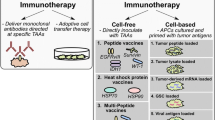
Abstract
Malignant gliomas are the most common primary brain tumor in adults, with an incidence of five cases per 100,000 persons per year. Grade IV glioblastoma is the most aggressive form and prognosis remains poor despite the current gold-standard first-line treatment—maximal safe resection and combination of radiotherapy with temozolomide chemotherapy—resulting in a median survival of approximately 15–17 months. Tumor recurrence occurs in virtually all glioblastoma patients, and there currently exists no accepted treatment for these patients. Recent advances in novel directed treatment options are showing efficacy and have entered into clinical trials. This review gives an overview of current front-line immunotherapy research in the fields of antibodies, including BiTEs and checkpoint inhibitors, and tumor vaccinations, including peptide and dendritic cell vaccinations. Furthermore, we discuss challenges specific to high-grade gliomas as well as opportunities for combination therapies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Glioblastoma
- Malignant glioma
- Immunotherapy
- Tumor vaccine
- Bispecific antibody
- Checkpoint modulator
- Dendritic cell vaccine
- Peptide vaccine
- Clinical trials
Introduction
Anti-cancer immunotherapies which activate the patient’s own immune system have shown efficacy and specificity in a variety of cancers, promising safer and more effective therapies. FDA approval of the anti-CD20 antibody rituximab for the treatment of lymphoma, the anti-HER2 antibody herceptin for treatment of breast cancer, and breakthrough checkpoint inhibitors such as anti-CTLA4 and anti-PD-1 have validated the field of immunotherapy and herald the start of an immunotherapy age that is revolutionizing cancer treatment [1–3].
The Not-So-Privileged Blood-Brain Barrier
Traditionally, literature describing the central nervous system (CNS) portrays a limited immune response marked by the blood–brain barrier, lack of a conventional lymphatic drainage system, and low levels of T cells, antigen-presenting cells, and major histocompatibility complexes [4]. However, recent findings provide evidence that while CNS entry is limited, there is a fully developed immune response in the brain. These findings include a lymph node-like drainage system which drains CNS antigens from the cerebrospinal fluid into the cervical lymph nodes, thereby facilitating immune surveillance of the CNS [5]. In addition, evidence shows that some immune cells are fully able to migrate into the CNS, where they are involved in diseases such as multiple sclerosis, CNS infections, and are also found in gliomas [4, 6]. In addition to the not-so-privileged blood–brain barrier, angiogenesis around the growing brain tumor leads to deterioration of brain microvasculature, increasing leakage [7]. That is, barrier functions such as tight junctions between the endothelial or transcytosis mechanisms may be relaxed, allowing increased penetration by immune cells [8].
Both the inherent control of the immune system over the brain and the deterioration of the blood–brain barrier during cancer growth warrant the potential of immunotherapy to redirect and activate immune cells that specifically recognize tumor cells within the brain.
Targets for Immunotherapies
The premise of immunotherapy rests on the idea that tumor cells are foreign and that the immune system can be taught to recognize the foreign cells or that a pre-existing immune response can be augmented. In order for such recognition to take place, antigens must be found that identify a specific tumor type and elicit an immune response. Broadly speaking, there are two types of immunologic targets, (i) tumor-associated antigens and (ii) tumor-specific antigens.
Tumor-Associated Antigens
Tumor-associated antigens (TAAs) are normal proteins that are overexpressed in tumor cells and can thereby serve to direct the immunologic response. Commonly, these antigens are lineage-differentiation antigens such as colorectal cancer antigens (CEA) [9] and alpha-fetoprotein (AFP) [10]. The major concern is that the expression of these antigens on healthy tissue, even if limited, could lead to autoimmunity if a potent immune response is elicited.
In glioblastoma, several studies have shown the overexpression of numerous proteins that could serve as immunological targets and for some antigens, clinical efficacy has been shown [11, 12]. Numerous studies have tested TAAs as potential immunotherapeutic targets for malignant brain tumors, including survivin, HER2neu, EphA2, EGFR, and telomerase [12–18].
Cancer/testis antigens (CTAs) represent a unique class of TAAs with normal expression restricted to germ cells in the testis but not in adult somatic cells. The melanoma-associated CTAs (MAGE, CAGE) are extensively expressed in a wide range of different cancers [19, 20].
An extensive expression analysis by Freitas et al. analyzed 153 cancer/testis antigens (CTAs), a class of differentiation antigens shown to be variably expressed within GBM tumors, and identified 4 CTAs (ACTL8, CTCFL, OIP5, and XAGE3) uniquely expressed within GBM tumors when compared to normal brain [21].
As with all TAAs, the question remains whether an approach targeting these antigens will yield a therapeutic window that shows efficacy yet limits adverse effects on healthy tissue expressing low levels of the antigen.
Tumor-Specific Antigens
In contrast to TAAs, which are normal proteins upregulated in cancer, tumor-specific antigens (TSAs) arise as mutations of normal proteins during the course of tumor progression and result in antigens that are exclusively expressed on malignant cells, albeit often on a subset of tumor cells. These antigens serve as prime targets for immunotherapies, as possible side effects such as cytotoxicity to healthy tissue are avoided.
In glioblastoma, a number of TSAs have been identified, of which some have already progressed into the clinic. Recent advances in genetic sequencing are rapidly identifying new mutations that identify subgroups of patients expressing a certain histologic type of brain cancer [22]. These neoantigens will need to be tested for their immunogenic potential to determine which can be used to develop future immunotherapies.
Currently, there are two TSAs that are highly prevalent and have shown immunogenicity in numerous studies. EGFRvIII is a conserved mutation of the epidermal growth factor receptor that is seen in approximately 31–50% of patients with GBM as well as in other cancers [23–28]. In those patients positive for EGFRvIII, the mutation is expressed in 37–87% of tumor cells.
A conserved mutation of isocitrate dehydrogenase type 1 (IDH1), which occurs at the critical arginine residue (Arg 132) in the catalytic pocket, results in a neomorphic enzymatic function, genetic instability, and malignant transformation [29]. This mutation, termed IDH1(R132H), occurs in more than 70% of grade III gliomas and, from a therapeutic viewpoint, represents an ideal candidate for a tumor-specific treatment of malignant glioma [30].
In addition, viral antigens, when upregulated specifically on malignant cells, may also serve as TSAs and have the unique advantage of being intrinsically foreign to the host and thus immunogenic. Therefore, while viruses may not be exclusively restricted to tumor cells, their expression is often undetectable in normal tissue of patients harboring virus-associated cancers. Our laboratory and others have recently shown human cytomegalovirus infection and low-level viral gene expression in malignant glioma [31, 32]. Given the success and safety of cellular immunotherapeutics targeting CMV in immunocompromised patients, immunodominant CMV antigens such as immediate early 1 (IE1), phosphoprotein 65 (pp65), and glycoprotein B (gB) have been shown to be expressed in GBM tumors and represent possible tumor-specific targets for the development of immunotherapies [33–35].
Antibodies for the Treatment of Intracerebral Malignancies
Monoclonal Antibodies Target Tumor Epitopes
The development of monoclonal antibodies (mAbs) recognizing specific epitopes has been used for the immunological treatment of many diseases, including cancer [36]. By recognizing and binding to specific epitopes, mAbs expose intruding cells and target them for uptake by phagocytic cells of the immune system, such as macrophages and dendritic cells. Furthermore, mAbs can target cellular components, such as secreted proteins, and thereby interfere with cell signaling.
Advances in technology over the past decades have made it possible to produce fully human affinity-matured antibodies via phage display directed evolution, transgenic mice, or mRNA and ribosome display, thereby resolving complications associated with murine antibodies such as human anti-mouse antibody formation and cytokine release syndrome [37–41].
Although antibodies can be found in the central nervous system at physiologic levels, GBM-induced disruptions of the blood–brain barrier facilitate antibody penetration. Several studies have shown that injecting antibodies IV in GBM patients results in significant therapeutic benefit [42–45]. In murine GBM models, an antibody directed against tenascin, a component of the tumor stroma, given systemically was shown to selectively localize to the tumor [42, 43].
The epidermal growth factor receptor (EGFR) is a well-studied and versatile signal transducer that is involved in cell proliferation, differentiation, survival, and metastasis [46]. EGFR is overexpressed in a number of tumors and plays an important role in the development of high-grade gliomas, especially in glioblastoma where it is commonly (40–60% of patients) amplified up to hundreds of gene copies [47, 48]. Anti-EGFR antibodies approved for the treatment of colorectal and head and neck cancers have been shown to inhibit ligand binding, receptor dimerization, and downstream signaling [49]. Sym004, a recently developed anti-EGFR antibody with enhanced effectiveness, is being tested in a phase II trial in recurrent glioblastoma in both patients that failed and did not fail bevacizumab treatment (Table 12.1).
Bispecific Antibodies Redirect and Activate Effector Immune Cells
Various solid tumors show infiltration with T cells and increased T cell infiltration often correlates with a good clinical outcome [50]. T cell infiltration has also been shown in glioma and is increased in high-grade tumors [51]. Substantial evidence suggests that the redirection of these T cells to specifically recognize and kill tumor cells is able to eradicate well-established tumors [52, 53]. Furthermore, clinical data have shown that mABs suffer from major limitations in their mode of action, including alternative Fc glycosylation, leading to suboptimal effector cell interaction, competition with circulating IgG, and activation of inhibitory receptors [54].
Bispecific antibodies (bsABs) are capable of binding two distinct targets and can be used to link T cells to tumor cells. Bispecific T cell engagers (BiTEs) consist of two antibody-derived linked single-chain Fv fragments (scFv) that are translated in tandem. One arm of the BiTE recognizes, for instance, the CD3 epsilon subunit on the T cell and the other arm binds a tumor antigen (Fig. 12.1). Upon binding, the BiTE causes crosslinking between adjacent tumor cells and T cells, regardless of the T cell receptor recognition, leading to T cell activation, synapse formation, and tumor lysis via perforin and granzyme secretion. Following BiTE-mediated tumor cell lysis, the T cells proliferate, express surface activation markers, and undergo serial rounds of killing [53, 55–57]. Furthermore, since crosslinking depends on binding to CD3 epsilon, T cell subsets implicated with tumor progression, such as Tregs, are also activated to lyse tumor cells [58, 59].
BiTE mode of action. The anti-CD3-EGFRvIII bispecific T cell engager (BiTE) is able to bind the CD3 epsilon subunit of the T cell receptor with one of its single-chain Fv (scFv) fragments and EGFRvIII on the glioma cell with the other scFv fragment. This leads to spatially restricted crosslinking and activation of the T cell, resulting in T cell-mediated tumor cell cytotoxicity via synapse formation and the release of perforin and granzyme
Since T cell activation requires physical linking to a tumor antigen, the immune activation is spatially and temporally restricted and highly specific for the chosen antigen. Furthermore, the small size of the BiTE results in a short half-life that allows quick regulation of antibody-mediated toxicity [60].
A recent clinical trial aims to treat patients with recurrent or refractory glioblastoma with a bispecific antibody made by the heteroconjugation of anti-EGFR and anti-CD3 antibody. Autologous activated T cells are loaded with the anti-EGFR-CD3 BiTE and injected intravenously into the patient with the goal of increasing T cell-mediated cytotoxicity toward tumor expressing EGFR [61]. The aim in this trial will be to determine whether a therapeutic window exists that will allow cell killing of EGFR overexpressing tumor cells without afflicting normal tissue (Table 12.1).
Our laboratory recently developed a BiTE produced by the heteroconjugation of an anti-EGFRvIII and anti-CD3 antibody. Experiments in mice show that systemic administration of the BiTE activates T cells in mice, resulting in extended survival and durable complete cures at rates of up to 75% [62]. Given the tumor specificity of the EGFRvIII antigen, treatment of patients with this antibody may have fewer side effects and increased efficacy.
Immune Checkpoint Modulators
The growth of a tumor is marked by significant changes to the microenvironment, leading to cancer-associated immunosuppression. This means that despite the presence of tumor-specific endogenous T cells, tumors escape destruction by upregulating inhibitory ligands that bind to inhibitory receptors on T cells, secretion of inhibitory cytokines (including TGF-beta and IL-10), and other mechanisms. This immunosuppression is particularly pronounced in glioma patients and leads to T cell dysfunction and an increase in the regulatory T cell phenotype [63–66].
Novel strategies for dealing with tumor-associated immunosuppression are the development of antagonistic mABs which block inhibitory ligands, such as CTLA-4, PD-1 and PD-L1, and agonistic mABs that stimulate the immune response by binding agonistic cell surface molecules, such as OX40 and 4-1BB (Fig. 12.2). Recent advances, in particular the FDA approval of the nivolumab–ipilimumab combination for the treatment of metastatic melanoma, highlight the powerful effect and curative potential of immune checkpoint modulators [67].
Immune checkpoint modulators. Monoclonal antibodies directed against the immune checkpoint inhibitors CTLA4 and PD1/PD-L1 are used to prevent downregulation of T cell activity and show high potential in GBM. OX40 and 4-1BB are agonistic molecules that, when bound by an antibody, stimulate T cell activity. Both mechanisms lead to a broad upregulation of immune cell activity. APC, antigen-presenting cell
Using anti-CTLA4 antibodies, our laboratory was able to show that systemic CTLA-4 blockade leads to long-term survival in 80% of treated mice with established gliomas without eliciting experimental allergic encephalomyelitis. Furthermore, treatment resulted in the recovery of normal CD4+ T cell counts and proliferative capacity and also suppressed increases in CD4+ CD25+ Foxp3+ GITR+ regulatory T cell fractions [68].
The first clinical trials with anti-CTLA4 and anti-PD-1 antibodies have recently begun for the treatment of newly diagnosed and recurrent GBM and are being tested alone or in combination with other checkpoint modulators, small molecules, and mAbs (Table 12.1). In one study comparable to the recent approval of ipilimumab–nivolumab combination for melanoma, anti-CTLA4 and anti-PD-1 are being tested separately or in combination in a three-armed study in patients with newly diagnosed GBM (Table 12.1).
However, even though trials using checkpoint inhibitors and agonists or combinations thereof have shown unprecedented potential for treating various cancer types, only a certain percentage of patients respond and toxicities are significant [3, 69]. The reasons for this are still unclear but are likely to also occur in GBM, emphasizing the need for in-depth diagnosis and hinting at the future of personalized medicine where certain checkpoint modulators or combinations thereof are prescribed based on patient-specific cancer and genetic traits.
Vaccinations for Tumor Control
The goal of vaccination is to sensitize the immune system against a target antigen and thereby elicit a potent and specific immune response that includes a memory response to the target. While vaccination has been used to successfully prevent and eradicate numerous diseases such as polio, tetanus, and typhoid, anti-tumor vaccinations have not shown the same efficacy and a lot of research is currently ongoing in this field.
Peptides
The major determinant for peptide vaccine-mediated immunogenicity is antigen choice. TAAs, given their expression on normal cells, usually elicit a subdued immune response due to central tolerance. On the other hand, TSAs, given their exclusive presentation on tumor cells, generally elicit a robust immune response similar to the immune response seen against antigens of infectious diseases.
The advantage of TAAs is their high frequency of expression in gliomas, making it possible to give most patients off-the-shelf synthetic tumor antigen peptides. Furthermore, by giving patients a cocktail of peptides, a broader immune response targeting multiple tumor subsets can be elicited. In contrast, TSAs are unique to the tumor and thereby peptides from these antigens may result in a highly tumor-focused immune response.
The mutated protein EGFRvIII, as discussed previously, represents an ideal target for anti-tumor immunotherapy. Our laboratory constructed a 13-amino-acid peptide spanning the vIII mutation and conjugated it to keyhole limpet hemocyanin (KLH). A phase II clinical trial showed that patients with EGFRvIII-positive newly diagnosed GBM, when vaccinated with rindopepimut, the EGFRvIII peptide, had a median survival of 26 months compared with the control historical cohort, which had a median survival of 15 months [70]. These positive results led to the start of a currently ongoing phase III clinical trial with the EGFRvIII peptide vaccine (Table 12.2).
However, given the heterogeneous nature of malignant brain tumor and peptide HLA restrictions, the drawback of single peptide vaccinations is that they may only be effective in a percentage of patients, and in the case of tumor-specific peptides only in the subset of patients expressing the mutated peptide. Trials are ongoing to determine whether combinations of multiple peptides will result in clinically effective peptide vaccination strategies (Table 12.2). Furthermore, increased research on neoantigens, antigens that spontaneously arise in individuals during the course of tumor progression, may lead to personalized solutions in which a patient’s tumor is sequenced after resection and peptide vaccinations are constructed based on the mutanome. Even though major challenges remain, such as locating immunogenic mutations and quickly constructing immunogenic peptides, clinical trials employing a personalized peptide pool approach have commenced (Table 12.2).
Whole Tumor Lysate
Whole tumor lysate can be used as a source of antigen and has the advantage of providing a tumor-specific repertoire of all potentially immunogenic epitopes. The rich repertoire of tumor-associated antigens contains epitopes for both CD8+ and CD4+ T cells, which is important as the parallel presentation of MHC Class I and II antigens could result in a stronger anti-tumor response and boost CD8+ T cell memory [71]. The use of tumor lysate and its encompassing antigen repertoire could also eliminate the time-consuming task of discovering strongly immunogenic antigens.
Tumor lysates can either be obtained from autologous tumor cells, which are taken from the patient, or from an allogenic cell line. Autologous tumor cells are only useful in patient-specific anti-tumor immunotherapies while allogenic tumor cells can be stored at cell banks and vaccines can be created en masse at GMP facilities [72]. Given alone, tumor lysates are administered with a strong adjuvant hapten to provoke a strong inflammatory response and increase their immunogenicity. In a murine glioma model, a CpG-tumor lysate vaccine given subcutaneously had a cure rate of up to 55% and showed significantly longer survival times than tumor lysate or CpG alone. Given their potential to be immunosuppressive, an alternative approach, discussed below, is to create dendritic cell vaccinations by pulsing dendritic cells with tumor lysate [73].
Dendritic Cells
Dendritic cells (DCs), with their powerful antigen-presenting function and unique ability to activate naïve T cells, form a crucial link between the innate and adaptive immune system. As sentinel members of the innate immune system, DCs scavenge for foreign antigens (PAMPs) and in response release cytokines. As members of the adaptive immune response, DCs take up pathogenic antigens, process them internally, and present them on their cell surface, thereby activating naïve, effector, and memory T cells and B cells, as well as maintaining tolerance against self-antigens [74]. In fact, DCs are described as the most potent endogenous activators of de novo T cell and B cell responses [75].
DC vaccination in GBM is based on the premise that patient-derived DCs can be generated ex vivo, stimulated to present immunogenic antigen, and reinfused into the patient where the cells will activate the adaptive immune response to destroy malignant cells (Fig. 12.3). Tumor-specific stimulation can be achieved by loading DCs with tumor cell lysate, peptides, viral vectors, DNA, or RNA [76–82].
Dendritic cell vaccine production. Patients first undergo leukapheresis to isolate PBMCs, followed by a period of differentiation to obtain immature dendritic cells (DCs). These cells are then loaded with antigens in the form of RNA, DNA, viral vectors, tumor lysate, or peptides. The DCs endogenously process the antigen and present it on their MHC molecules and, after a maturation step, the DCs are reinfused into the patient where they home to the lymph node and activate a tumor-specific immune response
In addition to loading DCs with the optimal tumor antigen, numerous components of the DC vaccine production process are undergoing investigation to produce potent immune responses. DCs can be matured in vitro to amplify the immune response using adjuvants or pro-inflammatory molecules. Though the optimal DC maturation is still under investigation, the current “gold standard” is a cytokine cocktail containing GM-CSF, IL-4, TNF-α, IL-1β, IL-6, and, in some instances, prostaglandin E2 (PGE2) [83, 84]. Subsequently, cytokines and chemokines have been used as adjuvants to increase antigen presentation and boost T cell expansion. Specifically, GM-CSF has been the most frequently used adjuvant and has shown efficacy in various systemic cancers and experimental brain tumors [85]. The therapeutic mechanism of GM-CSF involves the paracrine-mediated local release of GM-CSF at the vaccine/tumor antigen presentation interface and the resulting recruitment and activation of APCs [86]. These APCs consequently prime CD8+ and CD4+ T cells which recognize the tumor antigen, infiltrate the tumor cells, and lead to tumor regression [87].
Our laboratory has shown clinical efficacy in treating GBM patients with mRNA-transfected DCs [88]. RNA-transfected DCs have the major advantage that this approach is applicable to a wide range of patients as RNA can be amplified from a small number of tumor cells, meaning very little tumor sample is needed to prepare the therapy. In terms of safety, stimulating DCs with mRNA poses no risk of integration and is therefore a transient therapy, as compared to viral or DNA vectors [74]. In a recent randomized clinical trial, our group generated a dendritic cell vaccine using pp65 mRNA for treating glioblastoma (NCT00639639). Given its high and specific expression in glioblastoma, this viral antigen is ideal for eliciting a specific tumor response. By pre-conditioning patients with tetanus/diphtheria toxoid, lymph node homing and efficacy of the tumor antigen-specific DCs as well as patient survival was significantly increased [88]. A confirmatory double-blinded clinical trial is now testing the effects of tetanus preconditioning on survival in patients with newly diagnosed GBM (Table 12.2).
Alternatively, DCs can be pulsed with whole tumor lysate, which has a number of (theoretical) advantages over peptide loading, including the availability of the full repertoire of tumor-associated antigens, thereby allowing the DCs to “choose” the immunogenic antigen, and increasing the patient-response rate. Using autologous tumor cell lysate from each patient to load the DCs could represent an important step toward personalized medicine in the treatment of GBM. The DCVax-L vaccine (autologous dendritic cells pulsed with autologous tumor cell lysate) showed a 3-year overall survival rate, 2.5 times the usual period of survival, in a phase I/II clinical trial in newly diagnosed GBM, extended survival by 5 months or more for recurrent GBM, and is currently being tested in a blinded randomized phase III trial (Table 12.2) [89–91].
Considerations for the Future
Despite years of dedicated research, diagnosis with malignant gliomas, especially glioblastoma, remains a death sentence and places a heavy burden upon society. With a median survival of 15–17 months, traditional tumor treatments for GBM are of limited use and the need for directed therapy is dire. Recent developments in the field of immunotherapy, such as the peptide vaccine rindopepimut and the dendritic cell vaccine DCVax-L, have seen significant increases in overall survival and give hope that immunotherapy will play a major role in the treatment of malignant gliomas in the upcoming years.
The recent stunning success of checkpoint modulators, particularly the FDA approval of nivolumab–ipilimumab combination for treating metastatic melanoma, further validates immunotherapeutic approaches and is driving a number of ongoing clinical trials testing checkpoint inhibitors alone or in combination in high-grade glioma patients. However, issues such as serious toxicities and the large fraction of non-responders seen in other tumors will need to be addressed in glioma treatment.
Ultimately, long-term treatment of malignant gliomas may require approaches that combine traditional cancer therapies with various immunotherapeutics that serve to activate a tumor-specific immune response and maintain a tumor-suppressive milieu. The optimal combination of treatments could include peptides, mAbs, checkpoint modulators, and loaded DCs as well as activated immune cells and viral vectors and may require patient-specific personalization based on glioma subgroups, heterogeneity profiling, genetic sequencing, and current immune cell counts. Clinical trials testing such extensive combination approaches will need to use high-powered multi-armed approaches to discern therapeutic efficacy.
References
Dotan E, Aggarwal C, Smith MR. Impact of rituximab (rituxan) on the treatment of B-Cell non-hodgkin’s lymphoma. P T. 2010;35(3):148–57.
Baselga J. Clinical trials of herceptin(R) (trastuzumab). Eur J Cancer. 2001;37(Suppl 1):18–24.
Kim T, Amaria RN, Spencer C, Reuben A, Cooper ZA, Wargo JA. Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma. Cancer Biol Med. 2014;11(4):237–46.
Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11(9):504–14.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41.
Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12(9):623–35.
Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53(6):637–44.
Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32.
Sarobe P, Huarte E, Lasarte JJ, Borras-Cuesta F. Carcinoembryonic antigen as a target to induce anti-tumor immune responses. Curr Cancer Drug Targets. 2004;4(5):443–54.
He Y, Hong Y, Mizejewski GJ. Engineering alpha-fetoprotein-based gene vaccines to prevent and treat hepatocellular carcinoma: review and future prospects. Immunotherapy. 2014;6(6):725–36.
Tandon M, Vemula SV, Mittal SK. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin Ther Targets. 2011;15(1):31–51.
Okada H, Low KL, Kohanbash G, McDonald HA, Hamilton RL, Pollack IF. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88(3):245–50.
Fenstermaker RA, Ciesielski MJ. Challenges in the development of a survivin vaccine (SurVaxM) for malignant glioma. Expert Rev Vaccines. 2014;13(3):377–85.
Liu R, Mitchell DA. Survivin as an immunotherapeutic target for adult and pediatric malignant brain tumors. Cancer Immunol Immunother. 2010;59(2):183–93.
Komata T, Kanzawa T, Kondo Y, Kondo S. Telomerase as a therapeutic target for malignant gliomas. Oncogene. 2002;21(4):656–63.
Akiyama Y, Komiyama M, Miyata H, Yagoto M, Ashizawa T, Iizuka A, et al. Novel cancer-testis antigen expression on glioma cell lines derived from high-grade glioma patients. Oncol Rep. 2014;31(4):1683–90.
Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16(2):474–85.
Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70(2):588–97.
Zendman AJ, Ruiter DJ, Van Muijen GN. Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol. 2003;194(3):272–88.
Bolli M, Kocher T, Adamina M, Guller U, Dalquen P, Haas P, et al. Tissue microarray evaluation of melanoma antigen E (MAGE) tumor-associated antigen expression: potential indications for specific immunotherapy and prognostic relevance in squamous cell lung carcinoma. Ann Surg. 2002;236(6):785–93 (discussion 93).
Freitas M, Malheiros S, Stavale JN, Biassi TP, Zamuner FT, de Souza Begnami M, et al. Expression of cancer/testis antigens is correlated with improved survival in glioblastoma. Oncotarget. 2013;4(4):636–46.
Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC, et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63(7):700–7.
Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60(5):1383–7.
Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57(18):4130–40.
Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA. 1992;89(7):2965–9.
Cunningham MP, Essapen S, Thomas H, Green M, Lovell DP, Topham C, et al. Coexpression, prognostic significance and predictive value of EGFR, EGFRvIII and phosphorylated EGFR in colorectal cancer. Int J Oncol. 2005;27(2):317–25.
Garcia de Palazzo IE, Adams GP, Sundareshan P, Wong AJ, Testa JR, Bigner DD, et al. Expression of mutated epidermal growth factor receptor by non-small cell lung carcinomas. Cancer Res. 1993;53(14):3217–20.
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–7.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73.
Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10(1):10–8.
Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62(12):3347–50.
Sampson JH, Mitchell DA. Vaccination strategies for neuro-oncology. Neuro Oncol. 2015;17(Suppl 7):vii15–25.
Fuji S, Kapp M, Grigoleit GU, Einsele H. Adoptive immunotherapy with virus-specific T cells. Best Pract Res Clin Haematol. 2011;24(3):413–9.
Riddell SR, Greenberg PD. Cellular adoptive immunotherapy after bone marrow transplantation. Cancer Treat Res. 1995;76:337–69.
Reichert JM, Valge-Archer VE. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6(5):349–56.
Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23(9):1105–16.
Lonberg N. Fully human antibodies from transgenic mouse and phage display platforms. Curr Opin Immunol. 2008;20(4):450–9.
Gaston RS, Deierhoi MH, Patterson T, Prasthofer E, Julian BA, Barber WH, et al. OKT3 first-dose reaction: association with T cell subsets and cytokine release. Kidney Int. 1991;39(1):141–8.
Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325–38.
Reynolds JC, Del Vecchio S, Sakahara H, Lora ME, Carrasquillo JA, Neumann RD, et al. Anti-murine antibody response to mouse monoclonal antibodies: clinical findings and implications. Int J Rad Appl Instrum B. 1989;16(2):121–5.
Bourdon MA, Coleman RE, Blasberg RG, Groothuis DR, Bigner DD. Monoclonal antibody localization in subcutaneous and intracranial human glioma xenografts: paired-label and imaging analysis. Anticancer Res. 1984;4(3):133–40.
Bullard DE, Adams CJ, Coleman RE, Bigner DD. In vivo imaging of intracranial human glioma xenografts comparing specific with nonspecific radiolabeled monoclonal antibodies. J Neurosurg. 1986;64(2):257–62.
Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA. 2007;104(10):4071–6.
Zalutsky MR, Moseley RP, Coakham HB, Coleman RE, Bigner DD. Pharmacokinetics and tumor localization of 131I-labeled anti-tenascin monoclonal antibody 81C6 in patients with gliomas and other intracranial malignancies. Cancer Res. 1989;49(10):2807–13.
Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25.
Kang CS, Zhang ZY, Jia ZF, Wang GX, Qiu MZ, Zhou HX, et al. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006;13(5):530–8.
Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p 53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6(3):217–23 (discussion 23-4).
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7(4):301–11.
Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4(5):522–6.
Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17(13):4296–308.
Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–7.
Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115(1):98–104.
Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220–33.
Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43(6):763–71.
Mack M, Gruber R, Schmidt S, Riethmuller G, Kufer P. Biologic properties of a bispecific single-chain antibody directed against 17-1A (EpCAM) and CD3: tumor cell-dependent T cell stimulation and cytotoxic activity. J Immunol. 1997;158(8):3965–70.
Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100(6):690–7.
Choi BD, Gedeon PC, Herndon JE, Archer GE, Reap EA, Sanchez-Perez L, et al. Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol Res. 2013;1(3):163.
Choi BD, Gedeon PC, Sanchez-Perez L, Bigner DD, Sampson JH. Regulatory T cells are redirected to kill glioblastoma by an EGFRvIII-targeted bispecific antibody. Oncoimmunology. 2013;2(12):e26757.
Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009;1(6):539–47.
Zitron IM, Thakur A, Norkina O, Barger GR, Lum LG, Mittal S. Targeting and killing of glioblastoma with activated T cells armed with bispecific antibodies. BMC Cancer. 2013;13:83.
Choi BD, Kuan CT, Cai M, Archer GE, Mitchell DA, Gedeon PC, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci USA. 2013;110(1):270–5.
Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100(1–2):216–32.
Roszman T, Elliott L, Brooks W. Modulation of T-cell function by gliomas. Immunol Today. 1991;12(10):370–4.
Morford LA, Elliott LH, Carlson SL, Brooks WH, Roszman TL. T cell receptor-mediated signaling is defective in T cells obtained from patients with primary intracranial tumors. J Immunol. 1997;159(9):4415–25.
Roszman TL, Brooks WH. Immunobiology of primary intracranial tumours. III. Demonstration of a qualitative lymphocyte abnormality in patients with primary brain tumours. Clin Exp Immunol. 1980;39(2):395–402.
Mullard A. FDA approves first immunotherapy combo. Nat Rev Drug Discov. 2015;14(11):739.
Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–67.
Grimaldi AM, Marincola FM, Ascierto PA. Single versus combination immunotherapy drug treatment in melanoma. Expert Opin Biol Ther. 2015.
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–9.
Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189(5):753–6.
Chiang CL, Benencia F, Coukos G. Whole tumor antigen vaccines. Semin Immunol. 2010;22(3):132–43.
Wu A, Oh S, Gharagozlou S, Vedi RN, Ericson K, Low WC, et al. In vivo vaccination with tumor cell lysate plus CpG oligodeoxynucleotides eradicates murine glioblastoma. J Immunother. 2007;30(8):789–97.
Batich KA, Swartz AM, Sampson JH. Enhancing dendritic cell-based vaccination for highly aggressive glioblastoma. Expert Opin Biol Ther. 2015;15(1):79–94.
Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186(7):1177–82.
Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, et al. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186(8):1247–56.
Yamaguchi S, Tatsumi T, Takehara T, Sasakawa A, Hikita H, Kohga K, et al. Dendritic cell-based vaccines suppress metastatic liver tumor via activation of local innate and acquired immunity. Cancer Immunol Immunother. 2008;57(12):1861–9.
Hatfield P, Merrick AE, West E, O’Donnell D, Selby P, Vile R, et al. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J Immunother. 2008;31(7):620–32.
Courreges MC, Benencia F, Conejo-Garcia JR, Zhang L, Coukos G. Preparation of apoptotic tumor cells with replication-incompetent HSV augments the efficacy of dendritic cell vaccines. Cancer Gene Ther. 2006;13(2):182–93.
Benencia F, Courreges MC, Coukos G. Whole tumor antigen vaccination using dendritic cells: comparison of RNA electroporation and pulsing with UV-irradiated tumor cells. J Transl Med. 2008;6:21.
Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–6.
Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuno MA, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62(1):125–35.
Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97(11):3466–9.
Nair S, Archer GE, Tedder TF. Isolation and generation of human dendritic cells. Curr Protoc Immunol. 2012;Chapter 7:Unit7.32.
Gardner TA, Elzey BD, Hahn NM. Sipuleucel-T (provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother. 2012;8(4):534–9.
Gordon EM, Levy JP, Reed RA, Petchpud WN, Liu L, Wendler CB, et al. Targeting metastatic cancer from the inside: a new generation of targeted gene delivery vectors enables personalized cancer vaccination in situ. Int J Oncol. 2008;33(4):665–75.
Ojima T, Iwahashi M, Nakamura M, Matsuda K, Naka T, Nakamori M, et al. The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen. Int J Oncol. 2006;28(4):947–53.
Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–9.
Hdeib A, Sloan AE. Dendritic cell immunotherapy for solid tumors: evaluation of the DCVax(R) platform in the treatment of glioblastoma multiforme. CNS Oncol. 2015;4(2):63–9.
Polyzoidis S, Ashkan K. DCVax(R)-L–developed by northwest biotherapeutics. Hum Vaccin Immunother. 2014;10(11):3139–45.
Bosch M, Prins R, Liau L. Abstract 2491: treatment with tumor lysate-pulsed autologous dendritic cells prolongs survival in patients with recurrent glioblastoma multiforme. Cancer Res. 2015;75:2491.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Schaller, T.H., Sampson, J.H. (2017). Immunotherapy for High-Grade Gliomas. In: Moliterno Gunel, J., Piepmeier, J., Baehring, J. (eds) Malignant Brain Tumors . Springer, Cham. https://doi.org/10.1007/978-3-319-49864-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-49864-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49863-8
Online ISBN: 978-3-319-49864-5
eBook Packages: MedicineMedicine (R0)