Abstract
6-Mercaptopurine (6-MP) and its parent drug azathioprine (AZA) are well known for their immunosuppressive and lymphocytotoxic properties. These antimetabolite drugs have been shown to suppress disease activity in up to 70% of children with moderate to severe IBD; among these patients, 50% will achieve a clinical response after 4 months of continuous maintenance 6-MP therapy suggesting that inherent differences in drug metabolism or immunomodulation may influence clinical responsiveness to therapy. Moreover, although the overall risk of 6-MP-induced toxicity is low, these same presumed pharmogenomic determinants can only explain in part susceptibility to untoward antimetabolite-associated side effects, including the risk of malignancy. The risk of EBV-associated lymphoma among patients with leukemia on maintenance 6-MP therapy is well known, and now has also been shown in patients with IBD on long-term antimetabolite therapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- 6-Mercaptopurine
- Azathioprine
- Thiopurine
- Pediatric inflammatory bowel disease
- Crohn’s disease
- Ulcerative colitis
Introduction
6-Mercaptopurine (6-MP) and its parent drug azathioprine (AZA) are well known for their immunosuppressive and lymphocytotoxic properties [1, 2]. These antimetabolite drugs have been shown to suppress disease activity in up to 70% of children with moderate to severe Inflammatory Bowel Disease (IBD); among these patients, 50% will achieve a clinical response after 4 months of continuous maintenance 6-MP therapy [3, 4], suggesting that inherent differences in drug metabolism or immunomodulation may influence clinical responsiveness to therapy [5]. Moreover, although the overall risk of 6-MP-induced toxicity is low [6], these same presumed pharmogenomic determinants can only explain in part susceptibility to untoward antimetabolite-associated side effects [7], including the risk of malignancy [8]. The risk of EBV-associated lymphoma among patients with leukemia on maintenance 6-MP therapy is well known and now has also been shown in patients with IBD on long-term antimetabolite therapy. Although the absolute risk for lymphoma remains low [9], many pediatric gastroenterologists have now been compelled to consider alternate forms of immunosuppression. Unfortunately, the commercial availability of drug monitoring has not provided pediatricians any reassurance on potentially further minimizing the overall risk of antimetabolite-associated toxicity. Herein, we will review the use of AZA and 6-MP in the management of pediatric patients with IBD. Furthermore, the application of pharmacogenetic and 6-MP metabolite testing will also be discussed based on an analysis of the literature. Several recommendations will also be provided on applying this technology in clinical practice.
Clinical Indication
Maintenance Therapy
6-MP and AZA are often considered the immunosuppressant drugs of choice in the management of patients with steroid-dependent IBD. Pearson and coworkers published a meta-analysis of nine controlled clinical trials in adult patients with CD that showed clinical responsiveness to either 6-MP or AZA therapy was largely dependent on duration of therapy. In that study, 40–70% of patients successfully achieved corticosteroid withdrawal after a median delay in clinical response time of 16 weeks [10]. On account of this delay in clinical response time, many clinicians are either reluctant to prescribe these slow-acting agents or will prematurely discontinue antimetabolite drug therapy in favor of the more rapid onset biological therapies [11].
Although the delay in clinical response precludes the use of 6-MP as an induction therapy, the notion of using prednisone as a bridge to antimetabolite therapy was first studied by Markowitz and coworkers. In that study, 55 children with newly diagnosed (<6 weeks) CD were randomized in a prospective placebo controlled clinical trial to receive corticosteroids either with or without 6-MP therapy. All patients were placed on a corticosteroid weaning schedule. Patients on combination 6-MP and corticosteroid therapy had achieved clinical remission more effectively, and with a lower cumulative dose of corticosteroids than patients on placebo. Indeed, 92% of patients on 6-MP, and just 6% of patients on placebo, maintained clinical remission after 12 months of follow-up [12]. Interestingly, these investigators chose not to use mesalamine in either treatment arm, despite a probable therapeutic benefit in using slow released 5-ASA formulations in patients with mild to moderate CD [13]. Although, this study would support the notion of initiating antimetabolite as a first line therapy in patients with severe and aggressive disease phenotypes, most pediatric gastroenterologist will try to determine steroid dependency prior to instituting antimetabolite therapy. Future studies are needed to improve our understanding of genotype-phenotype correlations in clinical practice. This would allow clinicians to identify aggressive disease phenotypes and tailor medical therapy more effectively while minimizing the overall need for corticosteroids [14].
Adjunct Therapy
It has been the practice in many institutions, including our own, to initiate maintenance anti-TNF alpha therapy in patients that have proven refractory to either long-term 6-MP or AZA therapy. In a double blind randomized control trial of maintenance infliximab therapy over a 54 weeks trial period (ACCENT 1), 41% of all adult patients with CD achieved and maintained a favorable clinical response. Patients on maintenance infliximab therapy were also more likely to discontinue corticosteroids (29%) and sustain a protracted clinical response than patients on placebo (9%). This study was the first large multicentered study to have shown that retreatment with infliximab was more effective than placebo for maintaining clinical remission in patients with CD. Although 29% of all the patients recruited into the study were on concurrent immunosuppressive therapy, it should be noted that most patients had previously been unsuccessful in achieving disease remission on either 6-MP or AZA [15].
Several important questions come to mind when reviewing the ACCENT I study, including whether all individuals with CD who are treated with infliximab should receive concurrent immunosuppressive therapy despite having not benefited from them in the past. The answer to this question is best addressed by the apparent need for some form of adjunct immunosuppressive therapy in order to prevent antibody against infliximab formation (HACA). The concurrent use of immunosuppressive therapy has in the past been shown by Rutgeerts and coworkers to maintain a favorable clinical response to maintenance infliximab therapy, presumably due to the prevention of HACA antibody formation. In that study, 75% (12/16) of patients on concurrent 6-mercaptopurine maintained a favorable clinical response, compared to 50% (9/18) on no concurrent immunosuppressive therapy [16]. In the ACCENT 1 study, only 18% of the patients on neither concurrent prednisone nor immunosuppressive drug therapy developed HACA, compared to just 10% of patients on concurrent azathioprine or methotrexate therapy [15].
The SONIC [17] and SUCCESS [18] studies have clearly shown an improved overall maintenance of clinical response to combination anti-TNF and antimetabolite therapy in patients with moderate to severe Crohn’s disease and ulcerative colitis, respectively. In both double-blinded clinical trials, the efficacy of infliximab monotherapy was compared to AZA and combination AZA and infliximab in patients that were naïve to either immunosuppression or biologic therapy. Overall, the combination of AZA and infliximab showed an improved corticosteroid free remission (26 wks: SONIC [17]; 16 wks: SUCCESS [18]) compared to monotherapy alone.
Despite an evidence-based improvement in overall clinical response with combination therapy, the increased risk of lymphoma has precluded the concurrent use of antimetabolites with anti-TNF therapy. A recent meta-analysis showed a standardized incidence ratio of about 5, ranging from 2.8 in the analysis of 8 population studies to >9 in 10 referral studies. The risk was most noteworthy among ongoing users of antimetabolite therapy, but not former users. The most noteworthy risk was among patients after 1 year of exposure and less than 30 years of age. It is important to note that although the relative risks of lymphoma in active users was high, there remains a very low absolute risk of malignancy. Among patients <30 years, the risk was shown to remain 1:2000 patient years [9].
The notion of using methotrexate in lieu of either 6-MP or AZA has been downplayed on account of its limited usefulness in patients with UC [19], and its limited effectiveness when used in combination with infliximab compared to infliximab alone in achieving an overall improvement in steroid free clinical remission in patients with Crohn’s disease [20]. Although the risk of teratogenicity in males is low, the concern for birth defects among our adolescent female patients with IBD on methotrexate therapy must be underscored [21].
Although these results of SONIC and SUCCESS clearly demonstrate the steroid-sparing effects of a “top down” therapeutic approach to patients with severe active disease, physicians must appreciate that CD is a lifelong disease, and the risk for lymphomas and other neoplasia with combination immunosuppression must be considered [22, 23]. This is of critical importance, especially in a pediatric population who would be anticipated to receive maintenance infliximab therapy for many years. Albeit low, the risk of hepatosplenic T-cell lymphoma in pediatric patients on combination infliximab and 6-MP remains most disconcerting [24, 25].
Postoperative Prophylaxis
In patients with ileocolonic disease, the reoperation rate after surgical correction ranges from 25–60% after 5 years to up to 91% after 15 years. The recurrence risk after colocolic reanastomosis is much less. A good predictor of disease recurrence is disease behavior. Patients with perforating disease are twice more likely than patients with nonperforating disease to require subsequent surgeries [26]. It still remains difficult to accurately predict the course of disease in any particular patient with CD, and the clinical value of prophylactic therapy remains unclear.
Hanauer et al. described 131 adult patients with CD who underwent intestinal resection and ileocolonic anastomosis and were randomized to receive either placebo, 6-MP (50mmg/day) or mesalamine (3 g/day) as prophylactic therapy. Patients were evaluated prospectively with serial colonoscopies and small bowel barium enemas. In that study, 6-MP was shown to significantly (p <0.05) lower the clinical (50%) and endoscopic recurrence (43%) of CD compared to the mesalamine (clinical (58%); endoscopic (63%)) and placebo (clinical (77%); endoscopic (64%)) treatment groups. Although the dose of 6-MP used in this study was lower than that used for maintenance therapy, a well-defined prophylactic dose has yet to be defined. Despite the fact that clinical recurrence was similar between the 6-MP and mesalamine treatment groups, this study may support the continued use of antimetabolite drugs postoperatively in patients with aggressive disease phenotypes [27]. However, more recently Savarino et al. described 51 adult patients who were randomized to receive adalimumab (ADA), AZA, or mesalamine after ileocolonic resection. At 2 years, rate of endoscopic occurrence was lower in the ADA group (6.3%) compared with AZA (64.7%) or mesalamine (83.3%), with clinical recurrence also less frequent with ADA compared to AZA or mesalamine. There is no prospective pediatric data regarding the use of these medications for postoperative prevention [54].
Drug Monitoring
Most often, pediatricians will measure drug efficacy based on either an improvement in their patients’ clinical symptoms and quality of life or their ability to maintain remission while weaning off of corticosteroid therapy. In general, most physicians will tend to rely on their clinical judgment and experience in determining the dosage of AZA (2–2.5 mg/kg/day) [28, 29] or 6-MP (1–1.5 mg/kg/day) [30, 31] in treating patients with IBD. However, not all patients achieve clinical remission with this treatment approach despite presumed therapeutic drug dosing. This has led some physicians to adopt a dose escalation treatment strategy beyond traditional dosages. In these patients, 6-MP-induced leukopenia is used as a clinical end point to gauge drug dosing. Although Colonna and coworkers have shown that clinical response time is less in those patients with 6-MP-induced leukopenia, overall clinical response to therapy is independent of either 6-MP dose or total leukocyte count. In that study, none of their patients developed clinical signs of bone marrow suppression, required hospitalization for concurrent infection or required transfusions despite total leukocyte counts <5000 [32]. A true separation between immunosuppression [1] and cytotoxicity [2] has yet to be defined since the dosing of 6-MP and AZA has been based largely on clinical outcome. Indeed, the wide range in antimetabolite drug doses used in clinical practice would suggest that a safe and established therapeutic dose has yet to be determined [29–32]. As a consequence, the clinician must always remain aware of potential adverse effects, including allergic reactions, hepatitis, pancreatitis, bone marrow suppression, and lymphoma while attempting to achieve an optimal therapeutic response [6].
In comparison, the pediatric oncologist will effectively tailor the dose of either 6-MP or AZA based on the measure of erythrocyte 6-MP metabolite levels. Indeed, the notion of a therapeutic window of clinical efficacy and toxicity based on the measurement of AZA therapy has now become the standard of care in most pediatric practices treating patients with leukemia [33–35].
6-Mercaptopurine Metabolism
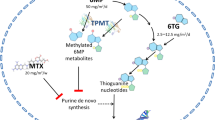
6-MP and its prodrug AZA are by themselves inactive, and must be transformed into their active ribonucleotides that function as purine antagonists. These antimetabolites are then incorporated into DNA, thereby interfering with ribonucleotide replication [36, 37]. The metabolism of 6-MP and AZA occurs intracellularly along the competing routes catalyzed by hypoxanthine phosphoribosyl transferase and thiopurine S-methyltransferase (TPMT), giving rise to 6-thioguanine nucleotides (6-TGn), and 6-methyl-mercaptopurine (6-MMP), respectively (Fig. 30.1) [5]. 6-TGn is the active ribonucleotide of 6-MP that functions as a purine antagonist inducing lymphocytotoxicity and immunosuppression [1, 2].
An apparent genetic polymorphism has been observed in TPMT activity in both the Caucasian and African-American populations. Negligible activity was noted in 0.3%, and low levels (<5 U/mL of blood) in 11% of individuals [5]. TPMT enzyme deficiency is inherited as an autosomal recessive trait, and to date, 10 mutant alleles and several silent and intronic mutations have been described [38]. In patients with the heterozygous TPMT genotype, 6-MP metabolism is shunted preferentially into the production of 6-TG nucleotides. Although 6-TG nucleotides are thought to be lymphocytoxic, and beneficial in the treatment of patients with leukemia and lymphoma, patients with low (<5) TPMT activity are at risk for bone marrow suppression by achieving potentially toxic erythrocyte 6-TGn levels on standard doses of 6-MP [39]. Despite low TPMT enzyme activity levels, therapeutic erythrocyte 6-TGn metabolite levels can still be achieved without untoward cytotoxicity by lowering the dose of 6-MP by about 50% [40].
6-MP Metabolite Monitoring in IBD
The measurement of the erythrocyte 6-MP metabolites 6-TGn and 6-MMP have been proposed as a useful clinical tool for measuring clinical efficacy, documenting patient compliance to therapy and explaining some drug-induced toxicity in patients with IBD. In our preliminary study in 25 adolescent patients with CD on long-term 6-MP therapy, high performance liquid chromatography measurement of erythrocyte 6-TG metabolite levels showed an inverse correlation with disease activity. Although a wide range of metabolite levels was associated with a favorable clinical response, patients with high 6-TGn levels (>250 pmoles/8 × 10 [8] RBCs) were uniformly asymptomatic [41]. Similar results have been reported in 93 pediatric and 45 adult patients with IBD in whom disease remission correlated well with erythrocyte 6-TGn levels between 230 and 260 pmoles/8 × 10 [8] RBCs, respectively [42, 43]. Although these studies and others would support the notion of a therapeutic index of clinical responsiveness based on the measurement of erythrocyte 6-TGn levels [44], several studies have shown no clinical correlation with metabolite monitoring (Table 30.1) [45–48]. This lack of consensus may in part be due to the heterogeneous nature of CD and ulcerative colitis, and may also be dependent on disease severity. Moreover, erythrocyte 6-TGn metabolite levels have not correlated with either lean body mass, adiposity, or surface to volume differences in children (personal observation). As a consequence, conventional weight-based drug therapy has been neither reliable nor predictive. Despite all of its limitations, 6-MP metabolite monitoring still remains the only objective means of monitoring patient compliance, toxicity, and clinical responsiveness to antimetabolite therapy.
In a study of adult patients with CD, high (>290) erythrocyte 6-TGn level showed a positive predictive value of 86% of obtaining a favorable clinical response to induction AZA therapy in patients with steroid refractory CD [49].
TPMT Activity
Genetic polymorphism in TPMT enzyme activity can be quantitatively measured by TPMT phenotype testing. One in 300 patients have absent TPMT enzyme activity, and are at risk for severe bone marrow suppression [39]. There have been a number of cases of irreversible bone marrow suppression both in patients with IBD and in patients with leukemia on standard doses of 6-MP or AZA therapy (personal communications). 6-MP metabolism is clearly influenced by inherent differences in TPMT activity present within the population. In a prospective open-labeled study in patients with IBD, the response rate to induction AZA therapy was highest in patients with less than average (<12 U/mL blood) TPMT activity. Clinical response also correlated well with achieving high erythrocyte (>250) 6-TGn metabolite levels. Indeed, the knowing of the low (<5) TPMT activity before initiating AZA therapy in two patients led to a low dosing strategy (1 mg/kg/day) with a favorable clinical response without untoward side effects [39]. Moreover, Kaskas and coworkers suggested that an effective low (0.25 mg/kg/day) treatment approach can be safely and effectively adopted in patients with the homozygous recessive genotype [40]. Both of these studies would support the contention that a low dose treatment strategy may be used to treat those patients with low TPMT activity.
In comparison, 10% of the population is considered to be rapid metabolizers’ of 6-MP, and in theory would require larger than standard doses of drug in order to achieve any therapeutic drug benefit. In these patients, 6-MP metabolism is shunted away from 6-TGn production and into the formation of 6-MMP. A study in children with IBD showed that a subgroup of these patients remain refractory to therapy despite a dose optimizing treatment strategy [50]. This may in part be due to high hepatic TPMT activity that may draw most of the 6-MP from the plasma, thereby limiting the amount of substrate available for the bone marrow and peripheral leukocytes. A similar study in adult patients with IBD was also able to identify these rapid metabolizers based on the measure of erythrocyte TPMT activity levels. In that study, patients with above average (>12) TPMT activity levels were less likely to respond to AZA therapy, and more likely to require higher dosages (2 mg/kg/day) of AZA from the outset in order to optimize erythrocyte 6-TGn metabolite levels. Moreover, patients with above average (>12) TPMT activity had a mean erythrocyte 6-TGn level that leveled off below a presumed therapeutic (<250) treatment level after 8 weeks of continuous AZA therapy. Sixty-nine % patients with TPMT activity levels ≤12 U/mL blood achieved a clinical response compared to just 30% of patients with above average (>12) TPMT activity after 4 months of continuous therapy. This study was the first to suggest that the pretreatment knowledge of TPMT activity may allow physicians to predict clinical response, and effectively dose AZA in order to maximize efficacy while minimizing the risk of toxicity. Indeed, in that study, patients with a TPMT enzyme activity less than 15 U/mL of blood were six times (OR: 6.2) more likely to show a favorable response to AZA therapy [49]. A recent study exploited the use of allopurinol, a potent inhibitor of xanthine oxidase, in patients with high (>16) TPMT activity who shunt 6-MP metabolism away from the production of 6-TGn metabolites, and remain refractory to presumed therapeutic antimetabolite drug dosing. In that study, the concomitant use of allopurinol with either AZA or 6-MP (reduced to 25–50% of the initial dose) in patients with presumed high TPMT activity levels achieved a significant increase in erythrocyte 6-TGn metabolite levels and the subsequent induction of disease remission. Although this treatment strategy led to a decrease in total leukocyte count, no patient developed clinical signs of toxicity [51].
6-MP Toxicity
Many pediatricians have been reluctant to prescribe 6-MP on account of potential drug related toxicity including pancreatitis 3%, bone marrow depression 2%, super-infection 7%, and hepatitis 0.3% [52]. Severe side effects are either idiosyncratic or related to generic polymorphism as described above. Although Black and coworkers suggested the notion that pharmacogenetic differences in 6-MP metabolism influence a patient’s risk of drug toxicity [7], TPMT polymorphism accounts for only 25% of all 6-MP-induced side effects [53]. However, genetic polymorphisms may play a role in determining the long-term risk for malignancy. In several pediatric oncology studies, the risk for secondary malignancies, including acute myeloblastic leukemia and myelodysplasia was higher in those children with low TPMT activity levels with acute lymphocytic leukemia on maintenance 6-MP therapy [54]. Recent studies in IBD have now shown that past exposure to thiopurines also increases the risk of myeloid disorders 7 fold compared to patients with IBD naïve to antimetabolite therapy [8].
Conclusion
6-MP and AZA have proven efficacy in the maintenance of disease remission in children with IBD. The application of pharmacogenomic and metabolite testing in clinical practice has helped to improve the overall clinical response to antimetabolite therapy in children with IBD and reduce the risk of antimetabolite-induced side effects. The careful monitoring of complete blood counts, and erythrocyte 6-TG metabolite levels are indicated in patients with either low (<5) or above average (>12) TPMT levels; relying on either total leukocyte counts or mean corpuscular volume as the sole measure of dosing adequacy should be used with caution [53].
References
Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1122–4.
Carvalho RS, Mahoney JA, Oliva-Hemker MM, et al. Inherent resistance to 6-thioguanine induced apoptosis correlates with disease activity in children with IBD. Gastroenterology. 2006;265:A203.
Markowitz J, Rosa J, Grancher K, Aiges H, Daum F. Long-term 6-mercaptopurine treatment in adolescents with Crohn’s disease. Gastroenterology. 1990;99:1347–51.
Verhave M, Winter HS, Grand RJ. Azathioprine in the treatment of children with inflammatory bowel disease. J Pediatr. 1990;117:809–14.
Weinshilboum RN, Sladek S. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyl transferase activity. Am J Hum Genet. 1980;32:651–62.
Present DH, Meltzer SJ, Krumholz MP, et al. 6-mercaptopurine in the management of inflammatory bowel disease: short and long-term toxicity. Ann Intern Med. 1995;111:641–9.
Black AJ, McLeod HL, Capell HA. Thiopurine methyl transferase predicts therapy-limitingsever toxicity from azathioprine. Ann Intern Med. 1998;129:716–8.
Lopez A, Mounier M, Bouvier AM, et al. Increased risk of acute myeloid leukemia and myelodysplastic syndromes in patients who received thiopruine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1324–9.
Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015;13:847–58.
Pearson DC, May GR, Fick GH, Sutherland SR. Azathioprine and 6-mercaptopurine in Crohn’s disease: a meta-analysis. Ann Intern Med. 1995;122:132–42.
Markowitz J, Hyams J, Mack D, et al. Corticosteroid therapy in the age of infliximab: acute and 1-year outcomes in newly diagnosed children with Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1124–9.
Markowitz J, Grancher K, Kohn N, et al. A multi-center trial of 6-mercaptopurine and prednisone therapy in children with newly diagnosed Crohn’s disease. Gastroenterology. 2000;119:895–902.
Camma C, Giunta M, Rosselli M, Cottone M. Mesalamine in the maintenance treatment of Crohn’s disease: a meta-analysis adjusted for confounding variables. Gastroenterology. 1997;113:1465–73.
Brant SR, Panhuysen CI, Bailey-Wilson JE, et al. Linkage heterogeneity for the IBD1 locus in Crohn’s disease pedigrees by disease onset and severity. Gastroenterology. 2000;119:1483–90.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCECT I randomized trial. Lancet. 2002;359:1541–9.
Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–9.
Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95.
Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.
Carbonnel F, Colombel JF, Filippi J, et al. Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterology. 2016 Feb;150(2):380–8.
Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014;146(3):681–8.
Weber-Schoendorfer C, Hoeltzenbein M, Wacker E, Meister R, Schaefer C. No evidence for an increased risk of adverse pregnancy outcome after paternal low-dose methotrexate: an observational cohort study. Rheumatology. 2014;53:757–63.
Siegel CA, Hur C, Korzenik JR, et al. Risks and benefits of infliximab for the treatment of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;8:1017–24.
Thayu M, Markowitz JE, Mamula P, et al. Hepatosplenic T-cell lymphoma in an adolescent patient after immunomodulator and biologic therapy for Crohn disease. J Pediatr Gastroenterol Nutr. 2005;2:220–2.
Mamula P, Markowitz JE, Cohen LJ, et al. Infliximab in pediatric ulcerative colitis: two-year follow-up. J Pediatr Gastroenterol Nutr. 2004 Mar;38(3):298–301.
Baldassano RN, Han PD, Jeshion WC, et al. Pediatric Crohn’s disease: risk factors for postoperative recurrence. Am J Gastroenterol. 2001;7:2169–76.
Hanauer SB, Korelitz BI, Rutgeerts P, et al. Post-operative maintenance of Crohn’s disease remission with 6-mercaptopurine, mesalamine or placebo: a 2 year trial. Gastroenterology. 2004;127:723–9.
Ewe K, Press AG, Singe CC, et al. Azathioprine combined with prednisolone or monotherapy with prednisolone in active Crohn’s disease. Gastroenterology. 1993;105:367–72.
Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut. 1995;37:674–8.
Korelitz BI, Adler DJ, Mendelsohn RA, et al. Long-term experience with 6-mercaptopurine in the treatment of Crohn’s disease. Am J Gastroenterol. 1993;88:1198–205.
Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–7.
Colonna T, Korelitz BI. The role of leukopenia in 6-mercaptopurine-induced remission of refractory Crohn’s disease. Am J Gastroenterol. 1993;89:362–6.
Lennard L, Rees CA, Lilleyman JS, et al. Childhood leukemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br J Clin Pharmacol. 1993;16:359–63.
Zimm S, Collins JM, Riccardi R, et al. Variable bioavailability of oral mercaptopurine Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered. N Engl J Med. 1983;308:1005–9.
McLeod HL, Relling MV, Liu Q, Pui CH, Evans WE. Polymorphic thiopurine methyl transferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood. 1995;85:1897–902.
Christie NT, Drake S, Meyn RE. 6-thioguanine induced DNA damage as a determinant of cytotoxicity in cultured hamster ovary cells. Cancer Res. 1986;44:3665–71.
Fairchild CR, Maybaum J, Kennedy KA. Concurrent unilateral chromatid damage and DNA strand breaks in response to 6-thioguanine treatment. Biochem Pharmacol. 1986;35:3533–41.
Alves S, Prata MJ, Ferreira F, Amorim A. Screening of thiopurine methyl s-transferase mutations by horizontal conformation-sensitive gel electrophoresis. Hum Mutat. 2000;15:246–53.
Evans WE, Horner M, Chu YQ, et al. Altered mercaptopurine metabolism, toxic effects, and dosage requirements in a thiopurine methyltransferase deficient child with acute lymphoblastic leukemia. J Pediatr. 1991;119:985–9.
Kaskas BA, Louis E, Hinderof U, et al. Safe treatment of thiopurine S-transferase deficient Crohn’s disease patients with azathioprine. Gut. 2003;52:140–2.
Cuffari C, Theoret Y, Latour S, et al. 6-mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut. 1996;39:401–6.
Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Theoret Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–13.
Achar JP, Stevens T, Brzezinski A, Seidner D, Lashner B. 6-Thioguanine levels versus white blood cell counts in guiding 6-mercaptopruine and azathioprine therapy. Am J Gastroenterol. 2000;95:A272.
Cuffari C, Hunt S, Bayless TM. Utilization of erythrocyte 6-thioguanine metabolite levels to optimize therapy in IBD. Gut. 2001;48:642–6.
Gupta P, Gokhlae R, Kirschner BS. 6-mercaptopurine metabolite levels in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:450–4.
Belaiche J, Desager JP, Horsman Y, Louis E. Therapeutic drug monitoring of azathioprine and 6-mercaptopurine metabolites in Crohn’s disease. Scand J Gastroenterol. 2001;36:71–6.
Lowry PW, Franklin CL, Weaver AL, Szumlanski C, Mays DC, Loftus EV, Tremaine WJ, Lipsky JJ, Weinshilboum RM, Sandborn WJ. Leukopenia resulting from a drug interaction between azathioprine or 6-mercaptopurine and mesalamine, sulphasalazine or balsalazide. Gut. 2001;49:656–64.
Goldenberg BA, Rawsthorne P, Berstein CN. The utility of 6-thioguanine metabolite levels in managing patients with inflammatory bowel disease. Am J Gastroenterol. 2004;99:1744–8.
Cuffari C, Dassoupolus T, Bayless TM. Thiopurine methyl-transferase activity influences clinical response to azathioprine therapy in patients with IBD. Clin Gastroenterol Hepatol. 2004;2:410–7.
Dubinsky MC, Yang H, Hassard PV, Seidman EG, Kam LY, Abreu MT, Targan SR, Vasiliauskas E. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002;122:904–15.
Sparrow MP, Hande SA, Friedman S, et al. Allopurinol safely and effectively optimizes thioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. 2005;22:441–6.
Markowitz J, Grancher K, Mandel F, Daum F. Immunosuppressive therapy in pediatric inflammatory bowel disease: results of a survey of the North American Society for pediatric Gastroenterology and Nutrition. Subcommittee on immunosuppressive use of the pediatric IBD collaborative research forum. Am J Gastroenterol. 1993;88:44–8.
Colombel JF, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology. 2000 Jun;118(6):1025–30.
Garza A, Sninsky CA. Changes in red cell mean corpuscular volume (MCV) during azathioprine or 6-mercaptopurine therapy for Crohn’s disease may indicate optimal dose titration. Gastroenterology. 2001;120:A3166.
Savarino E, Bodini G, Dulbecco P, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn’s disease: a randomized controlled trial. Am J Gastroenterol. 2013;108(1):1731–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Cuffari, C. (2017). 6-Mercaptopurine Therapy. In: Mamula, P., Grossman, A., Baldassano, R., Kelsen, J., Markowitz, J. (eds) Pediatric Inflammatory Bowel Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-49215-5_30
Download citation
DOI: https://doi.org/10.1007/978-3-319-49215-5_30
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49213-1
Online ISBN: 978-3-319-49215-5
eBook Packages: MedicineMedicine (R0)