Abstract
The amount of experimental studies on the toxicity of nanomaterials is growing fast. Interpretation and comparison of these studies is a complex issue due to the high amount of variables possibly determining the toxicity of nanomaterials.
Qualitative databases providing a structured combination, integration and quality evaluation of the existing data could reveal insights that cannot be seen from different studies alone. A few database initiatives are under development but in practice very little data is publicly available and collaboration between physicists, toxicologists, computer scientists and modellers is needed to further develop databases, standards and analysis tools.
In this case study the process of building a database on the in vitro toxicity of amorphous silica nanoparticles (NPs) is described in detail. Experimental data were systematically collected from peer reviewed papers, manually curated and stored in a standardised format. The result is a database in ISA-Tab-Nano including 68 peer reviewed papers on the toxicity of 148 amorphous silica NPs. Both the physicochemical characterization of the particles and their biological effect (described in 230 in vitro assays) were stored in the database. A scoring system was elaborated in order to evaluate the reliability of the stored data.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanotechnology results in the presence of a variety of different engineered NPs (NPs) in our environment. Due to their small size NPs behave differently from their larger counterparts of the same composition. Therefore adjusted safety assessment (hazard identification, hazard characterisation and exposure assessment) is needed. The last 10 years hazard identification and characterisation has mainly focused on finding the physicochemical properties of NPs that determine their interaction with biological systems and the underlying pathways causing these interactions, resulting in a large number of experimental data published. Drawing conclusions from these data is however difficult because of the large amount of variables possibly determining the toxicity outcome. Variables are associated with the nanomaterial itself and the exposure conditions, the biological test system (in vitro/in vivo) and the toxicological assay.
A structured combination and integration of the existing data could reveal variables which are important determinants of NPs toxicity and can eventually lead to predictive models and QSARs for nanotoxicity. Database initiatives relevant for nanotoxicology are under development: CaNanoLab [1], the Nanomaterial Registry [2], the NP Information Library [3] and the Nanomaterial-Biological Interactions Knowledge Base [4]. In practice very few data is publicly available and collaboration between physicists, toxicologists, computer scientists and modellers is needed to further develop databases, standards and analysis tools.
This case-study describes the construction of a database on the in vitro toxicity of amorphous silica NPs (including particles with a silica shell). A search of PubMed was performed to collect 68 peer-reviewed papers which were manually curated. Both the physicochemical characteristics of the NPs and their interaction with cellular systems were stored in an ISA-Tab-Nano compatible format. To assess the reliability of the stored data a scoring system was elaborated: variables associated with the nanomaterials, toxicity assay and biological system were scored to assess the reliability of the data.
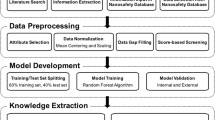
First the process of data collection (including criteria and literature search) (11.2.1), data storage (11.2.2) and data evaluation (11.2.3) is described in order to build a qualitative database. In paragraph 5 the database itself is described: the articles, in vitro assays and NPs. And some results of the reliability scoring system are presented. In paragraph 6 several issues that came up during the construction of the database and some future suggestions to overcome these issues are discussed.
This project is a subproject of the “Modelling Assays Platform “MAP” for hazard ranking of engineered metal-based NPs (MOD-ENP-TOX) ” project of the Seventh Framework Program (FP7) funded by the European Union.
2 Construction of the Database
2.1 Data Collection
2.1.1 Including Criteria
Data were collected from peer-reviewed papers which investigate the in vitro toxicity of amorphous silica NPs. This type of nanomaterial is well studied; a preliminary search gave more than 600 hits. And experiments have shown that different silica NPs, although it is one chemical identity, can give different biological effects [5].
Selected papers:
-
1.
study the toxicity of amorphous silica particles with a defined shape (aspect ratio <3), composition (all silica or a silica shell), crystallinity (amorphous) and primary size;
-
2.
study the effect of the particles on cell lines or primary cells (experimental in vitro studies),
-
3.
and include information on cell viability, apoptosis/necrosis, genotoxicity, oxidative stress and pro-inflammation.
The dose of NPs effectively reaching the cells depends on the exposure route/method (dry state, suspension or aerosol). In order to avoid dose-response curves which are difficult to compare, we only included experimental studies that administer particles to the cell in suspension.
2.1.2 Literature Search
A sensitive search strategy, to retrieve as many relevant papers as possible, was performed using PubMed. Searching “amorphous silica OR silica NPs AND toxicity NOT review” with a filter of 10 years and full text availability gave 624 hits (13th of May 2014).
Secondly the 624 papers were sorted by relevance. The relevance sort option is based on an algorithm that analyses each PubMed citation that includes the search terms. A “weight” is calculated for citations depending on how many search terms are found and in which fields they are found. In addition, recently-published articles are given a somewhat higher weight for sorting [6]. Only the titles of the 450 most relevant papers were further screened for retrieval (cfr. including criteria 11.2.1.1). The 117 retrieved articles underwent a second evaluation for which the including criteria were applied to the full article. Another 47 papers were excluded because they studied a different population (ecotoxicity study (n = 1), in vivo study (n = 5)), different intervention (used functionalised silica particles (n = 13), badly characterised particles (n = 15), crystalline particles (n = 1), microscale particles (n = 3), particles in dry state (n = 1)) and/or a different outcome (n = 6). Also one paper written in Chinese and three papers with no information on the statistics were excluded. Eventually 68 papers were retrieved for the construction of the database.
The selection procedure is depicted in Fig. 11.1.
2.2 Data Storage
Data are stored in ISA-Tab-Nano; an emerging standard format for sharing nanomaterial research data. The format supports the use of ontology terms to promote standardized descriptions, and facilitate search and integration of data. Four types of files are provided to store different types of data:
-
1.
The investigation file contains descriptive information (principle investigators, sponsor, link to full text paper,…) which lays the foundation for the other ISA-Tab-Nano files and links them together.
-
2.
The material file describes the materials used; nanomaterials but also other materials such as positive controls.
-
3.
The study file describes how samples (material and biological samples) are prepared for analysis (physicochemical, in vitro and in vivo characterisation).
-
4.
The assay file is designed to store the measured endpoints of the physicochemical, in vitro or in vivo characterisation of the nanomaterials [7].
Figure 11.2 depicts how the ISA-Tab-Nano files are linked to each other.
ISA-Tab-Nano recommends using the material file only to store the nominal characteristics and chemical composition of the NPs. The experimentally measured physicochemical characteristics are stored in the assay files. We chose to store all the physicochemical characteristics in the material file to make analysis and data integration more convenient afterwards in perspective of the MOD-ENP-TOX project.
The different fieldnames used in the material, investigation, assay and study file can be found in Table 11.1. The data of each article is stored in a set of these four types of files.
2.3 Data Reliability Evaluation
Klimisch et al. define the reliability of data as an evaluation of the inherent quality of a test report or publication relating to preferably standardized methodology and the way the experimental procedure and results are described to give evidence of the clarity and plausibility of the findings [8].
General guidelines on reliability assessment of toxicological data are described in literature. The following is a selected overview of the existing schemes relevant for the data reliability assessment within the MOD-ENP-TOX project. Both general schemes designed for the reliability assessment of chemicals and more specific schemes for nanomaterials are discussed (11.2.3.1). Afterwards a scheme is proposed to assess the reliability of the data in our database (11.2.3.2).
2.3.1 Schemes for Assessment of the Reliability of Toxicological Data
Klimisch et al. published a categorisation scheme to assign toxicological data of chemicals to one of four reliability categories: reliable without restrictions, reliable with restrictions, not reliable or not assignable. Distinction between these reliability categories are based on the amount of information provided on the testing procedure and analysis. Tests conducted and reported in accordance to international standards have the highest grade of reliability [8]. Unfortunately, the differentiation between reliability classes is not always clear. To make the decision process of assigning reliability categories more transparent and harmonised the ‘ToxRTool’ (Toxicological data Reliability Assessment Tool – [9]) was developed. The tool provides a detailed list of yes-no questions about the identification of the test substance, characterization of the biological system, description of the study design, documentation of the study results and the plausibility of the study design and data [10]. The answers to the yes-no questions are used to attribute the data to one of the Klimisch categories.
The evaluation criteria for toxicity need to be reconsidered for toxicological data of nanomaterials, taking into account the following complications:
-
1.
The toxicity of nanomaterials not only depends on the dose and the composition but also on the size, shape, specific surface area, surface coating, porosity, surface charge and the solubility of the nanomaterials [2, 11].
-
2.
No international standardized test procedures exist for both the physicochemical characterisation of the nanomaterials and their toxicity assessment. The OECD initiated a large programme on the safety of manufactured nanomaterials which will result in a set of guidelines supporting standardization [12]. Guidelines already published are: ‘Report of the OECD expert meeting on the physical chemical properties of manufactured nanomaterials and test guidelines’, ‘Guidance on Sample Preparation and Dosimetry for the Safety Testing of Manufactured Nanomaterials’,…
-
3.
Nanomaterials can interfere with toxicity assays resulting in false positive and false negative results [13, 14].
The Nanomaterial Registry provides a metric, the compliance level (CL), of the quality and quantity of characterization for each nanomaterial entry. In order to be compliant a nanomaterial characterization should include: the synthesis method or processing details, the DOI citation of the synthesis procedure, the manufacturer or synthesis laboratory name, the product name and lot number and the nanomaterial’s physical state. For each measurement the following should be reported: the technique, technique protocols and parameters and the best practice information. Compliance levels are only developed for data associated with the nanomaterial, not for the data on the interactions with biological systems [2].
Lubinski et al. published a scheme to assess the quality of nanotoxicity data in the context of developing QSPR’s/QSAR’s. A checklist of yes-no questions supports the user to assign data to one of five reliability classes. The checklist comprises questions related to the extent to which the nanomaterials were characterised, the degree to which the experimental assays and methods were described and the use of standardised protocols [15].
2.3.2 Scheme for Reliability Assessment of In Vitro Nanotoxicity Data
We developed a scoring system to assess the reliability of in vitro nanotoxicity data extracted from papers. Reliability scoring is based on the amount of information available on the different variables influencing in vitro toxicity outcome of the nanomaterials (which depends on the way of reporting, amount of detail given) and on how this information was obtained (methodology). The variables influencing in vitro nanotoxicity were recently listed by Krug [16]. He makes a differentiation between variables associated with the nanomaterial, with the toxicity assay and the biological system. The following is the list of variables copied from Krug and a proposition for a scoring system to assess the reliability of data of in vitro nanotoxicity data.
In the end a score will be assigned to each particle, assay and test system to give an indication of the reliability of the data.
Variables associated with the nanomaterial:
-
A.
sample purification for the removal of biologically relevant trace elements
-
B.
sample characterization of the raw material: composition and purity size shape agglomeration status etc.
-
C.
sample characterization regarding biological impurities: endotoxins etc.
-
D.
dispersion in biological media under relevant conditions: temperature humidity gas concentrations (O2, CO2) salinity etc.
-
E.
sample characterization in biological media: size and shape agglomeration status protein corona etc.
-
F.
the measurement device used for the characterisation (not listed by Krug)
In Table 11.2 is a concrete list of variables associated with the nanomaterials that should be reported because they influence the toxicity outcome. The more variables specified in the paper the more reliable the data.
Variables associated with the toxicity assay:
-
A.
selection of the correct test system regarding the biological endpoints
-
B.
different test systems for the same biological endpoint
-
C.
controls: adapted negative controls adapted positive controls comparison to reference materials
-
D.
testing of possible interferences of the NP with the biological test system binding of indicator molecules light absorption or fluorescence of the materials etc.
-
E.
not considered measurement uncertainty: round robins, calibration with standards or reference material
The following table (Table 11.3) is a concrete list of variables associated with the toxicity assay that should be reported because they influence the toxicity outcome.
Variables associated with the biological system:
-
A.
selection of the biological system
-
B.
cell lines: selection criteria identification age and storage number of passages etc.
-
C.
primary cells/organ systems: donor dependency donor variability culture conditions
-
D.
culture conditions during the experiments: temperature humidity gas concentrations (O2, CO2) salinity etc.
-
E.
biological parameter: cell density volume of the medium serum content of the medium compatibility of the solvent or dispersion medium
The following scoring system (Table 11.4) was elaborated for each biological system:
3 Description of the Database
3.1 The Articles
The 68 articles used to construct the database were from 35 different journals. The most represented journals were “Nanotoxicology” (9 papers), “Toxicology in vitro” (6 papers) “Tox Letters” (4 papers), and “Tox Sciences” (4 papers). Other high ranked journals e.g. “Particle & Fibre Toxicology” had a surprisingly low success rate (2 papers). This can be due to the fact that these higher ranked journals are less tolerant to publish studies with insufficient physicochemical data on the NPs or have a broader scope.
3.2 The In Vitro Assays
Articles are stored in ISA-Tab-Nano describing 148 different silica NPs (or NPs with a silica shell) and their biological impact in 230 different in vitro assays. Fourty three percent of the assays investigated viability, 16 percent apoptosis/necrosis, 26 percent oxidative stress, 10 percent pro-inflammation and 6 per cent genotoxicity (Fig. 11.3).
The different types of assays per type of toxicological endpoint represented in the database are listed in Table 11.5:
3.3 The Particles
One hundred and fourty eight particles are stored in the database. The characteristics stored in the database are believed to be important determinants of nanotoxicity: chemical composition, primary size, primary size distribution, shape, crystallinity, solubility, surface charge, surface area, and agglomeration/aggregation. Table 11.6 gives an overview of these characteristics, how these were measured and the frequency of these measurements in the database. As a preposition for an article to be accepted for storage in the database was the characterisation of the primary size, shape, composition and crystallinity all the 148 particles have these characteristics measured (relative frequency is 1).
Few simplifications were made to make that data more homogenous and comparable:
-
1.
diameter ranges were converted to a mean diameter by taking the arrhythmic mean of the upper and lower limit of the range
-
2.
for NPs with a bimodal size distribution only the smallest mean diameter was taken into account for analysis
-
3.
for ellipsoidal/cylindrical particles the average of the Feret min diameter and Feret max diameter is used as the mean diameter
3.3.1 Chemical Composition
All particles are made of amorphous silica or have an amorphous silica shell. The core of 15 particles is not pure silica but contains Fe3O4 (2), Fe3O4/Fe2O3 (2), dansylamide (1), rhodamine (7) or redF (3). Only eight particles have a coating: BSA (6), ethylene (1) and Al2O3 (1).
Of the 24 particles that were described in purity; 18 particles were said to have purity higher than 98 % or higher, the others were just mentioned to be pure.
The material synthesis is important for the composition of nanomaterials, especially the surface chemistry of the silica particles. The density of silanol groups on the particle surface depends on the temperature. At high temperature which is for example needed to make pyrogenic silica there is a dehydration of the silanol groups on the surface area resulting in a lower density of silanol groups on the surface and a possibly different reaction with the environment [17].
For 97 of the particles the way they were synthesised was specified in the article (Fig. 11.4): 36 % of these particles were made by the Stöber process, 16 % were ludox® silica, 16 % made by the reverse microemulsion process, 14 % is pyrogenic silica, 7 % precipitated.
3.3.2 Primary Size
The frequency distribution of the mean diameter of the particles is depicted in Fig. 11.5. For only 57 % of the particles a standard deviation is reported.
3.3.3 Shape
55.4 percent of the particles are spherical, 35.1 % polyhedral, 0.7 % cylindrical, 5.4 % irregular and 3.4 ellipsoidal. The particles were described with these terms in the article or the T/S EM pictures of the particles were used to assign the particles to one of these shape classes.
For the other measured characteristics there were too many missing values, it does not seem usefull to report any descriptive statistics on these.
3.4 Data Evaluation
3.4.1 Evaluation Variables Associated with Nanomaterials
The scoring scheme developed in 11.2.3.2 to evaluate variables associated with nanomaterials was filled out for each amorphous silica NP (Table 11.2). Figure 11.6 is a frequency table of the “Score characterisation” of the 148 amorphous silica nanomaterials. Articles were allowed in the database under the condition that the composition of the core and shell, the composition of the coating, the shape, the crystallinity and the primary size of the particles were known. Therefore, no characterisation scores less than five were observed. None of the particles has a maximum characterisation score of 14.
The percentage of characteristics for which the measurement device was specified is calculated for each particle (“Score measurement device”/“Score characterisation”*100 - Fig. 11.7).
The average of this calculation for all the NPs was only 45 % implying that in more than 50 % of the reported characteristics the method used was not clearly specified.
3.4.2 Evaluation Variables Associated with the Toxicity Assay and Test System
The two scoring schemes developed in to evaluate variables associated with the toxicity assay and test system were filled out for the MTT reduction assays (30 assays). Figure 11.8 are the frequency tables of the “Score toxicity assay” and the “Score test system” of the 30 MTT reduction assays included in the database.
4 Discussion
This case study describes the construction of an ISA-Tab-Nano formatted database, collecting data of in vitro toxicological studies published in peer reviewed journals. In total after triaging the data of 68 articles were included (starting from more than 600 hits). The characteristics (physicochemical and biological) of 148 different amorphous silica NPs are described in the database.
Several issues and according needs came up during construction of the database:
4.1 Purpose Made Materials vs Commercial (Standard/Benchmark) Materials
Many different particles were identified (although only amorphous silica NPs were included) and not much overlap in the use of nanomaterials was found. This is due to the fact that several silica NPs were “in house made” (or purpose made) and therefore only used in one experiment/study. On one hand this increases the scope of the database but on the other also increases the amount of data gaps and reduces the amount of overlap in the database hindering to create models generating data with high confidence. The use of a benchmark material would significantly increase the number of overlapping data points and make comparison between experiments, researchers, and particles possible.
4.2 Quality Evaluation of the Existing Data
In search of the critical properties that determine the toxicity of NPs, numerous investigations have been undertaken. Despite these efforts our knowledge on the possible hazardous effects of nanotechnology and its applications lags far behind the progress of nanotechnology. This is, mainly in the early years of nanotoxicology, caused by toxicity testing without appropriate material characterization and the lack of standardized dispersion and experimental protocols. Researchers underestimated the complexity of nanomaterials and experiments were set-up in similar ways as with chemicals. Inadequate characterisation made it impossible to link nanomaterials’ characteristics to their biological effect. Likewise, the question whether the existing (biological) assays for chemicals were appropriate for nanomaterials was not sufficiently addressed. Lately a lot of attention went to the importance of physicochemical characterisation of nanomaterials. Papers were published on minimal information criteria [2, 18]. Still little attention went to the validation of the (biological) assays for testing nanomaterials. Now, most researchers are aware of the importance of a detailed characterisation of both the nanomaterials and the toxicity assays, and the validation of the methods used to characterise them. The scientific community has started to fill the data and knowledge gaps.
This awareness has also initiated the search for good and workable quality criteria to assess the reliability of the already published toxicological data. The criteria used in our database are not integrated into the final database in ISA-Tab-Nano since these are not solely objective data but are the result of unavoidable subjective expert judgment. Researcher using the database can easily implement his/her own quality judgment to the database before applying it for modelling purposes.
4.3 Standardized Formats
Finally, it can be recommended that all researchers should deliver the data presented in a scientific paper as an annex in ISA-Tab-Nano format. Now we have curated manually 68 papers, which is a tedious job, prone on reporting errors. The process of curation of data from a scientific paper includes extracting data from text (in which often not all measured data are given), figures (the small scale does often not allow precise extraction of numeral data, and smoothing of curves does not allow correct extraction), and tables (providing in general the most detailed data). Not only the different sections (text vs tables and figures) deliver different quality of data, also the fact that most experiments, although repeated several times (n-value of repeated measures) are reported as one mean value (± SD); this data is still valuable in a database but it would be better to collect the data per experimental run. Therefore, if researchers are encouraged to deliver – together with a published paper/report – a small ISA-TAB Nano compatible database, this would deliver more useful details into the database and would reduce significantly the number of ‘human’ errors.
The current database will be made available to the other researchers within other modelling projects and with research institutes such as JRC in Italy. The database will be used in the MOD-TOX-ENP project, where algorithm(s) will be developed to identify those physicochemical properties determining the hazardous effects of NPs.
This study describes the construction of an ISA-Tab-Nano formatted database, collecting data of in vitro toxicological studies published in peer reviewed journals. The study revealed some issues and future needs:
-
Lately a lot of attention went to the importance of physicochemical characterisation of nanomaterials, to make the link between materials’ characteristics and biological effect. This should not result in a loss of quality of the biological characterization, including interference verification, of the nanomaterial.
-
Due to a general lack of good reference/benchmark data in publications it remains difficulty in comparing and combining data of different peer reviewed papers into a databases.
-
Standardised database formats will improve the exchange and integration of data – here we used ISA-Tab-Nano format.
-
It can be recommended that all researchers should deliver, as an annex to any publication, the data presented in ISA-Tab-Nano format (or another standard format).
References
caNanoLab [Internet]. Cited 6 Aug 2015. Available from: https://cananolab.nci.nih.gov/caNanoLab/#/
Mills KC, Murry D, Guzan KA, Ostraat ML (2014) Nanomaterial registry: database that captures the minimal information about nanomaterial physico-chemical characteristics. J Nanopart Res 16(2):1–9
NIOSH topic: nanotechnology : nanoparticle information library | CDC/NIOSH [Internet]. Cited 6 Aug 2015. Available from: http://www.nanoparticlelibrary.net/
NBI Knowlegebase [Internet]. Cited 6 Aug 2015. Available from: http://nbi.oregonstate.edu/analysis.php
Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH (2010) The nanosilica hazard: another variable entity. Part Fibre Toxicol 7(1):39
PubMed relevance sort [Internet]. Cited 1 Sep 2015. Available from: http://www.nlm.nih.gov/pubs/techbull/so13/so13_pm_relevance.html
Thomas DG, Gaheen S, Harper SL, Fritts M, Klaessig F, Hahn-Dantona E et al (2013) ISA-TAB-Nano: a specification for sharing nanomaterial research data in spreadsheet-based format. BMC Biotechnol 13(1):2
Klimisch H-J, Andreae M, Tillmann U (1997) A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol 25(1):1–5
ToxRTool – Toxicological data Reliability Assessment Tool — EURL ECVAM [Internet]. Cited 31 Jul 2015. Available from: https://eurl-ecvam.jrc.ec.europa.eu/about-ecvam/archive-publications/toxrtool
Schneider K, Schwarz M, Burkholder I, Kopp-Schneider A, Edler L, Kinsner-Ovaskainen A et al (2009) “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol Lett 189(2):138–144
Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K et al (2005) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol 2(1):8
Publications in the series on the safety of manufactured nanomaterials – OECD [Internet]. Cited 2015 Aug 7. Available from: http://www.oecd.org/science/nanosafety/publicationsintheseriesonthesafetyofmanufacturednanomaterials.htm
Monteiro-Riviere NA, Inman AO, Zhang LW (2009) Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol Appl Pharmacol 234(2):222–235
Wörle-Knirsch JM, Pulskamp K, Krug HF (2006) Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett 6(6):1261–1268
Lubinski L, Urbaszek P, Gajewicz A, Cronin MTD, Enoch SJ, Madden JC et al (2013) Evaluation criteria for the quality of published experimental data on nanomaterials and their usefulness for QSAR modelling. SAR QSAR Environ Res 24(12):995–1008
Krug HF (2014) Nanosafety research—are we on the right track? Angew Chem Int Ed 53(46):12304–12319
Vansant EF, Voort PVD, Vrancken KC (1995) Characterization and chemical modification of the silica surface. Elsevier, Amsterdam/New York, p. 573
Luyts K, Napierska D, Nemery B, Hoet PHM (2013) How physico-chemical characteristics of nanoparticles cause their toxicity: complex and unresolved interrelations. Environ Sci Process Impacts 15(1):23–38
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Vriens, H., Mertens, D., Regret, R., Lin, P., Locquet, JP., Hoet, P. (2017). Case Study III: The Construction of a Nanotoxicity Database – The MOD-ENP-TOX Experience. In: Tran, L., Bañares, M., Rallo, R. (eds) Modelling the Toxicity of Nanoparticles. Advances in Experimental Medicine and Biology, vol 947. Springer, Cham. https://doi.org/10.1007/978-3-319-47754-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-47754-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47752-7
Online ISBN: 978-3-319-47754-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)