance of a jump avoidance task. J Neurosci 28:6773–6786
Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI (2004) Distinct ensemble codes in hippocampal areas CA3 and CA1. Science 305:1295–1298
Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB (2005) Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309:619–623
MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H (2011) Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 71:737–749
MacDonald CJ, Carrow S, Place R, Eichenbaum H (2013) Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J Neurosci 33:14607–14616
Manns J, Eichenbaum H (2009) A cognitive map for object memory in the hippocampus. Learn Mem 16:616–624
Manns JR, Howard M, Eichenbaum H (2007) Gradual changes in hippocampal activity support remembering the order of events. Neuron 56:530–540
Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE (1994) Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus 4:410–421
Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA (1995) Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci 15:7079–7094
McClelland JL, McNaughton BL, O'Reilly RC (1995) Why are there complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102:419–457
McEchron MD, Disterhoft JF (1997) Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J Neurophysiol 78:1030–1044
McKenzie S, Eichenbaum H (2011) Consolidation and reconsolidation: two lives of memories? Neuron 71:224–233
McKenzie S, Robinson NTM, Herrera L, Churchill JC, Eichenbaum H (2013) Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. J Neurosci 33:10243–10256
McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, Eichenbaum H (2014) Hippocampal representation of related and opposing memories develop within distinct, hierarchically-organized neural schemas. Neuron 83:202–215
McNaughton BL, Barnes CA, O'Keefe J (1983) The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52:41–49
McNaughton BL, Barnes CA, Gerrard JL, Gothard M, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL (1996) Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol 199:173–185
McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB (2006) Path-integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci 7:663–678
Milivojevic B, Doeller CF (2013) Mnemonic networks in the hippocampal formation: from spatial maps to temporal and conceptual codes. J Exp Psychol Gen 142:1231–1241
Mizumori SJY (2007) Hippocampal place fields: relevance to learning and memory. Oxford University Press, Oxford
Modi MN, Dhawale AK, Bhalla US (2014) CA1 cell activity sequences emerge after reorganization of network correlation structure during associative learning. eLife 3:e01982
Moita MAP, Moisis S, Zhou Y, LeDoux JE, Blair HT (2003) Hippocampal place cells acquire location specific location specific responses to the conditioned stimulus during auditory fear conditioning. Neuron 37:485–497
Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT (2004) Putting fear in its place: remapping of hippocampal place cells during fear conditioning. J Neurosci 24:7015–7023
Moser EI, Kropff K, Moser MB (2008) Place cells, grid cells, and the brain’s spatial representation system. Ann Rev Neurosci 31:69–89
Muller RU (1996) A quarter of a century of place cells. Neuron 17:813–822
Muller RU, Kubie JL (1987) The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7:1951–1968
Muller RU, Kubie JL, Ranck JB Jr (1987) Spatial firing patterns of hippocampal complex spike cells in a fixed environment. J Neurosci 7:1935–1950
Muller RU, Bostock E, Taube JS, Kubie JL (1994) On the directional firing properties of hippocampal place cells. J Neurosci 14:7235–7251
Muzzio IA, Levita L, Kulkarni J, Monaco J, Kentros C, Stead M, Abbott LF, Kandel ER (2009) Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. PLoS Biol 7(6):e1000140. doi:10.1371/journal.pbio.1000140
Navratilova Z, Hoang LT, Schwindel CD, Tatsuno M, McNaughton BL (2012) Experience-dependent firing rate remapping generates directional selectivity in hippocampal place cells. Front Neural Circuits 6:6. doi:10.3389/fncir.2012.00006
Naya Y, Suzuki WA (2011) Integrating what and when across the primate medial temporal lobe. Science 333:773–776
O'Keefe J (1976) Place units in the hippocampus of the freely moving rat. Exp Neurol 51:78–109
O'Keefe J (1979) A review of hippocampal place cells. Prog Neurobiol 13:419–439
O'Keefe J, Burgess N (1996) Geometric determinants of the place fields of hippocampal neurons. Nature 381:425–428
O'Keefe J, Conway DH (1978) Hippocampal place units in the freely moving rat: why they fire when they fire. Exp Brain Res 31:573–590
O'Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34:171–175
O’Keefe J, Nadel L (1978) The Hippocampus as a Cognitive Map. Oxford University Press, New York
O’Keefe J, Speakman A (1987) Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res 68:1–27
Olds J, Disterhoft JF, Segal M, Kornblith CL, Hirsh R (1972) Learning centers of rat brain mapped by latencies of conditioned unit responses. J Neurophysiol 35:202–219
Olton DS, Branch M, Best PJ (1978) Spatial correlates hippocampal unit activity. Exp Neurol 58:387–409
Otto T, Eichenbaum H (1992) Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus 2:323–334
Pastalkova E, Itskov V, Amarasingham A, Buzsaki G (2008) Internally generated cell assembly sequences in the rat hippocampus. Science 321(5894):1322–1327
Paz R, Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I (2010) A neural substrate in the human hippocampus for linking successive events. Proc Natl Acad Sci U S A 107:6046–6051
Penick S, Solomon PR (1991) Hippocampus, context, and conditioning. Behav Neurosci 105:611–617
Pfeiffer BE, Foster DJ (2013) Hippocampal place cell sequences depict future paths to remembered goals. Nature 497:74–79
Pfeiffer BE, Foster DJ (2015) Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science 349:180–183
Quirk GJ, Muller RU, Kubie JL (1990) The firing of hippocampal place cells in the dark depends on the rat’s recent experience. J Neurosci 10:2008–2017
Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I (2005) Invariant visual representation by single neurons in the human brain. Nature 435:1102–1107
Ranck JB Jr (1973) Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. Part I. Behavioral correlates and firing repertoires. Exp Neurol 41:461–531
Ravassard P, Kees A, Willers B, Ho D, Aharoni D, Cushman J, Aghajan ZM, Mehta MR (2013) Multisensory control of hippocampal spatiotemporal selectivity. Science 340:1342–1346
Rawlins JNP (1985) Associations across time: the hippocampus as a temporary memory store. Behav Brain Sci 8:479–496
Rich PD, Liaw HP, Lee AK (2014) Place cells Large environments reveal the statistical structure governing hippocampal representations. Science 345:814–817
Levy WB (1989) A computational approach to hippocampal function. In: Hawkins RD, Bowers GH (eds) Computational models of learning in simple neural systems. Academic Press, Orlando, FL, pp 243–305
Robitsek JR, White J, Eichenbaum H (2013) Place cell activation predicts subsequent memory. Behav Brain Res 254:65–72
Rotenberg A, Muller RU (1997) Variable place-cell coupling to a continuously viewed stimulus: evidence that the hippocampus acts as a perceptual system. Philos Trans R Soc Lond B352:1505–1513
Rutishauser U, Schuman EM, Mamelak AN (2008) Activity of human hippocampal and amygdala neurons during retrieval of declarative memories. Proc Natl Acad Sci U S A 105:329–334
Save E, Nerad L, Poucet B (2000) Contribution of multiple sensory information to place field stability in hippocampal place cells. Hippocampus 10:64–76
Segal M, Olds J (1972) Behavior of units in hippocampal circuit of the rat during learning. J Neurophysiol 35:680–690
Shapiro ML, Tanila H, Eichenbaum H (1997) Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus 7:624–642
Shapiro ML, Kennedy P, Ferbinteanu J (2006) Representing episodes in the mammalian brain. Curr Opin Neurobiol 16:701–709
Sharp PE, Kubie JL, RU M (1990) Firing properties of hippocampal neurons in a visually symmetrical environment: contributions of multiple sensory cues and mnemonic processes. J Neurosci 10:3093–3105
Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM (2010) Experience dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J Neurosci 30:11586–11604
Singer AC, Carr MF, Karlsson MP, Frank LM (2013) Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron 77:1163–1173
Skaggs WE, McNaughton BL (1998) Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. J Neurosci 18:8455–8466
Smith DM, Mizumori SJ (2006) Learning-related development of context-specific neuronal responses to places and events: the hippocampal role in context processing. J Neurosci 26:3154–3163
Tanila H, Shapiro M, Gallagher M, Eichenbaum H (1997a) Brain aging: impaired coding of novel environmental cues. J Neurosci 17:5167–5174
Tanila H, Shapiro ML, Eichenbaum HE (1997b) Discordance of spatial representation in ensembles of hippocampal place cells. Hippocampus 7:613–623
Tanila H, Sipila P, Shapiro M, Eichenbaum H (1997c) Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci 17:5155–5166
Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, Schiller D (2015) A map for social navigation in the human brain. Neuron 87:231–243
Tolman EC (1948) Cognitive maps in rats and men. Psychol Rev 55:189–208
Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM (2007) Schemas and memory consolidation. Science 316:76–82
Tulving E (1983) Elements of Episodic Memory. Oxford University Press, New York
Vinnik E, Antopolskiy S, Itskov PM, Diamond ME (2012) Auditory stimuli elicit hippocampal neuronal responses during sleep. Front Syst Neurosci 6:49. doi:10.3389/fnsys.2012.00049
Wallenstein GV, Eichenbaum H, Hasselmo ME (1998) The hippocampus as an associator of discontiguous events. Trends Neurosci 21:315–365
Wang ME, Wann EG, Yuan RK, Ramos Álvarez MM, Stead SM, Muzzio IA (2012) Long-term stabilization of place cell remapping produced by a fearful experience. J Neurosci 32:15802–15814
Wang ME, Yuan RK, Keinath AT, Ramos Álvarez MM, Muzzio IA (2015) Extinction of learned fear induces hippocampal place cell remapping. J Neurosci 35:9122–9136
Wible CG, Findling RL, Shapiro M, Lang EJ, Crane S, Olton DS (1986) Mnemonic correlates of unit activity in the hippocampus. Brain Res 399:97–110
Wiebe SP, Stäubli UV (1999) Dynamic filtering of recognition codes in the hippocampus. J Neurosci 19:10562–10574
Wiener SI, Paul CA, Eichenbaum H (1989) Spatial and behavioral correlates of hippocampal neuronal activity. J Neurosci 9:2737–2763
Wills TJ, Lever C, Cacucci F, Burgess N, O’Keefe J (2005) Attractor dynamics in the hippocampal representation of the local environment. Science 308:873–876
Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA (2003) Single neurons in the monkey hippocampus and learning of new associations. Science 300:1578–1581
Wixted JT, Squire LR, Jang Y, Papesh MH, Goldinger SD, Kuhn JR, Smith KA, Treiman DM, Steinmetz PN (2014) Sparse and distributed coding of episodic memory ini neurons of the human hippocampus. Proc Natl Acad Sci U S A 111:9621–9626
Wood E, Dudchenko PA, Eichenbaum H (1999) The global record of memory in hippocampal neuronal activity. Nature 397:613–616
Wood E, Dudchenko PA, Robitsek JR, Eichenbaum H (2000) Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27:623–633
Young BJ, Fox GD, Eichenbaum H (1994) Correlates of hippocampal complex-spike cell activity in rats performing a nonspatial radial maze task. J Neurosci 14:6553–6563
Zeithamova D, Dominick AL, Preston AR (2012) Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron 75:168–179
Acknowledgements
NIH MH094263, MH51570, MH052090.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Eichenbaum, H. (2017). Elements of Information Processing in Hippocampal Neuronal Activity: Space, Time, and Memory. In: Hannula, D., Duff, M. (eds) The Hippocampus from Cells to Systems. Springer, Cham. https://doi.org/10.1007/978-3-319-50406-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-50406-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50405-6
Online ISBN: 978-3-319-50406-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)