Abstract
Pretreatments and enzymes have been a major hindrance to second-generation (2G) bioethanol production. As a result, most scientists have been focusing on the search for new enzymes and their subsequent characterization. Although this valuable knowledge has significantly improved the field generating initiatives of commercial production of 2G bioethanol, the cell walls themselves have received relatively little attention. In this chapter, we revise the work performed on sugarcane cell wall composition, structure, and architecture. From the status of looking exclusively to monosaccharide composition, research has evolved and several details about sugarcane cell wall polysaccharides and lignin were unrevealed. The studies about cell wall structure led to the proposition of the first model of sugarcane cell wall architecture in which macrofibrils (bundles of microfibrils) of cellulose are likely to be bound together by xyloglucan and arabinoxylans. These macrofibrils are covered with layers of more soluble hemicelluloses such as highly branched arabinoxylans and β-glucan. The lignin seems to be closely associated with the cellulose–hemicellulose domain, which is more hydrophobic than the other cell wall domains. Finally, lignin and cellulose–hemicellulose domains are embedded in a thin layer of pectin matrix. This model led to the proposition of a hypothesis that efficient cell wall degradation in the natural environment could be possible if the glycosyl hydrolases would sequentially degrade each layer at a time inwards towards cellulose microfibrils. This hypothesis was corroborated both during the attack of fungi to sugarcane biomass and during the aerenchyma formation in sugarcane roots. The highly complex sugarcane cell wall is now thought to be a result of a code, which is just starting to be unveiled. We believe that by further understanding the interactions among polymers and how endogenous enzymes attack cell walls, future strategies to induce endogenous biological pretreatments followed by the attack of enzyme consortia might significantly improve industrial processes for 2G bioethanol production from sugarcane.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Cell wall architecture
- Polysaccharides
- Cellulose
- Hemicellulose
- Pectins
- Lignin
- Bioenergy
- Glycosyl hydrolases
1 Introduction
Sugarcane breeding has promoted the development of varieties that are highly productive. This is one of the main factors that led Brazil to become the second largest world producer of first-generation (1G) bioethanol , which is produced from sucrose stored in culms of sugarcane. More recently, the advances in second-generation (2G) bioethanol science have endorsed the development of at least two Brazilian initiatives for commercial production of 2G bioethanol: GranBio and Raizen/Costa Pinto. Currently, the former utilizes sugarcane leaves as the main raw material for the 2G bioethanol production, while the second uses sugarcane bagasse, integrating 1G and 2G processes.
Second-generation bioethanol became a reality due to the significant progress that has been achieved in the development of processes to pretreat and hydrolyze the plant cell wall (Vohra et al. 2014; Healey et al. 2015; Saini et al. 2015). Despite the great advances, the hydrolysis step, reported to account for 9% of the cost of biomass conversion in 2002 (Aden et al. 2002), remains as a substantial parcel of the cost of 2G processes (Enzitec 2016). One of the factors that can help to reduce the costs in 2G processes is the formulation of more accurate enzymatic cocktails, designed to hydrolyze cell wall polysaccharides efficiently. For this purpose, it is crucial to understand how polysaccharides are structured and arranged into sugarcane cell walls, i.e., its architecture (Buckeridge et al. 2016—Fig. 2.1).
Hypothetical model of the sugarcane cell wall. Based on the information provided by the cell wall phenotyping procedures (De Souza et al. 2013; Buckeridge et al. 2015) different classes of polymers are arranged according to their solubility and topology based on antibodies and atomic force microscopy (Buckeridge et al. 2015). Sugarcane walls are of the type II (grasses), with relatively little pectin matrix and having arabinoxylans > β-glucan > xyloglucan > mannan as the main hemicelluloses . (a) Representative scheme of what could be the basic repetitive unit of the cell walls of sugarcane (possibly in primary and secondary walls, but with different proportions among domains). (b, c) Representative schemes of what could be a tridimensional portion of the primary cell wall of sugarcane. Secondary walls would probably have less of the hemicellulose domain and more cellulose macrofibrils. This tentative model does not include cell wall proteins, such as hydroxyproline-rich proteins that have not been studied in sugarcane to date
In this chapter, we review some of the work performed on sugarcane cell wall composition and architecture and discuss future scenarios of research to improve the efficiency of cell wall degradation in sugarcane.
2 Molecular Composition of Sugarcane Cell Walls
Sugarcane cell wall composition has been firstly estimated by analyzing monosaccharides. Peng et al. (2009) have shown that cell walls from sugarcane bagasse were composed of 38.3% glucose, 27.6% xylose, and 19.2% galactose. Later on, Masarin et al. (2011) reported the presence of 38–43% glucans, 25–32% hemicelluloses , as well as 1.6–7.5% extractives in bagasse of different sugarcane varieties produced in Brazil.
A more accurate appreciation of sugarcane cell wall composition was achieved by De Souza et al. (2013), who, besides monosaccharides, used a combination of different techniques such as oligosaccharide and polysaccharide profiling and Fourier transform infrared spectroscopy (FT-IR). Combined, these procedures can be defined as a cell wall phenotyping procedure. In this study, cell walls of leaves and culm were fractioned with a series of solvents so that polymers were extracted according to their solubility (Fig. 2.2). The composition of the polymers in each fraction was analyzed so that it was possible to estimate the average composition of each class of polymer (pectins , hemicelluloses , and cellulose ) of the cell wall of this plant. On average, sugarcane cell walls from both organs were shown to be composed of ~30% cellulose ; 40% arabinoxylan (AX); 10% β-glucan (BG); 8% xyloglucan (XG); 8% pectins including homogalacturonan, arabinogalactan, and arabinan; and 6% lignin .
Chemical fractionation of the cell wall as it occurs with sugarcane culm according to De Souza et al. (2013). Alcohol-insoluble residue (AIR) is prepared by washing dry biomass with 80% ethanol at 80 °C. AIR is then submitted to a sequence of extractions. β-Glucan (BG) and pectins are solubilized in ammonium oxalate, and lignin is retrieved from the material by sodium chlorite. A sequence of increasing concentrations of alkali (NaOH) will extract first arabinoxylans (AX) and some BG (0.1 and 1 M) and then xyloglucan (XG) with some arabinoxylan and (gluco)mannan (GM) (4 M). The residue ends up composed of 98% glucose, which is assumed to be all cellulose
Although the data compilation made by De Souza et al. (2013) led to a cell wall composition estimation that was quite different from other studies on bagasse (Rezende et al. 2011; Guilherme et al. 2015), one has to consider that both leaves and culm analyzed were from sugarcane in natura. The composition of in natura culms differs from bagasse since the latter loses some of the polysaccharides during the extraction of sucrose in the industry (Lara Azevedo, Amanda P. De Souza, and Marcos Buckeridge, unpublished results). The lignin content, though, might have been underestimated due to plant young age, possibly having a little interference in the hemicellulose proportion in the wall.
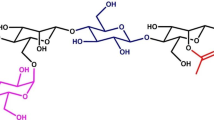
Some of the sugarcane cell wall polymers have been investigated in detail. For example, the identity of the carbohydrate linkages of the main hemicelluloses and pectins has been revealed through methylation analysis (see Fig. 2.3 for structures), and the cellulose nanostructure has been explored in depth (see Chap. 3 for further information). Also, the fine structure of the hemicelluloses has been determined by using endo-β-glycanases (De Souza et al., 2013). All of these features are known to contribute to the polysaccharide arrangement, and consequently to the cell wall architecture.
Structural features of the cell wall polysaccharides from sugarcane. Redesigned from Buckeridge and De Souza (2014). Molecular structures for each polysaccharide were based on methylation analysis of sugarcane cell wall fractions (Marcos Buckeridge and Nicolas C. Carpita, unpublished results)
3 Sugarcane Cell Wall Architecture
Cell walls can be thought of as a stratified composite formed by well-organized interactions (covalent and non-covalent ) among polymers. There seems to exist a basic module composed of cellulose , hemicelluloses , and pectins that is replicated within the wall (Fig. 2.1a), forming the network of compounds that emerge as the cell wall becomes proper (Fig. 2.1b, c).
In sugarcane, the model proposed for the basic module is based on the pattern of solubilization of polysaccharides described by De Souza et al. (2013), and on recent results obtained in our laboratory (Arthur Cambler, Amanda De Souza, and Marcos Buckeridge, unpublished results). The sugarcane basic model (Buckeridge et al. 2016) is formed by cellulose microfibrils that are probably bound together by arabinoxylans (AX) whose branches are esterified with phenylpropanoids (Fig. 2.1). The presence of phenylpropanoids , as well as acetylations, in the AX chains might confer extreme hydrophobic properties to this inner part of the cell wall module. The macrofibrils seem to be covered with more branched (and possibly less acetylated) AX that confers relative hydrophobicity to the wall. A small proportion of mannan (or glucomannan ) has been detected in the less soluble sugarcane cell wall fractions, suggesting that this polysaccharide might also interact with microfibrils and participate in the complex that forms macrofibrils. However, this hypothesis needs further investigation. The most soluble hemicellulosic polymers in the walls are mostly linear, but slightly arabinosylated xylan and the mixed linkage β-glucan (BG) . It has been proposed that BG plays a structural role in the wall, mainly by forming a scaffold for deposition of other wall polymers during development (Buckeridge et al. 2004). Alternatively, BG has been found to play a storage reserve role in wheat (Roulin and Feller 2001) and barley (Roulin et al. 2002).
Lignin of sugarcane is mostly associated with vascular bundles (mainly in its fibers) and a little—primarily in rind cells—is located in the walls of the parenchyma (composed mainly of p-coumaric acid), followed by significant amounts of hydroxycinnamic and ferulic acids (Masarin et al. 2011) (Fig. 2.4). However, it is not well known how these compounds are chemically linked to each other. What is known is that hydroxycinnamic and ferulic acids are esterified to hemicelluloses and pectins , nucleating the polymerization of lignin in the wall (see Chap. 4). Thus, there seems to exist a trend towards the presence of lignin in cell walls that contain more cellulose , whereas pectins , probably in the middle lamella, also contain some lignification. Recent evidence strongly suggests that AX is the main polymer bound to lignin , probably via its arabinosyl branching residues. Because a portion of the branched AX is retrieved from the walls of sugarcane only after extraction with sodium chlorite (Cambler, De Souza, and Buckeridge, unpublished results), we believe that lignin bridges are more frequent among AX molecules and somehow between them and cellulose .
Distribution of lignin in the culm of sugarcane. Lignin was stained with floroglucinol (a–c) and visualized by autofluorescence (d–f). Section of the culm was obtained from mature culm of sugarcane (cv. SP80-3280). Lignin is more concentrated in the vascular system, especially in the fibers (see mainly c), but is also detected in parenchyma cell walls, although staining less strongly in these cells. There is proportionally higher lignin concentration in the periphery of the culm due to higher incidence of vascular bundles. Pictures taken by Viviane C. Lopes
Although chemistry has helped understanding some aspects of the interactions among cell wall polymers, their topology within the wall is not yet accessible by any technique. The application of modern techniques to unveil cell wall features related to the physics, chemistry, and biochemistry of the cell wall of sugarcane has led to a working model of its architecture. However, we still lack tools that can show how the polymers are arranged in the native wall. Those tools are essential to advance the knowledge of the cell wall architecture. For that purpose, probes such as DNA aptamers could be developed to provide identification of polymer domains (Boese and Breaker 2007; Low et al. 2009). Nonetheless, the barriers to be crossed are enormous. For instance, the use of such probes, which could bypass the problem of the low wall porosity, would still face the hydrophobicity barrier of the deep regions of the wall, representing some technical issues that would be quite complex to deal with.
4 The Cell Wall Architecture Results from a Glycomic Code
From the discovery of the grass wall architecture to the modern days, a plethora of genes related to plant cell wall biosynthesis and hydrolysis have been described and characterized (see Wang et al. 2016 for a review). The knowledge about mechanisms of synthesis and hydrolysis of the cell walls in general highlights the fact that carbohydrate polymers are not composed of randomly linked monosaccharides, but by polymers that display very strict fine structures (Buckeridge and De Souza 2014). The fact that encoded polymers form such a complex structure raised the question whether cell walls could have a code that would be encrypted by the biosynthetic mechanisms of different classes of polymers (Buckeridge and De Souza 2014; Tavares and Buckeridge 2015).
The idea that cell wall polymers may display a code—named the glycomic code—has been put forward to explain how seed storage cell wall polysaccharides are degraded (Buckeridge 2010). In this case, XG clearly displays encoded fine structure that determines the action of glycosyl hydrolases (Tiné et al. 2003, 2006) as well as the level of binding to the surface of cellulose (Lima and Buckeridge 2001).
In the case of sugarcane cell walls, AX, the major component among hemicelluloses , seems to be encrypted by branching with arabinosyl residues, which are positioned at carbons 2 and/or 3 on xylosyl residues in the main chain. Many of the unbranched hydroxyls are supposedly acetylated making most of the polysaccharide hardly accessible to enzymes (Crivellari 2012).
The glycomic code may be the ultimate barrier to efficient hydrolysis. If so, by breaking the glycomic code of all cells in a tissue, one could supposedly control cell wall assembly and, by knowing precisely the mapping of polymer interactions, be able to hydrolyze cell walls with much higher precision.
5 What Structure and Architecture Mean for Hydrolysis
The level of complexity of the sugarcane cell walls is not surprising since some features of the architecture of cell walls of grasses have been known for decades (Carpita and Gibeaut 1993). However, the idea of hydrolyzing its components for bioenergy purposes remains a major challenge. Part of this challenge can be attributed to the lack of knowledge about cell wall architecture.
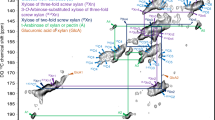
One of the main features of the cell wall architecture relies on the fine structure of the polysaccharides , as it determines how the polymers can be arranged in the wall. The study of the fine structure can be made using endo-hydrolases. When in low concentration, the endo-enzyme action is analogous to a DNA restriction enzyme. Thus, the use of endo-enzymes such as lichenase for BG, xyloglucan-endo-glucanase for XG, and GH10 or GH11 for AX produces “restriction maps” of oligosaccharides that can be viewed in HPAEC-PAD chromatograms. When such maps are obtained for cell wall fractions, they might reveal differences in polysaccharide fine structure that may be relevant for hydrolysis (De Souza et al., 2013, 2015). For instance, AX from sugarcane leaves is more branched with arabinose and seems to bind more strongly to cellulose in comparison to culm; that is, the AX from leaves remains bound up to the 4 M NaOH fraction during fractionation. On the other hand, XG seems to have more soluble fractions in culm than in leaves (Fig. 2.5), which may reflect differences in saccharification between the two organs since xyloglucan may interfere with the attack of enzymes to cellulose .
Comparison of the fine structures (restriction profiles) of the main hemicelluloses of sugarcane leaves (a) and culm (b). Adapted from De Souza et al. (2013). Xylanase used for detection of AX oligosaccharides and xyloglucan endo-glucanase (XEG) for detection of XG oligosaccharides. a xylose, b xylobiose, c xylotriose, d arabinoxylated oligosaccharides, un unknown xyloglucan oligosaccharides
Differences in the glycome profiling, i.e., identification of polysaccharide epitopes through monoclonal antibodies (Patthathil et al. 2012), can also suggest alterations in the fine structure since they give information about changes in the populations of the exposed epitopes present in the cell wall fractions (Zhu et al. 2010). In miscanthus, a closely related species to sugarcane, subtle differences in pectins were identified by glycome profiling such as the presence of arabinogalactans that were relevant to saccharification (De Souza et al. 2015).
The arrangement of the polymers into an architectural framework also raises issues related to pore size in the biomass, which apparently limits the penetration of enzymes on it (Buckeridge et al. 2016). Based on the polysaccharide structure and composition, we assume that at least 24 distinct linkages would require enzymes to be broken. For this hydrolysis process, at least 18 classes of enzymes would be needed (Table 2.1). Thus, to overcome the limitation of pore size and have an efficient hydrolysis, the basic architectural unit (Fig. 2.1a) would have to be attacked by enzymes acting from the surface towards the inner side of the macrofibril. Following this idea, De Souza et al. (2013) proposed a hypothetical mechanism by which a group of enzymes in a cocktail would have their action in a sequence of attacks. This attack would start with esterases (pectin methyl and acetyl esterases, xylan acetyl esterases, feruloyl esterases), followed by the action of endo- and exo-hydrolases that would attack the hemicelluloses within the wall (AX, BG, XG, and mannan). After such attack, cellulose microfibrils would end up naked and could then be attacked by lytic oxidases, endo-glucanases, and cellobiohydrolases.
This hypothesis received some experimental support by the experiment reported by Borin et al. (2015). These authors used proteomics and biochemistry to show that the production of enzymes in the forecasted sequence took place when Trichoderma reesei and Aspergillus niger were grown on sugarcane culm and bagasse. A similar sequence of enzyme attacks was also observed in an endogenous cell wall degradation during aerenchyma formation in sugarcane roots (refer to Grandis et al. 2014 and Tavares et al. 2015 for further information regarding endogenous mechanisms in plants that include cell wall degradation).
Together, these discoveries led us to propose that it could be more appropriate to use three enzyme consortia in which synergic properties are taken into consideration. The first consortium would target the most soluble polymers of the wall (pectins and BG debranching enzymes). The second one should be capable of degrading the main hemicelluloses that are bound to the surface of macrofibrils (endo- and exo-xylanases, xylosidases, mannanase, and lichenase in certain cases). Finally, the third consortium would contain mostly expansin, cellulose , lytic oxidases, and xyloglucan-degrading enzymes, possibly with some endo-xylanase as well (see Chap. 5 for details about each enzyme). If the consortia were added sequentially, mimicking how the hydrolysis happens in vivo, perhaps much less enzyme would have to be used in the process. The use of lower amounts of enzymes could be achieved, since the enzymes that degrade cellulose , for example, would be freshly added to the mixture instead of being present in the process for several hours without being able to act on their substrates.
The discovery that natural degradation mechanisms display sequential action of glycosyl hydrolases on biomass can also help to design biological pretreatments. One could design plants capable of activating certain cell wall-degrading enzymes just before harvesting, thus facilitating the pretreatment step. Altogether, this could have a potential to decrease significantly the higher costs associated to pretreatment and hydrolysis in the 2G processes.
6 Conclusions and Perspectives
In spite of the significant advances in sugarcane biomass hydrolysis , the difficulty to access the high level of complexity of the cell walls clearly shows that there is much more to be studied to unveil hidden details of polysaccharide structures as well as the ways in which they interact within the wall, giving rise to architectural features. The fine structures of sugarcane AX and XG, for instance, remain unknown. The fact that it so happens with the fine structural details of pectin branching is important since we know that pectins play important roles in recalcitrance (Latarullo et al. 2016).
The discovery that a sequential action of glycosyl hydrolases occurs in vivo brings a new perspective to how efficient cell wall dismantling and polysaccharide hydrolysis could be achieved. By imitating natural processes, but at the same time speeding them up, it is possible that biomass could be degraded faster and at the same time with lower enzyme concentrations. By understanding the mechanisms of synthesis and also the forces involved in the assembly of the cell wall composites, one would be able to hydrolyze them efficiently. Furthermore, it would be possible to gain control on how polymers assemble within the cell wall, so that it would be useful not only for bioenergy production but also to design new materials with much higher aggregated value for industry.
References
Aden A, Ruth M, Ibsen K, Jechura J, Neeves K, Sheehan J, Wallace B, Montague L, Slayton A, Lukas J. 2002. Lignocellulosic biomass to ethanol process design and economics utilizing co-current dilute acid prehydrolysis and enzymatic hydrolysis for corn stover. NREL Technical Report, TP-510-32438
Boese BJ, Breaker RR (2007) In vitro selection and characterization of cellulose-binding DNA aptamers. Nucleic Acids Res 35(19):6378–6388
Borin GP, Sanchez CC, De Souza AP, Santana ES, Souza AT, Leme AFP, Squina FM, Buckeridge M, Goldman GH, Oliveira JVC (2015) Comparative secretome analysis of Trichoderma reesei and Aspergillus niger during growth on sugarcane biomass. PLoS One 10(6):e0129275
Buckeridge MS, Santos WD, Tiné MS, De Souza AP (2015) The cell wall architecture of sugarcane and its implications to cell wall recalcitrance. In: Lam E, Carrer H, Silva JA (eds) Compendium of bioenergy Plants: sugarcane. CRC Press, 125p
Buckeridge MS, dos Santos WD, Tiné MAS, De Souza AP (2016) The cell wall architecture of sugarcane and its implications to cell wall recalcitrance. In: Lam E, Carrer H, da Silva JA, Kole C (eds) Compendium of bioenergy plants: sugarcane. CRC Press—Taylor and Francis Group, Boca Raton, pp 31–50
Buckeridge MS, Rayon C, Urbanowicz B, Tiné MAS, Carpita NC (2004) Mixed linkage (1-3),(1-4)-beta-d-glucans of grasses. Cereal Chem 81(1):115–127
Buckeridge MS, De Souza AP (2014) Breaking the “glycomic code” of cell wall polysaccharides may improve second-generation bioenergy production from biomass. Bioenergy Res 7:1065–1073
Buckeridge MS (2010) Seed cell wall storage polysaccharides: models to understand cell wall biosynthesis and degradation. Plant Physiol 154:1017–1023
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the cell wall during growth. Plant J 3:1–30
Crivellari AC (2012) Caracterização estrutural das hemiceluloses de paredes celulares de cana-de-açúcar. Dissertação de Mestrado, Instituto de Biociências, Universidade de São Paulo, São Paulo. Pdf download: http://www.teses.usp.br/teses/disponiveis/41/41132/tde-10102012-084959/. Accessed 23 Aug 2016
De Souza AP, Grandis A, Leite DCC, Buckeridge MS (2014) Sugarcane as a bioenergy source: history, performance, and perspectives for second-generation bioethanol. Bioenergy Res 7:24–35
De Souza AP, Kamei CLA, Torres AF, Pattathil S, Hahn MG, Trindade LM, Buckeridge MS (2015) How cell wall complexity influences saccharification efficiency in Miscanthus sinensis. J Exp Bot. doi:10.1093/jxb/erv183
De Souza AP, Leite DCC, Pathatil S, MG H, MS B (2013) Composition and structure of sugarcane cell wall polysaccharides: implications for second-generation bioethanol production. Bioenergy Res 6:564–579
De Lima DU, Buckeridge MS (2001) Interaction between cellulose and storage xyloglucans: the influence of degree of galactosylation. Carbohydr Polym 46(2):157–163
Enzitec (2016) Anais do XII Seminário Brasileiro de Tecnologia Enzimática. http://www.ucs.br/site/eventos/enzitec-2016/anais/. Accessed 8 Aug 2016
Grandis A, De Souza AP, Tavares EQP, Buckeridge MS (2014) Using natural plant cell wall degradation mechanisms to improve second generation bioethanol. In: McCann M, Buckeridge MS, Carpita NC (eds) Plants & bioenergy. Springer, New York, pp 211–230
Guilherme AA, Dantas PVF, Santos ES, Fernandes FAN, Macedo GR (2015) Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugarcane bagasse. Brazil J Chem Eng 32(1):23–33
Healey AL, Lee DJ, Furtado A, Simmons BA, Henry RJ (2015) Efficient eucalypt cell wall deconstruction and conversion for sustainable lignocellulosic biofuels. Front Bioeng Biotechnol 3:190
Latarullo MB, Tavares EQ, Maldonado GP, Leite DC, Buckeridge MS (2016) Pectins, endopolygalacturonases and bioenergy. Front Plant Sci 7:1401
Low SY, Hill JE, Peccia J (2009) DNA aptamers bind specifically and selectively to (1→3)-β-d-glucans. Biochem Biophys Res Commun 378(4):701–705
Masarin F, Gurpilhares DB, Bafa DCF, Barbosa MHP, Carvalho W, Ferraz A et al (2011) Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnol Biofuels 4:55
Patthathil S, Avci U, Miller JS, Hahn MG (2012) Immunological approaches to plant cell wall and biomass characterization: glycome profiling. In: ME H (ed) Biomass conversion: methods and protocols. Springer, New York, pp 61–72
Peng F, Ren JL, Xu F, Bian J, Peng P, Sun RC (2009) Comparative study of hemicelluloses obtained by graded ethanol precipitation of sugarcane bagasse. J Agric Food Chem 57:6305–6317
Rezende CA, de Lima MA, Maziero P, de Azevedo ER, Garcia W, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:54
Roulin S, Feller U (2001) Reversible accumulation of (1,3, 1,4)-β-d-glucan endohydrolase in wheat leaves under sugar depletion. J Exp Bot 52(365):2323–2332
Roulin S, Buchala AJ, Fincher GB (2002) Induction of (1,3, 1,4)-beta-d-glucan hydrolases in leaves of dark-incubated barley seedlings. Planta 215(1):51–59
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5(4):337–353
Tavares EQP, De Souza AP, Buckeridge MS (2015) How endogenous plant cell wall degradation mechanisms can help achieve higher efficiency in saccharification of biomass. J Exp Bot 66:4133–4143
Tiné MAS, Lima DU, Buckeridge MS (2003) Galactose branching modulates the action of cellulase on seed storage xyloglucans. Carbohydr Polym 52:135–141
Tiné MAS, Silva CO, Lima DU, Carpita NC, Buckeridge MS (2006) Fine structure of a mixed-oligomer storage xyloglucan from seeds of Hymenaea courbaril. Carbohydr Polym 66:444–454
Vohra M, Manwar J, Manmode R, Padgilwar S, Patil S (2014) Bioethanol production: feedstock and current technologies. J Environ Chem Eng 2:573–584
Wang Y, Fan C, Hu H, Li Y, Sun D, Wang Y, Peng L (2016) Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops. Biotechnol Adv 34:997–1017
Zhu X, Patthatil S, Mazumber K, Brehm A, Hahn MG, Dinesh-Kumar SP, Joshi CP (2010) Virus-induced silencing offers a functional platform for studying plant cell wall formation. Mol Plant 3:818–833
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Buckeridge, M.S., De Souza, A.P., Tavares, E.Q.P., Cambler, A.B. (2017). Sugarcane Cell Wall Structure and Degradation: From Monosaccharide Analyses to the Glycomic Code. In: Buckeridge, M., De Souza, A. (eds) Advances of Basic Science for Second Generation Bioethanol from Sugarcane. Springer, Cham. https://doi.org/10.1007/978-3-319-49826-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-49826-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49824-9
Online ISBN: 978-3-319-49826-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)