Abstract
Lakes Shira and Shunet (South Siberia, Russia) are saline ectogenic meromictic lakes. This meromixis is annually sustained by ice formation in winter. In spring due to melting of ice the second halocline is formed in the near-surface layers of mixolimnion that prevents the lakes from full circulation. During our observations (1998–2014) the mixolimnion in both the lakes was monomictic and undergone full circulation in autumn. Purple and green sulphur bacteria are abundant in the chemocline ; the phytoplankton diversity is relatively low; the major constituents of the zooplankton community are several genera of ciliates, calanoid copepod Arctodiaptomus salinus, rotifers Brachionus plicatilis and Hexarthra sp.; Paleolimnological study of the content of organic matter in sediments and the concentration of okenone demonstrated that Lake Shira was holomictic about 90 years ago when the water level was low. It became strongly meromictic just after the water level increase in the 1940s.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 General Description of the Lakes and Historical Overview
1.1 Lakes Physical Surrounding and Historical Overview of Lakes Level/Salinities
Saline lakes are numerous in Chebakovo-Balakhtinskaya depression of Minusinsk trough in Altai-Sayan Mountain region of South Siberia (Republic of Khakassia, Russian Federation). This is a lowland semiarid steppe region surrounded by mountain ridges (Parnachev and Degermendzhy 2002). In this region, the average annual precipitation (about 300 mm yr−1) is significantly lower than potential annual evaporation (about 600 mm yr−1) (Parnachev and Degermendzhy 2002). Such a marked difference with twice higher evaporation than precipitation is a prerequisite for the formation of saline lakes. Because of the continental climate of this territory, the mean annual temperatures vary greatly, i.e. between +18 °C in July and −19 °C in January.
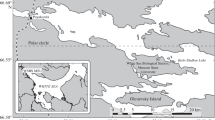
The saline lakes Shira (90.11 E, 54.30 N, 355 m ASL) and Shunet (90.13 E, 54.25 N, 418 m ASL) are the most studied lakes of this Siberian region (Fig. 5.1). Lake Shira is a closed elliptical (9.3 × 5.3 km) waterbody with an area of 35.9 km2 and maximum depth of 24 m (2007–2014). The main inflow to the lake is from the River Son, which provides about 42 % of freshwater supply to lake; other sources of water to the Lake Shira are precipitation and bottom seepage. Salinity in summer ranges from 14 g l−1 to 15 g l−1 in epilimnion and about 18 g l−1 in monimolimnion, based on data from 2007–2009 (Rogozin et al. 2010a). Lake Shunet is located about 8 km to the southeast of Lake Shira. This lake is also elliptical (1.2 × 0.4 km) but is both much smaller in area (0.47 km2) and shallower (6.2 m) than Lake Shira (max. depth 24 m). The lake has no surface outflow, but an inflow from a small stream that enters the lake on its south-west part. The salinity of the Lake Shunet in the mixolimnion (17–20 g l−1) and monimolimnion (up to 66 g l−1) differs markedly (Rogozin et al. 2009). The main anions in Lake Shira are sulphate>chloride>bicarbonate, and main cations are sodium>magnesium>potassium (Kalacheva et al. 2002). In Lake Shunet the chemical composition is similar to that in Lake Shira, but magnesium predominates over sodium. Ice cover persists in both lakes for 5–6 months, from November to early May, and thickness of ice is about 1 m at the middle of March (Rogozin et al. 2009). Both lakes are meromictic: based on studies during 1998–2014 (Zotina et al. 1999; Rogozin et al. 2010a, 2012).
The surface elevation of both lakes has noticeably changed over the observation period. Between the 1920 and 1940, the water level of Lake Shira decreased by about 7 m and salinity increased, reaching a maximum of 27 g l−1 in 1926 (Krivosheev and Khasanov 1990). The lake’s salinity increase was inversely related to the decrease in water volume (Krivosheev and Khasanov 1990). Also, at the beginning of the twentieth century, Lake Shunet was just 0.5 m deep, and salinity was as high as 380 g l−1 (Parnachev and Degermendzhy 2002). Since all the closed lakes in the region were observed to decrease in their water level (Krivosheev and Khasanov 1990; Parnachev and Degermendzhy 2002), it is suggested that fluctuation in balance of regional precipitation-evaporation and predominance of the latter were the cause of water level decreases in the lakes. We have no available information on the lakes’ stratification patterns for the period before 1940, and it is also unclear if the lakes were then meromictic or holomictic.
1.2 Lakes Stratification
The lakes have been shown to be meromictic with autumnal overturn restricted to mixolimnion (Rogozin et al. 2010a, 2012). The depth of mixolimnion and position of oxic-anoxic interface varied annually in Lake Shira whereas it was stable in Lake Shunet . Spring mixing processes contribute to the autumnal formation and positioning of mixolimnion.
The exceptionally windy spring of 2007 caused the deepening of mixolimnion in Lake Shira in the winter of 2008 (Rogozin et al. 2010a). The winter position of oxic-anoxic interface was affected by the position of lower boundary of mixolimnion. The salinity in the winter mixolimnion increases compared with the autumn because of freezing out of salts from the upper water layers during ice formation and their dissolution in the water below.
During May the salinity in the upper 2-m layer of Lake Shira decreases drastically due to melting of the ice on the lake surface. Therefore, the halocline, i.e. step of salinity represented by the maximum salinity gradient, is formed in the near-surface layers of mixolimnion (Fig. 5.2). The depth of that halocline coincides with the thermocline and pycnocline, indicating the formation of a warmer epilimnion. The oxic-anoxic interface remains near the former mixolimnion depth (Fig. 5.2).
Seasonal dynamics of stratification in Lake Shira . Grey colour indicates sulphide-rich water (from Rogozin et al. 2010a)
Even though the thickness of the measured profiles was qualitatively similar, in winter it differed for mixolimnion noticeably from winter to winter. In the years 2003, 2008, 2009, 2013 and 2014, the winter depth of lower boundary of mixolimnion ranged from 15.5 m to 16.7 m. In contrast, in other winters in this period, the mixolimnion depth varied between 9.5 m and 12.5 m (Rogozin et al. 2009; Rogozin et al. 2016).
A similar dynamics of vertical distribution of temperatures and salinity was also observed for Lake Shunet (Rogozin et al. 2012). The vertical distribution of salinity in the depth range from the surface to 4.5 m varied depending on the season; the salinity varied between 10 g l−1 and 25 g l−1 (Fig. 5.3). The salinity in the lower part of the water column hardly changed during the seasons indicating the absence of full circulation of water in Lake Shunet. The sharply heterogeneous distribution of dissolved salts combined with the invariably high sulphide concentration in the bottom layers indicates meromictic character of Lake Shunet. Thus, the water column of the lake is divided into aerobic mixolimnion and anaerobic monimolimnion.
Seasonal dynamics of Lake Shunet stratification. Grey colour indicates sulphide-rich water (from Rogozin et al. 2012)
The seasonal dynamics of salinity and temperature in the mixolimnions of both lakes is significantly affected by the formation of the ice cover and its thawing. Thus, the mixolimnion in both lakes was monomictic during the study period, undergoing full circulation in autumn.
Freshwater inflows during the 1930s and 1940s were considerable due to an increase in atmospheric precipitation, and they probably contributed to the formation of the stable salinity gradient observed in Lake Shira nowadays. A similar dramatic increase in water level was observed in 1930–1940s for Lake Shunet (see above). Therefore, lake’s meromixis, according to the generally accepted Hutchinson’s classification of lake stratification (Hutchinson 1957), has an ectogenic origin (i.e. induced by freshwater covering the saline water) rather than a crenogenic origin (i.e. induced by supply of mineral-rich water from the lake bottom).
1.3 Mathematical Modelling of Lake Shira Vertical Structure
The profiles of water temperature, salinity and density in the central deepest part of the lake during an open-water period are based on a 1-D mathematical model (Belolipetskii and Genova 2008). For the subglacial period we used a simplified model of ice formation based on the single-phase Stefan problem (Vuik 1993) with the linear temperature distribution in solid phase (Genova et al. 2010). We isolated an under-ice convective mixing layer, which was formed due to an increase in salinity during ice formation. To obtain analytical solutions concerning the vertical distributions of temperature, we used a vertical structure diagram in the form of several layers (Belolipetskii and Genova 2008). Ice melting processes, from both below and above the ice cover, were modelled for spring period. The calculated profiles of salinity and temperature of Lake Shira were in good agreement with the field data for autumnal and winter periods 2002–2003 and 2003–2004 (Genova et al. 2010).
The calculated and measured profiles of temperature and salinity were quite similar for most study dates. We used the depth of turbulent mixing layer as an indicator of the both calculated and observed mixing patterns (Fig. 5.4). Obviously, the turbulent mixing layer always spread downward to the upper boundary of the pycnocline, i.e. to the depth of maximal salinity gradient and was positioned near thermocline during the warm period and gradually descended to chemocline during autumnal cooling and mixing and winter stratification. Therefore, the sharp maxima indicate the time of ice melting and formation of thermocline in the upper layer (Fig. 5.4). The “shoulders” of solid lines indicate the calculated thermocline position during the stable summer stratification , and lateral straight portions indicate the calculated winter mixolimnion .
Upper Panel (a) Depth of maximal gradient of salinity: calculated for odd years (solid line), the same for even years (grey solid line); measured (filled triangle); sharp maxima indicate the moments of ice melting and formation of thermocline in the upper layer; (b) Depth of observed oxic-anoxic interface (open triangle). Arrows indicate “deep” mixolimnions (from Rogozin et al. 2010a)
Generally, the calculated depth of winter mixolimnion was in good accord with the observed depth for the winters of both 2002–2003 and 2004–2005. In other winters, except 2005–2006, when we did not measure, the agreement between the measures and calculated profiles was not so good. The calculated positions of thermocline in summer 2007 and mixolimnions in 2008 and 2009 were well “shallower” than the observed ones (Fig. 5.4a). The effects of internal currents, wind directions, lake morphology and water level fluctuations cannot be explained by the one-dimensional model. We, therefore, assume that the 2-D and 3-D models provide more adequate prognoses of lake dynamics. In addition, the available data of wind force are based on four preset time measurements per day, but the strong transient storms as observed in spring 2007 will produce noticeable mixing effects between the periods of wind force measurements (Rogozin et al. 2010a).
2 Biology and Biogeochemistry
2.1 Ecology of Phototrophic Sulphur Bacteria in Lakes Shira and Shunet
2.1.1 Species Composition of Phototrophic Sulphur Bacteria
In Lake Shira the purple sulphur bacteria (PSB) dominated in anoxic phototrophic community whereas green sulphur bacteria (GSB) are a minor group. On the contrary, both these bacterial types inhabit the redox zone and monimolimnion of Lake Shunet (Lunina et al. 2007a, b; Rogozin et al. 2009, 2010b). Culture-independent method of denaturing gradient gel electrophoresis of PCR-amplified fragments of 16S rRNA genes with universal eubacterial primers has shown that Thiocapsa sp. Shira_1 (Chromatiaceae) (AJ633676) isolated from chemocline of Lake Shira was dominant PSB in redox zones of both lakes in 2005 (Rogozin et al. 2010b). According to spectrophotometry analysis, the “green” GSB containing bacteriochlorophyll c or d and carotenoid chlorobactene inhabit the anaerobic zone of Lake Shunet (Lunina et al. 2007a, b; Rogozin et al. 2010b). One GSB strain related to Chlorobium limicola (Chlorobiaceae) and another to Prosthecochloris sp. were isolated in 2003 from both lakes Shira and Shunet, respectively (Lunina et al. 2007a). Nevertheless, PCR/DGGE show that another GSB phylotype dominated in chemocline of Lake Shunet in 2005 (Rogozin et al. 2010b).
2.1.2 Stratification of Phototrophic Sulphur Bacteria in Lake Shunet
Intensive “purple layer” of PSB was detected at the oxic-anoxic interface in Lake Shunet by precision sampling with multi-syringe sampler (Fig. 5.5) (Rogozin et al. 2005; Lunina et al. 2007a). This layer is observed in 5 cm depth interval at the oxic-anoxic interface, and persisted in all seasons during 2003–2012, though PSB abundance decreased for ice periods (Rogozin et al. 2012; Degermendzhy et al. 2010). PSB were extremely abundant ( >108 cell ml−1) during the open-water periods. Green sulphur bacteria developed mostly under the “purple layer” (Fig. 5.6). The spatio-temporal organization of the bacterial community inhabiting the chemocline of the stratified meromictic Lake Shunet (Khakassia, Russia) was investigated from May to September 2005 using microscopical analysis of photosynthetic pigments and PCR-DGGE with subsequent 16S rDNA analysis. The samples were collected with a multi-syringe stratification sampler at every 5 cm (Rogozin and Degermendzhy 2008). There were no large changes observed in the bacterial community of the chemocline, at least among the detected forms (Fig. 5.6). During the entire study, purple sulphur bacteria phylogenetically and morphologically close to Lamprocystis purpurea (Chromatiaceae) predominated in the chemocline. In the layers below the layer of purple bacteria, green sulphur bacteria were observed. Cryptomonas is a phytoflagellate developed in the upper zone of the chemocline (Fig. 5.6). Mahoney Lake (Canada) is the only other lake that is known to have similar abundances of PSB (Overmann 1997).
Multi-syringe thin-layer sampler (Rogozin and Degermendzhy 2008). The photo shows 15 simultaneous, vertical samples taken from Lake Shunet (Siberia, Russia) in August 2004 at 5-cm intervals. The “purple layer” can be easily distinguished (the 9th syringe from the top) (From Degermendzhy et al. 2010)
Vertical distribution of the physicochemical characteristics in Lake Shunet and the number of microorganisms in the chemocline in 2005. The total number of bacterial cells (DAPI) does not include the number of PSB cells (from Rogozin et al. 2010b)
In chemoclines of both lakes, optimal light conditions and sulphide concentration provide favourable conditions for photosynthetic production, whereas high-density gradients and high hydrophysical stability inhibit the sedimentation and turbulent mixing, respectively. Therefore, the combination of relatively shallow position of chemocline and high-density gradient in both lakes favours dense accumulation of PSB at the chemocline (Rogozin et al. 2012).
2.1.3 Effect of Winter Conditions on Phototrophic Sulphur Bacteria in Lakes Shira and Shunet
Anoxic phototrophic bacteria are generally known to persist in chemoclines of temperate meromictic lakes under ice (Lawrence et al. 1978; Overmann et al. 1994; Tonolla et al. 2003). In both lakes we studied vertical distribution and biomass of anoxic phototrophic bacteria and profiles of physical and chemical characteristics during under-ice periods from 2003 to 2008, except in 2006 (Rogozin et al. 2009). The bacterial layers in chemocline of both lakes were sampled with a thin layer using a hydraulic multi-syringe sampler (Rogozin and Degermendzhy 2008). Winter biomass of purple sulphur bacteria in both lakes varied depending on amount of light penetrating into the chemocline through the snow cover and on ice at the lake surface. The quantity of light reaching deeper layers depended on snow cover characteristics. The nature of snow cover could be remotely sensed by reflectance of the lake surface (Rogozin et al. 2009). Therefore, the under-ice light conditions could be roughly assessed by monitoring of snow cover dynamics in studied lakes. In addition, in the relatively less strongly stratified Lake Shira , the vertical position of chemocline in winter can vary resulting in considerable changes in light conditions in chemocline. Hence, the under-ice light conditions of anoxic phototrophic bacteria depend on chemocline position. Interestingly, in Lake Shira due to an increase in transparency of mixolimnion in winter, the combination of shallower chemocline and absence of snow resulted in light intensity exceeding summer values in the chemocline. Therefore, the biomass of purple sulphur bacteria in chemocline of the lake exceeded the summer values under these conditions (Rogozin et al. 2009).
In Lake Shunet , the light intensities in the chemocline and biomasses of PSB were always lower in winter than in summer but the biomass of GSB was similar over the season. In the chemocline of Lake Shira , temperature conditions in winter and summer were similar in most years. The PSB populations developed at temperatures ranging from 0 °С to 1.7 °С for most seasons, except during warm period in June 2007. In contrast, in Lake Shunet, the summer temperatures in chemocline were 5–15 °C higher than in winter.
Previous researchers (Pimenov et al. 2003; Savvichev et al. 2005; Lunina et al. 2007a, b) observed that rates of photosynthesis (14C Method) in these two lakes are lower in winter than in summer. Moreover, contribution of the anoxic photosynthesis to total primary production is rather low in all seasons (Pimenov et al. 2003; Savvichev et al. 2005). In both lakes, the oxic photosynthesis contribute from 93 % to 100 % of total assimilation of photosynthetic inorganic carbon during both summer and winter periods (Pimenov et al. 2003; Savvichev et al. 2005). In each lake the differences in primary production between summer and winter are more due to differences in oxic photosynthesis. However, the winter measurements are based only on data of 2003 (Savvichev et al. 2005) when light conditions in both lakes were very poor. Indeed, in the winters of 2003 and 2008, the light intensity measured in chemocline of Lake Shira was <0.4 μE m−2 s−1. This appears to be the lowest light intensity known to support growth of pure PSB cultures (van Gemerden et al. 1989). Thus, anoxic photosynthesis is negligible in the winters in Lake Shira and low in Lake Shunet . Obviously, in winter 2007, the rates of oxic and anoxic photosynthesis in both lakes were higher because of considerably higher under-ice light intensities. We have shown that variation in under-ice light intensities suggest that under-ice photosynthetic production may vary significantly depending on environmental conditions.
2.2 Vertical Distribution of Ciliates , Zooplankton and Amphipods
Geographical proximity of the salt lakes Shira and Shunet suggests a similar species composition because they are probably colonized by species from the same regional pool and because of the likely migration of species between the adjacent water bodies. Moreover, both lakes are meromictic having a similar composition of salts and broadly comparable levels of salinities. Both lakes are fishless in pelagic parts. It is not surprising that both lakes are dominated by the same species of pelagic zooplankton. Among the ciliates—representatives of the genera Cyclidium sp. and Strombidium sp.—and among crustacean zooplankton, both lakes are dominated by calanoid copepod Arctodiaptomus salinus. Rotifers are represented by two taxa—Brachionus plicatilis and Hexarthra sp. The largest invertebrate species in these lakes is the amphipod Gammarus lacustris, which inhabits both the littoral and pelagic zones of the lakes. Despite the similarity in species composition of zooplankton, the morphometric differences and the patterns of summer stratification in the lakes affect the vertical structure of zooplankton.
Ciliates
The pelagic zone of Lake Shunet comprises several ciliate genera: Oligotrichida, Scuticociliatida, Hypotrichida, Prostomatida, Cyclidium sp., Euplotes sp., Prorodon sp., Balanion sp., Strombidium sp. and Oxytricha sp. Most of these genera are adapted to poor light conditions , anoxia and hydrogen sulphide (Khromechek et al. 2010).
The most distinctive feature of ciliate community in the pelagic zone of Lake Shunet is their clear preference for a specific region in the water column: both abundance and biomass were much higher in the chemocline zone than in the mixolimnion (Fig. 5.7a). The protozooplankton maximum was observed in the 10–35 cm layer of the chemocline region, above or sometimes in the layer of PSB, where hydrogen sulphide concentration varied from 0 mg l−1 to 5 mg l−1 with little or no oxygen. The ciliate abundance in the chemocline (50–400 cells ml−1) was significantly higher than in the mixolimnion (0–36 cells ml−1). Ciliates in this zone do not seem to migrate and their seasonal dynamics in the chemocline is insignificant.
In contrast with Lake Shunet , the species composition of ciliates in the pelagic zone of Lake Shira is extremely poor, being represented by only two species—Cyclidium sp. and Strombidium sp. Unlike Lake Shunet, ciliates in Lake Shira are exposed to significant seasonal fluctuations. In spring and summer, they also form the deep maximum near the chemocline zone. Ciliate maxima were observed at 12–13 m depth, and their concentration was up to 200 ind. ml−1 (Fig. 5.7b), with an average of 80 ind. ml−1 in the water column. During early fall period, the ciliate maximum drops to about 20 cells ml−1, which is one-tenth the average value in summer when their mean concentration in the water column was only about 10 ind. ml−1; in winter, the ciliates are virtually absent in Lake Shira.
Zooplankton
Vertical distribution of different developmental stages and sexes of Arctodiaptomus salinus, rotifers and cladocerans, as well as temperature in mixolimnion divided in summer to epi-, meta- and hypolimnion in the two lakes are depicted in Fig. 5.8. The borders of metalimnion are the depths where the water temperature changes by at least 1 °C/m or more (Garneau et al. 2013). The obtained data on the average zooplankton densities (ind. l−1) and temperature distribution are for the four selected periods of the season: ice period (March), period of early stratification of mixolimnion (May–June), the period of stratification of mixolimnion (July–August) and autumn period of extensive mixing of mixolimnion. The data of 32 vertical profiles of zooplankton abundances and water temperature were averaged for the period 1999–2011 (No. of samples annually in parentheses) in Lake Shira (1999 (1), 2001 (6), 2003 (1), 2007 (4), 2008 (6), 2009 (5), 2010 (4), 2011 (5)) and 16 profiles for the period 2003–2011 in Lake Shunet (2003 (1), 2004 (3), 2007 (4), 2008 (4), 2009 (2), 2011 (2)).
Average concentrations (bars) of A. salinus and rotifers in different depth strata and temperature profiles (vertical solid line) in pelagic part of Lake Shira (1999–2011) and Lake Shunet (2003–2004, 2007–2009 and 2011). Horizontal dash lines indicate the borders of epi-, meta- and hypolimnion zones in the thermally and chemically stratified mixolimnion , except March in Lake Shira when the mixolimnion is uniform and lines simply indicate three equal in size zones
March. Under the ice the temperature of both lakes was uniform in the oxygenated water zone. Also copepods A. salinus lacked a persistent vertical structure. Copepodites of C1–3 and C4–5 stages dominated. In Lake Shunet the temperature above the chemocline zone was slightly elevated but without an increase of zooplankton. Nauplii were rare in both lakes but the concentration of zooplankton in Lake Shira was higher than in Lake Shunet—average 35 ind. l−1 versus 19 ind. l−1, respectively.
May–June. After melting of the ice, there was a mass development of copepodites C4–C5 to adults and increase in reproduction rates, as well as a development of rotifer Hexarthra sp. in both lakes. The zooplankton was characterized by a marked predominance of nauplii and rotifers in epi- and metalimnion. In Lake Shira, zooplankton abundance in the hypolimnion declined, unlike in Lake Shunet where all life stages of A. salinus, except nauplii, were distributed evenly in the different layers. In this period, concentration of zooplankton in Lake Shunet exceeded that in Lake Shira.
July–August. In mid-summer the epilimnion zone of the lakes expanded. Moreover, metalimnion of Lake Shunet sank to the chemocline zone. The peaks of copepodites C1–C3 and C4–C5 were separated: copepodites C1–C3 were encountered in epilimnion while the C4–C5 mostly occurred in metalimnion. Like in May–June, nauplii preferred to develop in the epilimnion. The high numbers of rotifers in this period were typical for B. plicatilis in Lake Shira , but this species was limited in its distribution to the epilimnetic water. In Lake Shunet the B. plicatilis massively developed in a thin layer adjacent to the chemocline zone. Other species of zooplankton (cladocera Moina sp., rotifers Hexarthra sp.) were found in samples occasionally.
In October, vertical distribution of temperature dramatically differed between the lakes. In Lake Shira the mixing zone extended almost to the depth where the anoxic zone began. The Lake Shunet had inverse stratification—the surface waters were colder, while the temperature at the depth of chemocline and below was higher (see Fig. 5.3). In Lake Shira concentration of A. salinus in a narrow zone of metalimnion was slightly higher than in the epilimnion above. The relative proportions of the adult stages, copepodites C4–5 and C1–3, were similar to that in the period July–August, except for naupliar stages—their contribution to the total number decreased to almost zero. Rotifer Hexarthra was still abundant, with a maximum in epilimnion. B. plicatilis was also present but in small numbers.
In the Lake Shunet , A. salinus was represented mostly by adult stages and copepodites C4–C5. Nauplii and copepodites C1–3 in the population were rare. The concentration of copepods in the metalimnion despite the higher temperature was lower than in the upper mixed layer of water. The numbers of B. plicatilis near the chemocline were comparable with summer numbers.
Interestingly, throughout the season the average numbers of A. salinus, including nauplii, varied within a rather narrow range, 31–34 ind. l−1 from March to October. Throughout the season, the average numbers of copepods A. salinus in the Lake Shunet were generally higher than in Lake Shira and were 49 ind. l−1 with a maximum in May–June.
The vertical distributions of A. salinus in both lakes were similar. Nauplii and copepodites C1–3 preferred to stay in epilimnion. However, copepodites C4–5 in Lake Shira preferred metalimnion, while in Lake Shunet they moved deeper into the hypolimnion. Hexarthra sp., a rotifer, in Lake Shira was present in all zones during entire study period. But in Lake Shunet it was limited to the period May–June and to epi- and metalimnion. B. plicatilis inhabited the epilimnion in Lake Shira, but in Lake Shunet it developed a rather thin layer only above the chemocline .
Gammarus
The amphipod Gammarus lacustris is generally considered as a benthic organism in lakes. However, there is growing evidence that the ecological niche of Gammarus is not only benthic but benthoplanktonic (e.g. Wilhelm et al. 2000). Lake Shira and Lake Shunet are both meromictic lakes, where Gammarus is one of the few dominant species among macro-zooplankton. In meromictic lakes, anoxic deeper layers restrict the vertical distribution of zooplankton (Zadereev and Tolomeyev 2007). It is clear that in the pelagic zone of the meromictic lakes there is no bottom habitat for the benthic animals. Repeated zooplankton sampling in central parts of these both lakes demonstrated that G. lacustris is common in samples from the mixolimnion . To understand the ecology of Gammarus in meromictic lakes, we studied the vertical distribution and abundance of Gammarus lacustris in the pelagic zone of two meromictic lakes during stable thermal stratification (July–August).
In both lakes during summer stratification, Gammarus was distributed non-homogenously, with a stable peak in the metalimnion (Zadereev et al. 2010). The average depth of Gammarus population in the pelagic zone was significantly correlated with the depth of the thermocline (Fig. 5.9). Our results shown that Gammarus is regularly present in the pelagic zone of two meromictic lakes, Shira and Shunet, during summer stratification. The gammarid exhibited high densities in the thermocline on all sampling dates. Thus, this type of vertical distribution is stable during summer stratification and typical for these lakes. The vertical distribution of Gammarus apparently reflects its physiological or ecological preferences, or both. The absence of fish in the pelagic zone, high oxygen concentration, low water temperature, increased seston concentration, elevated water density in the metalimnion and the anoxic hypolimnion can be the most probable combination of factors responsible for the peak abundance of Gammarus in the metalimnion of these lakes.
The correlation between the depth of the thermocline and the average depth of G. lacustris population in the pelagic zones of Lake Shira and Lake Shunet (Zadereev et al. 2010)
The peak abundance of Gammarus in the pelagic zone of both lakes amounted up to 400 ind. m−2, while the peak animal densities in the metalimnion reached 50 ind. m−3. These data are comparable with abundances of the same Gammarus species in the littoral of Lake Shira (Yemelyanova et al. 2002). Thus, both littoral and pelagic zones can be equally important habitats for amphipods in meromictic lakes.
It is also important that Gammarus summer biomass in the pelagics of both lakes (up to 15 g m−2) was comparable to that of dominant zooplankton grazers (20 g m−2 for Arctodiaptomus salinus and 15 g m−2 for Brachionus plicatilis) (Zadereev and Tolomeyev 2007). High pelagic densities of Gammarus suggest that this amphipod may have an important effect on the food web in this zone. Gladyshev et al. (2000), who studied the fatty acids in the stomach contents of Gammarus from Lake Shira , show that the animals probably feed on freshly sedimenting seston. We determine the role of Gammarus in the pelagic woodweb using its diet and growth rates. Based on a survival study of Gammarus on the lake’s seston in a 20-day mesocosm experiment (Tolomeyev et al. 2006), we can conclude that Gammarus can survive for long periods on in situ lake seston particles. We used these assumptions about feeding spectra to simulate with the hydrophysical-ecological model of Lake Shira the effect of Gammarus on the pelagic seston in the lake (Degermendzhy et al. 2010). It appears that Gammarus does play a role in regulating the seston carbon in lake water. For example, according to model simulations, Gammarus present in the water column at the peak densities can control the seston biomass by 30–40 %.
Thus, the presence in abundance of Gammarus in pelagic water seems to be an intriguing but an important feature of meromictic lakes, e.g. lakes Shira and Shunet, which lack fish due probably to high salinity. However, a more specific research directed to trophic and population dynamics of Gammarus, the role of littoral zone and the coupling of littoral and pelagic trophic budgets in order to examine the role this “predominantly” benthic animal plays in the lake’s ecosystem, is needed to quantify its importance in energy flow.
2.3 Food Web Structure and Interactions
Food web structure in the lakes Shira and Shunet is quite similar due mainly to similarity of species composition of bacterio-, phyto- and zooplankton (Degermendzhy et al. 2010). This similarity can be explained by the geographical proximity of the two lakes and by their similar salt composition, annual stratification pattern and their simple short food chain structure: mainly because of the absence of fish as a top predator in the pelagial of these both lakes. To understand the food web structure and interactions in the lakes, it is necessary to take into account the vertical stratification of physical, chemical and biological components in these lakes.
2.3.1 The Microbial Loop in Mixolimnion and Chemocline
Heterotrophic bacterioplankton is the main constituent of the planktonic microbial community in the mixolimnion of Lake Shira (Kopylov et al. 2002). Both the numbers and biomass of bacteria associated with detrital particles and microcolonies in the water column are considerable (up to 18 % of the total bacterial biomass). They may serve as a source of food for the zooplankton. Heterotrophic flagellates are dominant among the protozoa. Within the microbial loop, a food chain consisting of bacteria and heterotrophic flagellates appears to be a possible pathway transferring bacterial production to higher levels of the planktonic food web, i.e. they facilitate linking the microbial and macrobial food webs in these lakes.
Bacterial community consisting of purple sulphur and heterotrophic bacteria inhabits the chemocline of the Lake Shira . However, we consider the bacterial community in the chemocline as a not essential part of the trophic chain in Lake Shira. Because the depth of the chemocline is variable, the bacterial community does not reach high densities and the densities of ciliates and phytoflagellates are also low (Degermendzhy et al. 2010).
In contrast, the depth of the chemocline in Lake Shunet is stable; the mixolimnion is relatively thin, and the chemocline inhabited by the extremely dense bacterial community, a population of Cryptomonas sp. and ciliate community comprising several species (Khromechek et al. 2010). The trophic interactions in Lake Shunet partially differ from those in Lake Shira as both ciliates and zooplankton feed on bacterial community in the chemocline. Ciliates and calanoid copepods (A. salinus) also feed on Cryptomonas spp. (Tolomeev et al. 2010), which are most probably mixotrophic in the chemocline and consume bacteria.
2.3.2 Phytoplankton and Zooplankton
The phytoplankton diversity in both lakes is relatively low. In Lake Shira the dominant species in different seasons and at different depths were cyanobacteria Lyngbya contorta Lemm. and Microcystis pulverea (H.C. Wood) Forti, diatoms Cyclotella choctawhatcheeana (formerly identified as Cyclotella tuberculate), green algae Dictyosphaerium tetrachotomum Printz and Oocystis submarina Lagerheim and cryptophytic algae Cryptomonas salina Wislouch. During spring and at the beginning of the summer, the dominant species of phytoplankton in Lake Shira were L. contorta and C. choctawhatcheeana. They developed in the upper layers of the lake. The peak of C. salina near the oxic-anoxic interface was also typical for the spring and early summer periods. From late June to September, the phytoplankton in Lake Shira was stratified, with a maximum in the lower part (8–12 m) of the thermocline . In these strata the chlorophyll concentration reached about 23 μg l−1. This peak value mainly consisted of vertically segregated peaks of L. contorta, M. pulverea, D. tetrachotomum and O. submarina var. schiriensis (Gaevsky et al. 2002).
In Lake Shunet the composition of phytoplankton slightly differs. The dominant species are cyanobacteria Synechocystis sp.; diatoms Cyclotella sp. that is observed at all depths of the mixolimnion and Nitzschia acicularis, which is typical for surface waters; and green alga Dictyosphaerium tetrachotomum Printz which is observed at all depths of the mixolimnion (Degermendzhy et al. 2003). Population density of Cryptomonas salina Wislouch in the chemocline is high (Khromechek et al. 2010).
The pelagic zone of these lakes is dominated by identical species of zooplankton. The major constituents of the zooplankton community in both lakes are ciliates represented by the genera Cyclidium sp. and Strombidium sp., calanoid copepod Arctodiaptomus salinus, rotifers Brachionus plicatilis and Hexarthra sp. and amphipod Gammarus lacustris.
The similarity of the vertical structure of the distribution of A. salinus in both lakes consists mainly of nauplii and copepodites C1–3 in the epilimnion. Vertical distribution of copepodites C4–5 slightly differs: in Lake Shira copepodites prefer metalimnion zone and in Lake Shunet they move deeper in hypolimnion. Rotifer Hexarthra sp. in Lake Shira is present in all lake zones and present in all observation periods. In contrast, in Lake Shunet Hexarthra was limited to May–June period alone, only in epi- and metalimnion. Differences in vertical positioning in the lakes showed that whereas B. plicatilis inhabited the epilimnion in Lake Shira, in Lake Shunet this rotifer developed as a thin layer above the chemocline .
The feeding spectra of Arctodiaptomus salinus (Calanoida, Copepoda) populations inhabiting lakes Shira and Shunet are based on fatty acid (FA) trophic markers measured by Tolomeev et al. (2010). This study revealed the calanoid to feed on Cryptomonas and sulphur purple bacteria in Lake Shunet and on ciliates and colonial picoplankton in both lakes. The analysis of markers in storage lipids—triacylglycerols (TAG) of A. salinus—reflected the differences in seston composition of the lakes and, consequently, in the feeding preferences. FA composition of seston in the lakes moderately differed: levels of diatom FA markers were higher in Lake Shunet, and those of cyanobacteria and green algae markers were higher in Lake Shira . Moreover, A. salinus in Lake Shira had significantly higher concentrations of bacterial FA markers, while in Lake Shunet, contribution of cryptophytes and/or flagellates or both to the diet of A. salinus was higher. The analysis of markers in storage lipids also revealed high food selectivity by A. salinus. This conclusion is based on the detection in the storage lipids of the trophic markers of the minor components of seston, which must be selectively ingested by the animals in order that it can be detected in the storage lipids.
The presence of Gammarus lacustris in the pelagic part of both lakes is a specific feature of their food webs. The study of gut contents of G. lacustris and fatty acid trophical markers revealed that gammarids ingest primarily fresh seston (Gladyshev et al. 2000). The 20-day mesocosm experiments demonstrated that G. lacustris could grow on in situ concentrations of seston in the lakes. Moreover, the mesocosm experiments revealed that gammarids and copepods are most probably the other competitors for seston in the pelagic part of Lake Shira (Tolomeyev et al. 2006).
Thus, the difference in food web structure and trophic interactions (Fig. 5.10) is mainly due to differences in mean and maximum depths of the two lakes and in their salinity levels that control the stability of permanent stratification. Lake Shira , which is deeper and less saline, has a deeper redox zone with variable depth. Depending on the weather conditions, the oxic-anoxic interface can sink to about16 m or ascend to 11 m depth. The depth of the chemocline is variable and the bacterial densities in the chemocline are not high. The mixolimnion in summer also creates different habitats for various species. The distribution of phytoplankton is non-uniform with peak of biomass in the metalimnion. The distribution of zooplankton is also patchy with rotifers and juvenile copepods in the warm epilimnion and older copepods in the cold oxic hypolimnion (Zadereev and Tolomeyev 2007). The zooplankton that is comprised of calanoids A. salinus and rotifers B. plicatilis and H. oxiuris feed mostly on phytoplankton, while the bacterial community in the chemocline does not significantly contribute to the food web.
The main food web components (boxes) and trophic interactions (solid arrows) in two saline lakes Shira and Shunet, (South Siberia, Russia) in summer. The position of boxes demonstrates the location of the given species in the water column. Exact depths, physical and chemical values and concentrations of biological components are described in the other parts of this chapter (Degermendzhy et al. 2010)
The chemocline in Lake Shunet is located at 5 m depth, which unlike in Lake Shira is very stable, with the annual variations of <20 cm. This stability of the chemocline depth is based on the sharp salinity gradient between the mixolimnion (salinity ranges from 17 g l−1 to 20 g l−1) and monimolimnion (salinity up to 66 g l−1). Because the depth of the chemocline in Lake Shunet is stable and the mixolimnion is relatively shallow, the chemocline is inhabited by an extremely dense bacterial community, and by a population of Cryptomonas sp. and several ciliate species. As the mixolimnion of Lake Shunet is thermally not stratified for long periods, the vertical distributions of phytoplankton and zooplankton are also not vertically well defined. The trophic interactions in Lake Shunet partially differ from those in Lake Shira; ciliates, rotifers and zooplankton in the lake feed on bacterial community in the chemocline. Both ciliates and A. salinus also feed on Cryptomonas sp., which is most probably mixotrophic in the chemocline and consumes bacteria.
We conclude that the food web in the pelagic part of the Lake Shira is mainly based on the transfer of organic matter from the phytoplankton to zooplankton, which can be considered as a classical food web based on the autotrophic production of organic matter by the phytoplankton. In contrast, in Lake Shunet the food web is a combination of classical phytoplankton-zooplankton trophic link and trophic interactions based on the production of organic matter in the chemocline of the lake.
3 Mathematical Modelling to Understand the Ecosystem Functioning
3.1 General Information on the Lake Shira Model
Lake Shira was selected to develop coupled hydrophysical-biochemical model as the lake has a long history of observations and is of considerable economic value for the region. We developed model of Lake Shira to simulate and explain the thermal structure (the position of thermocline and halocline, vertical mixing rates and the vertical distributions of temperature, salinity and density) and the vertical spatial distribution of the main components of ecosystem (Fig. 5.11). The model was developed to simulate the processes in the pelagic part of the lake during the summer season. The thermal structures of the lake were calculated using the meteorological data, % cloud cover, air temperatures, wind speed and direction and vapor pressure. The model was developed taking into account general principles and approaches which were used in developing similar imitation instruments as well as on the basis of the extensive lake model sexperience (Mooij et al. 2010).
Schematic overview of the structure of Lake Shira one-dimensional imitation model (Prokopkin et al. 2014). The arrows show the main processes and interactions in ecosystem
Phytoplankton in the model is represented by the dominating species of green alga, Dictyosphaerium tetrachotomum, and cyanobacterium, Lyngbya contorta (Zotina et al. 1999). Also, the supplementary module describing the cryptophytic alga population was added recently (Prokopkin et al. 2014). Zooplankton is represented by a population of calanoid copepod Arctodiaptomus salinus, a species that dominates the biomass of pelagic zooplankton (Zadereev and Tolomeyev 2007), which is divided into two age classes: young nauplii and juvenile copepodite stages and old copepodite stages and adult copepods. The amphipod G. lacustris that occupies benthic-planktonic niche in the lake was also introduced in the model. The transformation of sulphur in the model is carried out by two groups of microorganisms: light-dependent purple sulphur bacteria oxidizing hydrogen sulphide (H2S) and sulphate-reducing bacteria decomposing detritus in the water column. The processes calculated for all biological components of the model are growth and death rates, sedimentation, consumption by other components (if any) and optionally respiration (excretion rates).
The model simulates organic matter cycling in the water column in units of dry weight, phosphorus and nitrogen and processes of diffusion and transformation (e.g. oxidation of hydrogen sulphide and nitrification). The model was calibrated and validated with the field data for the time period 2000–2010. Hydrophysical and biochemical modules of the model were described in detail by Belolipetsky et al. (2010) and Prokopkin et al. (2010, 2014) and compared with other lake models, e.g. the one of Mooij et al. (2010).
The model has wide range of applications—from general simulation of ecosystem development to the analysis of specific processes as well as scenarios of lake behaviour under the effect of different driving forces. Below we provide several examples of how the model was used to understand the stratified meromictic lake ecosystem.
3.2 Analysis of the Mechanisms of Formation of Deepwater Peak of Phytoplankton
In summer, the deepwater peak of phytoplankton biomass is typical for Lake Shira . The model simulates the general trends of the development of the phytoplankton (Fig. 5.12a) in agreement with the field observations: the dominating species of green alga Dictyosphaerium peaked in July at 8 m depth and in August at 10–12 m depth (Gaevsky et al. 2002); during the second part of summer, cyanobacterium Lyngbya contorta begins to dominate in phytoplankton community with the biomass peak above the peak of green alga (Zotina et al. 1999).
Model calculations (Prokopkin et al. 2010) of the vertical distribution of green alga (thick line) and cyanobacteria (thin line) biomasses. Left panels show the results for July 15; the right panels show the results for August 06. (a) Calculations under standard conditions; (b) results obtained in the absence of trophic pressure exerted by zooplankton; (c) calculations in the absence of phytoplankton sedimentation
To understand the driving forces for the changes in phytoplankton community, we selected several factors and processes that control phytoplankton dynamics: irradiance; water temperature; nutrient concentration; growth, mortality and respiration; consumption of zooplankton and amphipods; and sedimentation rates of organic matter. Sensitivity analysis of the model based on the different calculations, where these factors and processes were switched on/off or their effects were increased/decreased, was performed. This analysis allows ranking the model parameters, factors and processes according to degree of their influence on the phytoplankton community.
The model calculations show that filter-feeding calanoid A. salinus considerably affects the vertical distribution of phytoplankton and change of the dominant species (Fig. 5.12b). The analysis revealed that the sedimentation rate of phytoplankton seems to be the most important factor that determines the deep peak of phytoplankton biomass in the water column, i.e. the biomass would be concentrated in the subsurface water layers if sedimentation is absent (Fig. 5.12c) (Prokopkin et al. 2010).
We conclude that the changes in dominant species in phytoplankton community during summer occur in response to temperature and nutrient factors and grazing activity of zooplankton. The model demonstrates that the change of the vertical positions of the biomass of different phytoplankton species first of all is the result of sedimentation of phytoplankton that is either sinking of live phytoplankton based on gravity or senescence connected sedimentation (Prokopkin et al. 2010).
3.3 The Effect of Weather Parameters on the Thermal Stratification of the Lake
To understand the relative importance of the effect of weather parameters on the thermal stratification of Lake Shira , we obtained the temperature profiles of the water column using different weather scenarios. The reference scenario was calculated using the meteorological data set for the year 2002. Then, we compared the output of the reference scenario with the outputs of the calculations performed with altered meteorological data sets. To obtain the altered data sets, the values of wind speed or air temperature or % cloud cover in the reference year weather data set were increased or decreased by several values equal to the unit of measurement of corresponding weather parameter (by 1 m s−1 for wind speed, 1 °С for air temperature and 1 relative unit based on % for cloud cover).
The characteristic parameters of the temperature profile of the water column were (a) the depth of the thermocline, (b) the average temperature of water in the epilimnion and (c) the temperature of water at 10 m depth (hypolimnion) (Table 5.1). The values of these parameters were calculated as of June 1 and 15, July 1 and 15 and August 1, 15 and 31. The average of these values was used for analysis.
Changes in the values of weather parameters had strong effect on the temperature of the epilimnion, while for the hypolimnion this effect was less pronounced. We observed similar to Arvola et al. (2010) that in thermally stratified lakes, the increase in summer air temperatures will only result in an increase in hypolimnetic temperatures if winds are strong enough to trigger mixing. Also, Liu et al. (2014) demonstrated that increasing the air temperature warms up the epilimnion but does not effect the hypolimnion temperature. Our calculations support these observations.
The greatest variations in the depth of the thermocline in our calculations were caused by variations in the wind speed and air temperature. Opposite variations in the values of these parameters (increase in air temperature and decrease in wind intensity or vice versa) initiated two scenarios in the water column: (1) the increase in the temperature in the epilimnion together with an increase in the depth of thermocline or (2) a decrease in the temperature of the epilimnion with a decrease of the thermocline depth. The second scenario predicts less stable thermal stratification.
De Stasio et al. (1996) and some others state that in response to the ongoing global climate change , the lakes will become warmer and stratification will occur earlier and will be more pronounced. These hypotheses support our calculations if we associate the climate change with an increase in air temperature. The model study by Robertson and Ragotzkie (1990) showed that when the air temperature is increased by 1 °С, the temperature of the epilimnion rises by 0.4–0.85 °С, but the temperature of the hypolimnion in the middle of the summer season would not change significantly. Hondzo and Stefan (1993) showed that because of climate change (scenario of a 100 % increase of CO2 content in the atmosphere), the temperature of epilimnion will also increase, but to a lesser extent than the air temperature, and hypolimnetic temperature would be determined by the lake morphometry and spring mixing rather than by weather in summer alone. Based on our calculations, we estimated the increase in the water temperature in the epilimnion by 0.73 °С and in the hypolimnion by 0.01 °С, when the air temperature increases by 1 °С, which is in very good agreement with above-mentioned published results.
3.4 The Effect of Weather Parameters on the Lake Shira Ecosystem
The model analysis revealed the link between the variations of weather parameters and related changes in temperature profiles in the water column and response of ecosystem components both in the mixolimnion and the monimolimnion (Fig. 5.13).
A diagram of weather induced changes in the stratification of the water column and related effects on the food web of Lake Shira. Sign “+” denotes the significant positive correlations between parameters, sign “–”—significant negative correlations. *—the correlation is significant only for the values calculated with the variations in air temperature and wind speed
Response of mixolimnion and monimolimnion components to the weather changes can be depicted by two sets of correlations that influence the content of hydrogen sulphide in the monimolimnion and the depth of the chemocline . What is essential for the ecosystem behaviour is the response of two light-dependent and separated in space primary producers (phytoplankton and purple sulphur bacteria ) to the effect of weather parameters and interrelation between them.
The first interrelated set of links is between phytoplankton biomass and detrital mass in the water column and hydrogen sulphide content in the monimolimnion. Phytoplankton is the main source of dead organic matter (detritus) sinking to the monimolimnion, where it participates in the sulphur cycle and production of hydrogen sulphide.
The second interrelated set of links is between the biomass of phytoplankton in the water column, and of purple sulphur bacteria in the chemocline , and the content of hydrogen sulphide in the monimolimnion. Phytoplankton and purple sulphur bacteria are spatially separated light-dependent components; there is a negative correlation between their biomasses. A decrease in phytoplankton biomass probably leads to an increase in water transparency, more favourable underwater light conditions , and an increase in the biomass of purple sulphur bacteria. To support this finding, we note that the maximal biomasses of bacteria in winter were observed in winters of 2006 and 2007 when the light intensity in chemocline was highest because of snow-free weather conditions (Rogozin et al. 2009). As hydrogen sulphide is the main substrate for the growth of purple sulphur bacteria (Rogozin et al. 2012), an increase in their biomass leads to a decrease in the content of hydrogen sulphide in the monimolimnion. The content of hydrogen sulphide in the monimolimnion determines the depth of the chemocline.
Thus, we demonstrated how biological processes control the depth of the chemocline during the growing season. In our calculations, we did not use the depth of the chemocline at the beginning of the season as a factor influencing the ecosystem properties. Our studies on the lake during 2002–2009 showed (Rogozin et al. 2010a) that the depth of the chemocline in spring differs from year to year. This is because this depth in the years to come is likely to be affected by the weather conditions in the preceding year. Moreover, this depth in spring largely depends on the mixing depth of the mixolimnion in the preceding autumn period (Zadereev et al. 2014).
4 Paleolimnology. Reconstruction of Local Climate Lake Properties Based on Bacterial Pigments and Other Markers
4.1 Geochemical Characteristics of Lake Shira Sediments
We did not study the composition of Lake Shunet sediment because of its non-laminated semi-liquid consistency that prevented detailed analyses and determination of age of the layers. In contrast, Lake Shira sediment is a proper object for paleolimnological studies because of its laminated and consolidated structure (Figs. 5.14 and 5.15).
Carotenoids in upper sediments of Lake Shira . Right diagram shows the dynamics of the water level (maximum depth) in the lake. Shaded area shows carbonate (white) layer from Zykov et al. (2012)
Carotenoids and LOI550 in bottom sediments of Lake Shira . The absence of okenone in the lower sample is shown by an arrow (from Zykov et al. 2012; see explanation in text)
The water level of a closed saline lake is highly sensitive to climate changes: it depends first of all on humidity. Therefore, reconstruction of the changes in lake’s water level may help us understand the climate changes in the past better. The laminated structure of the bottom sediments is best preserved in meromictic lakes, because the stable stratification of the water column prevents sediment stirring and resuspension. In addition, high concentrations of sulphide and anoxia in bottom waters inhibit the activity of benthos, i.e. bioturbation is absent and, hence, the original progression of the sediment layering remains preserved. Therefore, the bottom sediments in the meromictic lakes are easier to date, and therefore they represent excellent “archives” of the climatic change (Overmann et al. 1993).
In 2009 a paleolimnological study of Lake Shira was first initiated by Rogozin et al. (2011). Isotope dating was carried out using 137Cs, 210Pb and 14C measurements together with direct counting the annual layers (Kalugin et al. 2013). Geochemical analyses show that Lake Shira has two contrasting mixing states: meromictic and holomictic. In upper sediment the period in which the first white layer was formed corresponds to the period of decrease in water level (1900–1930s), indicating that the water level had decreased (1920s) (Fig. 5.14). The content of organic matter is lower in the white sediment layers than in the dark sediment layers (Fig. 5.15), perhaps because of accelerated decomposition rate in the white layers because white layers indicate the oxic conditions in near-bottom waters (Kalugin et al. 2013)
This accelerated decomposition rate of organic matter was probably caused by oxic conditions prevailing in near-bottom waters during the low water-level period of the lake (1900–1930s). Therefore, Lake Shira was presumably holomictic during this period.
Meromictic periods, as in more recent time, are comprised of black thin laminated mud coloured by organics and hydrotroilite (FeSnH20), which are predominant in the core. For holomictic conditions , white organic matter-free mud appeared in the periods BC 220–75, AD 480–565, AD 900–1045, AD 1437–1603 and AD 1853–1947 (Fig. 5.15) (Kalugin et al. 2013). Presumably, most organic matter produced in the waterbody and sediment was decomposed by dissolved atmospheric oxygen due to mixing at that time. Simultaneously, the biomass of phototrophic sulphur bacteria seems decreased or disappeared due to lack of hydrogen sulphide.
4.2 Photosynthetic Pigments in Bottom Sediments
The fossil molecular remains of APB (such as carotenoids , bacteriochlorophylls and their derivatives (i.e. phaeopigments and DNA) serve as indicators of anaerobic conditions in the photic zone of the lake at some stage of its existence (Overmann et al. 1993). For the first time, in 2011, we analyzed the composition of carotenoids in the bottom sediments of Lake Shira . Okenone , the main carotenoid of purple sulphur bacteria inhabiting Lake Shira, was common in all sediment layers except from deepest layers located at a depth of 1430 m (about 2350 years old) (Fig. 5.14). Other carotenoids were found in all layers without exception. Clear maximum of okenone and local maxima of other carotenoids were detected near the upper limit of the carbonate layer at 13 cm in the sediment core. The concentration maximum of okenone was much higher than the maxima of all other carotenoids.
The presence of okenone allows one to construe that the anaerobic zone existed in the lake from fourth century BC to the present. Nevertheless, the absence of okenone in the earlier sample indicated that during the earlier period, there was no anaerobic activity in the near-bottom water layers. As known from studies on other lakes, okenone is found in bottom sediments only during meromixis/holomixis only if anoxia develops in summer hypolimnion (Schmidt et al. 2002; Dressler et al. 2007). As a rule, meromixis favours the high production of PSB and the better preservation of carotenoids (Overmann et al. 1993; Schmidt et al. 2002; Leavitt 1993). Therefore, a peak in the concentration of okenone in the layers forming 110–130 mm stratum (1945–1970) indicates pronounced meromictic properties of the lake in this period. The most probable (Rogozin et al. 2010a) cause for the current meromixis of Lake Shira is the influence of an ectogenic factor (freshwater layer lying above the saline water), which is in agreement with Hutchinson’s lake typology based on type of meromixis in lakes (Hutchinson 1957). Due to the inflow of large amounts of freshwater with the surface run-off into the saltier lake, which was the case during the 1920s and 1930s, in this period the water level was minimum, but a difference in salinity probably favoured a stable density gradient and permanent meromixis (Rogozin et al. 2010a). The low content of okenone in the sediment of Lake Shira until the 1920–1930s indicates that meromixis at a high water level either was quite weak or absent.
In the 1950s when the concentration of okenone was higher, the water level of the lake was about 2 m lower than at present (Fig. 5.14). Thus, it is quite possible that the depth of the mixolimnion (i.e. the depth position of the chemocline ) was lower than it is now. Consequently, light conditions in chemocline were better than now. At present, very low light intensity (about 2 μE m−2 s−1) in the chemocline of the lake limits light conditions for PSB (Rogozin et al. 2009).
5 Conclusions
Both lakes Shira and Shunet are examples of ectogenic meromictic lakes (meromixis is induced by freshwater lying on top of the saline water). This condition is sustained annually by ice formation in winter. In spring due to melting of ice, the second halocline is formed at the near-surface layers of mixolimnion that prevents the lakes from full circulation in spring. The mixolimnion in the both lakes is monomictic (based on the regular observations from year 2000 to year 2014) and undergoes full circulation in autumn.
Both these lakes because of their geographical proximity, similar chemical composition, stable stratification and the absence of fish and other plankton predators have quite similar species compositions of bacterio-, phyto- and zooplankton. However, there are also notable differences in the food web structure and interactions because of the differences in morphometry (mean and maximum depths) of these lakes and in the salinities that control the stability of permanent stratification. In Lake Shira , the zooplankton feed mostly on phytoplankton, while the bacterial community in the chemocline does not significantly contribute to the food web. In the shallower Lake Shunet , the extremely dense bacterial community, a population of Cryptomonas sp. and several species of ciliates inhabit the chemocline. The food spectra of zooplankton in Lake Shunet and Lake Shira differ. Ciliates , rotifers and zooplankton in Lake Shunet feed on bacterial community in the chemocline. Both ciliates and the calanoid A. salinus also feed on Cryptomonas sp. which is most probably mixotrophic in the chemocline and consumes bacteria.
We used the coupled biochemical-hydrophysical model of Lake Shira to study the effect of weather parameters on the ecosystem of this meromictic lake. We show that although different weather parameters have opposite effects, i.e. quantitatively their effects on the different ecosystem components are similar but opposite. Thus, even for estimating the consequences of global climate change on a specific ecosystem, local characteristics of weather variations should be taken into account, because they may considerably alter the ecosystem structure and functioning.
The major factors influencing the stability of stratification of the saline meromictic lakes studied by us are the salinity and water level. The maximal depth of Lake Shira in the last 120 years has increased from 16 m to 24 m presumably due to an increase in freshwater inflow into the lake due to a negative annual water budget based on precipitation-evaporation balance. With the increase in the depth of the lake, water salinity decreased. The differences between salinity of upper and bottom waters (i.e. salinity gradient), maximal depth and lake area influence the stability of permanent stratification. According to sediment composition, Lake Shira was holomictic about 90 years ago when the water level was low, but it became strongly meromictic just after the water level increase in the1940s. Therefore, the transition between holomictic and meromictic regimes can be caused by changes in the lake’s water level.
References
Arvola L, Glen G, Livingstone DM, Järvinen M, Blenckner T, Dokulil MT, Jennings E, Aonghusa CN, Nõges P, Nõges T, Weyhenmeyer GA (2010) The impact of the changing climate on the thermal characteristics of lakes. In: Glen G (ed) The impact of climate change on European lakes, Aquatic ecology series, vol 4. Springer, Dordrecht, New York, pp 85–102
Belolipetskii VM, Genova SN (2008) Calculation of vertical profiles of temperature and salinity in Shira Lake. Computational Technologies. Bull KazNU (mathematics, mechanics and informatics issue) 3(58):261–266 (in Russian)
Belolipetsky PV, Belolipetskii VM, Genova SN, Mooij WM (2010) Numerical modelling of vertical stratification of Shira Lake in summer. Aquat Ecol 44:561–570
De Stasio B, Hill D, Kleinhans J, Nibbelink N, Magnuson J (1996) Potential effects of global climate change on small north-temperate lakes: physics, fish and plankton. Limnol Oceanogr 41:1136–1149
Degermendzhy AG, Gaevsky NA, Belonog NP, Ivanova EA, Rogozin DYu, Koltahsev AA, Gribalev ES (2003) Izychenie physico-chimicheskix I biologicheskix charakteristik dvux balneologicheskix ozer (Matarak, Shunet, Khakassia). Vestnik Krasnoyarskogo gosudarstvennogo universiteta 5:107–115 (In Russian)
Degermendzhy AG, Zadereev YS, Rogozin DY, Prokopkin IG, Barkhatov YV, Tolomeev AP, Khromechek EB, Janse JP, Mooij WM, Gulati RD (2010) Vertical stratification of physical, chemical and biological components in two saline lakes Shira and Shunet (South Siberia, Russia). Aquat Ecol 44:619–632
Dressler M, Hubener T, Gors S, Werner P, Selig U (2007) Multi-proxy reconstruction of trophic state, hypolimnetic anoxia and phototrophic sulphur bacteria abundance in a dimictic lake in Nothern Germany over past 80 years. J Paleolimnol 37:205–219
Gaevsky NA, Zotina TA, Gorbaneva TB (2002) Vertical structure and photosynthetic activity of Lake Shira phytoplankton. Aquat Ecol 36:165–178
Garneau M-E, Posch T, Hitz G, Pomerleau F, Pradalier C, Siegwart R, Pernthaler J (2013) Short-term displacement of Planktothrix rubescens (cyanobacteria) in a pre-alpine lake observed using an autonomous sampling platform. Limnol Oceanogr 58:1892–1906
Genova SN, Belolipetskii VM, Rogozin DY, Degermendzhy AG, Mooij WM (2010) A one-dimensional model of vertical stratification of Lake Shira focused on winter conditions and ice cover. Aquat Ecol 44:571–584
Gladyshev MI, Emelianova AY, Kalachova GS, Zotina TA, Gaevsky NA, Zhilenkov MD (2000) Gut content analysis of Gammarus lacustris from a Siberian lake using biochemical and biophysical methods. Hydrobiologia 431:155–163
Hondzo M, Stefan HG (1993) Regional water temperature characteristics of lakes subjected to climate change. Clim Chang 24:187–211
Hutchinson GE (1957) A Treatise on Limnology, Geography, Physics and Chemistry, vol 1. Wiley, New York
Kalacheva GS, Gubanov VG, Gribovskaya IV, Gladchenko IA, Zinenko GK, Savitsky SV (2002) Chemical analysis of Lake Shira water (1997–2000). Aquat Ecol 36:23–141
Kalugin I, Darin A, Rogozin D, Tretyakov G (2013) Seasonal and centennial cyclesof carbonate mineralization during the past 2500 years from varved sediment in Lake Shira, South Siberia. Quat Int 290–291:245–252
Khromechek EB, Barkhatov YV, Rogozin DY (2010) Densities and distribution of flagellates and ciliates in the chemocline of saline, meromictic Lake Shunet (Siberia, Russia). Aquat Ecol 44:497–511
Krivosheev AS, Khasanov AP (1990) Therapeutic lakes of Krasnoyarsk Region. Krasnoyarsk Publishing House, Krasnoyarsk (In Russian)
Kopylov AI, Kosolapov DB, Romanenko AV, Degermendzhy AG (2002) Structure of planktonic microbial food web in a brackish stratified Siberian lake. Aquat Ecol 36:179–204
Lawrence JR, Haynes RC, Hammer UT (1978) Contribution of photosynthetic green sulphur bacteria to total primary production in a meromictic saline lake. Verh Int Verein Limnol 20:201–207
Leavitt PR (1993) A review of factors that regulate carotenoids and chlorophyll deposition and fossil pigment abundance. J paleolimnol 9:109–127
Liu W, Bocaniov SA, Lamb KG, Smith REH (2014) Three dimensional modeling of the effects of changes in meteorological forcing on the thermal structure of Lake Erie. J Great Lakes Res 40:827–840
Lunina ON, Bryantseva IA, Akimov VN, Rusanov II, Rogozin DY, Barinova ES, Lysenko AM, Pimenov NV (2007a) Seasonal changes in the structure of the anoxygenic photosynthetic bacterial community in Lake Shunet, Khakassia. Microbiology (Translated from Mikrobiologiya) 76:368–379
Lunina ON, Bryantseva IA, Akimov VN, Rusanov II, Barinova ES, Lysenko AM, Rogozin DY, Pimenov NV (2007b) Anoxigenic phototrophic bacteria community of Lake Shira (Khakassia). Microbiology (Translated from Mikrobiologiya) 76:469–479
Mooij WM, Trolle D, Jeppesen E, Arhonditsis G, Belolipetsky PV, Chitamwebwa DBR, Degermendzhy AG, DeAngelis DL, De Senerpont Domis LN, Downing AS, Elliott JA, Fragoso CR, Gaedke U, Genova SN, Gulati RD, Håkanson L, Hamilton DP, Hipsey MR, ‘t Hoen J, Hülsmann S, FH L, Makler-Pick V, Petzoldt T, Prokopkin IG, Rinke K, Schep SA, Tominaga K, Van Dam AA, Van Nes EH, Wells SA, Janse JH (2010) Challenges and opportunities for integrating lake ecosystem modelling approaches. Aquat Ecol 44:633–667
Overmann J, Beatty JT, Hall KJ (1994) Photosynthetic activity and population dynamics of Amoebobacter purpureus in a meromictic saline lake. FEMS Microbiol Ecol 15:309–320
Overmann J (1997) Mahoney Lake: a case study of the ecological significance of phototrophic sulfur bacteria. Adv Microb Ecol 15:251–288
Overmann J, Sandmann G, Hall KG, Northcote T (1993) Fossil carotenoids and paleolimnology of meromictic Mahoney Lake, British Columbia, Canada. Aquat Sci 55:1015–1621
Parnachev VP, Degermendzhy AG (2002) Geographical, geological and hydrochemical distribution of saline lakes in Khakasia, Southern Syberia. Aquat Ecol 36:107–122
Pimenov NV, Rusanov II, Karnachuk OV, Rogozin DY, Bryantseva IA, Lunina ON, Yusupov SK, Parnachev VP, Ivanov MV (2003) Microbial processes of the carbon and sulphur cycles in Lake Shira (Khakasia). Microbiology (Translated from Mikrobiologiya) 72:221–229
Prokopkin IG, Barkhatov YV, Khromechek EB (2014) A one-dimensional model for phytoflagellate distribution in the meromictic lake. Ecol Model 288:1–8
Prokopkin IG, Mooij WM, Janse JH, Degermendzhy AG (2010) A general one-dimensional vertical ecosystem model of Lake Shira (Russia, Khakasia): description, parametrization and analysis. Aquat Ecol 44:585–618
Robertson DM, Ragotzkie R (1990) Changes in the thermal structure of moderate to large sized lakes in response to changes in air temperature. Aquat Sci 52:360–380
Rogozin DY, Pimenov NV, Kosolapov DB, Chan’kovskaya YV, Degermendzhy AG (2005) Thin layer vertical distributions of purple sulfur bacteria in chemocline zones of meromictic Lakes Shira and Shunet (Khakassia). In: Doklady Biological Sciences. Proceedings of the Russian Academy of Sciences, vol 400, pp 54–56 (Translated from Doklady Akademii Nauk (2005) 400: 426–429)
Rogozin DY, Degermendzhy AG (2008) Hydraulically-operated thin-layer sampler for sampling heterogeneous water columns. J Sib Fed Univ 1:111–117
Rogozin DY, Genova SV, Gulati RD, Degermendzhy AG (2010a) Some generalizations on stratification and vertical mixing in meromictic Lake Shira, Russia, in the period 2002–2009. Aquat Ecol 44:485–496
Rogozin DY, Trusova MY, Khromechek EB, Degermendzhy AG (2010b) Microbial community of the chemocline of meromictic Lake Shunet during summer stratification. Microbiology (Translated from Mikrobiologiya) 79:253–261
Rogozin DY, Zykov VV, Chernetsky MY, Degermendzhy AG, Gulati RD (2009) Effect of winter conditions on distributions of anoxic phototrophic bacteria in two meromictic lakes in Siberia, Russia. Aquat Ecol 43:661–672
Rogozin DY, Zykov VV, Degermendzhi AG (2012) Ecology of purple sulfur bacteria in the highly stratified meromictic Lake Shunet (Siberia, Khakassia) in 2002–2009. Microbiology 81:727–735
Rogozin DY, Zykov VV, Kalugin IA, Daryin AV, Degermendzhy AG (2011) Carotenoids of phototrophic organisms in bottom sediments of meromictic Lake Shira (Siberia, Russia) as an indicator of past stratification. Dokl Biol Sci (Proc Russ Acad Sci) 439:228 (Translated from Doklady Akademii Nauk 439:282–285)
Rogozin DY, Zykov VV, Tarnovsky MO (2016) Dynamics of purple sulfur bacteria in saline meromictic Lake Shira (Khakasia, Siberia) for the period 2007–2013. Microbiology (Translated from Mikrobiologiya) 81:93–101
Savvichev AS, Rusanov II, Rogozin DY, Zakharova EE, Lunina ON, Yusupov SK, Pimenov NV, Degermendzhy AG, Ivanov MV (2005) Microbiological and isotopic–geochemical investigations of meromictic lakes in Khakasia in winter. Microbiology (Translated from Mikrobiologiya) 74:477–485
Schmidt R, Psenner R, Muller J, Indinger P, Kamenik C (2002) Impact of late glacial variations on stratification and trophic state of the meromictic lake Landsee (Austria): validation of a conceptual model by multi proxy studies. J Limnol 61:49–60
Tolomeev AP, Sushchik NN, Gulati RD, Makhutova ON, Kalacheva GS, Zotina TA (2010) Feeding spectra of Arctodiaptomus salinus (Calanoida, Copepoda) using fatty acid trophic markers in seston food in two salt lakes in South Siberia (Khakasia, Russia). Aquat Ecol 44:513–530
Tolomeyev AP, Zadereev ES, Degermendzhy AG (2006) Fine stratified distribution of Gammarus lacustris Sars (Crustacea: Amphipoda) in the pelagic zone of the meromictic Lake Shira (Khakassia, Russia). Dokl Biochem Biophys 411:346–348
Tonolla M, Peduzzi S, Hahn D, Peduzzi R (2003) Spatio-temporal distribution of phototrophic sulphur bacteria in the chemocline of meromictic Lake Cadagno (Switzerland). FEMS Microbiol Ecol 43:89–98
Van Gemerden H, Tughan CS, de Wit R, Gerbert RA (1989) Laminated microbial ecosystems on sheltered beaches in Scapa Flow, Orkney Islands. FEMS Microbiol Ecol 62:87–102
Vuik C (1993) Some historical notes about the Stefan problem. Nieuw Archief voor Wiskunde 11(2):157–167
Wilhelm FM, Schindler DW, McNaught AS (2000) The influence of experimental scale on estimating the predation rate of Gammarus lacustris (Crustacea: Amphipoda) on Daphnia in an alpine lake. J Plankton Res 22:1719–1734
Yemelyanova AY, Temerova TA, Degermendzhy AG (2002) Distribution of Gammarus lacustris Sars (Amphipoda, Gammaridae) in Lake Shira (Khakasia, Siberia) and laboratory study of its growth characteristics. Aquat Ecol 36:245–256
Zadereev ES, Tolomeev AP, Drobotov AV, Kolmakova AA (2014) The effect of weather variability on the spatial and seasonal dynamics of dissolved and suspended nutrients in the water column of meromictic Lake Shira. Contem Probl Ecol 7:384–396
Zadereev ES, Tolomeyev AP (2007) The vertical distribution of zooplankton in brackish meromictic lake with deep-water chlorophyll maximum. Hydrobiologia 576:69–82
Zadereev ES, Tolomeyev AP, Drobotov AV, Emeliyanova AY, Gubanov MV (2010) The vertical distribution and abundance of Gammarus lacustris in the pelagic zone of the meromictic lakes Shira and Shunet (Khakassia, Russia). Aquat Ecol 44:531–539
Zotina TA, Tolomeyev AP, Degermendzhy NN (1999) Lake Shira, a Siberian salt lake: ecosystem structure and function. 1. Major physico-chemical and biological features. Int J Salt Lake Res 8:211–232
Zykov VV, Rogozin DY, Kalugin IA, Dar’in AV, Degermendzhi AG (2012) Carotenoids in bottom sediments of Lake Shira as a paleoindicator for reconstruction of lake states in Khakssiya, Russia. Contemp Probl Ecol 5:434–442
Acknowledgement
The research on lakes Shira and Shunet was partially supported by the Council on grants from the President of the Russian Federation for support of leading scientific schools (grant NSh-9249.2016.5).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Rogozin, D. et al. (2017). Comparative Study of the Stability of Stratification and the Food Web Structure in the Meromictic Lakes Shira and Shunet (South Siberia, Russia). In: Gulati, R., Zadereev, E., Degermendzhi, A. (eds) Ecology of Meromictic Lakes. Ecological Studies, vol 228. Springer, Cham. https://doi.org/10.1007/978-3-319-49143-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-49143-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49141-7
Online ISBN: 978-3-319-49143-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)