Abstract
Acute kidney injury (AKI) is a common complication in critically ill patients, and is associated with increased morbidity and mortality. Sepsis is the most common cause of AKI in the critically ill. Considerable evidence has shown that AKI can occur in the absence of overt clinical signs of shock, and in the setting of increased renal blood flow. This has challenged the traditional paradigm that renal dysfunction was solely on the basis of hypoperfusion and ischemia. Animal and human data have further shown that sepsis-induced AKI is characterized not by acute tubular necrosis, but by a paucity of apoptosis and necrosis in the context of a very bland histology, by inflammation, by microvascular dysfunction, and cellular bioenergetics adaptive responses. These novel findings suggest that other potential mechanisms centered in these three domains may help explain the pathophysiology of sepsis-induced AKI. Furthermore, the extreme functional changes seen in sepsis-induced AKI and the response of the tubular epithelial cells to inflammation and injury may be adaptive. This chapter focuses on the recent advances in this area and discusses possible therapeutic interventions that might derive from these new insights into the pathogenesis of sepsis-induced AKI.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Sepsis is thought to be the primary etiology of acute kidney injury (AKI) in 40–50% of cases, making sepsis the most common cause of AKI in the critically ill [1]. Importantly, the development of AKI in the setting of sepsis increases the risk of death in hospital six to eightfold [1, 2], and among survivors, the risk of developing chronic kidney disease is also increased [3]. Despite this, the mechanisms by which sepsis causes AKI are not well understood, and hence current therapy remains reactive rather than preventive, and rather nonspecific. Given that the leading clinical conditions associated with AKI, namely, sepsis, major surgery, heart failure, and hypovolemia [1], are all associated with hypoperfusion, it is tempting to attribute all AKI to ischemia. However, an increasing body of evidence suggests that at least in a proportion of patients, AKI can occur in the absence of overt signs of hypoperfusion . Langenberg et al. showed, for example, that AKI developed in septic animals despite normal or increased renal blood flow [4]. In a human study, Prowle et al. were able to demonstrate that decreased renal blood flow (RBF) was not a universal finding even in patients with well-established sepsis-induced AKI [5]. Furthermore, in a large-scale study, including more than 1800 patients with community-acquired pneumonia, Murugan et al. found that a fifth to a quarter of patients with non-severe pneumonia, who were never admitted to an ICU, and who never displayed overt signs of shock or hypoperfusion, still developed AKI [6]. Complementary to the insights from clinical and in vivo studies, in vitro experiments where hemodynamics are no longer relevant, have shown that incubation of human renal tubular epithelial cells with plasma from septic patients induces damage of tubular epithelial cells evidenced by the increased release of tubular enzymes, elevated permeability, and the decreased expression of key molecules for tubular functional integrity [7]. Taken together these data provide evidence that, at least in some patients, renal injury cannot be explained solely on the basis of the classic paradigm of hypoperfusion and that other mechanisms must come into play.
One of the limitations in advancing the understanding of sepsis-induced AKI is the lack of pathologic specimens available, given that the risk of performing biopsies in this patient population outweighs any potential benefit. Recent studies in septic animals and postmortem observations in septic humans have provided evidence of what sepsis-induced AKI actually looks like. Despite representing the latest stages of the disease, these kidneys were characterized by a strikingly bland histology with focal areas of tubular injury, which was also entirely discordant with the profound functional impairment seen pre-mortem. In addition, and contrary to prior understanding, necrosis and apoptosis were largely absent [8, 9], which not only argues in favor of the notion that sepsis-induced AKI is not equivalent to acute tubular necrosis (ATN) , but supports the hypothesis that at least in the early stages, this phenotype may represent a concerted, organized, common underlying adaptive mechanism [9]. A consistent observation in these studies, regardless of species, disease stage, severity, or organ examined, appears to be the presence of three main alterations: inflammation [10, 11], diffuse microcirculatory flow abnormalities [12], and cellular bioenergetic adaptive responses to injury [9, 13]. The study and understanding of these three domains may provide a roadmap to unravel the mechanisms by which sepsis causes AKI and perhaps organ injury in general and may facilitate the development of more targeted therapies. In this chapter, we will first consider the current classification system for AKI and then briefly review the epidemiology; then we will review the roles various mechanisms may play in the genesis of sepsis-induced AKI and discuss potential therapeutic implications.
Definition of AKI in the Clinical Setting
The definition of AKI has undergone important transformations in recent years. The definition of AKI has been traditionally based on the assessment of renal function, and in particular, on the assessment of changes in glomerular filtration rate (GFR) . Although practical at the bedside, this approach is limited by the fact that functional changes not necessarily reflect structural alterations [3]. This is particularly true in sepsis-induced AKI, where a dramatic alteration in renal function is associated with very bland histology [8, 9]. An additional limitation is the assessment of GFR through the quantification of creatinine. Although creatinine levels correlate well with GFR in steady-state conditions, AKI usually occurs in the setting of changing physiologic or pathologic conditions. Finally, the assessment of renal dysfunction based on glomerular function does not take into account the presence of tubular dysfunction, which has been increasingly recognized as an important pathophysiogic event, and to be at least as important as the alterations in GFR. Despite these limitations, the standardization of two measures of glomerular function has provided the scientific community with a tool, in a common language, to assess the occurrence of AKI. These measures are serum creatinine and urine output. Today, the evaluation of the presence and degree of severity of AKI can be standardized with tools like the KDIGO criteria [14].
The Epidemiology of Sepsis-Induced AKI
Sepsis is the leading cause of acute kidney injury (AKI) in acutely ill patients. Acute kidney injury occurs in as much as 40–50% of septic critically ill patients, which increases the risk of death six- to eightfold [1, 2, 15, 16], and also the risk of advancing to renal fibrosis and chronic kidney disease [3]. Importantly, a large proportion of patients who are usually considered to be less severely compromised and thus at lower risk, still develop AKI. Murugan et al. showed in a large cohort of patients admitted to the emergency department with non-severe community acquired pneumonia that 34% of these patients developed AKI many of whom never required admission to an ICU [6]. This suggests that AKI is not only related to shock states or critical illness, and that patients with non-life-threatening infections may also be at high risk of developing renal dysfunction and its short and long-term consequences.
Novel Concepts in the Pathophysiology of Sepsis-Induced AKI
Recent evidence suggests that the origin of most cases of AKI is multifaceted and that several, concurrent mechanisms may be at play. These mechanisms include inflammation, profound, heterogeneous distortion of microvascular flow at the peritubular and glomerular levels, and tubular epithelial cell injury and impairment. Given that these three major events occur early in the course of sepsis, and that cell death seldom occurs, we conceptualize early sepsis-induced AKI as the clinical and biochemical manifestation of tubular cell responses to injury. We further hypothesize that such response is, at least in part, adaptive in that it is driven by metabolic down-regulation and reprioritization of energy expenditure to avoid energy imbalance and favors individual cell survival processes (such as maintenance of membrane potential and cell cycle arrest), at the expense of organ function (i.e., tubular absorption and secretion of solutes).
The Renal Microcirculation during Sepsis-Induced AKI
Sepsis causes a profound alteration in microvascular blood flow distribution [12, 17]. Such alteration is characterized by an increase in the heterogeneity of regional blood flow distribution, a decrease in the proportion of capillaries with “nutritive” (or continuous) blood flow, and an increase in the proportion of capillaries with intermittent or no flow [12, 18]. The renal microcirculation is disturbed in a similar fashion, as has been recently described in different models of sepsis-induced AKI [11, 19, 20], even in the setting of normal or even increased RBF [21]. Multiple mechanisms seem to frame this characteristic microcirculatory derangement, including endothelial dysfunction, impaired red blood cell deformability, thinning and damage of the glycocalyx layer, increased leukocyte activation and recruitment, and activation of the coagulation cascade with fibrin deposition [18]. Importantly, these alterations in microcirculatory flow and endothelial function are thought to contribute directly to the development of organ dysfunction through multiple mechanisms.
Uncoupling of microcirculatory blood flow distribution from metabolic demand, with the creation of microvascular shunts, has been proposed to result in areas of hypoperfusion and hypoxia [22, 23]. In relation to this, the endothelium also provides an essential system of retrograde communication that allows the microcirculation to fine tune and couple blood flow distribution to metabolic demand, which is in essence the concept of regional autoregulation . Tyml et al. have shown that LPS-induced endothelial injury results in loss of such retrograde communication rate between microvessels 500 μm apart [23, 24], suggesting that sepsis may not only impair the response to vasoactive mediators but also, the capacity of peripheral microvascular beds to autoregulate.
Similarly, endothelial dysfunction results in increased vascular permeability and worsening interstitial edema [25, 26], with two important consequences. First, edema increases the diffusion distance oxygen has to travel to reach target cells [27] further creating areas at risk for hypoxia. Second, given that the kidney is an encapsulated organ, tissue edema contributes to increased venous output pressures, aggravating congestion and perpetuating microvascular perfusion alterations [28, 29].
Endothelial cells are also important determinants of vascular tone and play an important role in the responsiveness to vasoactive mediators [30]. Injury to the arterial and arteriolar endothelium has consistently shown to result in impaired responsiveness to vasoactive substances, which may explain the loss of vasomotor tone during sepsis.
Nitric oxide (NO) has also been shown to have a potential role in the genesis of microvascular dysfunction and in the pathophysiology of AKI . Although sepsis is characterized by global increased NO production [31], the expression of one of the most important catalyzers of its production, inducible NO synthase (iNOS) , is rather heterogeneous [31]. Accordingly, it is possible that the heterogeneous expression of iNOS may result in heterogeneous regional concentrations of NO, which could result in the presence of vascular beds deprived of NO even in the setting of elevated systemic levels [32]. This is important as it is reminiscent of the characteristic heterogeneous pattern of microvascular dysfunction described in sepsis, and may relate pathophysiologically with areas of shunting and hypoxia [32]. Importantly, selective inhibition of iNOS not only can restore the renal microcirculatory derangements during sepsis, but is also associated with decreased functional manifestations of renal injury, suggesting that microcirculatory abnormalities may be in the mechanistic pathway of sepsis-induced AKI [19]. However, the interactions between NO, microvascular dysfunction, and AKI are not straightforward, as sepsis is also known to result in iNOS-dependent decrease in endothelial-derived NO synthase activity, which will also alter microvascular flow homeostasis [33, 34].
During sepsis, inflammation, oxidative stress, and the uncoupled eNOS [35] not only induce endothelial cell dysfunction but also damage the glycocalyx. The glycocalyx is a layer of organized glycosaminoglycan branches that protrudes from the surface of the endothelial cell membrane into the capillary lumen, and that has important biomechanical functions including maintenance of adequate capillary flow, oncotic and hydrostatic pressure gradient balance to limit filtration, and avoiding red and white cell adhesion [36]. Damage of the glycocalyx is thought to result in capillary leak, altered red blood cell flow, and increased adhesion and rolling of leukocytes after endothelial adhesion molecules are exposed, all of which contribute to the microvascular dysfunction phenotype characteristic of sepsis and to further inflammation.
Finally, sluggish peritubular flow may also result in amplification of the inflammatory signal. As demonstrated by Goddard et al. [37] in myocardial capillaries during a porcine model of endotoxemia, leukocytes decrease their velocities and increase their transit time in these areas of sluggish flow. In addition, there is evidence of upregulation of inflammatory molecules, such as intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 [38, 39] in these peritubular capillaries that would contribute to leukocyte activation and prolonged leukocyte transit. This prolonged transit may directly translate into a greater time of exposure of the endothelium and neighboring tubular epithelial cells to activated, cytokine secreting leukocytes and to other pathogen and damage-associated molecular patterns (PAMPs and DAMPs, respectively) that ultimately amplify the inflammatory signal, and induce focal oxidative stress and tubular injury. The tubular epithelial cells exposed to this amplified signal then act as primary targets for this alarm, and trigger a response in the adjacent segments of the proximal tubule evidenced by the induction of oxidative stress and vacuolization. The lack of apoptosis and necrosis suggests this is an organized, adaptive response that ultimately signals other tubular cells to shut down in a paracrine fashion. Importantly, this provides an explanation for why only a few heterogeneous groups of tubular epithelial cells demonstrate the typical histopathologic changes.
Inflammation Propagates Renal Damage During Sepsis
A strong association between cytokine levels (interleukin (IL)-6, IL-10, and macrophage migration inhibitory factor) and the development of sepsis-induced AKI [6, 40] supports the hypothesis that systemic inflammation is an important mediator of this process. During sepsis, although the inflammatory response is fundamental to clear the infection and later promote tissue recovery, it can also result in tissue damage and organ dysfunction [41]. In addition to leukocytes, dendritic cells, and resident macrophages, tubular epithelial cells are capable of recognizing and responding to pathogens-associated molecular patterns (PAMPs) through pattern-recognition receptors including toll-like receptors (TLR), C-type lectin receptors, retinoic acid inducible gene 1-like receptors, and nucleotide-binding oligomerization domain-like receptors [42], which results in the up-regulation of inflammatory gene transcription and initiation of innate immunity. This response is also stimulated by endogenous substances released by injured cells and tissues known as damage-associated molecular patterns (DAMPs) , which include DNA, RNA, histones, HMGB1, and S100 proteins, and which are recognized by these same receptors [43].
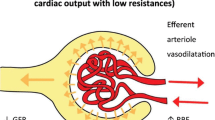
Pro-inflammatory mediators activate endothelial cells and induce up-regulation of adhesion molecules like E-selectin , which has been demonstrated to play a major role in leukocyte recruitment into the kidney during the late stages of sepsis-induced AKI [44]. Although not seen in all models of sepsis-induced AKI [45], elimination of neutrophils or blocking adhesion molecules that are required for neutrophil recruitment into the kidney completely abolished sepsis-induced AKI in a cecal ligation and puncture (CLP)-induced sepsis model [44]. This observation can be explained by the fact that leukocytes leaving peritubular capillaries have a close proximity to tubular epithelial cells and can directly activate tubular epithelial and dendritic cells by releasing pro-inflammatory mediators and DAMPs. The cycle is then perpetuated by the release of mediators like leukotriene B4, and platelet-activating factor which increase vascular permeability and up-regulate the expression of adhesion molecules that promote further inflammation [46–48]. In addition, DAMPs, PAMPs, and pro-inflammatory cytokines that are readily filtered through the glomerulus can activate these tubular epithelial cells from within the tubule (Fig. 8.1) [46, 49]. It has been recently shown that mammalian tubular epithelial cells (including human) express TLR2 and TLR4, and that these cells are capable of recognizing inflammatory mediators such as lipopolysaccharide (LPS) in a TLR4-dependent manner [50–53]. Furthermore, Krüger et al. [50] demonstrated that damaged human tubules stain positively for the TLR4 ligand, HMGB1, and that in vitro stimulation of human tubular epithelial cells with HMGB1 stimulates pro-inflammatory responses through TLR4 [50], suggesting that such mediators can act in an autocrine and paracrine fashion and may contribute to further tubular cell damage. The recognition that tubular epithelial cells are actually equipped with machinery to recognize the inflammatory signal supports the hypothesis that their response may be organized and not random. In support of this, Kalakeche et al. [51] have elegantly shown that TLR4-dependent LPS recognition in the tubular epithelial cells occurs in the S1 segment of the proximal tubule, that assembly of LPS with TLR-4 in the tubular epithelial cell produces internalization of LPS through fluid-filled endocytosis, and that this triggers an organized oxidative outburst in epithelial cells of the adjacent tubular segments (S2 and S3) but not in the S1 segment. These findings have led Kalakeche et al. [51] to suggest that the S1 segment of the proximal tubule may act as a sensor of danger that activates a series of events resulting in oxidative stress within distal tubular segments (S2, S3) and that could potentially explain tubular dysfunction in the setting of sepsis.
Alterations in the Kidney During Sepsis . These alterations are characterized by increased heterogeneity of flow, as well as an increase in the proportion of capillaries with sluggish or stop flow (represented in the figure by darker hexagons in the peritubular capillary). We have conceptualized that these areas of sluggish peritubular flow increase the transit time of activated leukocytes and that this may set the stage for an amplification of the “danger signal” in such areas. Note that the expression of TNF receptors in the S2 segment tubular cells has led to the hypothesis that S1 cells may actually signal distal segments in a paracrine fashion through secretion of TNF. Finally, there are also data suggesting that this paracrine signal may include mediators of cell cycle arrest, namely, TIMP-2 and IGFBP-7. Source: Gomez et al. Shock. 2014;41:3–11
The (Adaptive) Responses of Tubular Cells to Inflammation
With the exception of T lymphocytes and intestinal epithelia , and despite multiple triggering stimuli [54], significant necrosis or apoptosis does not occur during sepsis [8, 9], which suggests that during the acute phase, regardless of the consequences at the organ level, the cellular response is successful at preventing death. This denotes a possible underlying adaptive mechanism [9, 46, 55], and an opportunity to understand the response of the tubular epithelial cells to sepsis. Accordingly, it is reasonable to think that the tubular epithelial cell response to injury may be characterized at least in part by processes that limit pro-apoptotic triggers, by (a) prioritizing energy consumption and maintaining energy homeostasis, (b) maintaining cellular organelle function through quality control processes (general autophagy and mitophagy), and (c) limiting cell cycling and DNA replication.
Repriotitization of Energy Consumption
Energy balance dysregulation and mitochondrial injury are two major triggers of apoptosis and consequently, two of the most highly regulated cellular defense mechanisms to injury [56]. Although still controversial, sepsis seems to be associated with maintenance of ATP levels in the kidney [57] albeit with a decrease in production [58, 59], suggesting a significant decrease in ATP utilization. Furthermore, analogous to the evolutionarily conserved defense response to hypoxia, where nonvital functions are limited to avoid overtaxing energy expenditure [56], sterile inflammation by administration of lipopolysaccharide has been shown to induce downregulation of renal tubular cell ion transporters [60], which account for more than 70% of ATP cellular consumption [61]. Furthermore, there is evidence that experimental sepsis induces similar effects. Gupta et al. [62] showed that, in the presence of LPS, proximal tubules of mice have a delayed uptake of low-molecular-weight dextran, a sign of reduced endocytic capacity. Good et al. [63] have shown in an LPS-induced rodent sepsis model that LPS inhibits NHE1 (Na+/H+ exchanger 1) and thus blocks bicarbonate reabsorption in the medullary thick ascending limb of the loop of Henle. Finally, Hsiao et al. have shown that sodium transport (tubular sodium reabsorption) is decreased as early as 9 h after induction of sepsis by cecal ligation and puncture [64]. Taken together this evidence suggests that during sepsis, the response of the tubular epithelial cell may be characterized by an organized, hierarchical downregulation of major energy sinks like ion transport, while only fueling processes necessary to cell survival (i.e., maintenance of membrane potential) [65]. This is a highly conserved mechanism across species that seems to frame the core strategy of cellular response to threatening circumstances. It also provides the conceptual ground to suggest that cellular metabolic downregulation and reprioritization of energy consumption are pillars of the tubular epithelial response to sepsis and furthermore explains why organ function may be sacrificed in benefit of individual cell survival [62, 63].
Mitochondrial Quality Control Processes: Mitophagy
Mitophagy is an evolutionarily conserved, quality control mechanism, by which eukaryotic cells remove and digest dysfunctional mitochondria from the cytoplasm [66, 67]. During sepsis, TLR-mediated inflammation [68], oxidative stress [69, 70], and alterations in the electron transport chain that uncouple respiration and depolarize the mitochondrial membrane are potent triggers of mitophagy [67]. This early mitochondrial uncoupling characterized by an increment in O2 consumption (VO2) is not to be confused with the adaptive response it triggers, which is framed by the activation of mitophagy, and is characterized by a decrement in VO2 and conservation of energy. In the kidney, mitophagy is activated as early as 3 h after CLP-induced sepsis [64], suggesting it is part of the early response of tubular epithelial cells to injury. Importantly, insufficient activation of mitophagy has been associated with worse outcome in critically ill patients, and it has been postulated to contribute to cell and organ dysfunction [71]. On the other hand, stimulation of autophagy has been shown to be effective at protecting cells [64] and organ function [71] in the setting of experimental inflammatory insults. Furthermore, in the setting of experimental sepsis induced by CLP, decreased autophagy has been associated with increased blood urea nitrogen and creatinine levels and a decline in proximal tubular sodium transport [64]. As a protective response, mitophagy offers several advantages, namely, removal of dysfunctional mitochondria, with subsequent decrement in ROS/RNS production, energy conservation, limiting oxidative stress damage , and importantly, intercepting proapoptotic signals at the mitochondrial level impeding triggering of apoptosis [67, 72–74]. Indeed, Carchman et al. have shown that inhibition of mitophagy results in a robust apoptotic signal in hepatocytes of animals subjected to CLP [58]. It is unknown, however, what mitophagy-induced maintenance of renal function really means. The adaptive response, framed by metabolic downregulation, would most likely decrease tubular and renal function and not promote it, just as hibernation promotes the loss of function. Indeed, increased or preserved renal function in the setting of stress may result harmful in the long run. Yet, animal and human data associate acute stimulation of autophagy with preserved renal function, and its faulty activation or decline with worse outcome. It is possible that the interplay of autophagy and tubular cell function varies with time and that persistence of the initial protective response may ultimately be deleterious in the subacute or chronic phases.
Cell Cycle Arrest
There is a growing body of evidence indicating that mitochondria are intimately involved in the regulation of the cell cycle [67]. The ability of mitochondria to move within the cell, change shape, and coalesce in different ways has recently emerged as an important feature, which may influence the cell cycle [75]. Briefly, the cell cycle is the progression of cells through a number of steps in preparation for mitosis (G0, G1, S, G2, M). This preparation portrays several checkpoints in which the cell seems to evaluate whether it is prepared to advance to the next phase. Of particular interest to renal tubular injury in sepsis and the involvement of mitochondrial regulation is the G1-S checkpoint. Only at and during this stage, mitochondria have been shown to coalesce into a single, tubular network of mitochondria. This mesh seems to act as syncytia, with electrical coupling and unusual hyperpolarization [76], which fits well with prior studies showing an increase in O2 consumption during the G1-S transition of the cell cycle [77]. This also relates to the finding that a reduction in ATP production induced by specific ETC mutations produces cell cycle arrest at the G1-S checkpoint [78]. Together, these data indicate that the formation of this giant tubular network is necessary to meet the energy requirement needed to synthesize all the components for adequate cell division. It also suggests that the G1-S border is an important checkpoint of the cycle, whereby the inability to meet such energy requirements induces cell cycle arrest presumably to prevent a potentially lethal energy imbalance [75]. Yang et al. [79] recently showed in a rodent model of CLP-induced sepsis that G1-S cell cycle arrest was associated with kidney injury and that recovery of renal function paralleled cell cycle progression 48 h after CLP. These findings have become even more clinically relevant as tissue inhibitor of metalloproteinases 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP-7), two markers involved in G1-S cycle arrest, have been identified as the most sensitive and specific markers to predict risk of development of AKI in critically ill patients [80–82]. We speculate that the renal cell cycle arrest in the epithelial tubular cell may provide an advantage by avoiding replication because (a) it conserves energy and prevents triggering apoptosis or necrosis and (b) limiting replication diminishes the probability of DNA damage, reducing not only energy consumption employed in DNA repair, but also decreases the chances of triggering apoptosis.
Potential for Diagnostic and Therapeutic Targets
To date, no therapeutic measures are available to prevent or treat sepsis-induced AKI. A potential reason for this may be that often therapy is started too late in the disease process. The development of new biomarkers, which also provide insights into the pathophysiology of the disease, makes it possible to detect kidneys at risk for injury and thus enable earlier initiation of interventions [80–82].
The knowledge that inflammation, microvascular dysfunction , and adaptive responses of tubular cells are involved in the development of sepsis-induced AKI provides new diagnostic and therapeutic avenues. As these mechanisms are closely interlinked with each other, modulating one of these components simultaneously alters other components. As increased levels of pro-inflammatory mediators (e.g., IL-6) are associated with the development of AKI [40], it is tempting to speculate that eliminating these mediators or endotoxin can prevent sepsis-induced AKI. Experimentally, it has been shown that removal of such mediators by hemoadsorption completely protects against AKI in a CLP model of sepsis [7, 83, 84], and a clinical study demonstrated that reducing endotoxin by polymyxin-B hemoperfusion reduced RIFLE scores and urine tubular enzymes [7]. Along the same lines, Alkaline phosphatase (AP) is an endogenous enzyme that exerts detoxifying effects through dephosphorylation of endotoxins and pro-inflammatory extracellular ATP and is reduced during systemic inflammation. Heemskerk and colleagues [85] demonstrated that administration of AP was associated with a decreased expression of iNOS synthase in proximal tubule cells isolated from urine and that this related to an attenuated urinary excretion of glutathione S-transferase A1-1, a proximal tubule injury marker. In a small, randomized trial, Pickkers et al. showed that the administration of exogenous AP in septic patients improved endogenous creatinine clearance and reduced the requirement and duration of renal replacement therapy [86]. Modulating TNF-α signaling might be yet another therapeutic option, because a polymorphism in the promoter region of the TNFA gene is associated with markers of kidney disease severity and distant organ dysfunction [87].
To improve microcirculatory perfusion, vasodilators in the setting of sepsis are currently under investigation including nitroglycerin [17, 88], NO administration, and modulation of NO production [32, 34]. Furthermore, drugs with pleiotropic effects on the vasculature, such as statins [89] and erythropoietin [90], have the potential to prevent kidney injury by enhancing eNOS expression and decreasing vascular permeability. However, it is important to consider that regional microcirculatory autoregulation is only possible if sufficient perfusion pressure is attained, and thus early resuscitation goals still need to focus on achieving a mean arterial pressure sufficient enough to ensure perfusion. Asfar et al. have shown that such a goal must be a mean arterial pressure of 65–70 mmHg, and that higher levels of MAP only result in improved outcomes (decreased need for RRT) in the subpopulation of patients with chronic hypertension [91].
However, it is important to explore these treatment options bearing in mind that these mechanisms are part of the natural host response to sepsis, and that although known perpetrators of injury, they are also necessary for bacterial clearance, tissue protection and repair, and ultimately survival. Accordingly, the reader must not expect a single treatment modality to emerge as a magic bullet to prevention and/or treatment sepsis-induced AKI.
Conclusions
Close examination of the histology of various organs of patients dying from sepsis has dramatically changed the way we think of sepsis-induced organ dysfunction. The recognition that in the case of the kidney, sepsis-induced AKI cannot be entirely explained by the traditional concept of acute tubular necrosis, and that sepsis does not cause overt apoptosis and necrosis in failing organs, has challenged the notion that ischemia is the only mechanism explaining organ dysfunction. Importantly, it has also prompted many to suggest that the response to the septic environment may early on be adaptive in nature. In this review, we have now put forth a conceptual model that cellular energy regulation is fundamental to the adaptive response, and that such regulation is driven at least in part by metabolic down-regulation and re-prioritization of energy utilization and by mitochondrial quality control processes like mitophagy. Further work is warranted to better understand the role, timing, and reach of these multiple mechanisms in the pathogenesis of sepsis-induced AKI, and if this can be translated into novel diagnostic and therapeutic interventions to improve outcome in this patient population.
References
Uchino S, Kellum, JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8. doi:10.1001/jama.294.7.813.
Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37(9):2552–8. doi:10.1097/CCM.0b013e3181a5906f.
Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol. 2011;7(4):209–17. doi:10.1038/nrneph.2011.13.
Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69(11):1996–2002. doi:10.1038/sj.ki.5000440.
Prowle JR, Ishikawa K, May CN, Bellomo R. Renal blood flow during acute renal failure in man. Blood Purif. 2009;28(3):216–25. doi:10.1159/000230813.
Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–35. doi:10.1038/ki.2009.502.
Cantaluppi V, Assenzio B, Pasero D, et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive Care Med. 2008;34(9):1638–45. doi:10.1007/s00134-008-1124-6.
Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27(7):1230–51.
Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187(5):509–17. doi:10.1164/rccm.201211-1983OC.
Wang Z, Holthoff JH, Seely KA, et al. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. AJPA. 2012;180(2):505–16. doi:10.1016/j.ajpath.2011.10.011.
Seely KA, Holthoff JH, Burns ST, et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;301(1):F209–17. doi:10.1152/ajprenal.00687.2010.
De Backer D, Creteur J, Preiser J-C, Dubois M-J, Vincent J-L. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166(1):98–104.
Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364(9433):545–8. doi:10.1016/S0140-6736(04)16815-3.
KDIGO. Section 2: AKI definition. Kidney Int Suppl. 2012;2(1):19–36. doi:10.1038/kisup.2011.32.
Hoste EAJ, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med. 2008;36(4 Suppl):S146–51. doi:10.1097/CCM.0b013e318168c590.
Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–23. doi:10.1007/s00134-015-3934-7.
Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360(9343):1395–6.
De Backer D, Donadello K, Taccone FS, Ospina-Tascon G, Salgado D, Vincent J-L. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011;1(1):27. doi:10.1186/2110-5820-1-27.
Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol. 2005;289(6):F1324–32. doi:10.1152/ajprenal.00124.2005.
Holthoff JH, Wang Z, Seely KA, Gokden N, Mayeux PR. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012;81(4):370–8. doi:10.1038/ki.2011.347.
Bezemer R, Legrand M, Klijn E, et al. Real-time assessment of renal cortical microvascular perfusion heterogeneities using near-infrared laser speckle imaging. Opt Express. 2010;18(14):15054–61. doi:10.1364/OE.18.015054.
Dyson A, Bezemer R, Legrand M, Balestra G, Singer M, Ince C. Microvascular and interstitial oxygen tension in the renal cortex and medulla studied in a 4-h rat model of LPS-induced endotoxemia. Shock. 2011;36(1):83–9. doi:10.1097/SHK.0b013e3182169d5a.
Almac E, Siegemund M, Demirci C, Ince C. Microcirculatory recruitment maneuvers correct tissue CO2 abnormalities in sepsis. Minerva Anestesiol. 2006;72(6):507–19.
Tyml K, Wang X, Lidington D, Ouellette Y. Lipopolysaccharide reduces intercellular coupling in vitro and arteriolar conducted response in vivo. Am J Physiol Heart Circ Physiol. 2001;281(3):H1397–406.
Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6(2):107–15. doi:10.1038/nrneph.2009.213.
Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12(4):169. doi:10.1186/cc6948.
Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32(9):1928–48.
Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2013;10(1):37–47. doi:10.1038/nrneph.2013.232.
Rajendram R, Prowle JR. Venous congestion: are we adding insult to kidney injury in sepsis? Crit Care. 2014;18(1):104. doi:10.1186/cc13709.
Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539–52. doi:10.1016/j.bcp.2009.04.029.
Cunha FQ, Assreuy J, Moss DW, et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology. 1994;81(2):211–5.
Trzeciak S, Cinel I, Phillip Dellinger R, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008;15(5):399–413. doi:10.1111/j.1553-2712.2008.00109.x.
Chauhan SD, Seggara G, Vo PA, Macallister RJ, Hobbs AJ, Ahluwalia A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J. 2003;17(6):773–5. doi:10.1096/fj.02-0668fje.
Heemskerk S, Masereeuw R, Russel FGM, Pickkers P. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat Rev Nephrol. 2009;5(11):629–40. doi:10.1038/nrneph.2009.155.
Rabelink TJ, van Zonneveld A-J. Coupling eNOS uncoupling to the innate immune response. Arterioscler Thromb Vasc Biol. 2006;26(12):2585–7. doi:10.1161/01.ATV.0000250932.24151.50.
Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9(1):121–67. doi:10.1146/annurev.bioeng.9.060906.151959.
Goddard CM, Allard MF, Hogg JC, Herbertson MJ, Walley KR. Prolonged leukocyte transit time in coronary microcirculation of endotoxemic pigs. Am J Physiol. 1995;269(4 Pt 2):H1389–97.
Wu L, Tiwari MM, Messer KJ, et al. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol. 2007;292(1):F261–8. doi:10.1152/ajprenal.00263.2006.
Wu X, Guo R, Wang Y, Cunningham PN. The role of ICAM-1 in endotoxin-induced acute renal failure. Am J Physiol Renal Physiol. 2007;293(4):F1262–71. doi:10.1152/ajprenal.00445.2006.
Payen D, Lukaszewicz AC, Legrand M, et al. A multicentre study of acute kidney injury in severe sepsis and septic shock: association with inflammatory phenotype and HLA genotype. PLoS One. 2012;7(6):e35838. doi:10.1371/journal.pone.0035838.
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–51. doi:10.1056/NEJMra1208623.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20. doi:10.1016/j.cell.2010.01.022.
Chan JK, Roth J, Oppenheim JJ, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122(8):2711–9. doi:10.1172/JCI62423.
Herter JM, Rossaint J, Spieker T, Zarbock A. Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J Innate Immun. 2014;6(5):597–606. doi:10.1159/000358238.
Singbartl K, Bishop JV, Wen X, et al. Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int. 2011;80(6):633–44. doi:10.1038/ki.2011.201.
Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi:10.1097/SHK.0000000000000052.
Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157–69. doi:10.1016/S0140-6736(06)69005-3.
Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. AJPA. 2008;172(1):1–7. doi:10.2353/ajpath.2008.070502.
El-Achkar TM, Hosein M, Dagher PC. Pathways of renal injury in systemic gram-negative sepsis. Eur J Clin Invest. 2008;38:39–44. doi:10.1111/j.1365-2362.2008.02007.x.
Krüger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106(9):3390–5. doi:10.1073/pnas.0810169106.
Kalakeche R, Hato T, Rhodes G, et al. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol. 2011;22(8):1505–16. doi:10.1681/ASN.2011020203.
Mudaliar H, Pollock C, Komala MG, Chadban S, Wu H, Panchapakesan U. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Renal Physiol. 2013;305(2):F143–54. doi:10.1152/ajprenal.00398.2012.
Lin M, Yiu WH, Wu HJ, et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23(1):86–102. doi:10.1681/ASN.2010111210.
Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Arch Biochem Biophys. 2007;462(2):210–9. doi:10.1016/j.abb.2007.02.006.
Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5(1):66–72. doi:10.4161/viru.26907.
Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci U S A. 1996;93(18):9493–8.
May CN, Ishikawa K, Wan L, et al. Renal bioenergetics during early gram-negative mammalian sepsis and angiotensin II infusion. Intensive Care Med. 2012;38(5):886–93. doi:10.1007/s00134-012-2487-2.
Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011;53(6):2053–62. doi:10.1002/hep.24324.
Brealey D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R491–7. doi:10.1152/ajpregu.00432.2003.
Schmidt C, Höcherl K, Schweda F, Bucher M. Proinflammatory cytokines cause down-regulation of renal chloride entry pathways during sepsis. Crit Care Med. 2007;35(9):2110–9.
Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981;240(5):F357–71.
Gupta A, Rhodes GJ, Berg DT, Gerlitz B, Molitoris BA, Grinnell BW. Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. Am J Physiol Renal Physiol. 2007;293(1):F245–54. doi:10.1152/ajprenal.00477.2006.
Good DW, George T, Watts BA. Lipopolysaccharide directly alters renal tubule transport through distinct TLR4-dependent pathways in basolateral and apical membranes. Am J Physiol Renal Physiol. 2009;297(4):F866–74. doi:10.1152/ajprenal.00335.2009.
Hsiao H-W, Tsai K-L, Wang L-F, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37(3):289–96. doi:10.1097/SHK.0b013e318240b52a.
Carré JE, Singer M. Cellular energetic metabolism in sepsis: the need for a systems approach. Biochim Biophys Acta. 2008;1777(7–8):763–71. doi:10.1016/j.bbabio.2008.04.024.
Vanhorebeek I, Gunst J, Derde S, et al. Mitochondrial fusion, fission, and biogenesis in prolonged critically ill patients. J Clin Endocrinol Metab. 2012;97(1):E59–64. doi:10.1210/jc.2011-1760.
Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333(6046):1109–12. doi:10.1126/science.1201940.
Waltz P, Carchman EH, Young AC, et al. Lipopolysaccaride induces autophagic signaling in macrophages via a TLR4, heme oxygenase-1 dependent pathway. Autophagy. 2011;7(3):315–20. doi:10.4161/auto.7.3.14044.
Frank M, Duvezin-Caubet S, Koob S, et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012;1823(12):2297–310. doi:10.1016/j.bbamcr.2012.08.007.
Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8(10):1462–76. doi:10.4161/auto.21211.
Gunst J, Derese I, Aertgeerts A, et al. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013;41(1):182–94. doi:10.1097/CCM.0b013e3182676657.
Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–75. doi:10.1038/nature06639.
Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115(10):2679–88. doi:10.1172/JCI26390.
Ciechomska IA, Goemans CG, Tolkovsky AM. Molecular links between autophagy and apoptosis. Methods Mol Biol. 2008;445(Chapter 12):175–193. doi:10.1007/978-1-59745-157-4_12.
Finkel T, Hwang PM. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. Proc Natl Acad Sci U S A. 2009;106(29):11825–6. doi:10.1073/pnas.0906430106.
Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A. 2009;106(29):11960–5. doi:10.1073/pnas.0904875106.
Schieke SM, McCoy JP, Finkel T. Coordination of mitochondrial bioenergetics with G1 phase cell cycle progression. Cell Cycle. 2008;7(12):1782–7.
Mandal S, Guptan P, Owusu-Ansah E, Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell. 2005;9(6):843–54. doi:10.1016/j.devcel.2005.11.006.
Yang Q-H, Liu D-W, Long Y, Liu H-Z, Chai W-Z, Wang X-T. Acute renal failure during sepsis: potential role of cell cycle regulation. J Infect. 2009;58(6):459–64. doi:10.1016/j.jinf.2009.04.003.
Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9(3):e93460. doi:10.1371/journal.pone.0093460.t005.
Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189(8):932–9. doi:10.1164/rccm.201401-0077OC.
Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi:10.1186/cc12503.
Kellum JA, Venkataraman R, Powner D, Elder M, Hergenroeder G, Carter M. Feasibility study of cytokine removal by hemoadsorption in brain-dead humans. Crit Care Med. 2008;36(1):268–72. doi:10.1097/01.CCM.0000291646.34815.BB.
Peng Z-Y, Wang H-Z, Carter MJ, et al. Acute removal of common sepsis mediators does not explain the effects of extracorporeal blood purification in experimental sepsis. Kidney Int. 2012;81(4):363–9. doi:10.1038/ki.2011.320.
Heemskerk S, Masereeuw R, Moesker O, et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med. 2009;37(2):417–23–e1. doi:10.1097/CCM.0b013e31819598af.
Pickkers P, Heemskerk S, Schouten J, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care. 2012;16(1):R14. doi:10.1186/cc11159.
Susantitaphong P, Perianayagam MC, Tighiouart H, Liangos O, Bonventre JV, Jaber BL. Tumor necrosis factor alpha promoter polymorphism and severity of acute kidney injury. Nephron Clin Pract. 2013;123(1–2):67–73. doi:10.1159/000351684.
Boerma EC, Koopmans M, Konijn A, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38(1):93–100. doi:10.1097/CCM.0b013e3181b02fc1.
Liakopoulos OJ, Choi Y-H, Haldenwang PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. Eur Heart J. 2008;29(12):1548–59. doi:10.1093/eurheartj/ehn198.
Song YR, Lee T, You SJ, et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. Am J Nephrol. 2009;30(3):253–60. doi:10.1159/000223229.
Asfar P, Meziani F, Hamel J-F, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583–93. doi:10.1056/NEJMoa1312173.
Acknowledgments
The authors declare no conflicts of interest. This work was funded by NIH/NHLBI grant number 1K12HL109068-02 awarded to H.G., and research grant from the German research foundation (ZA428/10-1) and Else-Kröner Fresenius Stiftung awarded to A.Z.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Gomez, H., Zarbock, A., Murugan, R., Kellum, J.A. (2017). Sepsis-Induced AKI. In: Ward, N., Levy, M. (eds) Sepsis. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-48470-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-48470-9_8
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-48468-6
Online ISBN: 978-3-319-48470-9
eBook Packages: MedicineMedicine (R0)