Abstract
Nanomaterial research has recently gained importance due to prospective applications in human life and environment. However, scientific research on nano- and micro-sized materials has reached a saturation state. As a result, researchers planning to further develop nanomaterials, need an outlook on recent advances in synthesis, classification and characterization of nanomaterials. There is a need in particular for an overview of synthesis using biological materials namely bacteria, fungi, yeast, and plants, in order to design eco-friendly nanomaterials. Methods used to characterize these synthesized nanoparticles must also be reviewed to suggest the appropriate techniques in terms of spectroscopic and microscopic methods to study the physio-chemical properties of nanomaterials. Here we review the nature, types and synthesis of nanomaterials, with a detailed evaluation on biological synthesis. We also discuss in detail nanoparticle production by microorganisms including bacteria, fungi and yeasts. This chapter also provides updates on currently available techniques used to characterize nanoparticles.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Nanomaterials or nanoparticles represent a significant aspect of nanotechnology which deals with various studies involving particles ranging between 1 and 100 nm at least in one spatial dimension (Schneider 1997). Though several research groups have showed interest in nanotechnology in the recent years, their primary motivations regarding nanomaterials were towards its unique electronic, optical and mechanical properties compared to their bulk counterpart and molecular components. Production of nanoscale materials on a large scale is usually very difficult which has led to its synthesis and studies made solely in the lab scale conditions and later tried to be scaled-up for commercial purposes. Though nanotechnology has a high impact on many field of sciences including physics, engineering, biology, agriculture and food sciences, its importance in the agri-food sector is gaining momentum. Several government agencies of developing and developed countries are actively involved in research and development of nanomaterials in Agri-food sector owing to its importance in serving the growing demand for improved quality of common foods mainly food and water. The major scopes of agri-food based nanotechnology research is towards develop of functional food, effecient and rapid delivery of drugs, nutrients and gene functions at cellular level in plants and animals during stress (Nair et al. 2010), nano array based technology for detection and contamination of foods by pathogen (Senturk et al. 2013), nanocomposite or nanobiocomposite based plastic film coatings in food packaging (Arora and Padua 2010), nanoemulsion based material for decontamination of equipments and food packing (Chaudhry et al. 2008; McClements and Rao 2011), nanoparticles for effective increase and direct delivery of nutrients into the cells (Acosta 2009).
In all of these requisite or ongoing areas of nantoechnology research, the production of innovative and enhanced materials can be achieved by either top-down or bottom-up approach which are two approaches for the synthesis of nanomaterials. In the top-down approach, nanoscale devices are created by using larger, externally-controlled devices to direct their assembly. The top-down approach often makes use of traditional workshop or microfabrication methods in which externally-controlled tools are used to cut, mill and shape materials into the desired shape and order. Attrition and milling for making nanoparticles are typical top-down processes. In the bottom-up approach, molecular components arrange themselves into more complex assemblies like atom-by-atom, molecule-by-molecule and cluster-by-cluster from the bottom (e.g., growth of a crystal). Synthesis of nanoparticles by colloid dispersions is an example of the bottom-up approach. The bottom-up approach generally produces nanostructures with fewer defects as compared to nanostructures produced by the top-down approach. An approach where both these techniques are employed is known as an hybrid approach. Some of the materials commonly used for the synthesis of nanomaterials are outlined in Table 2.1.
In Agri-food industry, integration of nanoresearch is essential to select appropriate nanomaterials with desired chemical composition, controlled size, uniformity and stability. However, the common modes of synthesis of nanoparticles are by chemicals which are toxic to environment. Eco-friendly synthesis of nanoparticles is important keeping in mind its agricultural perspective. Extensive literature and database search reveals that several researchers in the field of nanoparticle synthesis and assembly have turned to the use of biological systems such as bacteria, yeasts, algae, fungi and actinomycetes, agricultural residues, biomass and plants. This chapter emphasizes on recent advances in synthesis, classification and characterization of nanoparticles synthesized using several biological materials namely bacteria, fungi, yeast, and plants and also summarizes the characterization techniques in terms of spectroscopic and microscopic methods to study the physio-chemical properties of nanomaterials.
2.2 Classification of Nanomaterials
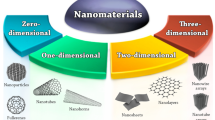
Nanomaterials are broadly classified into three categories, namely Nanoparticles, Nanoclays, Nanoemulsions (Fig. 2.1).
2.2.1 Nanoparticles
Nanoparticles can exist as nanostructures or as composites. All nanostructures can be built from elementary units (blocks) having low dimensionality – zero dimension, one dimension, two dimensions and three dimensions (Table 2.2). Depending on the dimension in which the size effect on the resultant property becomes apparent and the nanomaterials can be classified as zero dimensional (quantum dots) in which the movement of electrons is confined in all three dimensions, one-dimensional (quantum wires) in which the electrons can move freely only in the X-direction, two-dimensional (thin films) in which case the free electrons can move in the X-Y plane, or three dimensional (nanostructured material built of nanoparticles as building blocks) in which the free electron can move in the X, Y and Z directions (Siegel 1993).
Nanocomposites are found as a microcrystal matrix (micro-nano type), in which nanometer sized particles or inclusions (spherical, fiber-like, plate-like) of a second phase are dispersed in the intergranular regions or in both inter/intra granular spaces of matrix grains. Based on matrix material, three categories of nanocomposites can be named such as ceramic matrix nanocomposite, metal matrix nanocomposite and polymer matrix nanocomposite. Table 2.3 represents the materials, methods and application of different nanocomposite in agri-food sector.
Further nanoparticles can also be classified into organic and inorganic nanoparticles based on nature of material fabrication. Inorganic nanoparticles are typically shaped by the precipitation of inorganic salts, which are interconnected with molecules by covalent, metallic and so forth. The organic nanoparticles were assembled themselves in three dimensional form. Self-assembly, the vicinity of zwitterionic particles, with polar and nonpolar areas, as the primary segments of nanoparticles was key element for the fabrication of organic nanoparticles. Organic nanoparticles are synthesized using natural and synthetic organic molecules such as lipid bodies, protein aggregates, milk emulsion and other complex structure (Viruses, etc.). This form of nanoparticles plays a provital role in agri-food sector and cosmetic industries. Several food material for example, creams, chocolate, and cakes present nanoemulsions in their definition. Synthesis of organic nanoparticles can be fabricated by either top-down or bottom-up approach. The most common techniques are mechanical milling, microfluidics and lithography by top-down method. Precipitation, condensation were used to produce organic nanoparticles by bottom-up method. Inorganic nanoparticles on the other hand are more stable than organic nanoparticles but are limited by their stability in terms of either chemical or mechanical and nature of particle. Produced nanoparticles mainly differed in their consistency, size, yield or crystallinity and are often synthesized using various methods (Emulsion-Solvent Evaporation Method, Solvent Displacement/Precipitation method, hydrothermal, microemulsion, polyol process and aerosol pyrolysis). These nanoparticles can be broadly classified into nanostructure and nanocomposites based on the arrangement and their structures with combination of other material such as polymer, etc.
2.2.2 Nanoclays
Preparation of nanoclays and organoclays using charged (hydrophilic) nature of clay molecules such as alkyl/aryl ammonium, phosphonium or imidazolium in aqueous or solid state. The ion exchange reaction has two consequences; first, the gap between the single sheets is widened, enabling organic cations chain to move in between them and second, the surface properties of each single sheet are changed from being hydrophilic to hydrophobic or organophilic. It is easy and simple to characterize the chemical composition of nanoclays by gravimetric analysis, inductive coupled plasma, X-ray diffraction and fourier transform infrared spectroscopy.
2.2.3 Nanoemulsion
Dispersion of polymer, droplets and solid material in the form of a viscous liquid leads to an interesting soft material. The dispersed phase is also known as internal phase or the discontinuous phase while the outer phase is called dispersion medium, external phase or continuous phase. The emulsifying agent is also known as intermediate or interphase. Nanoemulsion can be synthesized by two methods; high (ultrasonification, high pressure homogenization, microfluidizer) and low energy emulsification (phase inversion temperature, solvent displacement and phase inversion composition).
Properties and usefulness of nanoparticles has been proved to various aspects, including size, polydispersity, dimensions and stability of nanoparticles and biomolecules (proteins and poly nucleic acids). Nanoparticles are produced by three different methods such as chemical, physical and bio-based.
2.2.4 Synthesis of Nanoparticles
This chapter mainly discusses the chemical and physical methods of nanoparticle synthesis.
2.2.4.1 Chemical Synthesis Methods
Chemical methods for nanoparticle production have been straightforward in nature and often allow synthesis of nanoparticles in large quantities. Moreover the possibility of controlling particle size even at nanometer scale is also possible during chemical synthesis of nanoparticles (Hyeon 2003). These exist a wide variety of methods in chemical synthesis of nanoparticles which include reduction, co-precipitation, nucleation, sol–gel method, flow injection, electrochemical, solvothermal, hydrothermal and microwave-assisted (Yu et al. 2009; Anbarasu et al. 2015; Umer et al. 2012; Chaki et al. 2015). Several methods of chemical synthesis, the reagents used and conditions are mentioned in Table. 2.4.
2.2.4.1.1 Chemical Reduction
The most commonly used approach for synthesis of nanoparticles is chemical reduction of organic and inorganic reducing agents such as sodium citrate, ascorbate, sodium borohydride (NaBH4), elemental hydrogen, polyol process, Tollens reagent, N, N-dimethylformamide (DMF), and poly (ethylene glycol)-block copolymers. This synthesis process results in agglomeration forming colloidal nanoparticles. Usage of protective or capping agents such as poly vinyl alcohol, poly vinylpyrrolidone, poly ethylene glycol, poly methacrylic acid and polymethylmethacrylate in addition to presence surfactants groups namely thiols, amines, acids and alcohols will able to stabilize the particles from sedimentation, agglomeration, or losing their surface properties.
2.2.4.1.2 Microemulsion
Nanoparticles are produced by initial spatial separation between two immiscible phases (metal and reducing agent) in two-phase aqueous organic systems and the interface between the two phases is mediated by quaternary ammonium salt. Metal clusters in the interface are stabilized, mainly due to capping of stabilizer molecules in the non-polar aqueous medium and transferred to organic phase.
2.2.4.1.3 Sol-Gel Process
In this method, wide variety of materials can be used for the synthesis of nanoparticles in desired shapes (particles, fibers or films). Primarily, sol formation is carried out by dissolving the metal alkoxide, metal-organic, or metal-inorganic salt precursors in a suitable solvent. Upon drying the sol, a polymeric network is formed in which the solvent molecules are trapped inside a solid (gel). Subsequent drying of the gel followed by the calcinations and sintering leads to the final ceramic product.
2.2.4.1.4 Polymerization
It is a very common method for preparation of nanomaterials. During polymerization the formation of microemulsion is a very much important factor, which has been the focus of extensive research worldwide due to its importance in a variety of technological applications. These applications include enhanced oil recovery, combustion, cosmetics, pharmaceuticals, agriculture, metal cutting, lubrication, food, enzymatic catalysis, organic and bio-organic reactions, chemical synthesis of nanoparticles and nanocapsules etc.
2.2.4.1.5 Oxidation Process
Oxidizes or deoxidizes of raw nanomaterial in the liquid or quasi-liquid phase state. The oxidation phases can be used to prepare the nanoparticles of metals, alloys or oxides, in either water solutions or organic solutions.
2.2.4.1.6 UV-Initiated Photoreduction
UV-initiated photoreduction induced nanoparticles were synthesized in the presence of protective and stabilizing agents (citrate, polyvinylpyrrolidone, poly (acrylic acid), and collagen) at room temperature. Synthesis and growth of nanorods and dendrites mainly depends on concentration of polyvinyl alcohol and silver nitrate.
2.2.4.1.7 Microwave-Assisted Synthesis
It is a most promising technique over other conventional method (Oil bath) for synthesis of nanoparticles with smaller sizes, narrower size distributions, and a higher degree of crystallization. Microwave heating has shorter reaction times, reduced energy consumption, and a better product yield which prevents the agglomeration of the particles formed.
2.2.4.1.8 Sonochemical Processing
Implementation of high intensity ultrasound wave as energy source in respective sol material has initiated a new field in processing technology. It results in collapse of the bubbles of the sol and it leads to very high temperature, high pressure, high heating and cooling rate. The extreme conditions during acoustic wave enable the reactants to cross the activation energy barrier in a very short amount of time to form the product phase.
2.2.4.1.9 Irradiation Method
Simple and effective technique, wide variety of irradiation (visible to high) is used to synthesis of nanoparticles. Irradiation of light source in an aqueous solution of silver salt and surfactant can produce silver NPs with a well defined shape and size distribution.
2.2.4.1.10 Electric Dispersion Reaction
The technique involves subjecting the reactor liquid (metal-alkoxide solution) to a pluse electric field and results in a precipitation reaction to synthesize ultra fine precursor powders of advanced ceramic materials. Under the applied electric field, the sol is shattered to micron-sized droplets, termed as microreactors, which contain hydrous precursor precipitate. The formed precursor powders can be thermally processed to obtain oxide nanoparticles.
2.2.4.2 Physical Method
Evaporation-condensation, Combustion synthesis, Arc discharge/plasma, Laser/electron beam heating, Laser ablation technique are the most prominent physical methods for the synthesizing of nanoparticles. Main advantage over chemical method is devoid of solvents and uniform distribution of nanoparticles.
2.2.4.2.1 Evaporation-Condensation
It is a technique evaporation of metals, alloys and ceramics using gases and allows reacting with each other. Later it condensed using cool gases results in the formation of nanoparticles or nanocapsules. It has many disadvantages; tube furnace occupies a large space, consumes a great amount of energy while raising the environmental temperature around the source material, and requires a lot of time to achieve thermal stability.
2.2.4.2.2 Combustion Synthesis
The major advantage of the method is that it is fast and required the least external energy input and gives high output with the possibility of producing wide variety of ceramic oxides. In combustion synthesis, there is the exploitation of an excess heat generating or exothermic reaction, to overcome the activation energy barrier for the formation of products using precursor organic compound (fuel) as the reducing agent and a metal salt as the oxidizing agent.
2.2.4.2.3 Arc Discharge/Plasma
This technique has been used widely for the synthesis of fullerenes and other related materials. There are two methods for the production of plasma; Direct current arc plasma and high frequency plasma. Direct current arc method is convert inert/active gases into ionized with the generation of high temperature and melt the materials. Further condensation of the evaporating matters lead to the formation of nanoparticles and nanocapsules.
2.2.4.2.4 Laser/Electron Beam Heating
Main principle of technique is to emit the electron from electron gun with high temperature due to application of high voltage and thus creates vacuum in the electron gun. Conveniently, using transmission electron microscope can be used for electron beam heating and irradiating of the materials for developing different form of nanomaterials such as carbon nanotubes, carbon nanocapsules, and carbon nanoparticles. It has several advantages like heating source is outside the evaporation system, any materials, including metals, compounds, ceramics, etc., there is no contamination from the heating source.
2.2.4.2.5 Laser Ablation Process
Laser ablation technique is recent development for the synthesis of nanoparticles in controlled particle size and compositions. The technique involves the vaporization of a target using pulsed laser, which is then followed by the controlled condensation in a diffusion cloud chamber under well-defined conditions of temperature and pressure. A wide variety of metal oxides, carbides and nitrides can be synthesized in nanoscale dimensions.
2.2.5 Biological Synthesis of Nanoparticles
Despite the presence of extensive preparation techniques, most of these methods have complex protocols, extreme conditions of temperatures and toxic chemicals as reducing agents which not only result in high running costs but may also contain minor toxic contaminations on particle surface which question their direct application in biological systems (Hebbalalu et al. 2013). Biological nanoparticle synthesis on the other hand, makes use of biological catalysts which result in an environment friendly approach of synthesis thereby gaining precedence over conventional physical or chemical methods (Kruis et al. 2000; Sastry et al. 2003; Ahmad et al. 2003; Hebbalalu et al. 2013). Several existing methods of biological nanoparticle synthesis are explained in detail below.
2.2.5.1 Plant Mediated Synthesis of Nanoparticles
Noruzi (2015) has comprehensively reviewed the biosynthesis of gold nanoparticle using plant extracts in which he has covered various methods of green synthesis of nanoparticles and their characterization techniques in detail
The review also covers application of gold nanoparticle in various fields. With this note, we are including some more recent research reports on synthesis of nanoparticle using plant extracts. Silver and gold nanoparticles synthesis has been reported extensively using Neem (Azadirachta indica) leaf broth (Shankar et al. 2004). Chenopodium album (an obnoxious weed) leaf extract used for the synthesis of silver and gold nanoparticle and produced quasi-spherical shaped particle with size of 10–30 nm and in the same study the influence of leaf extract quantities, metal ion concentration, contact time, temperature and pH on the synthesis of nanoparticles were also evaluated. With increase of concentration of the leaf extract, the size of the particle becomes small. The particle size was increased with increase in metal ion concentration. At 5 and 1 mM concentration, 40–90 nm silver nanoparticle was formed and 50–100 nm of gold nanoparticles was formed at 1 mM concentration. The reaction time resulting in synthesis of nanoparticle ranges from 15 min to 2 h and sharp peaks were obtained upto 2 h following which a slight variation was observed. On studying the effect of temperature and pH on silver nanoparticle synthesis, increase in temperature increased the particle size but large sized nanoparticle only in case of a lower pH. However in case of gold nanoparticle, almost similar shape and size was observed from pH4 to pH10. Flavanones and terpenoids present in the plant or leaf extracts often been reported to be responsible for the synthesis of nanoparticles. Oxalate and aldehydic groups present in the leaf can be act as reducing agent as well as ligand (Dwivedi and Gopal 2010).
Aluminium oxide nanoparticles were synthesized from lemon grass leaf extract in the size of 9–180 nm which were spherical in shape (Ansari et al. 2015). Similarly, Prabhu et al. 2015 demonstrated the extracellular synthesis of copper nanoparticles in the size of 20–25 nm using Garcinia mangostana leaf extract. Garcinia mangostana leaf extract also examined for the production of silver nanoparticle. The optimum reaction time in both cases was 1 h and 1 mM concentration of substrate for the synthesis of nanoparticle. Based on temperature, initial increase in temperature led to the formation of increase in number of particles and at higher pH, smaller sized particles were observed (Veerasamy et al. 2011).
Coleus amboinicus leaf extract produced spherical, triangular, truncated triangular, hexagonal and decahedral shaped gold nanoparticle with the size range of 4.6–55.1 nm. Aromatic amines, amides and secondary alcohols were reported to be responsible for the capping of the particle (Narayanan and Sakthivel 2010). Magnolia kobus and Diopyros kaki synthesized gold nanoparticle extracellularly, at lower temperature and decreased concentrations of leaf broths. They produced spherical, triangular, pentagonal and hexagonal nanoparticle in the size of 5–300 nm. At higher temperature and increase concentration of leaf broth, small spherical shaped nanoparticles were produced. gold nanoparticles synthesized using Magnolia kobus are bounded by some proteins and metabolites such as terpenoids having functional groups of amines, alcohols, ketones, aldehydes and carboxylic acids (Song et al. 2009). Similar results were observed in the synthesis of gold nanoparticle using olive leaf extract by Khalil et al. 2012. When Zingiber officinale extracts used, gold nanoparticles of size 5–15 nm were synthesized. Alkaloids, alkanoids, flavonoids in the extracts were reported to act as capping agents. The synthesized particles were used for blood biocompatibility (Kumar et al. 2011). These nanoparticles were non platelet activating and non-complement activating on contact with human blood. These nanoparticles are not aggregate with other blood cells.
Terminalia bellirica fruit extract have also been used to synthesize silver nanoparticle, and the nanoparticles evaluated for antibacterial and antioxidant activity. In their research, the authors have identified gallic acid to be responsible for the reduction and stability of the nanoparticles (Hoskote Anand and Mandal 2015). Further silver nanoparticle synthesis using beet root (Bindhu and Umadevi 2015) and A. indicum leaves extract have also been reported (Ashokkumar et al. 2015). The synthesized nanoparticles were spherical in shape and 10 nm in size. Gold nanoparticle production using cold and hot extract of Pedalium murex showed that both the extracts produced nanoparticles in the range of 180–200 nm. Hexagonal, triangular and spherical nanoparticle was seen in TEM analysis and semi-spherical nanoparticle was observed in SEM analysis (Peter et al. 2014).
In recent studies, seed extract of Pistacia atlantica was utilized for the synthesis of silver nanoparticle which produced spherical shaped particles with 27 nm in size. The particles were stable in the pH range of 7–11 and OH group acts as a reducing/ capping agent (Sadeghi et al. 2015). In the same year, the same authors used the leaf extract of Ziziphora tenuior for the synthesis of silver nanoparticle which formed spherical particles with 8–40 nm size. The alkaloids, phenolic compounds, terpenoids and co-enzymes present in the leaf have been hypothesized to play a major role in the reduction and stabilization of the nanoparticles (Sadeghi and Gholamhoseinpoor 2015). When Ficus carica leaf extracts were used to synthesize silver nanoparticles irradiances between 6.5 and 13.3 mW/cm2 in the range of 330–550 nm wavelength were used and the results were compared in the dark and under direct sunlight (Ulug et al. 2015). Available data on synthesis of nanoparticles using plant extract is represented in the Table 2.5.
2.2.5.2 Bacteria in Synthesis of Nanoparticle
Inorganic materials are produced by microbes either intra or extracellularly. Among microorganisms, bacteria have been extensively studied by researchers for the synthesis of metallic nanoparticles (Table 2.6). Bacteria can be cultivated in shorter intervals of time and are easy targets for the recovery of nanoparticles. Several researchers are working on the synthesis of nanomaterials for more than decades. Recent developments and current scenario of the nanoparticle research has been reviewed by many scientists. A comprehensive explanation of extracellular and intracellular silver nanoparticle synthesis of bacteria or bacterially derived components has been reported by Singh et al. 2015. They also proceed further and mention in detail, the mechanism behind the extra and intracellular synthesis, as well as the role of reducing as well as capping agents in the synthesis (Fig. 2.2).
Several bacterial strains, regardless of their taxonomic groups have been reported to synthesize silver nanoparticles (Mohanpuria et al. 2008; Narayanan and Sakthivel 2010; Sweet et al. 2012). On a careful observation, it can be understood that most of these isolates are soil and marine origin, thus they were involved in several biogeochemical cycles of metals (Sweet et al. 2012). These bacteria are metabolically accustomed to presence of metals in their environment and detoxify them by reduction and/or precipitation mechanism of inorganic ions to metal nanoclusters (Narayanan and Sakthivel 2010). Strain Pseudomonas stutzeri AG259 which was primarily isolated from silver mines showed synthesis of silver nanoparticles and magnetotactic bacteria Magnetospirillum magneticum produced magnetic (Fe3O4) nanoparticles (Mohanpuria and Rana 2008). However there are also a few reports where strains of Lactobacillus sp., Bacillus sp., Shewanella algae and Rhodobacter sphaeroides are able to synthesize nanoparticles of metals such as gold, silver, titanium, platinum and zinc (Sweet et al. 2012). Therefore, one microorganism can form nanoparticles of different metals and the nanoparticle synthesizing capacity of a given bacterial strain dependent on the physiological properties of the strain but its lineage or taxonomic group to which they belong.
Until now, specific bacterial secretions responsible for bio-reduction of silver have not been reported, with the exception of a NADH dependent nitrate reductase that involved in microbial nanoparticle synthesis (Durán and Seabra 2012). Under conditions of high pH, EPS from bacteria, made of glucose, delivered reducing power to reduce metal ions into nanoparticles (Sintubin et al. 2009). However, in psychrotolerant bacteria, a direct evidence of proteins or EPS production promoting AgNPs synthesis at low temperatures remains to be documented. Interestingly, one psychrotolerant bacteria Pseudoalteromonas antarctica NF3, produced EPS and certain outer membrane vesicle proteins that were linked to survival of this bacteria under low temperature (Nevot et al. 2006a, b). Regardless of the putative carbohydrate and protein based mechanisms, specific cellular proteins or polymers based AgNP synthesis remains unclear at present (Sintubin et al. 2009; Shivaji et al. 2011).
The techniques involved in the characterization and application of nanoparticle have also been illustrated extensively in the past (Fedlheim and Foss 2001; Oskam 2006). As bacterial synthesis of nanoparticle has been described in detail by so many authors, in this chapter, we have tried to emphasize synthesis of nanoparticles by extremophilic bacteria as well bacteria colonizing niche environments. One of the primary reports on nanoparticle synthesis by extremophilic bacteria was on Pseudomonas stuzeri AG259. The bacterium was able to synthesize silver nanoparticles of size 35–46 nm intracellularly and accumulate the synthesized particles in its periplasmic space (Slawson et al. 1992). Morganella sp. RP-42, an insect midgut bacterium produced extracellular silver nanoparticle of size 20±5 nm and was identified to host three homologous silver resistant gene (silE, silP and silS) in its plasmids. The gene SilE was found to encode a periplasmic silver-binding protein (Parikh et al. 2008).
In other studies, cadmium sulfide (CdS) nanocrystals were produced intracellularly by E. coli (Sweeney et al. 1998) and extracellularly at the cell surface by Clostridium thermoacetium (Cunningham and Lundie 1993). In a study by Watson et al. 1999, Magnetic iron sulfide (FeS) nanoparticles of size about 20 nm were observed on the cell surface of sulfate-reducing bacteria. Though FeS nanoparticles are commonly found to be synthesized under anaerobic condition, a later study showed FeS nanoparticle production by Actinobacter sp. under aerobic conditions (Bharde et al. 2005). In another report, Konishi et al. (2004) demonstrated the effect of pH in influencing the size of nanoparticles. Under pH 1, the extracellularlly synthesized particles were 50–500 nm in size whereas at pH7 there was significant size reduction upto 10–20 nm. Similar results were observed in Rhodopseudomonas capsulate. Moreover nanoparticle synthesis at pH 7 was found to result in more spherical shaped particles with size ranging from 10 to 20 nm whereas at pH 4, triangular nanoplates were observed (He et al. 2007). Therefore pH plays a vital role in the synthesis of nanomaterials, by regulating the size, morphology and location of nanoparticles.
In a report by Juibari et al. (2011), an extremophilic bacterium, Ureibacillus thermosphaericus isolated from Ramsan geothermal hot springs located in Mazandatan province, Iran exhibited extracellular silver nanoparticle synthesize at higher temperatures (60–80 °C) under varying concentrations of AgNO3 (0.001–0.1 M). Maximum nanoparticle synthesis was achieved at 0.01 M AgNO3 at 80 °C. The biomolecules produced by the extremophile under extreme temperatures may play a major role in the synthesis of nanoparticles even under unfavorable temperature conditions. Halomonas maura, a salt tolerant bacterium produced mauran, a polysaccharide which plays a major role in synthesis of MR(muran)/CH(chitosan) nanoparticle, by polyelectrolyte complexation of CH and MR solution through strong magnetic stirring (Raveendran et al. 2015).
Another thermophilic bacterium, Geobacillus stearothermophilus was reported to synthesize nanoparticles of silver and gold nanoparticle. Gold nanoparticles synthesized by this extremophilic bacterium were spherical shaped and monodispersed with a size range of 5–8 nm. In case of silver nanoparticles, the particles were polydispersed, spherical shaped with a size range of 5–35 nm (Mohammed Fayaz et al. 2011). The authors stated that the free amine groups or cysteine residues present in the proteins were key components responsible for the synthesis of nanoparticles. They have also identified seven different proteins in the range of ascending 98 kDa-12 kDa that are bearing free amine or cysteine bearing molecules aiding nanoparticle synthesis. Similarly, gold nanoparticle synthesis by Shewanella oneidensis has been reported by Suresh et al. (2011). An extracellularly synthesized spherical gold nanoparticle particles of size 2–50 nm by S. oneidensis was found to be stable for several months. The particles were hypothesized to be synthesized by the reducing agents present in the cell membrane and were capped by the detachable protein or peptide coat. In 2012, Dhandapani et al. synthesized TiO2 (Titanium oxide) nanoparticles using Bacillus subtilis which was reported to destroy biofilm forming microbes. In another study, 11 marine bacteria were screened for the synthesis of gold nanoparticle (Sharma et al. 2012). Among those strains, Marinobacter pelagius isolated from solar saltern in Kakinada (India), produced monodispersed gold nanoparticle of size 10 nm that were highly stable. This is one of the initial reports on the synthesis of gold nanoparticle by marine bacteria. They confirmed the role of extracellular biomolecules produced by the bacteria in reducing and capping of nanoparticles. A study by Dehnad et al. (2015) demonstrated gold nanoparticle synthesis by Arthrobacter nitroguajacolicus, isolated from Andaliyan gold mine in north-west of Iran. It exhibited intracellular synthesis of spherical shaped nanoparticles of about 40 nm in 24 h. However, not all bacteria are found to be capable of gold nanoparticle synthesis in shorter time periods. An alkalothermophilic actinobacterium, Thermomonospora sp. synthesized gold nanoparticle extracellularly taking 120 h for reduction of aurum ion. In the case of strains Rhodococcus, it reduced the aurum ion in 24 h, but the particles were synthesized intracellularly which complicated the extraction and also destroys the bacterial cells (Kalabegishvili et al. 2012).
MnO2 nanoparticles were synthesized by the marine bacteria Saccharophagus degradans (Sde 2–40) using KMnO4 as the substrate. Salunke and his colleagues (2015) ruled out the possibility of negative outcome on nanoparticle synthesis under the presence of several media components used for microbial growth. Recently, Srivastava et al. 2013 reported the synthesis of Tellurium nanoparticles (TeNPs) by haloarchea on Halococcus salifodinae BK3, which was isolated from the salt pan. The enzyme responsible for the synthesis of TeNPs was identified as tellurite reductase. The archea H. salifodinae was orange-red pigmented and the pigmentation was found to decrease with increased concentration of tellurite which turned the organism black against being orange-red. TEM analysis exhibited hexagonal needle-shaped nanoparticle with an average diameter of 10 nm and length of 44 nm. In E. coli, α-NADPH dependent sulfite reductase was extracted and used for the synthesis of gold nanoparticle. The particles were spherical in shape with 10 nm in size which was found to act as an antifungal agent (Gholami-Shabani et al. 2015).
Silver nanoparticle synthesis by psychrotolerant bacteria, Pseudomonas mandelii was reported by Mageswari et al. (2015). Its stability under long-term storage, and larvicidal activity under low-temperature conditions were investigated. Electron and atomic force microscopy studies revealed that 6 among 22 psychrotolerant isolates synthesized silver nanoparticles with an average diameter of 1.9–14.1 nm. Pseudomonas mandelii SR1 synthesized the least-sized silver nanoparticles with an average diameter of 1.9–10 nm, at temperatures as low as 12 °C without aggregate formation, and the synthesized nanoparticles were stable for up to 19 months of storage period. In larvicidal studies, LC90 (lethal concentration) values against Anopheles subpictus and Culex tritaeniorhynchus larvae were at 31.7 and 35.6 mg/L, respectively. The synthesis of nanoparticles on different temperature, pH, incubation time and storage stability in dark and light conditions was studied. In UV spectroscopy scan, the synthesized silver nanoparticle showed a peak around 420 nm, which is a characteristic of the surface plasmon resonance of silver nanoparticles. XRD analysis revealed the diffraction peaks at 46°, 54°, 68°, 26° and 32° corresponding to (2 1 1), (2 2 0), (2 2 2), (1 1 0) and (1 1 1) that were planes of silver and silver oxide. EDAX spectrum confirmed the presence of silver atom. In FTIR analysis, the presence of primary/secondary amines and sugar derivatives were confirmed. These results revealed that the protein and sugar molecules were responsible for the synthesis of silver nanoparticle. Further, the sugar derivatives were analyzed by HPLC. The synthesized silver nanoparticles showed larvicidal activity at low temperature which is non-toxic to environment.
Nanoparticle synthesis by psychrotolerant bacterium, M. psychrotolerans (Parikh et al. 2011) was investigated at temperatures as low as 12 °C and it showed enhanced synthesis at 22 °C reflect the cold-adapted physiology of the strain. Extracellular synthesis of silver nanoparticles using psychrophilic bacteria was investigated by Shivaji et al. 2011. The synthesized silver nanoparticles were characterized using UV–Visible spectroscopy, transmission electron microscopy and atomic force microscopy. The sizes of the silver nanoparticles ranged from 6 to 13 nm and were stable for 8 months in the dark. The synthesis and stability of silver nanoparticles appears to depend on the temperature, pH or the species of bacteria (P. antarctica or A. kerguelensis) from which the supernatant is used. It was observed that the A. kerguelensis supernatant could not produce silver nanoparticles at the temperature where the P. antarctica could synthesize silver nanoparticles. This study provides evidence that the factors in the cell-free culture supernatants that facilitate synthesis of silver nanoparticles vary from bacterial species to species.
2.2.5.3 Fungi Mediated Synthesis of Nanoparticles
In recent years, there has been an increase in research focusing on the use of fungi for the synthesis of different types of nanoparticles (Dhillon et al. 2012). Research on fungi has been found to be preferred as they offer several advantages. One such benefit is that they could secrete large quantities of enzymes. Other benefit includes ease of handling compared to other microorganisms, which make the biosynthesis of nanoparticles using fungi potentially exciting (Table 2.7). However, one major concern is the genetic manipulation of some specific enzymes for over expression is much more difficult than in prokaryotes.
Fusarium oxysporum, is a fungus that has been often used for the biosynthesis of nanoparticles. F. oxysporum synthesizes extracellular gold nanoparticles which are spherical or triangular in shape and are at a size of 20–40 nm and they are found to be well dispersed with no significant reunion was found even after a month (Mukherjee et al. 2002). In another report, stable cadmium sulfide nanoparticles of size range 5–20 nm was synthesized by F. oxysporum. This nano-CdS was produced with the help of enzyme sulfate reductase when exposed to CdSO4 solution. Similarly ZnS, PbS and MoS2 nanoparticles also could be synthesized by F. oxysporum when incubated with corresponding sulfate (Ahmad et al. 2002). They also used F. oxysporum for biosynthesis of cruciform-shaped CaCO3 crystals by exposing to aqueous calcium ions. In both of these studies the basic mechanism behind the synthesis is discussed as the role of enzymes present in fungi which played a crucial role during the synthesis (Rautaray et al. 2003).
Acidophilic fungus Verticillium sp. has been reported to produce intracellular silver nanoparticles in cytoplasm (Sastry et al. 2003). When F. oxysporum was utilized to synthesize alloy nanoparticles, surprisingly Au-Ag alloy nanoparticles were obtained which was the first reported on the fungus-mediated biosynthesis of Au-Ag alloy nanoparticles. The highly stable alloy nanoparticles in the size range of 8–14 nm were formed when the fungi were exposed to equimolar concentrations of AgNO3 and HAuCl4. The fungi F. oxysporum was demonstrated to secrete NADH-dependent enzymes and it was confirmed that the nano-alloy composition was different in the control of the amount of NADH (Senapati et al. 2005). In 2006, it was reported that the substantial amorphous hydrated silica in the rice husk could be bioleached and biotransformed into quasi-spherical crystalline silica nanoparticles in a size range of 2–6 nm. Further studies demonstrated that the amorphous hydrated silica could be bioleached by the proteins secreted by the cells. However, amorphous hydrated silica could not be biotransformed into silica nanocrystallites by these proteins alone (Bansal et al. 2006). The fungi Aspergillus flavus has been reported to synthesize silver nanoparticles on the surface of cell wall with size ranging in 8–9 nm. Proteins present on the cell wall were hypothesized to be responsible for the reduction and stability of the particle (Vigneshwaran et al. 2007).
Extracellular platinum nanoparticles with the size ranged 5–30 nm were obtained when the F. oxysporum incubated with H2PtCl6 which were stabilized by proteins (Syed and Ahmad 2012). Biosynthesis of spherical ZnS nanoparticles with the average size of 42 nm was also reported using F. oxysporum (Mirzadeh et al. 2013). It was found that other species of Fusarium such as F. acuminatum (Ingle et al. 2008) and F. semitectum (Basavaraja et al. 2008)could also be used to synthesize extracellular silver nanoparticles with size of 10–60 nm with spherical in shape. The synthesized particles were stable for weeks. In fungus V. luteoalbum, gold nanoparticle synthesis was found to occur at different conditions of pH. At pH 3, spherical shaped particles were formed. However at pH 5 with spherical, triangular, hexagons, spheres and rod shaped particles were observed and when the pH was increased to 7 and 9, small spherical and irregular shaped particles were formed (Gericke and Pinches 2006).
Silver nanoparticles synthesis has also been reported when aqueous extracts of Agaricus bisporus were used. A study using Agaricus bisporus revealed that these nanoparticles were of spherical shape and size ranging between 8 and 20 nm (El-Sonbaty). Biosynthesis of gold nanoparticles by fungus Botrytis cinerea was reported first in 2014. This gold nanoparticle synthesis was found to result in nanoparticles of various shapes such as spherical, pyramidal, hexagonal, triangular and decahedral of sizes ranging between 1 and 100 nm. Biomolecules secreted by the fungus were responsible for the production of gold nanoparticles, but the exact mechanism remains to be elucidated (Castro et al. 2014).
2.2.5.4 Yeast in Synthesis of Nanoparticle
Studies on biosynthesis of metallic nanoparticles by yeast are few compared to bacteria and fungi (Table 2.8). Available research carried out on yeast mostly deal with the synthesis of quantum semiconductor nanocrystallites. Extremophilic yeasts isolated from the drainage of acid mine have been utilized for the synthesis of Ag and Au nanoparticles. Yeasts are reported to exhibit growth up to concentrations such as 1.5 mM Ag ion and 0.09 mM Au ion and nanoparticle synthesis is mainly extracellular in nature with the produced particles around 20–100 nm in size synthesized (Mourato et al. 2011). The most commonly studied yeast Sachharomyces cerevisae has also been used in the biosynthesis of metallic nanoparticles in recent years. Biosynthesis of TiO2 nanoparticles using the S. cerevisae yielded in nanoparticles of size 8–35 nm and it was proposed that the quinones and membrane bound oxidoreductases might affect the formation of TiO2 nanoparticles (Jha et al. 2009). The yeast S. cerevisae was investigated for the synthesis of MnO2 nanoparticle in both extra and intracellular manner. All experiments showed positive result with spherical and hexagonal shaped nanoparticle ranging 30 nm in diameter (Salunke et al. 2015). Report also exists on gold nanoparticle synthesis using the yeast S. cerevisae (Lin et al. 2005). When gold nanoparticles synthesis was studied in Yarrowia lipolytica, the particles were associated with the cell wall and were hexagonal or triangular in shape at varying pH of 2, 7 and 9. Reductases were reported to have a major responsibility in nanoparticle production by yeasts. A method to extract cell wall associated nanoparticles from yeasts using a freeze and thaw method was proposed (Agnihotri et al. 2009).
2.2.5.5 Actinomycetes in Synthesis of Nanoparticle
Actinomycetes are widely distributed in nature and have the characteristics of both fungi and bacteria. Gold nanoparticles have been reported to be synthesized successfully with Rhodococcus sp. When an alkalotolerant actinomycete was used for intracellular synthesis of gold nanoparticle particles with the dimension of 5–15 nm were produced (Ahmad et al. 2003). This was the very first report on the extracellular synthesis of gold nanoparticle with the use of bacteria. These thermoactinomycetes are able to restore gold ion into spherical NPs with good monodisperity at an average size of 8 nm. The FTIR analysis demonstrates that the surface of the gold nanoparticle was amide (N) and (O) protein, which are stable factors that promote these Nanoparticles.
A recent report demonstrates gold nanoparticle production by Streptomyces viridogens strain [HM10] a novel actinomyces. The strain was reported to follow an intracellular mechanism for synthesis of gold nanoparticles. Electron microscopy (TEM) study on the strain revealed the presence of spherical and rod shaped gold mycelium and X- ray diffraction analysis demonstrated that their average size to be 18–20 nm. At the same time, the strain HM10 showed increased growth at 1 and 10 mM concentration of HAuC14. In addition the gold nanoparticles produced by the strain HM10 possessed high antibacterial activity against S. aureus and E. coli (Balagurunathan et al. 2011). Nanoparticle production by S. albidoflavus has been found to be both extracellular and intracellular. An interesting phenomenon observed was that that the nanoparticles synthesized by this strain were reaction-time dependent. The average size of these nanoparticles from S. albidoflavus was found to be 14.5 nm. By varying pH and reaction time multidimensional gold nanoparticles were generated (Prakasham et al. 2012). Thirty five marine actinobacterial strains were isolated from marine sediment of Busan coast, South Korea and employed for the biosynthesis of gold nanoparticle. In that, Nocardiopsis sp. MBRC-48 exhibited the production of extracellular gold nanoparticles. The synthesized nanoparticles have been characterized using UV-visible spectral analysis, XRD and FTIR. In FTIR analysis, the results clearly evidenced that the nanoparticle formation occurred by proteins, enzymes and metabolites. EDAX spectroscopy revealed that the presence of elemental gold peak. TEM analysis confirmed that the particle were spherical in shape and non-uniformly distributed with size ranges from 7 to 15 nm and they evaluated the antibacterial, antioxidant and cytotoxicity of the nanoparticles (Manivasagan et al. 2015). Few reports for Actinomycetes-mediated synthesis of nanoparticles list in the Table 2.9.
2.2.6 Characterization of Nanomaterials
Several sophisticated techniques being continuously developed for characterization of the size, shape, stability, of nanomaterial as well as to determine and identify the presence of metal signals in the synthesized nanomaterials (Table 2.10). Characterization of nanomaterials can be carried out using two major strategies which are spectroscopy and microscopy techniques.
2.2.6.1 Spectroscopic Techniques
2.2.6.1.1 Ultraviolet-Visible Spectroscopy
Nanoparticles from metals such as gold and silver interact strongly at specific wavelengths of light (500–600 and 400–450 nm) based on their optical property and surface plasma resonance.
Such characteristics of the nanoparticles can be attributed to free flow electron oscillation in the elements composing the nanoparticles being induced by the electromagnetic field (Noruzi et al. 2011). However, these techniques are used only to detect metallic nanoparticles and are often not compatible with other nanomaterials. A change in color of the initial solution to red or violet for gold nanoparticles indicates the presence of synthesized nanomaterials in the extract and the solution turns brown in case of silver nanoparticles (Noruzi et al. 2012). Thus color change indicates and influences the absorption pattern which confirms the presence of synthesized nanomaterials in the extract. The plasma resonance property has been found to vary with size and shape of nanoparticles. A change in surface resonance due to variation in particle size may lead to a shift in wavelength from red (increase in particle size) and blue (reduction in particle size). However, in several cases adverse effect in the position of plasma resonance has been observed due to factors such as environment dielectric properties, interactions that are physical or chemical which happens on the particle surface, surface charge, interparticle distance as well as aggregation. Asymmetrical and broad plasma resonance bands indicate that diverse orientation of material often results in formation of anisotropic nanoparticles (Kasthuri et al. 2009; Noruzi et al. 2011).
2.2.6.1.2 X-Ray Diffraction
XRD is one of the most powerful techniques to study the structure of nanomaterial which provides us data regarding the width and shape of the nanomaterials (size of crystalline, lattice, dislodgment of particle structures, etc). Samples are often prepared as smear or compact flat or in capillary and are exposed to a monochromatic beam of X-ray which is diffracted by the nanomaterial and data on the diffracted beam is collected at an angle (2θ) with respect to the incident beam. A powder diffraction pattern supplies information from the nanomaterial on phases present, phase concentrations, structure, as well as the degree of crystallinity or amorphous content followed by details on crystallite size/strain. The peak widths in given phase pattern provides information on the average crystallite size where large crystallites give rise to sharp peaks and any increase in the peak width indicates a reduced crystallite size.
The measure of the crystallite size does not equal to the size of the particle as a single nanoparticle can be an aggregate of several crystallites. Peak broadening also occurs as a result of variation in d-spacing caused by microstrain. The average crystallite size can be determined from the breadth of the peaks in an X-ray diffractogram, assuming no lattice strain or defects are observed, through the Debye-Scherrer equation (Dubey et al. 2010). Comparison of average particle sizes either using a power XRD or TEM methods can help us obtain the crystal structures of nanoparticles. An important parameter to be considered is X-ray coherence length, which gives us an approximation of the average size of crystalline domain of nanomaterial (XRD-size), whereas total size of the particles (TEM-size) can be viewed from the TEM metaphors (Schmid 2004). A major disadvantage of using these techniques is that it is very difficult to find out the single conformation and binding state of growing crystals followed by occurrence of low intensity diffracted X-rays in low atomic number materials (Cao 2004). A recent study has proposed the use of a femtosecond pulse to determine the macromolecules for non-matured crystal (Chapman et al. 2011).
2.2.6.1.3 Fourier Transformed Infrared Spectroscopy
FT-IR is primarily used to determine the functional groups of the protein bound over the surface of nanomaterials or functional groups of exoploysaccharides which act as capping agents and thereby improve stability during biological synthesis of nanomaterials. Identifying such biomolecules is important to change or enhance the chemical structural composition which may leads to understand the development of their properties and probable future applications. These types of biomolecules in biological suspension falls into absorption spectrum in the range of 1000–1800 cm-1 (C=C, C=O, C-N, C-O) which indicate the possible role of those compounds in maintaining the nanoparticle stability. The stretching peak for amine and amide groups during an FT-IR analysis falls in the range between 3200 and 3500 cm-1 (N-H and O-H) (Philip 2010).
2.2.6.1.4 Dynamic Light Scattering
DLS technique is used to study the size distribution and hydrodynamic average particle size of nanoparticles. The basic principle behind this technique is that the movement of a particle depends on time of fluctuation in light scattering to the suspension which is based on Doppler effect (Brar and Verma 2011). Determination of particle size distribution is not completely known due to adverse effect on particle suspension, scattering angle and shape of nanoparticles. This technique is especially important in reuse of nanoparticles, as the samples can be used for other characterization. In addition, scattering of light is directly proportional to sixth power of particle radius; this reason helps extremely to determine the presence of small aggregates. This technique is quicker and less expensive than that of microscopy technique for analysis the size of nanomaterials (Bankar et al. 2010).
2.2.6.1.5 Zeta Potential
Zeta potential is an instrument to measure the electrostatic potential of nanomaterial by supplying electric field between dispersion medium and stationary layer of fluid. Zeta potential difference of nanomaterials between -10 to +10 mV are considered as neutral, whereas greater than +30 or less than -30 mV are considered as cationic or anionic charged particles. Almost all biologically synthesized nanomaterials were strongly cationic charged particles due to negatively charged cell membrane which displaying more toxic associated with cell wall disruption (Clogston and Patri 2011). Dilution of concentrated samples which does not affects the results of synthesized nanoparticles it may due to shift in zeta-potential value was attributed to an increase in contribution of the signal from extraneous particulate matter (Tantra et al. 2010). Zeta potential and hydrodynamic size can be altered by charging the pH of the solution. At low pH, nanomaterial have a positive surface and inversely, at high pH, a negative surface charge. The isoelectric focusing point is intermediate pH it results in net charge of particles will be zero. State and stability of nanoparticles depends on pH, surface charge and coating surface. From profound studies, pH can change the dispersion state by altering the zeta potential which influences the ionic strength of dispersion medium by altering the double layered surface electric charge of nanomaterial (Jiang et al. 2009).
2.2.6.1.6 Other Spectroscopy Techniques
To characterize the physio-chemical properties of nanomaterials, other techniques like X-ray photoelectron spectroscopy (XPS), Inductively coupled plasma atomic emission spectroscopy (ICP/AES), Raman scattering (RS), Fluorescence correlation spectroscopy (FCS), circular dichroism (CD), Mass Spectroscpy (MS) were used.
2.2.6.2 Microscopy Techniques
2.2.6.2.1 Transmission Electron Microscopy
The transmission electron microscope (TEM) is the most very powerful tool for probing the structure of nanosized particles. It is not only can give morphological information of shape and size of material even able to define at atom level of the nanomaterials. To analyse the structure of nanomaterials by two types of mode either Bright-field TEM of Dark field TEM. In Bright-field TEM, is most common type of mode to imaging the nanomaterials in which images are captured by direct exposure of transmitted electrons and any type of materials can be visualized in the form of liquid or solid. In dark-field TEM, directly use diffracted electron at specific set of crystal plane. Mainly, it is used to locate defect in the crystal. For imaging the nanomaterials using TEM depends on their contrast of sample relative to the background. Samples are prepared for imaging by drying nanoparticles on a copper grid that is coated with a thin layer of carbon. Materials with high electron densities can able visible directly than amorphous carbon such as metals (silver, gold, copper, aluminium) and most of the oxides and other materials as to be coats with tungsten-gold materials. Size and morphological distribution of nanomaterials is straight forward if it is individual non-aggregated samples. Otherwise, it is statistically difficult to image the particular size and shape of the nanomaterial. Since the presence of polymeric structure (irregular or more than one size) of nanomaterials in dispersion, the resulting statistical representation in average and standard deviation of the sample may not be accurate. For irregular shape nanomaterials an alternative method is used to image by cross sectional area or convert the area to an equivalent spherical diameter by using softwares.
The fast electrons used in a TEM are capable of penetrating many atomic planes and so are diffracted by crystalline regions of material just like X-rays. Their wavelength (~ 0.04 nm for Eo ~ 100 keV) is much less than a typical atomic-plane spacing (~ 0.3 nm) so that according to the Bragg equation nλ = 2dsinθ, Bragg angles θ are small. In addition the integer n is usually taken as unity, since nth order diffraction from planes of spacing d can be regarded as first order diffraction from planes of spacing d/n (Wittstock 2001). Diffraction represents elastic scattering of electrons in a crystal. The regularity of the spacing of these nuclei results in a redistribution of the angular distribution of the scattered intensity. Instead of continuous distribution over scattering angle, there are sharp peaks centered on certain scattering angles, each twice the corresponding Bragg angle θ. In the TEM this angular distribution can be displayed by magnifying the diffraction pattern first formed at the back focal plane of the objective lens. Examination of the TEM image of the polycrystalline specimen shows that there is a variation of electron intensity within each crystallite. This diffraction contrast arises either from atomic defects within the crystal or the crystalline nature of the material itself, combined with the wave nature of transmitted electrons.
The resolution of the TEM could be improved by special arrangements of the magnetic fields serving as focuses of the electron beam, by enhancing the energy of the electrons. The main advantages of the HRTEM are the direct observations of the detailed nanostructures, such as the core/shell structure, the interfaces and the surfaces, the atomic defects (including point defects,
dislocation, planar defects) the twin structures etc (West 2007). The nanocrystal characterization can be done using transmission microscopy. Here, an electron beam is used to image a thin sample in transmission mode. The resolution is a sensitive function of the beam voltage and electron optics: a low-resolution microscope operating at 100 kV might have a 2–3 Å resolution while a high voltage machine designed for imaging can have a resolution approaching 1 Å.
Three main issues are causing major problems during image process such as particle agglomeration, Impurities in the samples and few particles in images.
Particle Agglomeration
A number of factors influences how sample synthesized and preparation conditions namely, dynamic property of dispersion or diffusion of particles in the solvent during drying and concentrating process.
Impurities in the Sample
Due to electron density present in the sample and in the dispersion. i.e., presence of salt, silica, etc.
Few Particles in Image
It’s mainly due to the way of sample preparation, in that presence of low or dense particles in the gird.
Recently, Scanning Transmission Electron Microscope (STEM) has advanced to generate images by staging a scan of a narrow electron beam over the samples and coupled with high-angle annular dark-field (HAADF) detector. Other detectors such as Energy-dispersive X-ray spectroscopy (EDX) and Electron energy loss spectroscopy (EELS) used for analysis elements by qualitative and quantitatively. In addition, cryo-TEM an equipment to examine biological samples under cryogenic temperature, preventing them from structural deformation caused by electron beams and by the conventional sample treatment during ultra-sectioning.
2.2.6.2.2 Scanning Electron Microscopy
Scanning electron microscope (SEM), which is used to observe the morphology of a surface of sample and back scattering of electrons at higher magnification, higher resolution and depth of focus. SEM creates a low-energy beam of electrons (1–30 keV) and detecting the electrons scattered off the sample. Samples for SEM are mounted on aluminum or carbon stubs either in the liquid or solid form. Unlike TEM, in SEM, prepared samples can be introduced for imaging in bulk form, as there is no need to cut thin sections. Generally, sample preparation is less tedious for SEM imaging, compared to TEM. For example, liquid samples can be dried directly on the SEM stub (Lorenz et al. 2010), or closed in agar capsules that, after chemical treatment, undergo notch (Egelandsdal et al. 1999). In addition, Focused-ion beam scanning electron microscope (FIB-SEM) the ion beam can cut into the material and a solid specimen can be sectioned for 3D imaging and also gives the possibility of tilting the sample to allow observation from different angles and 3D imaging. A camera is used to photograph the image or it may be digitized and processed on a computer. The characteristic X-rays emitted may be analyzed for their energy and intensity (EDX), the energy being the signature of the element emitting them and the intensity as to how much of it is present. SEM while having lower resolution than TEM, is able to image nanoparticles on bulk surfaces and for direct visualization of nanocrystals in larger assemblies (Khler and Fritzsche 2004).
2.2.6.2.3 Scanning Probe Microscopy
Most advance equipment to determine the surface structures are the atomic force microscope (AFM) and scanning tunneling microscope (STM). These techniques are capable of imaging the local surface topography with atomic resolution (Wiesendanger 1994).
2.2.6.2.4 Atomic Force Microscopy
Atomic force microscope (AFM) and scanning force microscope (SFM) are the most common techniques from the SPM family and play a major role in research and development part of nanotechnology. Both these methods are based on minute but measurable forces between a sharp metallic tip and atoms present on a surface. Atomic force microscope (AFM) was invented by Gerd Binning in the year 1986. The tip is usually mounted on arm called cantilever which is flexible in nature, and the entire set up is placed at subnanometer distance from the sample surface. As the tip is brought closer to a sample placed on the surface, interatomic forces between the tip and the atoms in the samples causes the cantilever to curve finely which is detected optically by a laser beam reflecting off from the back of the cantilever. When the tip scans over the entire sample surface, an image which is proportional to the deflections of the cantilever can be obtained, which basically represents the three dimensional structure of the sample surface. When the tip is made to scan the sample in the X-Y plane, it senses the attractive or repulsive forces arising from the surface atoms which are then redirected to the Z direction (Sarid 1991). Primarily, there are two ways in which atomic force microscopy can be applied namely contact mode and non-contact/tapping mode. During the contact mode, the tip is placed within few angstroms from the sample surface, and there are direct interactions occurring between the atoms on the tip and on the sample surface. It is highly complex to describe the atomic force interactions occurring during the contact mode and requires molecular dynamics stimulation of the coulombic interactions taking place between charges or charge distribution, followed by polarization caused due to induced dipole moments as well as quantum mechanical forces when the electrons in the orbital start to interact, for each pair of atoms residing on the tip and the sample surface. Despite its complexity, the contact mode is the usual choice to study by researchers to study surface morphology of particles with atomic resolution (Sarid and Coratger 1991). The distance between the tip and the sample is increased in the non-contact mode, usually between 2 and 30 nm. The non-contact mode describes the forces between the sample surface and tip in terms of macroscopic interactions. The tapping mode requires the sample to have a flat surface and a spherical tip. Other forces may such as electrostatic force and magnetostatic force may also play a role in influencing the interactions between sample surface and tip. The forces in the non-contact mode are on average of 2–4 orders smaller in magnitude than forces occurring in the contact mode. Details on the sub nanometer scale are not possible to obtain in the non-contact methods as the interactions that occur are only between larger portions of the tip and the sample. The non-contact mode is mainly used for study and capture magnetic domains or electronic devices. An atomic force microscope is primarily used to identify the sample’s surface characteristics at the atomic level, including its topography as well as the magnetic and electrical properties. AFM creates a highly magnified 3D image of a surface which helps us to directly view surface features which may only have nanometer-sized dimensions including those such as single atoms and/or molecules on a surface. This gives researchers the facility to directly visualize objects that are of nanometers in size and measure various dimensions of the surface features (Binnig et al. 1982; Lang et al. 2002).
2.2.6.2.5 Scanning Tunneling Microscopy
Scanning tunneling microscope or STM, belonging to the SPM family was invented in 1982 by Gerd Binning and Heinrich Rohrer remains a key tool used in nanotechnology. STM is primarily used to study surface properties at an atomic level and works on the principle of measuring the tunneling current between a sharpened metallic tip and surface of a conducting material. When a small bias of voltage is applied between the atomically sharp tip and the sample, there is no flow of current if the distance separating the tip and the sample is large. On the contrary, current flows across the space when the tip is brought closer but without physical contact towards the sample. Such a current is termed as a tunneling current which is the consequence of overlapping wave functions occurring between the atom on the tip and opposite atom on the sample surface. When there is small bias voltage, the electrons are able to travel across a vacuum barrier from the tip and reach the sample. However, the degree of the tunneling current is extremely sensitive and relies on the distance that separates the tip from the sample, the local electron density in the sample as well as the local barrier height. STM is typically performed on conductive and semiconductive surfaces and the atomic information of the surface of the samples can be mapped out as well as the topography of surface electronic states measured. Some of the common applications of STM are atomic resolution imaging and electrochemical STM whereas scanning tunneling spectroscopy (STS) aids imaging of poorly conductive samples (Ong et al. 2013).
2.2.7 Conclusion
As an innovative branch among emerging sciences nanotechnology has the potential to completely revolutionize our lives. There exists a massive variety of nanomaterials today which posses a wide range of properties with the potential to be developed into endless applications. Despite its description as an emerging technology, several indications exist that are examples of human use of nanomaterials for centuries such as the ruby colour in crystals which are caused by gold nanoparticles that are trapped within the crystal matrix. Emerging studies describing the manipulation of materials at their atomic level has paved way for the development of nanomaterials ranging in the sizes scaling 1–100 nanometres. Modification of material properties on a molecular scale offers the prospective for upgrading performance for applications in every aspect of human activity. The potential applications for nanomaterials in the future are limitless, with chances of producing significant advances in the fields of electronics, food, medicine, computing, etc.
Thus the use of nanomaterials can hope to bring a range of benefits to society. On the economical front, science and technology are key participants in improving the economy and life quality, both of which are proposed to be improved by commercialization of positive nanomaterial technologies. In terms of energy efficiency, nanomaterials can have a huge impact as it is proposed that they are functional even at higher temperatures and hence can improve efficiency of power plants, thereby enabling the development of new energy generation systems. However, industrial-scale production of nanomaterials could be possible only when they provide performances similar to conventional materials at a much reduced cost and cause less environmental impacts compared to conventional materials. Thinking along similar lines, though its impact in the future and implications has debated over the last decade, products making use of nanomaterials and nanotechnology has gradually started to arrive. Keeping in mind several other technologies and innovations which faced initial reluctances and antagonism, nanotechnology follows the same path causing alarms in terms of its environmental impacts or the economy. Further, research related to production and applications of nanomaterials has gained significant improvements by receiving funding from both private and government categories in the recent years. Therefore until the benefits of nanomaterials are disproved, research on nanomaterials as well as nanotechnology must be encouraged.
References
Acosta E (2009) Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr Opin Colloid Interface Sci 14:3–15. doi:10.1016/j.cocis.2008.01.002
Agnihotri M, Joshi S, Kumar AR et al (2009) Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater Lett 63:1231–1234. doi:10.1016/j.matlet.2009.02.042
Ahmad A, Mukherjee P, Mandal D et al (2002) Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J Am Chem Soc 124:12108–12109. doi:10.1021/ja027296o
Ahmad A, Senapati S, Khan MI et al (2003) Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 19:3550–3553. doi:10.1021/la026772l
Anbarasu M, Anandan M, Chinnasamy E et al (2015) Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim Acta A Mol Biomol Spectrosc 135:536–539. doi:10.1016/j.saa.2014.07.059
Ansari MA, Khan HM, Alzohairy MA et al (2015) Green synthesis of Al2O3 nanoparticles and their bactericidal potential against clinical isolates of multi-drug resistant Pseudomonas aeruginosa. World J Microbiol Biotechnol 31:153–164. doi:10.1007/s11274-014-1757-2
Arora A, Padua GW (2010) Review: nanocomposites in food packaging. J Food Sci 75:R43–R49. doi:10.1111/j.1750-3841.2009.01456.x
Ashokkumar S, Ravi S, Kathiravan V, Velmurugan S (2015) Synthesis of silver nanoparticles using A. indicum leaf extract and their antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 134:34–39. doi:10.1016/j.saa.2014.05.076
Balagurunathan R, Radhakrishnan M, Rajendran RB, Velmurugan D (2011) Biosynthesis of gold nanoparticles by actinomycete Streptomyces viridogens strain HM10. Indian J Biochem Biophys 48:331–335
Bankar A, Joshi B, Kumar AR, Zinjarde S (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A Physicochem Eng Asp 368:58–63. doi:10.1016/j.colsurfa.2010.07.024
Bansal V, Ahmad A, Sastry M (2006) Fungus-mediated biotransformation of amorphous silica in rice husk to nanocrystalline silica. J Am Chem Soc 128:14059–14066. doi:10.1021/ja062113+
Basavaraja S, Balaji SD, Lagashetty A et al (2008) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater Res Bull 43:1164–1170. doi:10.1016/j.materresbull.2007.06.020
Bharde A, Wani A, Shouche Y et al (2005) Bacterial aerobic synthesis of nanocrystalline magnetite. J Am Chem Soc 127:9326–9327. doi:10.1021/ja0508469
Bindhu MR, Umadevi M (2015) Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 135:373–378. doi:10.1016/j.saa.2014.07.045
Binnig G, Rohrer H, Gerber C, Weibel E (1982) Surface studies by scanning tunneling microscopy. Phys Rev Lett 49:57–61. doi:10.1103/PhysRevLett.49.57
Brar SK, Verma M (2011) Measurement of nanoparticles by light-scattering techniques. TrAC Trends Anal Chem 30:4–17. doi:10.1016/j.trac.2010.08.008
Cao G (2004) Nanostructures and nanomaterials – synthesis, properties and applications. Imperial College Press, London
Castro ME, Cottet L, Castillo A (2014) Biosynthesis of gold nanoparticles by extracellular molecules produced by the phytopathogenic fungus Botrytis cinerea. Mater Lett 115:42–44. doi:10.1016/j.matlet.2013.10.020
Chaki SH, Malek TJ, Chaudhary MD et al (2015) Magnetite Fe3O4 nanoparticles synthesis by wet chemical reduction and their characterization. Adv Nat Sci Nanosci Nanotechnol 6:035009. doi:10.1088/2043-6262/6/3/035009
Chapman HN, Fromme P, Barty A et al (2011) Femtosecond X-ray protein nanocrystallography. Nature 470:73–77. doi:10.1038/nature09750
Chaudhry Q, Scotter M, Blackburn J et al (2008) Applications and implications of nanotechnologies for the food sector. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25:241–258. doi:10.1080/02652030701744538
Cho KH, Park JE, Osaka T, Park SG (2005) The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta 51:956–960. doi:10.1016/j.electacta.2005.04.071
Clogston JD, Patri AK (2011) Zeta potential measurement. Methods Mol Biol 697:63–70. doi:10.1007/978-1-60327-198-1_6
Cunningham DP, Lundie LL (1993) Precipitation of cadmium by Clostridium thermoaceticum. Appl Environ Microbiol 59:7–14
De Azeredo HMC (2009) Nanocomposites for food packaging applications. Food Res Int 42:1240–1253. doi:10.1016/j.foodres.2009.03.019
Dehnad A, Hamedi J, Derakhshan-Khadivi F, Abusov R (2015) Green synthesis of gold nanoparticles by a metal resistant isolated from gold mine. IEEE Trans Nanobioscience. doi:10.1109/TNB.2014.2377232
Dhandapani P, Maruthamuthu S, Rajagopal G (2012) Bio-mediated synthesis of TiO 2 nanoparticles and its photocatalytic effect on aquatic biofilm. J Photochem Photobiol B Biol 110:43–49. doi:10.1016/j.jphotobiol.2012.03.003
Dhillon GS, Brar SK, Kaur S, Verma M (2012) Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Crit Rev Biotechnol 32:49–73. doi:10.3109/07388551.2010.550568
Dubey SP, Lahtinen M, Sillanpää M (2010) Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 45:1065–1071. doi:10.1016/j.procbio.2010.03.024
Durán N, Seabra AB (2012) Metallic oxide nanoparticles: state of the art in biogenic syntheses and their mechanisms. Appl Microbiol Biotechnol 95:275–288. doi:10.1007/s00253-012-4118-9
Dwivedi AD, Gopal K (2010) Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A Physicochem Eng Asp 369:27–33. doi:10.1016/j.colsurfa.2010.07.020
Egelandsdal B, Christiansen KF, Høst V et al (1999) Evaluation of scanning electron microscopy images of a model dressing using image feature extraction techniques and principal component analysis. Scanning 21:316–325
El-Sonbaty SM (2013) Fungus-mediated synthesis of silver nanoparticles and evaluation of antitumor activity. Cancer Nanotechnol 4:73–79. doi:10.1007/s12645-013-0038-3
Evanoff DD, Chumanov G, Evanoff Jr. DD (2004) Size-controlled synthesis of nanoparticles. 2. Measurement of extinction, scattering, and absorption cross sections. J Phys Chem B 108:13957–13962. doi: 10.1021/jp0475640
Fedlheim DL, Foss CA (2001) Metal nanoparticles: synthesis, characterization, and applications. CRC Press, Boca Raton
Gan T, Sun J, Cao S et al (2012) One-step electrochemical approach for the preparation of graphene wrapped-phosphotungstic acid hybrid and its application for simultaneous determination of sunset yellow and tartrazine. Electrochim Acta 74:151–157. doi:10.1016/j.electacta.2012.04.039
Garland A (2009) The Global market for carbon nanotubes to 2015: a realistic market assessment, Nanoposts, http://www.nanoposts.com/index.php?mod=nanotubes. Accessed at 16 Oct 2009.
Gericke M, Pinches A (2006) Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132–140. doi:10.1016/j.hydromet.2006.03.019
Gholami-Shabani M, Shams-Ghahfarokhi M, Gholami-Shabani Z et al (2015) Enzymatic synthesis of gold nanoparticles using sulfite reductase purified from Escherichia coli: a green eco-friendly approach. Process Biochem 50:1076–1085. doi:10.1016/j.procbio.2015.04.004
Grouchko M, Kamyshny A, Ben-Ami K, Magdassi S (2009) Synthesis of copper nanoparticles catalyzed by pre-formed silver nanoparticles. J Nanopart Res 11:713–716. doi:10.1007/s11051-007-9324-5
He S, Guo Z, Zhang Y et al (2007) Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett 61:3984–3987. doi:10.1016/j.matlet.2007.01.018
Hebbalalu D, Lalley J, Nadagouda MN, Varma RS (2013) Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain Chem Eng 1:703–712. doi:10.1021/sc4000362
Holister P, Roman Vas C, Harper T (2003) Fullerenes: Technology white papers nr.7, Cinetifica, Ltd
Hoskote Anand KK, Mandal BK (2015) Activity study of biogenic spherical silver nanoparticles towards microbes and oxidants. Spectrochim Acta A Mol Biomol Spectrosc 135:639–645. doi:10.1016/j.saa.2014.07.013
Hyeon T (2003) Chemical synthesis of magnetic nanoparticles. Chem Commun. 927–934.
Ingle A, Gade A, Pierrat S et al (2008) Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci 4:141–144. doi:10.2174/157341308784340804
Peter J, Backialakshmi M, Karpagavinayagam P, Vedhi C (2014) Green synthesis and characterization of colloidal gold nanoparticles for optical properties. J Adv Chem Sci 1:1–5
Jaroenworaluck A, Sunsaneeyametha W, Kosachan N, Stevens R (2006) Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf Interface Anal 38:473–477. doi:10.1002/sia.2313
Jha AK, Prasad K, Kulkarni AR (2009) Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf B Biointerfaces 71:226–229. doi:10.1016/j.colsurfb.2009.02.007
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77–89. doi:10.1007/s11051-008-9446-4
Juibari MM, Abbasalizadeh S, Jouzani GS, Noruzi M (2011) Intensified biosynthesis of silver nanoparticles using a native extremophilic Ureibacillus thermosphaericus strain. Mater Lett 65:1014–1017. doi:10.1016/j.matlet.2010.12.056
Kalabegishvili TL, Kirkesali EI, Rcheulishvili AN et al (2012) Synthesis of gold nanoparticles by some strains of Arthrobacter genera. J Mater Sci Eng 2:164–173
Kasthuri J, Kathiravan K, Rajendiran N (2009) Phyllanthin-assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopart Res 11:1075–1085. doi:10.1007/s11051-008-9494-9
Kawahara K, Tsuruda K, Morishita M, Uchida M (2000) Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent Mater 16:452–455. doi:10.1016/S0109-5641(00)00050-6
Khalil MMH, Ismail EH, El-Magdoub F (2012) Biosynthesis of Au nanoparticles using olive leaf extract. Arab J Chem 5:431–437. doi:10.1016/j.arabjc.2010.11.011
Khler M, Fritzsche W (2004) Nanotechnology. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Kole C, Kole P, Randunu KM et al (2013) Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol 13:37. doi:10.1186/1472-6750-13-37
Konishi Y, Nomura T, Mizoe K (2004) A new synthesis route from spent sulfuric acid pickling solution to ferrite nanoparticles. Hydrometallurgy 74:57–65. doi:10.1016/j.hydromet.2004.01.007
Kruis FE, Maisels A, Fissan H (2000) Direct simulation Monte Carlo method for particle coagulation and aggregation. AIChE J 46:1735–1742. doi:10.1002/aic.690460905
Kumar KP, Paul W, Sharma CP (2011) Green synthesis of gold nanoparticles with Zingiber officinale extract: characterization and blood compatibility. Process Biochem 46:2007–2013. doi:10.1016/j.procbio.2011.07.011
Lagarón JM, Cabedo L, Cava D et al (2005) Improving packaged food quality and safety. Part 2: nanocomposites. Food Addit Contam 22:994–998. doi:10.1080/02652030500239656
Lang HP, Hegner M, Meyer E, Gerber C (2002) Nanomechanics from atomic resolution to molecular recognition based on atomic force microscopy technology. Nanotechnology 13:R29–R36. doi:10.1088/0957-4484/13/5/202
Lin Z, Wu J, Xue R, Yang Y (2005) Spectroscopic characterization of Au3+ biosorption by waste biomass of Saccharomyces cerevisiae. Spectrochim Acta – Part A Mol Biomol Spectrosc 61:761–765. doi:10.1016/j.saa.2004.03.029
Lisiecki I, Billoudet F, Pileni MP (1996) Control of the shape and the size of copper metallic particles. J Phys Chem 100:4160–4166. doi:10.1021/jp9523837
Lorenz C, Tiede K, Tear S et al (2010) Imaging and characterization of engineered nanoparticles in sunscreens by electron microscopy, under wet and dry conditions. Int J Occup Environ Health 16:406–428. doi:10.1179/oeh.2010.16.4.406
Mageswari A, Subramanian P, Ravindran V et al (2015) Synthesis and larvicidal activity of low-temperature stable silver nanoparticles from psychrotolerant Pseudomonas mandelii. Environ Sci Pollut Res Int 22:5383–5394. doi:10.1007/s11356-014-3735-5
Manivasagan P, Alam MS, Kang K-H et al (2015) Extracellular synthesis of gold bionanoparticles by Nocardiopsis sp. and evaluation of its antimicrobial, antioxidant and cytotoxic activities. Bioprocess Biosyst Eng. doi:10.1007/s00449-015-1358-y
Matsumura Y, Yoshikata K, Kunisaki S, Tsuchido T (2003) Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl Environ Microbiol 69:4278–4281. doi:10.1128/AEM.69.7.4278
McClements DJ, Rao J (2011) Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr 51:285–330
Merga G, Wilson R, Lynn G et al (2007) Redox catalysis on “naked” silver nanoparticles. J Phys Chem C 111:12220–12226. doi:10.1021/jp074257w
Mirzadeh S, Darezereshki E, Bakhtiari F et al (2013) Characterization of zinc sulfide (ZnS) nanoparticles biosynthesized by Fusarium oxysporum. Mater Sci Semicond Process 16:374–378. doi:10.1016/j.mssp.2012.09.008
Mohammed Fayaz A, Girilal M, Rahman M et al (2011) Biosynthesis of silver and gold nanoparticles using thermophilic bacterium Geobacillus stearothermophilus. Process Biochem 46:1958–1962. doi:10.1016/j.procbio.2011.07.003
Mohanpuria P, Rana NK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10:507–517
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10:507–517. doi:10.1007/s11051-007-9275-x
Mourato A, Gadanho M, Lino AR, Tenreiro R (2011) Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg Chem Appl 2011:546074. doi:10.1155/2011/546074
Mukherjee P, Senapati S, Mandal D et al (2002) Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chembiochem 3:461–463 doi: 10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X
Nair R, Varghese SH, Nair BG et al (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163. doi:10.1016/j.plantsci.2010.04.012
Nakamura E, Sawamura M (2001) Chemistry of η5-fullerene metal complexes. Pure Appl Chem. doi:10.1351/pac200173020355
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interf Sci 156:1–13. doi:10.1016/j.cis.2010.02.001
Narayanan KB, Sakthivel N (2011) Extracellular synthesis of silver nanoparticles using the leaf extract of Coleus amboinicus Lour. Mater Res Bull 46:1708–1713. doi:10.1016/j.materresbull.2011.05.041
Nevot M, Deroncele V, López-Iglesias C et al (2006a) Ultrastructural analysis of the extracellular matter secreted by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Microb Ecol 51:501–507. doi:10.1007/s00248-006-9065-5
Nevot M, Deroncelé V, Messner P et al (2006b) Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ Microbiol 8:1523–1533. doi:10.1111/j.1462-2920.2006.01043.x
Noruzi M (2015) Biosynthesis of gold nanoparticles using plant extracts. Bioprocess Biosyst Eng 38:1–14. doi:10.1007/s00449-014-1251-0
Noruzi M, Zare D, Davoodi D (2012) A rapid biosynthesis route for the preparation of gold nanoparticles by aqueous extract of cypress leaves at room temperature. Spectrochim Acta A Mol Biomol Spectrosc 94:84–88. doi:10.1016/j.saa.2012.03.041
Noruzi M, Zare D, Khoshnevisan K, Davoodi D (2011) Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc 79:1461–1465. doi:10.1016/j.saa.2011.05.001
Ong QK, Reguera J, Silva PJ et al (2013) High-resolution scanning tunneling microscopy characterization of mixed monolayer protected gold nanoparticles. ACS Nano 7:8529–8539. doi:10.1021/nn402414b
Oskam G (2006) Metal oxide nanoparticles: synthesis, characterization and application. J Sol-Gel Sci Technol 37:161–164. doi:10.1007/s10971-005-6621-2
Panacek A, Kvítek L, Prucek R et al (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110:16248–16253. doi:10.1021/jp063826h
Parikh RY, Ramanathan R, Coloe PJ et al (2011) Genus-wide physicochemical evidence of extracellular crystalline silver nanoparticles biosynthesis by Morganella spp. PLoS One 6:1–7. doi:10.1371/journal.pone.0021401
Parikh RY, Singh S, Prasad BLV et al (2008) Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: towards understanding biochemical synthesis mechanism. ChemBioChem 9:1415–1422. doi:10.1002/cbic.200700592
Philip D (2010) Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim Acta – Part A Mol Biomol Spectrosc 77:807–810. doi:10.1016/j.saa.2010.08.008
Prabhu YT, Venkateswara Rao K, Sesha Sai V, Pavani T (2015) A facile biosynthesis of copper nanoparticles: a micro-structural and antibacterial activity investigation. J Saudi Chem Soc. doi:10.1016/j.jscs.2015.04.002
Prakasham RS, Buddana SK, Yannam SK, Guntuku GS (2012) Characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus. J Microbiol Biotechnol 22:614–621
Rautaray D, Ahmad A, Sastry M (2003) Biosynthesis of CaCO3 crystals of complex morphology using a fungus and an actinomycete. J Am Chem Soc 125:14656–14657. doi:10.1021/ja0374877
Raveendran S, Palaninathan V, Nagaoka Y et al (2015) Extremophilic polysaccharide nanoparticles for cancer nanotherapy and evaluation of antioxidant properties. Int J Biol Macromol 76:310–319. doi:10.1016/j.ijbiomac.2015.03.001
Ray SS (2013) Environmentally friendly polymer nanocomposites: types, processing and properties. Elsevier
Rothschild A, Cohen SR, Tenne R (1999) WS2 nanotubes as tips in scanning probe microscopy. Appl Phys Lett 75:4025. doi:10.1063/1.125526
Sadeghi B, Gholamhoseinpoor F (2015) A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc 134:310–315. doi:10.1016/j.saa.2014.06.046
Sadeghi B, Rostami A, Momeni SS (2015) Facile green synthesis of silver nanoparticles using seed aqueous extract of Pistacia atlantica and its antibacterial activity. Spectrochim Acta – Part A Mol Biomol Spectrosc 134:326–332. doi:10.1016/j.saa.2014.05.078
Salunke BK, Sawant SS, Lee S-I, Kim BS (2015) Comparative study of MnO2 nanoparticle synthesis by marine bacterium Saccharophagus degradans and yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 99:5419–5427. doi:10.1007/s00253-015-6559-4
Sarid D (1991) Review of scanning force microscopy. J Vac Sci Technol B Microelectron Nanom Struct 9:431. doi:10.1116/1.585585
Sarid D, Coratger R (1991) Scanning force microscopy-with applications to electric, magnetic and atomic forces. Oxford University Press, New York
Sastry M, Ahmad A, Islam Khan M, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci 85:162–170
Schmid G (2004) Nanoparticles: from theory to application. Wiley-VCH, Weinheim
Schneider JJ (1997) Nanomaterials: synthesis, properties and applications. IOP Publishing, Bristol
Sekhon BS (2014) Nanotechnology in agri-food production: an overview. Nanotechnol Sci Appl 7:31–53. doi:10.2147/NSA.S39406
Senapati S, Ahmad A, Khan MI et al (2005) Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 1:517–520. doi:10.1002/smll.200400053
Senturk A, Yalcin B, Otles S (2013) Nanotechnology as a food perspective. J Nanomater Mol Nanotechnol 2013(2):6–13. doi:10.4172/2324-8777.1000125
Shankar SS, Rai A, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275:496–502. doi:10.1016/j.jcis.2004.03.003
Sharma N, Pinnaka AK, Raje M et al (2012) Exploitation of marine bacteria for production of gold nanoparticles. Microb Cell Factories 11:86. doi:10.1186/1475-2859-11-86
Shivaji S, Madhu S, Singh S (2011) Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem 46:1800–1807. doi:10.1016/j.procbio.2011.06.008
Siegel RW (1993) Synthesis and properties of nanophase materials. Mater Sci Eng A 168:189–197. doi:10.1016/0921-5093(93)90726-U
Silvestre C, Cimmino S (eds) (2013) Ecosustainable polymer nanomaterials for food packaging: innovative solutions, characterization needs, safety and environmental issues. CRC Press, Boca Raton
Singh R, Shedbalkar UU, Wadhwani SA, Chopade BA (2015) Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl Microbiol Biotechnol 99:4579–4593. doi:10.1007/s00253-015-6622-1
Sintubin L, De Windt W, Dick J et al (2009) Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol 84:741–749. doi:10.1007/s00253-009-2032-6
Slawson RM, Van Dyke MI, Lee H, Trevors JT (1992) Germanium and silver resistance, accumulation, and toxicity in microorganisms. Plasmid 27:72–79. doi:10.1016/0147-619X(92)90008-X
Song JY, Jang HK, Kim BS (2009) Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem 44:1133–1138. doi:10.1016/j.procbio.2009.06.005
Srivastava P, Bragança J, Ramanan SR, Kowshik M (2013) Synthesis of silver nanoparticles using haloarchaeal isolate Halococcus salifodinae BK3. Extremophiles 17:821–831. doi:10.1007/s00792-013-0563-3
Suresh AK, Pelletier DA, Wang W et al (2011) Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater 7:2148–2152. doi:10.1016/j.actbio.2011.01.023
Sweeney RY, Mao, Chuanbin XG, Justin L. Burt AMB (1998) Bacterial biosynthesis of cadmium sulfide nanocrystals. Development 125:2457–2467. doi: 10.1016/j.chembiol .2004.08.022
Sweet MJ, Chesser A, Singleton I (2012) Review: metal-based nanoparticles; size, function, and areas for advancement in applied microbiology. Adv App Microbiol 80:113
Syed A, Ahmad A (2012) Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B Biointerfaces 97:27–31. doi:10.1016/j.colsurfb.2012.03.026
Tagmatarchis N, Shinohara H (2001) Fullerenes in medicinal chemistry and their biological applications. Mini Rev Med Chem 1:339–348
Tantra R, Schulze P, Quincey P (2010) Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 8:279–285. doi:10.1016/j.partic.2010.01.003
Ulug B, Haluk Turkdemir M, Cicek A, Mete A (2015) Role of irradiation in the green synthesis of silver nanoparticles mediated by fig (Ficus carica) leaf extract. Spectrochim Acta A Mol Biomol Spectrosc 135:153–161. doi:10.1016/j.saa.2014.06.142
Umer A, Naveed S, Ramzan N (2012) Selection of a suitable method for the synthesis of copper nanoparticles. NANO: Brief Rep Rev 7:1230005–1230020
Veerasamy R, Xin TZ, Gunasagaran S et al (2011) Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 15:113–120. doi:10.1016/j.jscs.2010.06.004
Vigneshwaran N, Ashtaputre NM, Varadarajan PV et al (2007) Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett 61:1413–1418. doi:10.1016/j.matlet.2006.07.042
Vigneshwaran N, Kumar S, AA K et al (2006) Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology 17:5087–5095. doi:10.1088/0957-4484/17/20/008
Vogelson CT (2001) Modern drug discovery, 4th edn. Advances in drug delivery systems, pp 49–52
Watson JHP, Ellwood DC, Soper AK, Charnock J (1999) Nanosized strongly-magnetic bacterially-produced iron sulfide materials. J Magn Magn Mater 203:69–72 doi: http://dx.doi.org/10.1016/S0304-8853(99)00191-2
West AR (2007) Solid state chemistry and its applications. Wiley India Pvt. Limited
Wiesendanger R (1994) Scanning probe microscopy and spectroscopy. Cambridge University Press, Cambridge, p. 637
Wiley B, Sun Y, Mayers B, Xia Y (2005) Shape-controlled synthesis of metal nanostructures: the case of silver. Chem –A Eur J 11:454–463. doi:10.1002/chem.200400927
Wittstock G (2001) Z.L. Wang (ed): characterization of nanophase materials. J Solid State Electrochem 5:367–368. doi:10.1007/s100080000145
Xiong J, Wang Y, Xue Q, Wu X (2011) Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem 13:900. doi:10.1039/c0gc00772b
Yang Y, Gupta MC, Dudley KL (2007) Studies on electromagnetic interference shielding characteristics of metal nanoparticle- and carbon nanostructure-filled polymer composites in the Ku-band frequency. Micro Nano Lett 2:85–89. doi:10.1049/mnl:20070042
Yu CH, Tam K, Tsang ESC (2009) Chemical methods for preparation of nanoparticles in solution. Handb Met Phys 5:113–141
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mageswari, A., Srinivasan, R., Subramanian, P., Ramesh, N., Gothandam, K.M. (2016). Nanomaterials: Classification, Biological Synthesis and Characterization. In: Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Nanoscience in Food and Agriculture 3. Sustainable Agriculture Reviews, vol 23. Springer, Cham. https://doi.org/10.1007/978-3-319-48009-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-48009-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48008-4
Online ISBN: 978-3-319-48009-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)