Abstract
Acute brain injury is a dynamic process that frequently includes hemodynamic, electrical, and metabolic changes. Pathologic events such as intracranial hypertension, cerebral ischemia, brain tissue hypoxia or non-convulsive seizures can cause increased stress to the vulnerable brain resulting in additional brain injury. Early detection of these often subclinical changes and proactive management to optimize the delivery of oxygen and energy substrates is the goal of modern multimodality monitoring. In this chapter we will provide an overview and discuss how to interpret the results of the most commonly monitored physiologic parameters, including intracranial pressure, cerebral blood flow, jugular venous oxygen saturation, quantitative EEG measures, near-infrared spectroscopy, cerebral microdialysis and brain tissue oxygenation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Multimodality monitoring
- Intracranial pressure
- Cerebral blood flow
- Jugular venous oxygen saturation
- Cerebral microdialysis
- Brain tissue oxygen tension
- Near-infrared spectroscopy
20.1 Introduction
Brain multimodality monitoring (BMM) encompasses a variety of technologies that can provide real time information about the relative health or distress of the brain after various forms of acute injury. Multiple pathologic processes such as inflammation, brain edema and ischemia can lead to evolving brain damage. This so-called secondary brain injury significantly contributes to disability and long-term outcome. By optimizing cerebral hemodynamics, oxygenation and metabolism, BMM can help to create and maintain an optimal physiologic environment for the injured brain.
20.2 Case Presentation
A 44-year-old man fell and hit his head on a rock while skiing on an unmarked slope. The patient was not wearing a helmet at the time of his injury. At the scene, he intermittently opened his eyes, moaned and had flexion withdrawal of his arms (Glasgow Coma Sore 6). There was a contusion on his forehead but no other signs of injury. The patient was transported to the nearest emergency room where he no longer opened his eyes, had extensor posturing of his arms and made no verbal responses.
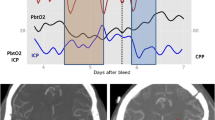
A non-contrast head CT revealed bilateral frontal contusions with evidence of transtentorial herniation (Fig. 20.1). An external ventricular device (EVD) was emergently placed and revealed an elevated opening pressure of 34 mmHg. At the same time a multi-lumen bolt was placed for monitoring of brain tissue oxygen and cellular metabolism. The patient was started on hyperosmolar therapy to counter the effects of the present ICP crisis. Once the patient arrived to the Neuro ICU, he was connected to continuous EEG monitoring, which revealed evidence of subclinical seizures. Data from microdialysis showed evidence of cerebrometabolic crisis. The patient was loaded with an antiepileptic agent to control the seizures. Once the subclinical seizures were controlled, the lactate/pyruvate ratio decreased and the patient’s ICP normalized. PbO2 levels in in the brain also stabilized.
An MRI of the brain revealed evolution of the bifrontal contusions, but no strokes were seen. After many days the patient was successfully extubated and was able to eat on his own. Clinically his exam was consistent with a bifrontal injury. He was discharged approximately 2 weeks after admission to an acute rehabilitation facility that specializes in acute brain injury.
20.3 Intracranial Pressure (ICP)
ICP reflects the global pressure in the intracranial vault. Measurement of ICP remains the most commonly performed type of monitoring in patients with acute brain injury and is routinely used to guide medical care. Elevated intracranial pressure can compromise cerebral blood flow and lead to brain herniation. The most common devices for ICP measurement are intraventricular catheters and fiberoptic intraparenchymal monitors. Intraventricular catheters are surgically placed into the frontal horn of the lateral ventricle and connected to an external pressure transducer. They are highly accurate and allow for therapeutic drainage of CSF. The primary disadvantage is a high rate of infection (5–10%) and difficult placement in case of small or compressed ventricles. Intraparenchymal monitors consist of a small catheter with a fiberoptic microtransducer at the tip. They have a lower complication rate and are not at risk for catheter occlusion or leakage. Their main drawbacks include the inability to drain CSF for diagnostic or therapeutic purposes and the potential to lose accuracy over several days, as catheters cannot be recalibrated after initial placement [1]. The decision to place an ICP monitor is generally made when a patient is suspected to have elevated ICP (imaging and exam findings) and coma (GCS of 8 or less). Normal values range between 7 and 15 mmHg. The threshold that defines intracranial hypertension is uncertain but generally values above 20–25 mmHg are considered pathological [2]. It is important to consider that when little or no CSF volume is left due to brain swelling, compartmentalized intracranial hypertension may exist. Uniformly distributed ICP requires that CSF can circulate freely between all its natural pools, thus equilibrating pressure everywhere. In circumstances where CSF becomes trapped in isolated areas, ventricular catheters may not reflect ICP in all intracranial compartments.
Continuous measurement of ICP can be use to calculate cerebral perfusion pressure (CPP). Under normal circumstances, the cerebral vasculature has the intrinsic ability to maintain a stable blood flow despite changes in cerebral perfusion pressure, a mechanism known as cerebral autoregulation [3, 4]. This mechanism ensures that the cerebral blood flow matches the brain’s metabolic demands and protects it from hypo- or hyperperfusion. After acute brain injury, this precise control of cerebral blood flow is frequently impaired, and as a result, acute changes in systemic pressure are passively transmitted to the cerebral circulation. This may lead to insufficiently low cerebral blood flow causing ischemia, or conversely, too high flow causing intracranial hypertension and cerebral edema. Although the optimal CPP for a given patient may vary, it is generally kept between 60 and 110 mmHg.
In addition to absolute pressure measurements, analysis of the ICP waveform can provide clues about reduced brain compliance (Δ volume/Δ pressure), which often precedes frank ICP elevations [5]. As compliance falls, the second peak of the ICP waveform (P2 or tidal wave) becomes elevated relative to the first peak (P1 or percussion wave) giving the wave a more rounded appearance (Fig. 20.2).
Elevated ICP is consistently associated with poor outcome and increased mortality [6]. Refractory ICP is associated with a drastic increase in risk of death [7]. However, a recent randomized controlled trial failed to demonstrate a benefit from ICP monitoring after traumatic brain injury [8]. This trial compared two management strategies, in which treatment was triggered by either ICP monitoring or by a combination of physical exam findings and neuroimaging. It must be emphasized that evaluation and treatment of elevated ICP was fundamental to both groups in this trial, and although there was a trend towards lower mortality and more efficient care, ICP monitoring alone may not be enough to enhance TBI outcome. Care of severe TBI is complex with several mechanisms contributing to secondary brain injury. Consequently, it may require additional monitors (i.e. brain multimodality monitoring) to gain better insight into patient specific pathophysiology and provide more targeted care in order to improve outcome.
20.4 Cerebral Blood Flow
Recent advancements in technology allow direct measurement of regional blood flow via thermal diffusion (TD-rCBF). The probe consists of small catheter that contains two metal plates at its distal tip (about 5 mm apart). The distal plate is minimally heated thereby generating a constant spherical temperature field. Temperature variations at the proximal sensor are a measure of the tissue’s ability to transport heat and correlate with cerebral blood flow. The probe is usually inserted into the brain parenchyma through a multi-lumen bolt next to ICP and PbtO2 probes. It provides a continuous and quantitative measure of blood flow in a small volume surrounding the catheter tip. Regional CBF values have shown good agreement with xenon enhanced CT [9]. The technique has been used in combination with PbtO2 to optimize CPP after TBI and guide blood pressure management in SAH patients with vasospasm [10, 11]. While the technology is promising, the available data is limited without clearly defined ischemia thresholds. Although the risks of probe placement are small (1–2% risk of bleeding, infection) and comparable to the complication rate of other intraparenchymal catheters, the utility of rCBF monitoring is limited due to its invasive nature as well as its small sample volume with uncertainty about where to place the probes. Furthermore, the accuracy of TD-rCBF probes may be influenced by elevations in temperature.
20.5 Jugular Venous Oxygen Saturation (SjvO2)
Venous blood from the brain drains via the cerebral sinuses and jugular veins to the right atrium. Measurement of the oxygen saturation in the draining blood provides information about the balance between oxygen delivery and the cerebral metabolic demand. Simplistically, when metabolic demand exceeds supply the brain extracts more oxygen resulting in a decreased SjvO2.
For the measurement of SjvO2, a catheter is placed retrograde via the internal jugular vein into the jugular bulb (dilated portion of the jugular vein just below the base of the skull). It is important that the catheter tip is positioned beyond the inlet of the facial vein and inferior petrosal sinus to avoid contamination with oxygen-rich, extracerebral blood. Placement of the catheter tip in the jugular bulb should be confirmed with a lateral skull radiograph. The tip of the catheter should be at the level of the mastoid air cells [12].
O2 saturation can be measured continuously using a fiberoptic catheter or intermittently by drawing and analyzing a blood sample.
Under physiologic conditions, cerebral blood flow matches metabolic rate of oxygen (CMRO2) and the difference in oxygen content between arterial and jugular venous blood (AVDO2) remains constant. If arterial oxyhemoglobin saturation and hemoglobin concentration remain stable, the SjvO2 is a good approximation of the AVDO2. A low SjvO2 (i.e. jugular desaturation) indicates increased oxygen extraction from the blood, suggesting inadequate cerebral blood flow in relation to CMRO2. A high SjVO2 indicates that oxygen supply is greater than demand (Table 20.1).
Monitoring of jugular venous oximetry can be considered in comatose patients (GCS ≤8) at risk for cerebral ischemia such as severe traumatic brain injury or high-grade subarachnoid hemorrhage [2]. In these patients monitoring of SjvO2 allows early detection of ischemia and can be used to guide hyperventilation therapy [13,14,15,16]. Robertson et al. reported that jugular venous desaturation (SjvO2 <50%) were a frequent occurrence in patients with severe traumatic brain injury and in the majority of cases could be attributed to elevated intracranial pressure, hypocarbia and hypotension. Furthermore, the number of desaturations was associated with an increased risk of death and poor neurologic outcome [14, 16]. Hyperventilation therapy is frequently used in the management of intracranial pressure. Reductions in PaCO2 lead to cerebral vasoconstriction resulting decreased intracranial volume and pressure. However, the reduction in blood flow can also cause cerebral hypoperfusion and thus precipitate or worsen ischemia. SvjO2 has been shown to correlate well with brain tissue oxygen (PbtO2) and SvjO2 monitoring can be useful to optimize hyperventilation therapy [15]. However, small regions of critically hypoperfused brain have been demonstrated even while SvjO2 remained above 50% [17].
It is important to emphasize that SjvO2 is a global measure of cerebral oxygenation and not very sensitive for detecting regional ischemia. For jugular venous desaturations <50% to occur, at least 13% (170 ml) of the brain has to become ischemic [18]. Further limitations include considerable variability in saturations measured from both sides of the brain [19]. The sensitivity to detect jugular venous desaturation can be increased by cannulating the side of the predominant lesion or for diffuse injury the side with the larger jugular foramen on CT imaging [20]. Alternatively, the dominant internal jugular vein can be determined by unilateral compression of the vessel and selection of the side that produces the greater rise in ICP as it likely represents the side of predominant venous drainage [21]. Contamination with extracranial blood can occur if the catheter is placed too proximally or in cases of intermittent sampling if blood is aspirated rapidly (>2 ml/min). Inaccuracies may also occur if catheter tip is thrombosed or impacted against the vessel wall. While the rate of infection and complications related to catheter insertion are rare, the incidence of subclinical thrombosis is reported in up to 40% of cases [22].
20.6 Continuous Electroencephalography
Continuous electroencephalopathy monitoring (cEEG) in the neurocritical intensive care unit is primarily used for the detection of non-convulsive seizures (NCSz) or status epilepticus (NCSE) in patients with unexplained changes in level of consciousness. EEG is also helpful for the characterization of sudden spells such as tremors, twitching, posturing, eye deviation or agitation. Other indications in include detection of cerebral ischemia, monitoring the level of sedation and titration of medication during anaesthetic coma as well as prognostication.
Seizures are seen in 10–30% of patients with acute brain injury [23, 24]. While the majority of them are not exhibiting motor features, subclinical seizures have been associated with increased intracranial pressure and disturbed brain metabolism possibly leading to secondary brain injury [25, 26]. Furthermore, patients with seizures have increased mortality and among patients with SAH seizure burden has been associated with poor functional and cognitive [27, 28].
There is limited data to support the use of cEEG compared to spot EEGs (approximately 30 min); however, spot EEG will not detect nonconvulsive seizures in about half of those having seizures compared to longer monitoring [23].
Another important application of EEG in neurocritical care is the detection of brain ischemia. Brain function is represented on EEG as an oscillating wave of various frequencies. EEG activity is conventionally divided into the following frequencies (number of waveforms per second):
-
Delta (0.5–3 Hz)
-
Theta (4–7 Hz)
-
Alpha (8–12 Hz)
-
Beta (>13 Hz).
Most of the waveforms are generated by pyramidal neurons within the cortex. These cells are very sensitive to hypoxia and ischemia and EEG abnormalities can be seen within seconds to minutes. With decreasing cerebral blood flow, the EEG progresses through predictable changes: (1) loss of faster beta frequencies, (2) slowing of background to theta and later delta range, and (3) background attenuation and finally suppression of all frequencies [29]. Therefore, EEG allows providers the opportunity to detect ischemia and to alter treatment before hypoperfusion leads to cell death and permanent injury. However, some of the early changes can be subtle and may not be appreciated on raw EEG. Mathematical processing of the raw data, also known as quantitative EEG (qEEG) analysis, can help to better visualize these changes. Using frequency analysis, the original EEG can be quantified in terms of frequency, amplitude and rhythmicity, which allows the calculation of numerical values or percentages. Furthermore, this digital form can compress long periods of EEG data into readable graphs allowing for prolonged monitoring of patients and aid detection of ischemia. The ideal qEEG measure to identify ischemia is still being debated and may depend of the clinical situation. A variety of parameters to measure slowing or attenuation have been suggested; they commonly include some ratio between fast and slow frequencies or a measure of the relative power (i.e. amount) of a specific frequency within the entire EEG spectrum.
Ischemia monitoring can be particularly useful in comatose or sedated patients when the clinical exam is limited. A reduction in alpha/delta ratio (8–13 Hz/1–4 Hz) and a decrease in the variability in relative alpha frequency have been shown to correlate with angiographic vasospasm or delayed cerebral ischemia (DCI) in patients with poor-grade SAH [30, 31]. For detection of focal ischemia, the relative delta percentage appears to provide the most robust correlation with CBF [32]. Clinically, qEEG has been correlated with stroke severity as measured by the National Institutes of Health Stroke Scale, infarct volume on MRI, and functional outcome [33,34,35,36]. Furthermore, qEEG can be used to assess treatment response, and given the correlation to cerebral perfusion pressure may be helpful to guide blood pressure management in individual patients after large-vessel acute ischemic stroke [37].
Despite the many advantages of cEEG, it does have its drawbacks. The monitoring systems, technician training, and the costs of disposable equipment can be expensive. Frequent review of many hours of data is necessary and can be time consuming. Quantitative EEG has the promise to reduce some of this time; however, having clinicians specially trained to interpret qEEG results is essential.
20.7 Near-Infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is a non-invasive technique to measure the oxygen saturation of hemoglobin in the brain tissue. Delivered via optodes placed on the skin, near-infrared light has the ability to penetrate through scalp, bone and brain tissue up to a depth of about 3 cm. NIRS operates on the principle that the majority of near-infrared light is absorbed intracranially by oxygenated hemoglobin (HbO2) and de-oxygenated hemoglobin (Hb). Assuming constant scattering and applying knowledge of the absorption spectra for HbO2 and Hb, light attenuation can be converted into concentrations of HbO2 and Hb using the modified Lambert–Beer Law [38]. Their sum provides the total hemoglobin concentration (HbT) and the ratio HbO2/HbT equals the oxygen saturation of hemoglobin in brain tissue (StO2 or ScO2). In the setting of stable arterial oxygenation and cerebral metabolic rate, StO2 has been used as a surrogate for cerebral blood flow [39].
NIRS has many clinical applications. For example, NIRS can be used to monitor for changes in cerebral blood flow and oxygenation without the need for invasive probes and has been used to provide information regarding cerebral autoregulation after TBI, stroke and subarachnoid hemorrhage [40,41,42].
Despite the increased utilization of NIRS, there are some important limitations. First and foremost, there is concern for extracerebral contamination of the NIRS signal. In order to measure brain tissue oxygen saturation photons must travel through scalp, skull and dura, which contain various concentrations of blood and other tissue-derived chromophores (light absorbing molecules), which potentially confound the signal derived from the cerebral cortex [43]. In addition, NIRS light attenuation is not just the result of absorbtion by target chromophores (Hg and HgO2), but also light scattering. Bone, hair, subgaleal collections, subdural hematomas, and differences in areas of subarachnoid spaces can result in in nonlinear relationships between absorption and attenuation changes [44].
20.8 Cerebral Microdialysis
Cerebral microdialysis is used to measure brain tissue chemistry thus providing important information about brain metabolism and more specifically the adequacy of energy supply and cellular function. A thin catheter is placed through a cranial bolt into an area of interest in the brain parenchyma. The catheter is lined with a semi-permeable dialysis membrane and constantly perfused at a very low rate with an isotonic solution (e.g. artificial cerebrospinal fluid). Molecules below a certain size (usually 20 kDa) diffuse from the extracellular space through the membrane into the perfusion fluid, which is collected at regular intervals (e.g. every 60 min) and analyzed at the bedside using the manufacturer’s equipment [45]. The analysis usually includes concentrations of glucose, pyruvate, lactate, glutamate, and glycerol. Many other metabolites as well as exogenous substances such as administered drugs can be studied, however, their clinical utility remains to be determined.
Glucose, pyruvate, and lactate provide information about the available fuel source of the brain as well as the brain’s ability to go through aerobic metabolism. When energy is needed, glucose undergoes a series of enzymatic conversions known as glycolysis. Under aerobic conditions, the resulting pyruvate enters the citric acid cycle and gets metabolized to ATP. During hypoxia and ischemia, the end product of pyruvate is lactate resulting in an increased lactate to pyruvate ratio (LPR). Ischemia also results in the release of glutamate, a marker of metabolic distress. High glycerol levels originating from glycero–phospholipid containing cell membranes indicate cellular breakdown. Normal values for the metabolites have been established to help guide clinicians in interpreting this data (Table 20.2), however, variations over time and changes in response to therapeutic interventions may be more useful [46]. Furthermore, analyzing trends for multiple microdialysis markers rather than looking at individual metabolites may allow for a more meaningful interpretation. For example, a typical pattern of cerebral ischemia includes a marked decrease in brain glucose, elevated lactate, increase in LPR and lactate to glucose ratio (LGR) and a moderate decrease in pyruvate [45].
Cerebral microdialysis has been used in various clinical situations and is indicated in patients at risk for cerebral ischemia, hypoxia, energy failure, and glucose deficiency [2]. In patients with severe traumatic brain injury, it may contribute to prognostication. A high lactate to pyruvate ratio has been shown to predict mortality and poor functional outcome [47]. For prognostication cerebral microdialysis should only be used in association with clinical indicators and other brain monitoring techniques [2]. After subarachnoid hemorrhage, cerebral microdialysis may identify ischemic tissue before it progresses to irreversible cell damage, thus providing an opportunity for therapeutic intervention. Cerebral microdialysis can assist titration of medical therapies such as blood pressure management or systemic glucose control. Whether treatments directed towards improving neurochemistry lead to improved outcomes remains to be determined.
Cerebral microdialysis has an excellent safety record and many of the same risks apply as with placing any type of catheter into the brain. However, just like brain tissue oxygen monitoring, cerebral microdialysis is a focal measurement and should be interpreted based on that capital location seen on postinsertion CT scan. Other limitations include the lack of real-time data given the time it takes for metabolite collection and sample analysis. Cerebral microdialysis is labor-intensive and unit staff need to be trained in catheter maintenance, sample collection, and analysis using the manufacturer’s equipment. Also, it may be necessary to involve hospital IT personnel in order to integrate this data into the electronic medical record.
20.9 Brain Parenchymal Oxygen Tension
Adequate oxygen delivery to the brain is important to prevent secondary brain injury. Oxygen content in a discrete area of brain tissue can be measured with a small catheter placed through a cranial bolt into the white matter about 2–3 cm below the dura. The catheter provides continuous measurements of the brain parenchymal oxygen tension (PbtO2), thus providing vital information about oxygen delivery and consumption. Some uncertainty exists regarding the ideal placement of the monitor. Because probes provide only regional information (~17 mm3), they are typically placed in the area of the brain at greatest risk for ischemia and secondary injury. In patients with focal injury such as ICH, well-demarcated cerebral contusions or infarction, a peri-lesional placement is preferred. For detection of ischemia related to vasospasm and DCI after subarachnoid hemorrhage (SAH), the probe is typically placed in the vascular territory supplied by the ruptured artery or hemisphere with the greatest clot burden. In patients with diffuse injury, probes are most commonly placed in the non-dominant frontal lobe.
-
A normal PbtO2 value is between 23 and 35 mmHg [48].
-
PbtO2 value of less than 20 indicates possible lack of brain oxygen and is considered a warning to clinicians that intervention may be necessary [2].
Most data regarding PbtO2 comes from patients with traumatic brain injury and SAH. Several observational studies have demonstrated an association between brain tissue hypoxia and unfavorable outcome [49]. PbtO2 can be influenced by a variety of local and systemic factors including arterial blood pressure (MAP), ICP, fraction of inspired oxygen (FiO2), arterial partial pressure of oxygen (PaO2), temperature, and blood hemoglobin concentration. Among them mean arterial pressure and FiO2 are most strongly correlated with PbtO2 [50]. However, a low PbtO2 can be seen despite maintenance of normal ICP and CPP [51]. Thus, strategies to improve PbtO2 should be tailored to the individual patient and additional monitoring devices (EEG, CBF monitor, brain temperature probe) may help to narrow the differential diagnosis of a low PbtO2. While one patient may respond to hemodynamic augmentation, another patient may require blood transfusion and an increase in oxygen transport capacity. Most common interventions include CPP optimization (treatment of intravascular volume depletion, augmentation of blood pressure and cardiac output), treatment of elevated ICP (CSF diversion, osmotherapy or surgical decompression), ventilator management (adjustment of FiO2 or other ventilator settings), or decreasing metabolic demand (sedation, treatment of fever and seizures, hypothermia) [52]. It is unknown at this time if PbtO2- directed therapy alters prognosis; however current data is promising and a phase II study to test this hypothesis is currently under way.
Probe placement is generally safe with a low complication rate and data is accurate for up to 10 days [49, 53]. There are some limitations and challenges in obtaining PbtO2 data. Because of substantial differences in PbtO2 values between manufacturing companies, the device should not be used interchangeably. Also, the currently used bedside technique does not allow for accurate placement of catheters in the peri-lesional tissue.
20.10 Bioinformatics
To capture the complex pathophysiology underlying acute brain injury, it is important to record and integrate multiple parameters of brain function. However, the systems currently in place lack data integration, as most devices capture only the data that they acquire. In 2009, the American Society for Testing and Materials envisioned an ‘integrated clinical environment’ that will help to better assimilate medical devices and understand the complex interaction of various parameters.
Another important aspect of capturing this data is to ensure that the data is captured in a synchronous fashion. All data should be timestamped to coincide with each other. This will help give the clinician insight into physiological changes and how they relate to each other. Finally, a system needs to be designed that can filter data that may contain artifacts. Oftentimes, systems may need to be zeroed or are frequently disconnected in order to provide routine clinical care. A system must be able to recognize gaps and resulting artifact, and separate these periods of data as they may mask underlying trends. By focusing on these key elements to data integration, providers will be able to better understand trends and begin to develop theories.
Currently, a small number of hospitals and institutions have designed the limited number of integrated systems that exist. The disadvantage of these systems is that data can only be collected on one patient at a time. However, these systems are less expensive than distributed systems. Distributed systems are more difficult to set-up because they must be placed in multiple patient rooms. Information must be transferred to a computer server that can handle sensitive patient information.
Despite the advances in monitoring the neurocritical care patient in the ICU, the ability to import multiple data points into a coordinated system has been challenging. As the barriers that limit the design of integrated system lessen, there is an opportunity for health care providers to interpret and understand data that was previously unknown. Hypothesizing about this physiological information may lead to a better understanding of the human brain and how it affects patient outcomes in the future.
Summary Points
-
Acute brain injury is a dynamic process that frequently includes hemodynamic, electrical, and metabolic changes.
-
A variety of developing modalities are available for evaluating the physiologic parameters of brain activity and metabolism.
-
Continued research and development of technology is needed to better integrate multimodality measurements and optimize impact on patient outcomes.
References
Piper I, Barnes A, Smith D, Dunn L. The Camino intracranial pressure sensor: is it optimal technology? An internal audit with a review of current intracranial pressure monitoring technologies. Neurosurgery. 2001;49:1158–64. discussion 1164–5.
Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014;21(Suppl 2):S1–26. doi:10.1007/s12028-014-0041-5.
Lassen NA. Autoregulation of cerebral blood flow. Circ Res. 1964;15(SUPPL):201–4.
Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52.
Cardoso ER, Rowan JO, Galbraith S. Analysis of the cerebrospinal fluid pulse wave in intracranial pressure. J Neurosurg. 1983;59:817–21. doi:10.3171/jns.1983.59.5.0817.
Badri S, Chen J, Barber J, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38:1800–9. doi:10.1007/s00134-012-2655-4.
Treggiari MM, Schutz N, Yanez ND, Romand J-A. Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: a systematic review. Neurocrit Care. 2007;6:104–12. doi:10.1007/s12028-007-0012-1.
Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–81. doi:10.1056/NEJMoa1207363.
Vajkoczy P, Roth H, Horn P, et al. Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J Neurosurg. 2000;93:265–74. doi:10.3171/jns.2000.93.2.0265.
Rosenthal G, Sanchez-Mejia RO, Phan N, et al. Incorporating a parenchymal thermal diffusion cerebral blood flow probe in bedside assessment of cerebral autoregulation and vasoreactivity in patients with severe traumatic brain injury. J Neurosurg. 2011;114:62–70. doi:10.3171/2010.6.JNS091360.
Muench E, Horn P, Bauhuf C, et al. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Crit Care Med. 2007;35:1844–51. quiz 1852 doi:10.1097/01.CCM.0000275392.08410.DD.
Bankier AA, Fleischmann D, Windisch A, et al. Position of jugular oxygen saturation catheter in patients with head trauma: assessment by use of plain films. AJR Am J Roentgenol. 1995;164:437–41. doi:10.2214/ajr.164.2.7839985.
Lewis SB, Myburgh JA, Reilly PL. Detection of cerebral venous desaturation by continuous jugular bulb oximetry following acute neurotrauma. Anaesth Intensive Care. 1995;23:307–14.
Gopinath SP, Valadka AB, Uzura M, Robertson CS. Comparison of jugular venous oxygen saturation and brain tissue Po2 as monitors of cerebral ischemia after head injury. Crit Care Med. 1999;27:2337–45.
Gupta AK, Hutchinson PJ, Al-Rawi P, et al. Measuring brain tissue oxygenation compared with jugular venous oxygen saturation for monitoring cerebral oxygenation after traumatic brain injury. Anesth Analg. 1999;88:549–53.
Robertson CS, Gopinath SP, Goodman JC, et al. SjvO2 monitoring in head-injured patients. J Neurotrauma. 1995;12:891–6. doi:10.1089/neu.1995.12.891.
Coles JP, Minhas PS, Fryer TD, et al. Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates. Crit Care Med. 2002;30:1950–9. doi:10.1097/01.CCM.0000026331.91456.9A.
Coles JP, Fryer TD, Smielewski P, et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24:202–11. doi:10.1097/01.WCB.0000103022.98348.24.
Beards SC, Yule S, Kassner A, Jackson A. Anatomical variation of cerebral venous drainage: the theoretical effect on jugular bulb blood samples. Anaesthesia. 1998;53:627–33.
Metz C, Holzschuh M, Bein T, et al. Monitoring of cerebral oxygen metabolism in the jugular bulb: reliability of unilateral measurements in severe head injury. J Cereb Blood Flow Metab. 1998;18:332–43. doi:10.1097/00004647-199803000-00012.
Dearden NM. Jugular bulb venous oxygen saturation in the management of severe head injury. Curr Opin Anesthesiol. 1991;4:279.
Coplin WM, O'Keefe GE, Grady MS, et al. Thrombotic, infectious, and procedural complications of the jugular bulb catheter in the intensive care unit. Neurosurgery. 1997;41:101–7. discussion 107–9.
Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8.
Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–5.
Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–6.
Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53–64. doi:10.1002/ana.23859.
De Marchis GM, Pugin D, Meyers E, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86:253–60. doi:10.1212/WNL.0000000000002281.
Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–12. doi:10.1385/NCC:4:2:103.
Jordan KG. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol. 2004;21:341–52.
Claassen J, Hirsch LJ, Kreiter KT, Du EY. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115(12):2699–710.
Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607–15.
Foreman B, Claassen J. Quantitative EEG for the detection of brain ischemia. Crit Care. 2012;16:216. doi:10.1186/cc11230.
Sheorajpanday RVA, Nagels G, Weeren AJTM, et al. Additional value of quantitative EEG in acute anterior circulation syndrome of presumed ischemic origin. Clin Neurophysiol. 2010;121:1719–25. doi:10.1016/j.clinph.2009.10.037.
Sheorajpanday RVA, Nagels G, Weeren AJTM, De Deyn PP. Quantitative EEG in ischemic stroke: correlation with infarct volume and functional status in posterior circulation and lacunar syndromes. Clin Neurophysiol. 2011;122:884–90. doi:10.1016/j.clinph.2010.08.020.
Sheorajpanday RVA, Nagels G, Weeren AJTM, et al. Reproducibility and clinical relevance of quantitative EEG parameters in cerebral ischemia: a basic approach. Clin Neurophysiol. 2009;120:845–55. doi:10.1016/j.clinph.2009.02.171.
Finnigan SP, Rose SE, Walsh M, et al. Correlation of quantitative EEG in acute ischemic stroke with 30-day NIHSS score: comparison with diffusion and perfusion MRI. Stroke. 2004;35:899–903. doi:10.1161/01.STR.0000122622.73916.d2.
Diedler J, Sykora M, Rupp A, et al. Impaired cerebral vasomotor activity in spontaneous intracerebral hemorrhage. Stroke. 2009;40:815–9. doi:10.1161/STROKEAHA.108.531020.
Wyatt JS, Cope M, Delpy DT, et al. Quantitation of cerebral blood volume in human infants by near-infrared spectroscopy. J Appl Physiol (1985). 1990;68:1086–91.
Fantini S, Sassaroli A, Tgavalekos KT, Kornbluth J. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophoton. 2016;3:031411. doi:10.1117/1.NPh.3.3.031411.
Zweifel C, Castellani G, Czosnyka M, et al. Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41:1963–8. doi:10.1161/STROKEAHA.109.577320.
Reinhard M, Wehrle-Wieland E, Grabiak D, et al. Oscillatory cerebral hemodynamics – the macro- vs. microvascular level. J Neurol Sci. 2006;250:103–9. doi:10.1016/j.jns.2006.07.011.
Diedler J, Zweifel C, Budohoski KP, et al. The limitations of near-infrared spectroscopy to assess cerebrovascular reactivity: the role of slow frequency oscillations. Anesth Analg. 2011;113:849–57. doi:10.1213/ANE.0b013e3182285dc0.
Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–13. doi:10.1093/bja/aep299.
Sen AN, Gopinath SP, Robertson CS. Clinical application of near-infrared spectroscopy in patients with traumatic brain injury: a review of the progress of the field. Neurophoton. 2016;3:031409. doi:10.1117/1.NPh.3.3.031409.
Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma. 2005;22:3–41. doi:10.1089/neu.2005.22.3.
Reinstrup P, Ståhl N, Mellergård P, et al. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery. 2000;47:701–9. discussion 709–10.
Timofeev I, Carpenter KLH, Nortje J, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 2011;134:484–94. doi:10.1093/brain/awq353.
Pennings FA, Schuurman PR, van den Munckhof P, Bouma GJ. Brain tissue oxygen pressure monitoring in awake patients during functional neurosurgery: the assessment of normal values. J Neurotrauma. 2008;25:1173–7. doi:10.1089/neu.2007.0402.
Maloney-Wilensky E, Gracias V, Itkin A, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review*. Crit Care Med. 2009;37:2057–63. doi:10.1097/CCM.0b013e3181a009f8.
Rose JC, Neill TA, Hemphill JC. Continuous monitoring of the microcirculation in neurocritical care: an update on brain tissue oxygenation. Curr Opin Crit Care. 2006;12:97–102. doi:10.1097/01.ccx.0000216574.26686.e9.
Oddo M, Levine JM, Mackenzie L, et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery. 2011;69:1037–45. discussion 1045. doi:10.1227/NEU.0b013e3182287ca7.
Bohman L-E, Heuer GG, Macyszyn L, et al. Medical management of compromised brain oxygen in patients with severe traumatic brain injury. Neurocrit Care. 2011;14:361–9. doi:10.1007/s12028-011-9526-7.
Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the international multidisciplinary Consensus conference on multimodality monitoring in Neurocritical care. Neurocrit Care. 2014;21:1–26. doi:10.1007/s12028-014-0041-5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Cassa, R., Petersen, N. (2018). Multimodality Monitoring. In: White, J., Sheth, K. (eds) Neurocritical Care for the Advanced Practice Clinician. Springer, Cham. https://doi.org/10.1007/978-3-319-48669-7_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-48669-7_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48667-3
Online ISBN: 978-3-319-48669-7
eBook Packages: MedicineMedicine (R0)