Abstract
Iodine is a necessary micronutrient and essential for the synthesis of the thyroid hormones. Iodine exposure may also occur as a result from iodine fortifications programs (through salt iodization, fortification of foods, or other routes), medications, dietary supplements, topical iodine antiseptics, radiographic iodinated contrast media, and other sources. Excess iodine exposure, particularly among individuals with underlying thyroid disease, has the potential for inducing hyperthyroidism and hypothyroidism. Iodine-induced thyroid dysfunction can be transient or permanent. With the exception of specific medical indications for the use of supraphysiologic iodine, excessive iodine ingestion and/or exposure should be avoided.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
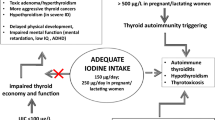
Iodine is an important micronutrient that is crucial for thyroid hormone production. As it is naturally present as a trace element primarily in coastal areas, iodine deficiency was historically and still continues to be a significant public health issue in many regions of the world. It is taken in primarily through the diet, and universal salt iodization and iodine fortification of foods have been adopted worldwide to prevent the adverse effects of iodine deficiency. The U.S. Institute of Medicine, the World Health Organization (WHO), the United Nations Children’s Emergency Fund (UNICEF), and the International Council for the Control of Iodine Deficiency Disorders (ICCIDD) [since renamed the Iodine Global Network (IGN)] recommend a daily iodine intake of 150 μg in adults [1, 2].
However, in addition to the diet, iodine exposure can also occur incidentally from medical procedures or medications and other sources, often in concentrations much higher than the recommended daily intake levels. Excess iodine intake or exposure, particularly if acute, can have adverse consequences, due to its potential for inducing thyroid dysfunction, and both iodine-induced hypothyroidism and hyperthyroidism can result. Based on limited supporting data for the threshold for iodine levels that would be deemed unsafe, the U.S. Institute of Medicine has set a Tolerable Upper Level (TUL) (the approximate threshold below which significant adverse effects are unlikely to occur in a healthy population) for iodine at 1,100 μg/day in adults [1], while the WHO, UNICEF, and IGN advise that pregnant and lactating women ingest no more than 500 μg of iodine per day [2] (Table 6.1).
Population Measures of Excess Iodine Exposure
Because of significant variations in dietary iodine intake, the iodine status of a given individual cannot be reliably measured [3]. Thus, iodine nutrition can only be measured in populations, which has historically been done using median urinary iodine concentrations (UIC). Median UICs are a good biomarker of iodine intake. As defined by the WHO, UNICEF, and IGN, median UICs greater than 300 μg/L are consistent with excessive iodine status among 6–12 year old children [2].
Alternatively, thyroglobulin levels measured from dried blood spots may potentially also serve as a measure of iodine status among school-aged children. In one report, the mean dried blood spot thyroglobulin concentration was significantly higher in healthy 6–12 year old children in whom the median UIC was greater than 300 μg/L, suggesting that dried blood spot thyroglobulin levels may serve as a sensitive marker for iodine excess in this population [4].
Thyroidal Response to Iodine Excess
The normal physiologic adaptation to excess iodine exposure is known as the acute Wolff-Chaikoff effect. From experiments during the 1940s, it was recognized that rats exposed to high amounts of iodide through the peritoneum have a reduction in thyroid hormone synthesis that persists for approximately 24 h [5] and thus a corresponding decreased risk for the development of iodine-induced thyrotoxicosis. Although the exact mechanism for this decrease in thyroid hormone production (the acute Wolff-Chaikoff effect) is not completely understood, it has been postulated that several compounds, including intrathyroidal iodolactones, iodoaldehydes, and/or iodolipids, on thyroid peroxidase activity, are formed and work to inhibit thyroid hormone synthesis in the thyroid follicular cell [6]. Furthermore, there may also be a reduction of deiodinase activity within the thyroid, as a result of the excess iodine exposure, which contributes toward decreased thyroid hormone production.
The physiologic phenomena of the acute Wolff-Chaikoff effect and decreased thyroid hormone production are transient in most individuals, as escape from the acute Wolff-Chaikoff effect occurs and normal thyroid hormone production resumes within a few days [7]. The mechanism for the escape was elucidated in 1999, when Eng and colleagues reported that there is a marked decrease in expression of the sodium-iodide symporter (NIS) present on the basolateral membrane of thyroid follicular cells 24 h after the excess iodine exposure occurs [8]. NIS is a 13-transmembrane glycoprotein which mediates the active transport of iodine from the circulation into the thyroid [9], and decreased thyroidal NIS expression thus results in lower intrathyroidal iodine concentrations. The reduction in intrathyroidal iodine stores results in decreased formation of the iodinated inhibitory substances on thyroid hormone synthesis, thereby enabling the resumption of normal thyroid hormone production.
However, there are certain instances in which the acute Wolff-Chaikoff effect or escape from the effect do not occur, resulting in iodine-induced thyrotoxicosis or iodine-induced hypothyroidism, respectively. Predisposing factors include those which result in dysregulation of the thyroid follicular cell. Thus, individuals with a history of Hashimoto’s thyroiditis, Graves’ disease (even if treated), postpartum thyroiditis, subacute thyroiditis, partial thyroidectomy, or use of lithium (which traps iodine in the thyroid gland), may be particularly susceptible. The developing fetus or neonate (in whom thyroid development is still occurring) is also particularly vulnerable. In these instances, the iodine-induced thyroid dysfunction may be transient or permanent.
Iodine-Induced Hyperthyroidism
In certain susceptible patients, the excess iodine load provides a rich substrate for increased thyroid hormone production. Iodine-induced hyperthyroidism (the Jod-Basedow phenomenon) was initially described in the early 1800s, when thyrotoxicosis was observed to be more common among those with endemic goiter treated with iodine supplementation, compared to non-goitrous individuals. The frequency of iodine-induced hyperthyroidism varies by the degree of endemic iodine deficiency in the local region and timecourse of iodine exposure; a summary by country has been published [10]. The development of hyperthyroidism following excess iodine exposure can occur within days to weeks [11].
Iodine-induced hyperthyroidism may be transient or permanent, and risk factors include nontoxic or diffuse nodular goiter, latent Graves’ disease, and longstanding iodine deficiency [12, 13]. Endemic iodine deficiency predisposes to but not is not necessary for the development of iodine-induced hyperthyroidism, as it has also been described among euthyroid patients with nodular goiter in iodine sufficient areas [13, 14].
Iodine-Induced Hypothyroidism
The underlying mechanism of iodine-induced hypothyroidism is inhibition of thyroid hormone synthesis due to failure to escape from the acute Wolff-Chaikoff effect, but hypothyroidism may also be due to the development of autoimmune thyroiditis resulting from the iodine load. Some data suggest that exposure to high iodine concentrations may also decrease thyroid hormone synthesis and release, as reported in several small studies which show mild decreases in serum thyroid hormone levels and increases in the serum TSH level to the upper limit of the normal range [15–17]. The development of iodine-induced hypothyroidism can occur as early as weeks following exposure to excess iodine [18].
The developing fetus and young infant are particularly vulnerable to the effects of iodine excess, as normal thyroid function is critical for neurodevelopment and somatic growth. It is thought that the ability to fully escape the Wolff-Chaikoff effect does not mature until around 36 weeks gestation, so that although pregnant women who receive a large iodine load might be able to maintain normal thyroid function, their fetuses could become selectively hypothyroid. One recent case series described three newborn infants who were found to be profoundly hypothyroid (serum TSH concentrations ranging up to 419 mIU/L) following maternal ingestion of an iodine-rich supplement (12.5 mg/day) daily during pregnancy [19]. However, although this is not routinely used (see section below regarding “Medical Uses of Excess Iodine”), there is experience in Japan with using high-dose iodine (as potassium iodide, KI) for the treatment of maternal Graves’ hyperthyroidism with few, if any, adverse outcomes. One report demonstrated no observed fetal thyroid dysfunction among 283 women treated with KI in the first trimester of pregnancy [20].
Iodine-Induced Thyroid Autoimmunity
Iodine status has also been thought to be an important contributor toward the development of thyroid autoimmunity in a dose-dependent manner that acts through both cellular and humoral responses [21]. In a study of three regions in China with mildly deficient iodine intake, more than adequate iodine intake, and excessive iodine intake, the prevalence of autoimmune thyroiditis was 0.2 %, 1.0 %, and 1.3 %, respectively [22]. Similarly, in the 4–5 years after the introduction of a mandatory program of salt and bread iodization Denmark in 2000 there was an increase in serum thyroid peroxidase and thyroid anti-thyroglobulin antibody positivity, compared to the period prior to the program [23]. The increase was seen in all age groups studied, but was particularly frequent among young women. Most individuals developed only low titers of the serum thyroid antibodies.
Potential Sources of Excess Iodine Exposure
Iodine can be present in concentrations of up to several thousand-fold higher than the recommended levels [1] from several common sources. These may include iodine-containing foods, medications, supplements, and as iodinated contrast agents used for radiologic studies. Estimates for the iodine content in some common foods and medical agents are shown in Table 6.2. Ingestion or exposure to iodine-rich substances can, in some susceptible individuals, result in thyroid dysfunction from the excess iodine load.
Iodine Fortification
Iodine fortification has been the primary method of ameliorating iodine deficiency, the most common cause of preventable intellectual impairment, on a global scale over the past century. Small amounts of iodine have been administrated as iodized oil orally and intramuscularly, introduced into the water supply, used in crop irrigation, incorporated into animal fodder, and introduced into food through salt iodization, bread iodophors, and other products [24]. Goyle et al reported the successful use of fortified micronutrient biscuits in raising the median urinary iodine levels of Indian schoolgirls [25].
Although iodine supplementation and food fortification efforts have decreased the number of those at risk for iodine deficiency and its associated sequelae, particularly in the most recent decades, the use of iodine has also led to concerns regarding excessive iodine exposure in selected individuals.
Iodine supplementation and food fortification have also been shown to be associated with increased incidences of thyroid dysfunction, thyroid autoimmunity, and in some reports, thyroid cancer. Subclinical hypothyroidism was more common in those supplemented with a 400 μg iodine daily tablet, compared to placebo, in a study of over 200 Chinese adults [26] similar to the results of other studies in Denmark [27] and New Zealand [28], which also showed an increased prevalence of transient hyperthyroidism. There has also been an increase in the incidence of thyroid autoimmunity [29] and thyroid cancer, particularly papillary thyroid cancer, following iodine supplementation in some studies. The rise in thyroid cancer over the past few decades is multifactorial, but has been discussed as perhaps a consequence of changes in iodine nutrition from many countries that have analyzed the trend before and after various iodine fortification programs [30–33].
Diet
National market surveys in the U.S. show that dairy products, some breads, seaweed and other seafood, and iodized salt are the most common sources of dietary iodine nutrition [34]. Salt iodization is viewed to be one of the safest and most effective methods of achieving population iodine sufficiency. Iodine fortification of all food-grade salt is mandated in approximately 120 countries, although the enforcement and degree of implementation of these efforts in individual countries are unknown [35]. Salt is not generally considered to be a source of iodine excess as long as appropriate monitoring of salt iodization programs is conducted.
The iodine content in some grain products stems from the use of iodate dough conditioners, which help to preserve the shelf stability of bread. In Tasmania, bread iodation was introduced in 1966 as a prophylactic measure against iodine deficiency and endemic goiter [36]. However, in the initial few years following this intervention, several reports demonstrated an increase in the incidence of thyrotoxicosis that was thought to result from excess iodine exposure [37, 38]. In the U.S., bread iodation was thought to be associated with the decreases in thyroidal radioactive iodine uptake values nationally during the 1960s [39]. In Australia, due to previous reports of low iodine status, iodization of bread was mandated in 2009, which resulted in a modest increase of the median urinary iodine concentration among pregnant women [40]; there are no known reports of iodine-induced thyroid dysfunction as a result of this action in this population.
One example of an iodine-rich food is seaweed, and the iodine content of seaweed can vary widely [41]. Cases of iodine-induced thyrotoxicosis have frequently been reported among seaweed users, including one woman who drank kelp-containing tea for four weeks [42], another patient who had a longstanding history of using kelp-containing dietary supplements [43], and a woman who was consuming a kelp-based diet plan [44]. Recently, Kasahara et al reported a case of delayed onset congenital hypothyroidism in an infant with a mutation in the dual oxidase (DUOX2) gene, which is known to be associated with transient congenital hypothyroidism, which was exacerbated by maternal ingestion of excessive seaweed during pregnancy [45]. In other reports, both short-term and chronic seaweed ingestion has been reported to be associated with modest elevations of serum TSH without overt thyroid dysfunction [46–48]. Seaweed soup is commonly ingested by postpartum women as a cultural practice in some regions and contains over 1,700 μg per 250 mL serving [49]. Japanese reports have shown the positive correlation between maternal iodine intake of seaweed soup during pregnancy and elevated serum TSH concentrations of their newborn infants [50, 51], similar to several Australian cases of neonatal hypothyroidism arising from maternal ingestion of seaweed soup and of soy milk manufactured with seaweed during pregnancy [52].
Amiodarone
Amiodarone is an iodine-rich medication which has been in use since the 1960s and which was approved for the management of ventricular tachyarrhythmias in the U.S. in 1985 [53]. It has a high iodine content (37 % by weight), so that one 200 mg tablet contains 75 mg iodine, equivalent to several thousand times the recommended daily requirement of 150 μg in adults [1]. Amiodarone has a long half-life (approximately 100 days) and can accumulate in adipose tissue, the liver, and the lungs.
Due to the its high iodine content, amiodarone use is associated with type 1 amiodarone-induced thyrotoxicosis (AIT) in 4–28 % of patients [54, 55], depending on whether preexisting thyroid disease is present and overall iodine status from nutritional intake. The two types of AIT are important to distinguish, as their recommended management is different. Type 1 AIT is iodine-induced and is associated with increased thyroid hormone synthesis and treated with thionamides, beta-blockers, and if available, perchlorate, while type II AIT is characterized by a destructive thyroiditis and usually managed with corticosteroids if it is severe [56]. Type 1 AIT is more commonly seen in individuals with underlying nodular goiter or Graves’ disease, whereas Type 1 AIT is more frequent in individuals residing in iodine-sufficient areas [56].
Amiodarone use can also result in iodine-induced hypothyroidism. Amiodarone-induced hypothyroidism (AIH) is more common than AIT in iodine-sufficient regions, and particularly among individuals with chronic lymphocytic thyroiditis [53]. As in other examples of iodine-induced hypothyroidism, AIH may spontaneously remit or become permanent.
Iodinated Contrast Media
Iodinated contrast media is a common iodine-rich substance used in radiographic diagnostic imaging, and a typical dose contains 13,500 μg of free iodide and 15–60 g of bound iodine [57], amounts which are equivalent to several hundred times the daily recommended intakes. Both ionic and nonionic contrast media contain approximately 300–370 mg iodine/mL [58]. Several case reports have demonstrated the effects of thyroid dysfunction arising after iodinated contrast use [59–61]. Studies in Germany and the U.S. showed that a small proportion of patients who received either coronary angiography or an iodinated CT scan developed subclinical hypothyroidism approximately one week after the exam [62, 63]. In a Turkish study of 101 patients who underwent coronary angiography, there was a small increased risk of subclinical hyperthyroidism at up to 8 weeks following the iodine exposure [64]. However, one small study showed that administration of intravenous iodine contrast during pregnancy did not result in a significantly increased incidence of fetal thyroid dysfunction [65].
Several large population studies have also examined the associations between iodinated contrast media use and the development of thyroid dysfunction. In a nested case-control study of adult patients at a Boston hospital over 20 years, there were significantly increased risks for both incident hyperthyroidism and hypothyroidism following iodinated contrast media exposure [57]. These findings were also confirmed in a community-based Boston cohort [66] and two general population cohorts in Taiwan [67, 68].
The effects of iodinated contrast media use in diagnostic imaging may not be immediate. In a prospective study of 54 euthyroid adults who received elective outpatient iodinated computed tomography (CT) scans, the mean time to achieve peak urinary iodine concentration (UIC) was 1.1 ± 0.5 weeks, and normalization of UIC was not achieved until 5.2 ± 4.0 weeks [69]. In this report, 22 % of the subjects developed incident thyroid dysfunction over the up to three months of follow up in the study. The effects of iodinated contrast media on inducing potential thyroid dysfunction are particularly important in the neonate. One case series reported three neonates who presented with profound hypothyroidism (serum TSH concentrations ranging from 30–175 mIU/L) within one month of having undergone cardiac catherization(s) which utilized iodinated contrast [70]. Another study analyzed pediatric patients (<18 years old) with thyroid dysfunction, matched 1:1 to euthyroid controls by age, sex, and race, and reported a three-fold increased risk of incident thyroid function abnormalities among those who had a history of iodinated contrast media exposure, with nearly 11 months as a the mean time period between iodine exposure and thyroid dysfunction [71]. These data confirm the several case reports that have been made known to the U.S. Food and Drug Administration concerning the potential for hypothyroidism resulting from use of iodine-containing contrast agents in infants [72].
Guidelines by the Contrast Media Safety Committee of the European Society of Urogenital Radiology advocate that high-risk patients be monitored for thyroid dysfunction following iodinated contrast use [73]. There are currently no guidelines for screening or following at-risk patients receiving iodinated contrast in the U.S.
Topical Iodine
The development of thyroid dysfunction related to transdermal iodine use has been most commonly reported in hospitalized neonates. A recent study in Israel reported significantly higher serum TSH concentrations in preterm neonates who had received topical iodinated antiseptic cleansers, compared to the preterm neonates who received alcohol-based topic cleansers (15.4 vs. 7.8 mIU/L, p<0.01) [74]. However, most neonatal intensive care units in the U.S. have phased out routine use of topical iodine given the concerns regarding excess iodine iodine exposure in this population [75]. Iodine is also commonly used as a topical antiseptic in many surgical settings (including mediastinal irrigation [76]) and among burn victims, whose ability to absorb topical iodine may be increased. Iodine-induced thyrotoxicosis and a review of the differential diagnosis was also described in a paraplegic woman in the U.S. who had applied topical povidone-iodine prior to urinary self-catheterization several times daily for many years [6].
Drinking Water
The iodine content of drinking water has also been a reported source of excess iodine exposure. Among Sahawari refugee children in the Algerian desert, where the median iodine content of household drinking water was 108 μg/L (interquartile range 77–297 μg/L) during a 2007 assessment, the median UIC was 565 μg/L (interquartile range 357–887 μg/L) [77]. Naturally high concentrations of iodine in drinking water have also been reported in some regions of China; one region of Hebei province reported a median UIC of 518 μg/L (interquartile range 347–735 μg/L) among children residing in the area [78].
Reversible elevations in serum TSH have been observed among U.S. astronauts drinking iodinated water [79] and individuals ingesting water purified by iodinated tablets [80]. In the 1990s, due to use of a faulty iodine-based water filtration system, small increases in serum TSH concentrations were detected in American Peace Corp workers in Niger; these changes resolved following discontinuation of the iodinated water source [81].
Miscellaneous Sources of Excess Iodine Exposure
Other sources of potentially excessive iodine exposure include various expectorants, vitamins and supplements, food preservatives, prescribed medications, parenteral preparations, topical antiseptics, mouthwashes [82], and vaginal douches [16]. Iodine-containing expectorants were historically a potentially concerning source of iodine excess, particularly in patients with cystic fibrosis who were treated with these agents routinely. However, iodine-containing expectorants are now longer regularly in use, and a recent study shows that overt iodine-induced thyroid dysfunction among cystic fibrosis patients is uncommon [83].
Medical Uses of Excess Iodine
There are specific clinical settings in which supraphysiologic iodine is medically indicated. Saturated solution of potassium iodide (SSKI), or Lugol’s solution, for which the generic formulation contains 1,000 mg potassium iodide (KI) per mL, can be used for the rapid treatment of hyperthyroidism. Use of SSKI is usually reserved for patients with thyroid storm or in patients who are managed preoperatively for Graves’ disease and administered in conjunction with antithyroidal and other therapies. In such patients, pharmacologic doses of iodine should be administered after a thionamide has been first dosed to block thyroid hormone synthesis. Also, supraphysiologic iodine ingestion may be recommended following a nuclear emergency to prophylax against the exposure to radioactive iodine.
Recommendations Cautioning Against Excess Iodine Exposure
TUL thresholds have been defined by U.S. Institute of Medicine as the highest average daily intake level of a nutrient which is unlikely to pose any adverse health effects (defined as a significant alteration to the human structure or its function) in the majority of population [1]. The range of intake between the Recommended Dietary Allowance (RDA) and TUL is not one in which there is a possible beneficial effect, but it is likely biologically tolerable. At chronic daily intakes above the TUL, adverse effects may increase. The RDA and TUL for iodine are 150 μg/day and 1,100 μg/day in adults, respectively [1] (Table 6.1).
The derivation of the TULs for any given nutrient is based on risk assessment models, in which the probability of ingestion/exposure to the nutrient having adverse health effects in human is systematically evaluated using both qualitative and quantitative available data from the animal and human literature [1]. Risk assessment of nutrients must acknowledge the scientific uncertainties of the information that may be present and make explicit the basis for judgments. Models are based on identifying the potential hazard, assessment of a dose response, assessment of the nutrient intake in humans, and characterization of the risk. This process involves identification of a no observed adverse effect level (NOAEL) or lowest observed adverse effect level (LOAEL). The LOAEL values which informed the US TUL were based on two small dose-response studies [17, 84]. The NOAEL is the most sensitive measure of a nutrient’s toxicity and takes into account areas of uncertainty (termed uncertainty factors [UF]) in the available literature. The TULs of all nutrients are based on the assumption of chronic daily exposure from all sources, including food, water, and supplements.
The WHO, UNICEF, and IGN also advise that pregnant and lactating women ingest no more than 500 μg of iodine per day [2] (Table 6.1). Recognizing that many iodine-containing supplements contain excessive iodine amounts, the American Thyroid Association advises against the ingestion of iodine supplements containing more than 500 μg of iodine per day in the general population [85].
Abbreviations
- AIH:
-
Amiodarone-induced hypothyroidism
- AIT:
-
Amiodarone-induced thyrotoxicosis
- CT:
-
Computed tomography
- ICCIDD:
-
International Council for the Control of Iodine Deficiency Disorders
- IGN:
-
Iodine Global Network
- KI:
-
Potassium iodide
- LOAEL:
-
Lowest observed adverse effect level
- NIS:
-
Sodium/iodide symporter
- NOAEL:
-
No observed adverse effect level
- RDA:
-
Recommended Dietary Allowance
- SSKI:
-
Saturated solution of potassium iodide
- TSH:
-
Thyroid stimulating hormone
- TUL:
-
Tolerable upper level
- UF:
-
Uncertainty factors
- UIC:
-
Urinary iodine concentration
- UNICEF:
-
United Nations Children’s Emergency Fund
- WHO:
-
World Health Organization
References
U.S. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes. Washington, DC: National Academy Press; 2006. p. 320.
WHO, UNICEF, and ICCIDD. Assessment of the iodine deficiency disorders and monitoring their elimination. Geneva: World Health Organization; 2007. Report No.: WHO/NHD/01.1.
Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53(5):401.
Zimmermann MB, Aeberli I, Andersson M, Assey V, Yorg JA, Jooste P, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100–299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab. 2013;98(3):1271.
Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174(2):555.
Pramyothin P, Leung AM, Pearce EN, Malabanan AO, Braverman LE. Clinical problem-solving. A hidden solution. New England J Med. 2011;365(22):2123.
Braverman LE, Ingbar SH. Changes in thyroidal function during adaptation to large doses of iodide. J Clin Invest. 1963;42:1216.
Eng PH, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N, et al. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140(8):3404.
Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379(6564):458.
Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83–100.
Roti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid Off J Am Thyroid Assoc. 2001;11(5):493.
Martin FI, Tress BW, Colman PG, Deam DR. Iodine-induced hyperthyroidism due to nonionic contrast radiography in the elderly. Am J Med. 1993;95(1):78.
Higgs M, Hull E, Lujan E. A case report of post-operative Jöd-Basedow phenomennon following oral and IV iodine contrast administration. Case Rep Endocrinol. 2014;2014:980283.
Vagenakis AG, Wang CA, Burger A, Maloof F, Braverman LE, Ingbar SH. Iodide-induced thyrotoxicosis in Boston. New England J Med. 1972;287(11):523.
Saberi M, Utiger RD. Augmentation of thyrotropin responses to thyrotropin-releasing hormone following small decreases in serum thyroid hormone concentrations. J Clin Endocrinol Metab. 1975;40(3):435.
Safran M, Braverman LE. Effect of chronic douching with polyvinylpyrrolidone-iodine on iodine absorption and thyroid function. Obstet Gynecol. 1982;60(1):35.
Paul T, Meyers B, Witorsch RJ, Pino S, Chipkin S, Ingbar SH, et al. The effect of small increases in dietary iodine on thyroid function in euthyroid subjects. Metab Clin Exp. 1988;37(2):121.
Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-Induced hypothyroidism. Thyroid Off J Am Thyroid Assoc. 2001;11(5):501.
Connelly KJ, Boston BA, Pearce EN, Sesser D, Snyder D, Braverman LE, et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr. 2012;161(4):760.
Yoshihara A, Noh JY, Watanabe N, Mukasa K, Ohye H, Suzuki M, et al. Substituting potassium iodide for methimazole as the treatment for graves’ disease during the first trimester may reduce the incidence of congenital anomalies: a retrospective study at a single medical institution in Japan. Thyroid. 2015;25(10):1155–61.
Teng X, Shan Z, Teng W, Fan C, Wang H, Guo R. Experimental study on the effects of chronic iodine excess on thyroid function, structure, and autoimmunity in autoimmune-prone NOD.H-2 h4 mice. Clin Exp Med. 2009;9(1):51–9.
Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354(26):2783–93.
Pedersen IB, Knudsen N, Carlé A, Vejbjerg P, Jørgensen T, Perrild H, et al. A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol. 2011;75(1):120–6.
Schiess S, Cressey PJ, Thomson BM. Predictive modelling of interventions to improve iodine intake in New Zealand. Public Health Nutr. 2012;15(10):1932.
Goyle A, Prakash S. Efficacy of multi-micronutrient fortified biscuits on urinary iodine levels of adolescent girls from Jaipur, India. Malaysian J Nutr. 2011;17(2):143.
Sang Z, Wang PP, Yao Z, Shen J, Halfyard B, Tan L, et al. Exploration of the safe upper level of iodine intake in euthyroid Chinese adults: a randomized double-blind trial. Am J Clin Nutr. 2012;95(2):367.
Laurberg P, Jorgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, et al. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol/Eur Fed Endocrine Soc. 2006;155(2):219.
Thomson CD, Campbell JM, Miller J, Skeaff SA. Minimal impact of excess iodate intake on thyroid hormones and selenium status in older New Zealanders. Eur J Endocrinol/Eur Fed Endocrine Soc. 2011;165(5):745.
Kahaly GJ, Dienes HP, Beyer J, Hommel G. Iodide induces thyroid autoimmunity in patients with endemic goitre: a randomised, double-blind, placebo-controlled trial. Eur J Endocrinol. 1998;139(3):290–7.
Dong W, Zhang H, Zhang P, Li X, He L, Wang Z, et al. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med Sci Monit Int Med J Exp Clin Res. 2013;19:49.
Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int J Cancer Journal international du cancer. 2012;131(10):2360.
Gomez Segovia I, Gallowitsch HJ, Kresnik E, Kumnig G, Igerc I, Matschnig S, et al. Descriptive epidemiology of thyroid carcinoma in Carinthia, Austria: 1984–2001. Histopathologic features and tumor classification of 734 cases under elevated general iodination of table salt since 1990: population-based age-stratified analysis on thyroid carcinoma incidence. Thyroid. 2004;14(4):277–86.
Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res. 2015;8:8.
Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration’s Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008;18(6):571.
Dasgupta PK, Liu Y, Dyke JV. Iodine nutrition: iodine content of iodized salt in the United States. Environ Sci Technol. 2008;42(4):1315.
Clements FW. Goitre prophylaxis by addition of potassium iodate to bread. Experience in Tasmania. Lancet. 1970;1(7645):489–92.
Stewart JC, Vidor GI, Buttifield IH, Hetzel BS. Epidemic thyrotoxicosis in northern Tasmania: studies of clinical features and iodine nutrition. Aust N Z J Med. 1971;1(3):203–11.
Adams DD, Kennedy TH, Stewart JC, Utiger RD, Vidor GI. Hyperthyroidism in Tasmania following iodide supplementation: measurements of thyroid-stimulating autoantibodies and thyrotropin. J Clin Endocrinol Metab. 1975;41(2):221–8.
Leung AM, Braverman LE, Pearce EN. History of U.S. iodine fortification and supplementation. Nutrients. 2012;4(11):1740.
Clifton VL, Hodyl NA, Fogarty PA, Torpy DJ, Roberts R, Nettelbeck T, et al. The impact of iodine supplementation and bread fortification on urinary iodine concentrations in a mildly iodine deficient population of pregnant women in South Australia. Nutr J. 2013;12:32.
Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid Off J Am Thyroid Assoc. 2004;14(10):836.
Mussig K, Thamer C, Bares R, Lipp HP, Haring HU, Gallwitz B. Iodine-induced thyrotoxicosis after ingestion of kelp-containing tea. J Gen Intern Med. 2006;21(6):C11.
Eliason BC. Transient hyperthyroidism in a patient taking dietary supplements containing kelp. J Am Board Fam Pract. 1998;11(6):478.
Di Matola T, Zeppa P, Gasperi M, Vitale M. Thyroid dysfunction following a kelp-containing marketed diet. BMJ Case Rep 2014;2014.
Kasahara T, Narumi S, Okasora K, Takaya R, Tamai H, Hasegawa T. Delayed onset congenital hypothyroidism in a patient with DUOX2 mutations and maternal iodine excess. Am J Med Genet Part A. 2013;161A(1):214.
Teas J, Braverman LE, Kurzer MS, Pino S, Hurley TG, Hebert JR. Seaweed and soy: companion foods in Asian cuisine and their effects on thyroid function in American women. J Med Food. 2007;10(1):90.
Miyai K, Tokushige T, Kondo M, Iodine RG. Suppression of thyroid function during ingestion of seaweed “Kombu” (Laminaria japonoca) in normal Japanese adults. Endocrine J. 2008;55(6):1103.
Clark CD, Bassett B, Burge MR. Effects of kelp supplementation on thyroid function in euthyroid subjects. Endocr Pract Off J Am Col Endocrinol Am Assoc Clin Endocrinol. 2003;9(5):363.
Rhee SS, Braverman LE, Pino S, He X, Pearce EN. High iodine content of Korean seaweed soup: a health risk for lactating women and their infants? Thyroid Off J Am Thyroid Assoc. 2011;21(8):927.
Nishiyama S, Mikeda T, Okada T, Nakamura K, Kotani T, Hishinuma A. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid Off J Am Thyroid Assoc. 2004;14(12):1077.
Emder PJ, Jack MM. Iodine-induced neonatal hypothyroidism secondary to maternal seaweed consumption: a common practice in some Asian cultures to promote breast milk supply. J Paediatr Child Health. 2011;47(10):750.
Crawford BA, Cowell CT, Emder PJ, Learoyd DL, Chua EL, Sinn J, et al. Iodine toxicity from soy milk and seaweed ingestion is associated with serious thyroid dysfunction. Med J Aust. 2010;193(7):413.
Jabrocka-Hybel A, Bednarczuk T, Bartalena L, Pach D, Ruchała M, Kamiński G, et al. Amiodarone and the thyroid. Endokrynol Pol. 2015;66(2):176–86.
Vorperian VR, Havighurst TC, Miller S, January CT. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol. 1997;30(3):791–8.
Theodoraki A, Vanderpump MP. Thyrotoxicosis associated with the use of amiodarone: the utility of ultrasound in patient management. Clin Endocrinol. 2015;175(6):1037–47.
De Leo S, Lee SY, LE. B. Hyperthyroidism. Lancet. 2016;388:906–18.
Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172(2):153.
Lee SY, Rhee CM, Leung AM, Braverman LE, Brent GA, Pearce EN. A review: radiographic iodinated contrast media-induced thyroid dysfunction. J Clin Endocrinol Metab. 2015;100(2):376–83.
Alkhuja S, Pyram R, Odeyemi O. In the eye of the storm: iodinated contrast medium induced thyroid storm presenting as cardiopulmonary arrest. Heart Lung J Crit Care. 2013;42(4):267.
Foppiani L, Cascio C, Lo PG. Iodine-induced hyperthyroidism as combination of different etiologies: an overlooked entity in the elderly. Aging Clin Exp Res. 2015;28(5):1023–7.
Iakovou I, Zapandiotis A, Mpalaris V, Goulis DG. Radio-contrast agent-induced hyperthyroidism: case report and review of the literature. Arch Endocrinol Metab. 2016;60(3):287–9.
Gartner W, Weissel M. Do iodine-containing contrast media induce clinically relevant changes in thyroid function parameters of euthyroid patients within the first week? Thyroid Off J Am Thyroid Assoc. 2004;14(7):521.
Koroscil TM, Pelletier PR, Slauson JW, Hennessey J. Short-term effects of coronary angiographic contrast agents on thyroid function. Endocr Pract Off J Am Col Endocrinol Am Assoc Clin Endocrinol. 1997;3(4):219.
Ozkan S, AS O, Kayatas K, Demirtunc R, Eren M, Uslu H, et al. Thyroid functions after contrast agent administration for coronary angiography: a prospective observational study in euthyroid patients. Anadolu kardiyoloji dergisi: AKD = The Anatolian J Cardiol. 2013;13(4):363.
Kochi MH, Kaloudis EV, Ahmed W, Moore WH. Effect of in utero exposure of iodinated intravenous contrast on neonatal thyroid function. J Comput Assist Tomogr. 2012;36(2):165.
Rhee CM, Lynch KE, Zandi-Nejad K, Pearce EN, Alexander EK, Brunelli SM. Iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism in a community-based cohort. Endocrinol Stud. 2013;3(e8):27–30.
Kornelius E, Chiou JY, Yang YS, Peng CH, Lai YR, Huang CN. Iodinated contrast media increased the risk of thyroid dysfunction: a 6-year retrospective cohort study. J Clin Endocrinol Metab. 2015;100(9):3372–9.
Hsieh MS, Chiu CS, Chen WC, Chiang JH, Lin SY, Lin MY, et al. Iodinated contrast medium exposure during computed tomography increase the risk of subsequent development of thyroid disorders in patients without known thyroid disease: a nationwide population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore). 2015;94(50):e2279.
Lee SY, Chang DL, He X, Pearce EN, Braverman LE, Leung AM. Urinary iodine excretion and serum thyroid function in adults after iodinated contrast administration. Thyroid. 2015;25(5):471–7.
Thaker VV, Leung AM, Braverman LE, Brown RS, Levine B. Iodine-induced hypothyroidism in full-term infants with congenital heart disease: more common than currently appreciated? J Clin Endocrinol Metab. 2014;99(10):3521–6.
Barr ML, Chiu HK, Li N, Yeh MW, Rhee CM, Casillas J, et al. Thyroid dysfunction in children exposed to iodinated contrast media. J Clin Endocrinol Metab. 2016;101(6):2366–70.
U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA advises of rare cases of underactive thyroid in infants given iodine-containing contrast agents for medical imaging. November 15, 2015. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm472782.htm.
van der Molen AJ, Thomsen HS, Morcos SK. Contrast media safety committee ESoUR. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol. 2004;14(5):902.
Linder N, Davidovitch N, Reichman B, Kuint J, Lubin D, Meyerovitch J, et al. Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants. J Pediatr. 1997;131(3):434.
Belfort MB, Pearce EN, Braverman LE, He X, Brown RS. Low iodine content in the diets of hospitalized preterm infants. J Clin Endocrinol Metab. 2012;97(4):E632.
Kovacikova L, Kunovsky P, Skrak P, Hraska V, Kostalova L, Tomeckova E. Thyroid hormone metabolism in pediatric cardiac patients treated by continuous povidone-iodine irrigation for deep sternal wound infection. Eur J Cardiothorac Surg. 2002;21(6):1037–41.
Henjum S, Barikmo I, Gjerlaug AK, Mohamed-Lehabib A, Oshaug A, Strand TA, et al. Endemic goitre and excessive iodine in urine and drinking water among Saharawi refugee children. Public Health Nutr. 2010;13(9):1472–7.
Lv S, Xie L, Xu D, Wang Y, Jia L, Du Y. Effect of reducing iodine excess on children’s goiter prevalence in areas with high iodine in drinking water. Endocrine. 2016;52(2):296–304.
McMonigal KA, Braverman LE, Dunn JT, Stanbury JB, Wear ML, Hamm PB, et al. Thyroid function changes related to use of iodinated water in the U.S. Space Program. Aviat Space Environ Med. 2000;71(11):1120.
Georgitis WJ, McDermott MT, Kidd GS. An iodine load from water-purification tablets alters thyroid function in humans. Mil Med. 1993;158(12):794.
Pearce EN, Gerber AR, Gootnick DB, Khan LK, Li R, Pino S, et al. Effects of chronic iodine excess in a cohort of long-term American workers in West Africa. J Clin Endocrinol Metab. 2002;87(12):5499.
Ader AW, Paul TL, Reinhardt W, Safran M, Pino S, McArthur W, et al. Effect of mouth rinsing with two polyvinylpyrrolidone-iodine mixtures on iodine absorption and thyroid function. J Clin Endocrinol Metab. 1988;66(3):632.
Lee SY, Chesdachai S, Lee MJ, He XM, Tangpricha V, Braverman LE. Thyroid function in patients with cystic fibrosis: no longer a concern? Thyroid. 2016;26:875–9.
Gardner DF, Centor RM, Utiger RD. Effects of low dose oral iodide supplementation on thyroid function in normal men. Clin Endocrinol. 1988;28(3):283–8.
Leung AM, Avram AM, Brenner AV, Duntas LH, Ehrenkranz J, Hennessey JV, et al. Potential risks of excess iodine ingestion and exposure: statement by the American Thyroid Association Public Health Committee. Thyroid. 2015;25(2):145–6.
Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10(3):136–42.
Acknowledgement
This work was supported in part by NIH 5K23HD068552.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Leung, A.M. (2017). The Effects of Iodine Excess. In: Pearce, E. (eds) Iodine Deficiency Disorders and Their Elimination. Springer, Cham. https://doi.org/10.1007/978-3-319-49505-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-49505-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49504-0
Online ISBN: 978-3-319-49505-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)