Abstract
In this chapter, we discuss respiratory outcomes of preterm infants and the impact later in life of bronchopulmonary dysplasia (BPD). After the neonatal period, the major respiratory problems of preterm-born infants (with or without BPD) that require hospitalization are respiratory exacerbations caused by infections, particularly viral infections. In older children who were born prematurely, the most common symptoms are coughing, wheezing, and/or other asthma-like symptoms. Overall, in comparison to full-term born children, the risk of preterm-born children developing asthma or a wheezing disorder during childhood is almost twice as high. Patients are, however, often labeled asthmatic even though the underlying mechanisms are likely to be very different. There is no evidence of the widespread use of bronchodilators or inhaled corticosteroids, although a component of variable airflow obstruction may be present. To achieve optimal treatment, additional evidence is required. In order to prevent either over- or undertreatment, it is important to characterize diseases of the airways in the survivors of preterm birth. This is done in terms of the extent and nature of airflow obstruction, the pattern of any inflammation, and the presence of airway reactivity. Symptoms become milder as children grow older. Nevertheless, a group of adolescents and adults remains, who still present with chronic airway obstruction defined by recurrent episodes of wheezing and decreased lung function tests, that is, decreased forced expiratory volume. The risk of wheezing disorders increases as the degree of prematurity increases. Putative mechanisms for wheezing may include early lung injury or maldevelopment during infancy, respiratory infections during the first year of life, and structural changes of the lung parenchyma.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Respiratory symptoms
- Wheeze
- Dyspnea
- Retractions
- Outcome
- Airway
- Hyperresponsiveness
- Airflow obstruction
- Maldevelopment
- Structural changes
- Epidemiology
Definitions
In accordance with the World Health Organization (WHO), preterm birth is defined as a live birth before 37 completed weeks of pregnancy. On the basis of gestational age, three subcategories of preterm birth are distinguished:
-
Extremely preterm (<28 weeks)
-
Very preterm (28 to <32 weeks)
-
Moderate to late preterm (32 to <37 weeks)
Bronchopulmonary Dysplasia
The classic diagnosis of BPD may be assigned at 28 days of life, provided the following criteria are met:
-
1.
Supplemental oxygen is required beyond 28 days of age in order to maintain PaO2 above 50 mmHg
-
2.
Chest radiograph with diffuse abnormal findings characteristic of BPD
Newer Criteria
The newer criteria for BPD, issued by the National Institute of Health (USA) and applicable to neonates treated with more than 21 % oxygen for at least 28 days, are as follows:
Mild
-
Breathing room air at 36 weeks’ postmenstrual age or at discharge (whichever comes first) for infants born before 32 weeks or
-
Breathing room air by 56 days postnatal age or at discharge (whichever comes first) for infants born after 32 weeks’ gestation
Moderate
-
The need for <30 % oxygen at 36 weeks’ postmenstrual age or at discharge (whichever comes first) for infants born before 32 weeks, or
-
The need for <30 % oxygen until 56 days’ postnatal age or at discharge (whichever comes first)
Severe
-
The need for >30 % oxygen, with or without positive pressure ventilation or continuous positive pressure, at 36 weeks’ postmenstrual age or at discharge (whichever comes first) for infants born before 32 weeks, or
-
The need for >30 % oxygen, with or without positive pressure ventilation or continuous positive pressure, at 56 days’ postnatal age or at discharge (whichever comes first) for infants born after 32 weeks’ gestation
Currently, the most commonly used clinical definition of BPD is the need for supplemental oxygen at 36 weeks’ corrected gestational age, often referred to as the Shennan definition [1]. Newer definitions, incorporating a physiological test of room air saturation at 36 weeks and a severity grade based on the extent of respiratory support required, have also been proposed and are used both clinically and for research purposes [2].
Wheeze
Wheezing is a high-pitched whistling sound heard during breathing caused by air being forced through airways that are narrower than normal. Wheezing is commonly more prominent when breathing out than when breathing in.
Asthma (WHO Definition; See http://www.who.int/respiratory/asthma/definition/en/)
Asthma is a disease characterized by recurrent attacks of breathlessness and wheezing that vary in severity and frequency from person to person. In one individual such attacks may occur every hour, and in another they are a daily occurrence. This condition is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they become easily irritated. During an attack, the lining of the passages swell, causing the airways to narrow and thus the flow of air in and out of the lungs is reduced.
Introduction
Worldwide, the WHO has estimated that more than 15 million infants are born preterm, that is, 11 % of live births. Rates of preterm births are increasing in most countries with reliable data [3]. Across 184 countries, the rate of preterm births ranges from 5 to 18 % of live births. In 2010, it was estimated that the rate of preterm births in the USA was 12 %, accounting for 42 % of all preterm births in developed regions [4]. Approximately, 90 % of preterm infants survived [5]. In 2014, in the USA, the preterm birth rate declined to 9.57 %, while the low birth weight rate remained essentially unchanged at 8 % [6].
In this chapter, we discuss respiratory outcomes of preterm infants and the impact of the diagnosis BPD later in life. It is important to realize, however, that not only respiratory problems may play a role later on, but cardiovascular diseases and developmental problems also play a role. Many survivors face a lifetime of disability, including learning disabilities, visual, and hearing problems. The significant associations of preterm birth with adult health and health-related problems have become increasingly recognized thanks to epidemiological research and clinical observations [7]. In the light of the increasing numbers of survivors of preterm birth, with or without BPD, and the lack of a clear understanding of the etiology of sequelae in later life, the goal of this chapter is to review the published literature to determine the effects of preterm birth on different stages during the course of life. We address the following questions:
-
1.
Which respiratory symptoms are described in preterm-born infants?
-
2.
Does wheezing occur in former preterm-born children, adolescents, or adults?
-
3.
Does gestational age matter?
-
4.
Which putative mechanisms are to be expected?
-
5.
Which risk factors are described for respiratory problems in former preterm-born children?
Which Respiratory Symptoms Are Described in Preterm-Born Infants?
Preterm birth predisposes individuals to the development of chronic respiratory diseases in infancy, childhood, and adulthood, including asthma and chronic obstructive pulmonary disease (COPD) [8]. Beyond the neonatal period, the major respiratory problems during infancy and early childhood that require hospitalization are respiratory exacerbations caused by infections, viral infections particular. Signs and symptoms of severe respiratory infections may include fever, a barking cough, wheezing, tachypnea, chest wall retractions, nasal flaring, difficulty with drinking, lethargy, and sometimes, cyanosis. These symptoms become milder in school-age children. Nevertheless, a group of children remains – even as they grow older – who have recurrent episodes of wheezing and decreased lung function tests, that is, decreased forced expiratory volume [9].
Other respiratory symptoms associated with preterm birth are noisy breathing and stridor due to laryngomalacia or postintubation, subglottic stenosis, vocal cord paralysis, or tracheomalacia. Although preterm infants do not necessarily have a higher incidence of laryngomalacia, they do tend to develop the more severe form of this condition [10]. Left vocal cord paralysis is not uncommon in patients exposed to persistent ductus arteriosus surgery as preterm infants. The condition may be easily overlooked, and the symptoms may be confused with those of other diseases. Laryngoscopy should be offered on the basis of liberal indications after persistent ductus arteriosus ligation [11].
BPD develops in approximately 10–40 % of infants born with very low birth weight (VLBW) and extremely low birth weight, amounting to 5000 to 10,000 new cases in the USA each year, depending on the definition applied [12]. Although mortality attributable to BPD has declined over the past decade [13], BPD places a significant demand on health services [12] and constitutes a significant health burden long after the neonatal period.
On first presentation in 1967, infants with BPD showed persistent respiratory signs and symptoms; they required supplemental oxygen to treat hypoxemia, displayed persistent abnormal lung fields on chest radiograph; and histopathological changes, such as interstitial thickening, lung fibrosis, airway epithelial metaplasia, and smooth muscle hypertrophy occurred [14]. Following the increased use of antenatal steroids for lung maturation and the development of exogenous surfactant replacement therapy, the severity of infant lung diseases decreased and the survival of preterm newborns improved, particularly at lower gestational ages. These medical advancements resulted in the evolution of the disorder to a new form of the condition, one that predominantly occurs in the group of extremely preterm infants. BPD, in the contemporary era of perinatal care, is characterized by persistent decreases in alveolar counts, with enlarged alveoli, resulting in an overall reduction of the surface area available for gas exchange. It is, therefore, considered a consequence of disrupted or arrested lung development [15, 16].
Several studies have shown that infants who develop BPD experience more problems during infancy, childhood, adolescence, and adult age than preterm-born infants who do not develop BPD and healthy controls [17–19]. These problems emerge in different areas of functioning. Chronic respiratory signs in infants with BPD include tachypnea with shallow breathing retractions, a paradoxical breathing pattern with rhonchi, crackles, and wheeze. Pulmonary function tests show decreased tidal volume, increased airway resistance, decreased dynamic lung compliance with increasing ventilation/perfusion mismatch, and decreased V’maxFRC (that worsen during the first year of life) [20, 21]. Uneven airway obstruction leads to gas trapping and hyperinflation with abnormal distribution of ventilation [20]. Because of increased vascular resistance in the lungs, children with BPD may develop right ventricular hypertrophy. Left ventricular hypertrophy is also seen, possibly associated with systemic hypertension, a condition commonly found in children with BPD [22–24]. With persisting lung impairment, survivors of BPD may be at risk of developing chronic obstructive physiologic impairments later in life, such as fixed airflow obstruction and hyperinflation.
Does Wheezing Occur in Former Preterm-Born Children, Adolescents, or Adults?
Been et al. performed a systematic review and a meta-analysis on preterm birth and childhood wheezing disorders. The symptoms they studied included wheezing, coughing, chest tightness, and shortness of breath [9].
The researchers identified 30 studies that investigated the association between preterm birth and asthma or wheezing disorders in more than 1.5 million children between 1995 and the present. This time span was chosen to allow for recent changes in the management of preterm birth. Of the 30 major studies on the association published worldwide, nearly a third reported no effect, whereas the others reported significant associations, with odds ratios (ORs) ranging from 1.2 to 4.9. Across the studies, 13.7 % of the preterm-born infants developed asthma or wheezing disorders during childhood, compared to only 8.3 % of infants born at term. Overall, therefore, the risk of preterm infants developing either asthma or a wheezing disorder during childhood was 1.71 times higher than the risk of full-term infants developing these conditions (an unadjusted OR of 1.71) [9]. Inconsistencies in the preterm birth–asthma association in part reflected differences among studies in three key domains: definitions of asthma, degree of prematurity, and the age at which asthma was assessed.
The pulmonary outcome of extremely preterm and very preterm children (born before 32 weeks’ gestational age) has been studied extensively [5, 25]. The residual respiratory problems of these children include cough and wheeze and/or other asthma-like symptoms [26, 27]. In contrast to the pulmonary problems of extremely and very preterm infants, the pulmonary outcomes of former moderate to late preterm children are largely unknown, even though this group is much larger [6]. It remains uncertain whether the risk of long-term respiratory morbidity is larger in late preterm- born children than in full-term born children [28]. There are reports of increased hospitalization for respiratory problems in the first year of life, a higher rate of respiratory symptoms, for example, nocturnal coughing or wheezing without having a cold during early childhood, and a higher likelihood of abnormal pulmonary function studies [28, 29].
The Lollipop study showed that moderate to late preterm-born children had more respiratory problems during their first 5 years of life than their full-term born counterparts [29]. At the age of 5 years, rates of respiratory symptoms between former moderate to late preterms and extremely and very preterm children were similar and both were higher than in full-term born children. The symptoms resulted in more medication used and more absenteeism from school (see Table 1).
Other studies, however, found that late preterm birth was not associated with a diagnosis of asthma in early childhood [30, 31].
The EPICure study is a good example of considering respiratory symptoms during preadolescence and adolescence in children born extremely preterm. This study defined extremely preterm as <25 completed weeks of gestation. It showed increased respiratory morbidity at 11 years of age, especially among those children who had been diagnosed with BPD [32]. When compared to classmates, children born extremely preterm were more likely to have a current diagnosis of asthma (25 % versus 13 %; P < .01), recent respiratory symptoms, and medication. Among members of the extremely preterm group, significantly more with prior BPD reported wheeze in the past 12 months (see Table 2).
For some former preterm-born adolescents, particularly those who suffered BPD, obstructive lung disease persists into adulthood. Wong et al. reported significantly increased respiratory symptoms in a young adult population born at a time prior to the routine use of surfactant and who had all survived moderate to severe BPD [33]. During adolescence and adulthood, the balance of evidence suggested that preterm infants either with or without subsequent BPD have excess respiratory symptoms, including cough, wheeze, and asthma [34]. Functional pulmonary abnormalities consist of airway obstruction, airway hyperreactivity, and hyperinflation as well as exercise restriction [14, 35]. In these patients, a program of lung function monitoring and pulmonary prophylaxis by means of elimination of specific risk factors, such as smoking, in adulthood is advisable.
Does Gestational Age Matter?
Complications of preterm birth (<37 completed weeks of gestation) are most often seen in very preterm infants (28–31 weeks’ gestation) and extremely preterm infants (<28 weeks’ gestation), although it is increasingly recognized that even moderate to late preterm infants are at increased risk of adverse health and developmental outcomes [36]. This increased risk of respiratory morbidity in late preterm infants is probably related to immature lung structure, since lung development of the terminal respiratory sacs and alveoli continues between gestational weeks 34 and 36.
Infants born most extremely preterm, during the late canalicular or saccular stage of lung development, carry the greatest burden of early respiratory disease, putting them at greatest risk of later pulmonary morbidity. Indeed, despite medical advances in neonatal care that has led to improvements in the survival rate of extremely preterm infants, the prevalence of the neonatal chronic lung disease, BPD, has not diminished [18]. It remains the most common complication of extremely preterm birth. Later in life, the risk of asthma or wheezing disorders increases as the degree of prematurity increases [9]. The risk was considerably higher among children born very preterm, (OR 3.00, 95 % CI 2.61–3.44), when compared to moderately preterm children (OR 1.49, 95 % CI 1.34–1.66) [9].
Which Putative Mechanisms Are to Be Expected?
What is the pathophysiology of respiratory symptoms, such as wheezing disorders, in former preterms? There is the assumption of asthmatics wheeze due to airflow obstruction as a result of the cumulative effects of smooth muscle constriction around airways, airway wall edema, intraluminal mucus accumulation, inflammatory cell infiltration of the submucosa, and basement membrane thickening [37]. Inflammation can cause airway hyperresponsiveness and airflow obstruction.
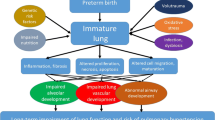
Is this also the case for preterms? They are often labeled asthmatic, although the underlying mechanisms are likely to be very different. Several mechanisms, such as lung growth, inflammation, and structural changes might play a role in putative mechanisms for wheezing in patients born prematurely.
Lung Growth
The respiratory system undergoes significant growth and development during the third trimester of fetal life and throughout the first year of infancy. Postnatally, the pattern of physiological airway development is best characterized by the global lung initiative (http://www.lungfunction.org/). In healthy children, lung volume and function (FVC and FEV1) continue to increase throughout childhood and reach a plateau at 20 to 25 years [38]. Subsequently, lung volume and function decline steadily with age. In individuals who experienced early lung injury or maldevelopment during infancy, a reduction in peak lung growth may appear [8]. The outcome of poor lung development depends on the type and severity of the insult as well as the developmental stage of the lung at the time it occurred [39]. Longitudinal studies show temporal tracking of small airway diseases among preterm-born individuals [40, 41].
The “new” BPD is often described with pathologic changes of large, simplified alveolar structures, a dysmorphic capillary configuration, and variable interstitial cellularity and/or fibroproliferation [15]. Airway and vascular lesions, when present, tend to be present in infants who develop more severe diseases over time [42]. The concept that “new” BPD results in an arrest in alveolization should be modified to that of an impairment in alveolarization since evidence shows that short ventilatory times and/or the use of nCPAP allow continued alveolar formation [42]. An area of emerging interest in the field of lung imaging is 3He diffusion MRI. With this technique, alveolar damage was studied in survivors of extreme preterm birth by comparing alveolar dimensions between full-term born and preterm-born school children [43]. Alveolar size at school age was similar in survivors of extreme prematurity and full-term born children. Because extreme preterm birth is associated with deranged alveolar structure in infancy, the most likely explanation for this finding is catch-up alveolarization.
Therefore, prematurity per se may have an impact on lung growth, but neonatal events and treatment, for example, supplemental oxygen or mechanical ventilation, may cause inflammatory responses followed by a repair process. The repair process may be “healing” but may also become chronic in response to continued inflammation, resulting in structural changes in the airways that are referred to as remodeling. These structural changes may result in irreversible narrowing of the airways. Over time, in most children, with or without BPD, pulmonary function improves. Whether this improvement represents repair of damaged lung tissue or growth of new lung tissue, or both, has yet to be determined.
Children and adolescents with severe BPD, however, show evidence of chronic obstructive pulmonary disease. Kotecha et al. systematically reviewed the literature to determine whether the percentage predicted forced expiratory volume during 1 s (%FEV1) is lower in preterm-born subjects, with or without BPD, in comparison to full-term born controls. They found that %FEV1 is decreased in preterm-born survivors, even in individuals who did not develop BPD. For the preterm-born group without BPD, the mean difference %FEV1 in comparison to full-term born controls is −7.2 %, and for the BPD groups, it was −16.2 % to −18.9 %, respectively (according to the definition of BPD) [44].
Inflammation
Former preterm-born children show little evidence of eosinophilic inflammation [45]. Exhaled nitric oxide concentrations are significantly lower in BPD survivors than in asthmatic cases, suggesting that different pathogenetic mechanisms characterize these two chronic obstructive lung diseases [46].
When inflammation plays a role, treatment with inhaled corticosteroids might be effective. A recent systematic review studied whether inhaled bronchodilators and inhaled corticosteroids improve long-term outcomes in neonates with BPD. No meta-analysis was attempted due to the large degree of heterogeneity and quality assessment of the studies included. The conclusion was that although these inhaled therapies seem to have some benefit, very limited data are available suggesting that these treatments at neonatal age improve long-term outcomes of infants with BPD [47].
Kotecha et al. systematically reviewed the evidence for bronchodilator treatment in former preterm-born children and adults. They concluded that the majority of the studies reported short-term effects of a single-dose administration with an improvement in %FEV1 after bronchodilator treatment. There is, however, a paucity of data on the effect of longer term administration of bronchodilators on the lung function of former preterm-born children [48]. The number of studies with inhaled corticosteroids is small in older preterm-born children and these studies show no effect [49]. There is no current evidence to advocate widespread use of bronchodilators or inhaled corticosteroids, even though a component of variable airflow obstruction may be present. Additional evidence for optimal treatment is required [50].
Acute inflammation due to respiratory tract infections, such as respiratory syncytial virus (RSV) infection, could be an important mechanism of recurrent wheeze during the first year of life in preterm-born infants [51]. A connection between viral infections and exaggerated cell death and inflammatory pathways in the developing lung was recently revealed [52].
Structural Changes
In contrast to early lung development, a process exemplified by the branching of the developing airways, the later development of the immature lung remains poorly understood. A key event in late lung development is secondary septation, in which secondary septa arise from primary septa, creating a greater number of alveoli of a smaller size. This phase in lung development dramatically expands the surface area over which gas exchange can take place [52]. Secondary septation, together with architectural changes to the vascular structure of the lung that minimize the distance between inspired air and blood, is the objective of late lung development. When late lung development is disturbed, lung architecture is malformed. Depending on the severity of the architectural malformation, there may be serious consequences in terms of respiratory function, as well as long-term consequences in later life [52]. It is not clear whether early preterm lung injury is associated with structural damage to surrounding lung tissue because it is difficult to obtain histological tissue for study purposes. Nevertheless, a crucial mechanism that secures airway patency and thus adequate maintenance of functional residual capacity (FRC) is airway tethering [53–55]. Tethering is the element that couples lung volume to airway patency. Thus, as lung volumes increase, airway diameter and hence expiratory flows increase. Maturation of the alveolar network improves parenchymal elastance and, as a consequence, airway tethering. Immaturity adds up to the elements constituting the vulnerability of preterm infants [53].
Radiologic studies in older and more severe cases with BPD showed structural changes such as emphysema on high-resolution CT scans [33, 56, 57]. There seems to be an association between the extent of radiological abnormality on high-resolution CT scans and the severity of lung function impairment [33].
Which Risk Factors Are Described for Respiratory Problems in Former Preterm-Born Children?
On the one hand, there are several factors that may cause a preterm birth and that, additionally, are likely to affect fetal lung development and adult outcome. On the other hand, there are several postnatal factors that affect the risk of continuing respiratory problems in a former preterm-born child.
Preterm delivery is the most common cause of abnormal lung development and can itself lead to lifelong sequelae. As described above, compared to full-term born children, children born very preterm (before 32 weeks’ gestation) approximately run a three times higher risk of developing asthma and/or wheezing disorders in unadjusted and adjusted analyses [9].
Factors that may result in poor lung development prenatally include inadequate nutrition or specific nutrient deficiencies, maternal alcohol consumption, exposure to tobacco smoke, chorioamnionitis, and intrauterine infection. Postnatal factors include being ventilated with high oxygen concentrations, BPD, respiratory infections, and exposure to environmental pollution [8, 52]. Hypoxia also represents a potent stimulus that has a negative impact on late lung development. The impact of corticosteroids on lung development is complicated, since the positive anti-inflammatory and lung maturation impact of corticosteroids is counterbalanced by growth retardation and other effects on the lung [52].
With regard to the respiratory tract infections, “the chicken or the egg” debate is ongoing. Human RSV is the most common cause of severe lower respiratory tract illnesses in both preterm and full-term newborns and young children, and it is associated with subsequent recurrent wheeze. Observational studies cannot determine whether RSV infection is the cause of recurrent wheeze or the first indication of preexistent pulmonary vulnerability in preterm infants. Several studies showed increased susceptibility of preterm-born children (with and without BPD) to RSV [58]. In the Lollipop study, the rates of hospitalization due to proven RSV infection are higher in both preterm groups than in full terms. No difference in disease severity was observed. Among moderate–late preterms, the rate of RSV hospitalization was higher for lower gestational ages and if the infants had been exposed to passive smoking [59].
Nonrandomized trials in preterm infants (approximately 30 ± 2 weeks’ gestational age) suggested that the prevention of lower respiratory tract illness caused by RSV reduces subsequent recurrent wheeze in infants without a family history of atopy, while no effect was found in infants with a family history of atopy [60, 61]. In another study, in otherwise healthy 33–35 weeks’ gestational age preterm infants, palivizumab treatment resulted in a significant reduction in wheezing days during the first year of life, even after treatment had stopped. These findings might implicate RSV infection as an important mechanism of recurrent wheeze during the first year of life in such infants [51]. Other risk factors for continuing respiratory problems in moderate to late preterm children were found to be eczema during the first year of life, passive smoking during the first year, higher social class, and a positive family history of asthma [29].
Conclusion
The major respiratory problems for preterm-born infants (with or without BPD) that need hospitalization are respiratory exacerbations caused by infections, particularly viral infections. The most common symptoms in former preterm-born children are cough, wheeze, and/or other asthma-like symptoms. Overall, the risk of former preterm children developing asthma or a wheezing disorder during childhood is almost twice that of full-term infants. As children grow older, the symptoms become milder. Nevertheless, a group of adolescents and adults remains who still present with chronic airway obstruction defined by recurrent episodes of wheezing and decreased lung function tests, that is, decreased forced expiratory volume. The risk of asthma and/or wheezing disorders increases as the degree of prematurity increases. Putative mechanisms for wheeze may include early lung injury or maldevelopment during infancy, respiratory infections during the first year of life, and structural changes of the lung parenchyma. Patients are often labeled asthmatic although the underlying mechanisms are likely to be very different. Characterizing airway diseases in adult survivors of preterm birth in terms of extent and nature of airflow obstruction, pattern of any inflammation, and presence of airway reactivity is very important to prevent over or under treatment.
References
Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82(4):527–32.
Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(1098–4275; 5):1305–11.
Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. 2014;384(9938):189–205.
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72.
Gibson AM, Doyle LW. Respiratory outcomes for the tiniest or most immature infants. Semin Fetal Neonatal Med. 2014;19(2):105–11.
Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2014. Natl Vital Stat Rep. 2015;64(12):1–64.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73.
Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1(9):728–42.
Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11(1):e1001596.
Adil E, Rager T, Carr M. Location of airway obstruction in term and preterm infants with laryngomalacia. Am J Otolaryngol. 2012;33(4):437–40.
Roksund OD, Clemm H, Heimdal JH, Aukland SM, Sandvik L, Markestad T, et al. Left vocal cord paralysis after extreme preterm birth, a new clinical scenario in adults. Pediatrics. 2010;126(6):e1569–77.
Van Marter LJ. Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14(6):358–66.
Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–40.
Northway Jr WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(0028–4793; 7):357–68.
Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46(0031–3998; 6):641–3.
Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003;8(1):63–71.
Northway Jr WH, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323(0028–4793; 26):1793–9.
Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118(1098–4275; 1):108–13.
Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med. 2006;173(8):890–6.
Watts JL, Ariagno RL, Brady JP. Chronic pulmonary disease in neonates after artificial ventilation: distribution of ventilation and pulmonary interstitial emphysema. Pediatrics. 1977;60(3):273–81.
Hofhuis W, Huysman MW, van der Wiel EC, Holland WP, Hop WC, Brinkhorst G, et al. Worsening of V’maxFRC in infants with chronic lung disease in the first year of life: a more favorable outcome after high-frequency oscillation ventilation. Am J Respir Crit Care Med. 2002;166(12):1539–43.
Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367(9520):1421–31.
Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120(6):1260–9.
Lenfant C. Lung biology in health and disease. New York: Marcel Dekker Inc.; 2000.
Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–9.
Vrijlandt EJ, Boezen HM, Gerritsen J, Stremmelaar EF, Duiverman EJ. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr. 2007;150(3):256–61.
Vrijlandt EJ, Gerritsen J, Boezen HM, Duiverman EJ. Gender differences in respiratory symptoms in 19-year-old adults born preterm. Respir Res. 2005;6:117.
Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013;132(4):741–51.
Vrijlandt EJ, Kerstjens JM, Duiverman EJ, Bos AF, Reijneveld SA. Moderately preterm children have more respiratory problems during their first 5 years of life than children born full term. Am J Respir Crit Care Med. 2013;187(11):1234–40.
Abe K, Shapiro-Mendoza CK, Hall LR, Satten GA. Late preterm birth and risk of developing asthma. J Pediatr. 2010;157(1):74–8.
Voge GA, Katusic SK, Qin R, Juhn YJ. Risk of asthma in late preterm infants: a propensity score approach. J Allergy Clin Immunol Pract. 2015;3(6):905–10.
Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182(2):237–45.
Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32(1399–3003; 2):321–8.
Narang I. Review series: What goes around, comes around: childhood influences on later lung health? Long-term follow-up of infants with lung disease of prematurity. Chron Respir Dis. 2010;7(4):259–69.
Bader D, Ramos AD, Lew CD, Platzker AC, Stabile MW, Keens TG. Childhood sequelae of infant lung disease: exercise and pulmonary function abnormalities after bronchopulmonary dysplasia. J Pediatr. 1987;110(0022–3476; 5):693–9.
Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Trends in severe bronchopulmonary dysplasia rates between 1994 and 2002. J Pediatr. 2005;146(4):469–73.
Fanta CH. Asthma. N Engl J Med. 2009;360(10):1002–14.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43.
Kallapur SG, Ikegami M. Physiological consequences of intrauterine insults. Paediatr Respir Rev. 2006;7(2):110–6.
Sonnenschein-van der Voort AM, Jaddoe VW, Raat H, Moll HA, Hofman A, de Jongste JC, et al. Fetal and infant growth and asthma symptoms in preschool children: the Generation R Study. Am J Respir Crit Care Med. 2012;185(7):731–7.
Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. 2013;68(8):767–76.
Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(0146–0005; 4):179–84.
Narayanan M, Beardsmore CS, Owers-Bradley J, Dogaru CM, Mada M, Ball I, et al. Catch-up alveolarization in ex-preterm children: evidence from (3)He magnetic resonance. Am J Respir Crit Care Med. 2013;187(10):1104–9.
Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, Dunstan FD, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. 2013;68(8):760–6.
Baraldi E, Bonetto G, Zacchello F, Filippone M. Low exhaled nitric oxide in school-age children with bronchopulmonary dysplasia and airflow limitation. Am J Respir Crit Care Med. 2005;171(1):68–72.
Carraro S, Piacentini G, Lusiani M, Uyan ZS, Filippone M, Schiavon M, et al. Exhaled air temperature in children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2010;45(12):1240–5.
Clouse BJ, Jadcherla SR, Slaughter JL. Systematic review of inhaled bronchodilator and corticosteroid therapies in infants with bronchopulmonary dysplasia: implications and future directions. PLoS One. 2016;11(2):e0148188.
Kotecha SJ, Edwards MO, Watkins WJ, Lowe J, Henderson AJ, Kotecha S. Effect of bronchodilators on forced expiratory volume in 1 s in preterm-born participants aged 5 and over: a systematic review. Neonatology. 2015;107(3):231–40.
Chan KN, Silverman M. Increased airway responsiveness in children of low birth weight at school age: effect of topical corticosteroids. Arch Dis Child. 1993;69(1468–2044; 1):120–4.
Bolton CE, Bush A, Hurst JR, Kotecha S, McGarvey L. Republished: lung consequences in adults born prematurely. Postgrad Med J. 2015;91(1082):712–8.
Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368(19):1791–9.
Madurga A, Mizikova I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2013;305(12):L893–905.
Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics. 2010;126(1):115–28.
Henschen M, Stocks J, Brookes I, Frey U. New aspects of airway mechanics in pre-term infants. Eur Respir J. 2006;27(5):913–20.
Plopper CG, Nishio SJ, Schelegle ES. Tethering tracheobronchial airways within the lungs. Am J Respir Crit Care Med. 2003;167(1):2–3.
Wong P, Murray C, Louw J, French N, Chambers D. Adult bronchopulmonary dysplasia: computed tomography pulmonary findings. J Med Imaging Radiat Oncol. 2011;55(4):373–8.
Aquino SL, Schechter MS, Chiles C, Ablin DS, Chipps B, Webb WR. High-resolution inspiratory and expiratory CT in older children and adults with bronchopulmonary dysplasia. AJR Am J Roentgenol. 1999;173(4):963–7.
Boyce TG, Mellen BG, Mitchel Jr EF, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–70.
Gijtenbeek RG, Kerstjens JM, Reijneveld SA, Duiverman EJ, Bos AF, Vrijlandt EJ. RSV infection among children born moderately preterm in a community-based cohort. Eur J Pediatr. 2015;174(4):435–42.
Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151(1):34–42. 42.e1.
Simoes EA, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR, et al. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126(2):256–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Vrijlandt, E. (2017). Why Do Preterm Infants Wheeze? Clues from Epidemiology. In: Hibbs, A., Muhlebach , M. (eds) Respiratory Outcomes in Preterm Infants. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-48835-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-48835-6_2
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-48834-9
Online ISBN: 978-3-319-48835-6
eBook Packages: MedicineMedicine (R0)