Abstract
This review focuses on megakaryocyte development, regulation of platelet production, and the role of thrombopoietin (TPO) in relation to thrombocytopenia and correction of thrombocytopenia in immune thrombocytopenia (ITP). It appears that molecules (antibodies) and cells (T cells) that attack platelets are also directed against megakaryocytes and, thus, ITP is characterized by not only by accelerated platelet destruction but also by inhibition of platelet production. There is also a relative TPO deficiency, meaning that correction can often be achieved by administration of TPO receptor agonists. Current understanding of the pathology of ITP is extremely complicated. It seems that T helper cells (CD4) are required, even to make antibodies to platelets. However, initial studies of plasma infusion from ITP patients into normal subjects suggested that, in 10 of 26 cases, infusion of such plasma does not result in thrombocytopenia. It is possible that, in some of these outliers, all the antiplatelet antibody is on the platelets themselves and there is no free antibody in the plasma. However, clinical experience indicates that cytotoxic and other T cells are heavily involved and that there are almost no functions (receptors, cell to cell interactions, cytokine releases) of T cells that have not been demonstrated to be abnormal in ITP. Several studies with the beta T cell receptor, demonstrating important olioclonal and clonal abnormalities, have reinforced this.
Treatment of ITP remains very complicated and uncertain after a course of steroids (even there, the choice of dexamethasone versus prednisone has not been settled). Rituximab-based treatment and use of TPO receptor agonists have strong studies favoring their use, but other studies show limited likelihood of a good response in patients. It is easier to increase the platelet count in the short term than to achieve the “Holy Grail” of a cure.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Mycophenolate Mofetil
- Immune Thrombocytopenia
- Platelet Production
- Antiplatelet Antibody
- Reticulin Fibrosis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Definition of ITP
ITP initially stood for idiopathic thrombocytopenic purpura. After being called immune thrombocytopenic purpura as a result of the studies by Shulman et al. (1965), ITP now stands for immune thrombocytopenia. The purpura was dropped when it was recognized that some thrombocytopenic patients did not have manifestations of bleeding. Other abbreviations included AITP (autoimmune thrombocytopenic purpura).
The current definition (unlike the initial one) excludes non-immune thrombocytopenias such as the inherited thrombocytopenias. Although the concept of autoimmune (autoantibody-mediated) thrombocytopenia is very clear and unanimously agreed upon, ITP remains a diagnosis of exclusion because of the lack of utility of platelet antibody testing. This is complicated because, for example, the only way to know that an apparent case of ITP is primary and not, for example, secondary to hepatitis C, CMV, H. Pylori, CVID, or CLL is to run tests to exclude these possibilities. However, such extensive testing is far from the standard of care. Which epidemiologic, clinical, or laboratory features to use as the basis for performing certain additional tests has never been thoroughly explored or validated.
The only way to confirm ITP positively is by a good response to ITP-specific therapy. The classic example is a dramatic but transient overnight platelet increase in response to intravenous gamma globulin (IVIG), but even a rapid and substantial response to steroids is very suggestive of ITP. These responses, if present, are believed to be useful if there is a response but do not distinguish primary from secondary ITP.
At present, there is clear consensus that there is little benefit of anti-platelet antibody testing to include or exclude the diagnosis of ITP. In the future, if preliminary findings are confirmed and the consensus shifts, then distinction of types of ITP between those with predominant anti-platelet antibodies directed against GPIb-IX-V and those with antibodies primarily directed against αIIbβ3 may become clinically important; or those with antibodies compared with those lacking antibodies.
Platelet Production and Regulation of Thrombopoietin Levels
Megakaryocyte development is driven by many different transcription factors (as shown in Fig. 1), a process further complicated by migration of megakaryocyte progenitors, which start in the stem cell niche and must get to the vascular niche. Migration is dependent on SDF1α and relies on collagen, von Willebrand factor, and fibrinogen interacting with the platelet and megakaryocyte counter-receptors GPIb-IX-V and αIIbβ3. Once the pro-platelets are extended into the blood vessel, shear forces from blood flow cause them to fragment off (see Italiano (2017) for more details). Even if they are still platelet aggregates, they become single platelets after they arrive at the pulmonary capillaries.
Megakaryocyte (MK) maturation and platelet development in ITP. Differentiation of megakaryocytes and mechanism of platelet release, with incomplete listing of key mediators. This figure is adapted from Pecci A, Balduini CL (2014) Lessons in platelet production from inherited thrombocytopenias. Br J Haematol 165(2):179–92
Traditionally, antibodies to platelets have been thought to be directed to αIIbβ3 and GPIb-IX-V. However, an interesting feature is that the Ashwell–Morell receptor in hepatocytes might play a role in platelet regulation. Antibodies to GPIb-IX-V can cause externalization of neuraminidase, which then desialylates GPIb on platelets. These desialylated platelets, like aged platelets, are cleared in the liver by interaction with the Ashwell–Morell receptor. Data from Karen Hoffmeister’s group suggests that, via a receptor that is similar to that of interleukin(IL)-6, these platelets then stimulate production of thrombopoietin (TPO) via the JAK2–STAT3 signaling cascade (Grozovsky et al. 2015). However, whether this happens rarely, all the time, or somewhere in between remains to be clarified (Fig. 2).
There is no correlation between platelet counts and TPO levels. Instead, the TPO levels seem to depend on the numbers of megakaryocytes in the bone marrow (Fig. 3). This is similar to the regulation of granulocyte colony-stimulating factor but not erythropoietin levels (Corbacioglu et al. 2000). Thrombocytopenic patients with aplastic anemia have very high TPO levels, whereas patients with ITP and very low platelet counts have only slightly elevated or normal circulating TPO (Kuter et al. 1994). This is the so-called Kuter hypothesis. What is less clear are the mechanisms of regulation, up or down, of TPO production. Current studies suggest that the role of inflammation is mediated primarily by IL-6 in the liver.
Kuter hypothesis of TPO regulation. The role of platelet count and marrow megakaryocyte number in determining thrombopoietin level: low platelet count in ITP (in which there are ample numbers of megakaryocytes in the marrow) is consistent with TPO binding to TPO receptors and thus a reduction in the level of TPO to normal or near normal. Adapted from Kosugi S et al (2003) Br J Haematol 93:704–706; Aledort LM et al (2004) Am J Hematol 76:205–213
Autoimmunity in ITP
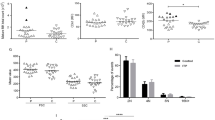
How do we know that ITP is an autoimmune disease? The classic studies of Harrington (Fig. 4a) showed that infusion of ITP patient’s plasma into normal controls mostly (but not always) causes thrombocytopenia in the recipients (Harrington et al. 1951). Follow-up studies have shown that this effect appears to be antibody mediated. Bob McMillan’s group and Mei Chang in Diane Nugent’s group (Fig. 4b) showed that plasma from ITP patients inhibited growth and development of megakaryocytes to variable extents; the plasma from different patients had different degrees of effect in both studies (Chang et al. 2003; McMillan et al. 2004). It thus appears clear that antiplatelet antibodies react not only with platelets but also with megakaryocytes. Whether this is important to the pathogenesis of ITP in all, most, or some cases is not clear.
ITP is an autoantibody-mediated disease. (a) Original experiment by Harrington et al. (1951), demonstrating the thrombocytopenic effect of plasma from ITP patients when infused into normal recipients. (b) Antibodies from patients with ITP inhibit the growth of megakaryocytes in culture (McMillan et al. 2004)
What do we know about T cells in ITP? There have been a multitude of studies demonstrating a multitude of abnormalities of T cells (Semple et al. 1991, 1996). One set involves the abnormalities of T cells as complex immune regulators of the immune response. The most sophisticated involve analyses of newly discovered cytokines or of cell populations such as follicular B cells in the spleen and their role in anti-platelet antibody regulation and/or production. Other studies have similar levels of sophistication and are very complicated.
Perhaps the clearest and most uniform findings have involved T regulatory cells (Tregs). Evidence for their deficiency in both number and function has been confirmed in a number of studies (Bao et al. 2010). Similarly, their restoration in response to therapy has been invoked not only with TPO agents but also using several different therapies (e.g., rituximab) (Stasi et al. 2007). Tregs can suppress the interaction of dendritic cells with T helper cells and prevent T helper cells from interacting with B cells to make antiplatelet antibodies by reducing their proliferation.
A different case is investigation of cytotoxic T cells to determine their role in direct destruction of platelets and megakaryocytes. A classic study of cytotoxic T cells was carried out by Wadenvik’s group. In a small number of patients with ITP, an in vitro natural killer-type assay showed that there was cytotoxicity to platelets, with lysis. Furthermore, the study pinpointed the mechanism (e.g., granzyme levels were increased) and showed that not only was there more lysis in patients with ITP than in controls but also more cytotoxicity with active ITP than for ITP in remission (Fig. 4b) (Olsson et al. 2003). Additional studies of this topic have been very limited.
In the 1990s, there were studies showing T cell proliferation in response to patients’ own platelets and studies consistent with an active TH1-mediated disease driven by IL-2, interferon γ, and perhaps even IL-15 (Fig. 4a). To date, hundreds of studies have been performed demonstrating a confusing variety of abnormalities of T cells and their cytokines (Fig. 5).
Role of T-cell-mediated platelet destruction in the immune pathology in ITP. (a) T cell proliferation in response to a patient’s own platelets (Semple et al. 1991). (b) Cytotoxic T cells play a role in destruction of platelets in active ITP (Olsson et al. 2003) (CD4 skewing to Th1 should be figure C, and comes from Semple JW and Provan D (2012) Curr Opin Hematol 19:357–362)
The role of CD8 cells seems more complicated than just to attack platelets and megakaryocytes in patients with ITP. It appears that CD8 cells are necessary for megakaryocyte proliferation and, when CD8-positive T cells are co-cultured with megakaryocyte progenitors, more megakaryocytes develop, there is less apotosis, and fewer platelets, which is somewhat surprising (Li et al. 2007; Fig. 6). Similarly, it appears that dexamethasone can increase the number of CD8-positive T cells and increase Tregs, which is a surprising finding given the expected immunosuppressive effect of steroids.
The role of CD8+ T cells in the bone marrow. Figure taken from Li S et al (2007) Br J Haematol 139:605–611
Some of the complexities of the pathogenesis of ITP, in addition to those mentioned, include epitope spreading (presentation of new epitopes of the platelet glycoproteins so that there is a broader spectrum of anti-platelet antibodies) in macrophages, which may contribute to chronicity and failure to respond to ITP therapies; the role of anti-platelet antibodies; and the role of these antibodies in impairing megakaryocyte production and maturation and in reducing platelet production. In addition, there are many co-stimulatory molecules involved between macrophages as antigen-presenting cells, helper T cells, and B cells including CD40, CD40 ligand (CD154), CD80, and CD28. These and other interactions could help determine whether the disorder is serious or not so serious and whether the patient is likely to get better over time (Cines and Blanchette 2002).
Management of ITP
There are many challenges in managing ITP. Diagnosis can be complicated, although not most of the time. Classic diagnostic practice involves excluding all other causes and having a normal complete blood count, except for thrombocytopenia, and no findings such as hepatosplenomegaly or lymphadenopathy on physical examination. Response to treatment can also affect the diagnosis; for example, if a patient receives IVIG (or steroids) and the platelets increase dramatically, albeit transiently, then that strongly suggests that this is a case of ITP. It should be noted, however, that such a response (as mentioned in Sect. “Introduction: Definition of ITP”) does not differentiate secondary from primary ITP (Fig. 7).
Pathogenesis can be very complicated and treatment decisions involve many different factors. These factors include the platelet count, the degree of symptoms especially bleeding and fatigue, and how the patient feels about treatment in general and a given treatment in particular. The risk of bleeding is an important factor for some patients (e.g., construction workers or athletes), as is travel (e.g., consideration of what to do when a patient does not have their usual source of emergency back-up available). Patients may be treated differently depending on the duration of their disease (newly diagnosed, persistent, or chronic) and can be treated with a wide variety of agents, as listed in the 2010 consensus document (Provan et al. 2010).
Decisions about treatment are not clearly defined. For adults, most are either asymptomatic or have minor bruising and perhaps a few scattered petechiae, despite relatively low platelet counts. Treatment is mostly given for platelet counts below 30,000/μL. There is increased mortality in elderly patients with persistently low counts (Cohen et al. 2000). Certain studies in the past have demonstrated that morbidity and mortality can derive from complications of immunosuppressive treatment given at too high a dose for too long, especially too much prednisone for months or years (Portielje et al. 2001).
The primary goal of treatment is to avoid intracranial hemorrhage, although improving health-related quality of life (HRQoL) is now recognized as very important and is more relevant for many patients. Data from Cortellazzo et al. (1991), summarized by Cohen et al. (2000), show that the rate of fatal hemorrhage per year for patients aged 40–60 years is 0.012 per year (i.e., just over 1 in 1000); for those aged over 60 years, the mortality is 0.13 per patient year (13 per 1000), which is thought to be driven to a large extent by comorbidities and other medications (Cortelazzo et al. 1991). In addition, patients with ITP have an increased risk of venous thromboembolism. In a long-term study of romiplostim, it was demonstrated that strokes occurred particularly in the over 70 year olds (Kuter et al. 2013).
For children, and possibly younger adults, treatment is required if the count is over 30,000/μL only in cases where there is bleeding, the patient requires surgery, the patient is on anticoagulation or antiplatelet agents, and possibly if other factors such fatigue and access to care are involved. In the 10,000–30,000/μL range, the issue is whether treatment is needed and, if so, what to treat. Factors to take into consideration are age, comorbidities, and past treatment, including how well it worked or did not work. For guidelines or information as of early June 2016, there is the international consensus report that was published in 2010 (Provan et al. 2010) and the second version of the American Society of Hematology (ASH) evidenced-based guidelines for ITP (Neunert et al. 2011). Both the consensus report and the ASH guidelines are under revision and new versions should appear within the next year (Fig. 8).
First and second line treatments in the consensus document. Treatments listed alphabetically (also by category for third line) divided by first, second, and third line from the 2010 consensus document (Provan et al. 2010)
The consensus report lists front line treatments, which everybody agrees with although there are differences about which treatments to use and when. The most recent study showed that the platelet count went up quicker with dexamethasone than with prednisone, but long-term outcome (the current Holy Grail of ITP treatment) was not significantly different between dexamethasone and prednisone treatments (Wei et al. 2016). The choice of second line treatments is more complicated because there is not much evidence other than some randomized trials of thrombopoeitic agents compared with placebo and a very large amount of open-label, single-arm data for splenectomy. A “How I Treat” article in 2012 focused on the selection of second line agents (Ghanima et al. 2012). For other agents, the issues are their efficacy and the side effects. For third line treatment, the only option that has sufficient data and category A evidence is use of thrombopoietic agents (Bussel et al. 2006, 2007; Saleh et al. 2013; Kuter et al. 2013).
For second line treatments, common morbidity for persistent ITP is caused by infection or other steroid-related complications and the most prominent steroid-sparing agents are rituximab; immunosuppression with danazol, azathioprine, or mycophenolate mofetil; TPO receptor agonists (TPO-RAs; romiplostim or eltrombopag); and splenectomy.
Looking at rituximab, the only study that assessed long-term outcome looked at responders 1 year after the initial rituximab infusion (Patel et al. 2012) and showed that the long-term outcome (more than 3 years) was only a 21 % persistent complete response. All other responding patients (approximately another 20–30 %), whether the response was partial or complete, relapse and require additional treatment (Patel et al. 2012). This number (21 %) could represent the percentage of people who would have improved over time anyway.
As this result became more evident, efforts were made to supplement rituximab treatment, the choice being high-dose dexamethasone. The initial concept was that rituximab would deplete intravascular B cells and plasma cells would die off. However, plasma cells lived longer than had hitherto been believed to be the case and, therefore, rituximab alone did not result in full depletion of antibody-making cells . This was consistent with the lack of development of hypogammaglobulinemia with rituximab alone (Cooper et al. 2004). Borrowing from experience with myeloma treatment, it was thought that dexamethasone would affect the plasma cells and rituximab would affect the B cells and, thus, there would be a better response to combined therapy. Two initial studies explored the addition of one 4-day cycle of dexamethasone to the standard dose of rituximab, but both compared this combination to one cycle of dexamethasone alone rather than to rituximab alone (Zaja et al. 2010; Gudbrandsdottir et al. 2013). The results were good, but only newly diagnosed patients (the easiest to treat patients) were entered in the studies and long-term follow up is not available. More recent results using three cycles of dexamethasone and standard treatment of four doses of rituximab demonstrate that the only subset of patients with a very good cure rate(>70 %) are women with duration of their ITP of less than 1 year. This finding in women with ITP was supported by an identical result in adolescent girls (Chapin et al. 2016). It is, however, important to realize that, even in the groups that eventually relapse, as many as 50 % are in continuing response more than 2 years after the initial rituximab treatment. This combination provides good results at a high short-term (1–3 year) rate but the eventual relapse lessens the positive impact of this treatment scheme.
The next consideration was to use mycophenolate mofetil (MMF) as a second line treatment option. There are at least 11 ITP studies of MMF treatment, none of which are very large in size or randomized (Taylor et al. 2015). Overall, the response rates are about 50 % and MMF is well tolerated. Whether MMF is better than azathioprine is not clear. A newly issued “black box warning” (package insert for the USA) is that MMF can alter the levels of oral contraceptive hormones and therefore result in their failing to prevent pregnancy. MMF is best used in either mild to moderate disease or as a sequel to dexamethasone–rituximab to enhance cure rates (Chapin et al. 2016).
Splenectomy has become a very infrequently used treatment (Boyle et al. 2013). Over the period 1991–2009, in a US population, the rate of splenectomy fell from 30 to <10 % and is probably even lower today. Note that splenectomy offers the greatest chance for clinical remission and is potentially the most cost-effective treatment. Taking all comers with splenectomy, approximately 60 % of patients have durable remissions if the operation is not performed too late in the course of the disease (Kojouri et al. 2004). Currently, many patients refuse splenectomy if it is only going to work 60 % of the time. There are perioperative complications including infection, bleeding, and thrombosis (post-op anticoagulation treatment may be appropriate) and the long-term complications of infection with overwhelming post-splenectomy sepsis and thrombotic complications (Thomsen et al. 2010). A recent study presented at the European Hematology Association (EHA) congress in June 2016 by the group of Waleed Ghanima suggested that there was no increase in myocardial infarctions or pulmonary hypertension in patients with ITP who underwent splenectomy. However, there was a significant approximately 50 % increase in venous thrombosis and this translated into a significant increase in the incidence of stroke (Rørholt et al. 2016).
Turning to the thrombopoietic agents, we know that TPO drives the pathway from stem cells all the way to megakaryocyte proliferation (Kaushansky 1998). Its role in platelet release from megakaryocytes is uncertain. As discussed above, we do not know what fraction of TPO production is constitutive and how much it can be upregulated (e.g., by binding of desialated platelets to the Ashwell–Morell receptor) (Fig. 9).
Both romiplostim and eltrombopag were developed by screening of compounds. Romiplostim is composed of four small dipeptides attached to each other by disulfide bridges and to an Fc portion of an IgG1 molecule to increase the half-life by allowing recirculation via FcRn. It is given once a week subcutaneously. In the USA, although not in Europe, it is still not approved for self-administration by the patient at home. Eltrombopag is given daily by mouth. Although there have been recent changes to the US package insert, we believe it should be taken on an empty stomach at least 2 h after eating and without eating for another 2 h afterwards. Furthermore, there should be no divalent cation intake (i.e., calcium in milk or other dairy or other supplements for 4 h before and 4 h after). Most people therefore take it at bedtime, do not eat after dinner, and do not have dairy with dinner, but this is not always possible. Both romiplostim and eltrombopag are expected to cross the placenta and thereby affect fetal bone marrow, so their use in pregnancy is strongly discouraged. Both agents have been used at the very end of pregnancy to increase the platelet count in either refractory ITP patients or in those with inherited thrombocytopenias so that the platelet count is good enough for delivery and, hopefully, for epidural anesthesia (personal experience).
Both molecules share similar mechanisms of action although it is thought that they bind at different places, with romiplostim binding at the TPO binding site and eltrombopag potentially binding in the intramembranous portion. The difference in binding could underlie anecdotal findings in patients who respond to one agent but not the other (Khellaf et al. 2013). A theoretical advantage of the lack of eltrombopag binding at the TPO receptor site is that TPO can bind there, creating an additive or even synergistic effect, but this remains theoretical. Eltrombopag is a chelator and is inactive when it has chelated a cation, which is why calcium, magnesium, and iron inactivate it if ingested in proximity to the drug.
Figure 10 compares two randomized controlled trials (Kuter et al. 2010; Cheng et al. 2011). There are also a number of other randomized controlled trials with each agent. Adult ITP patients appear to respond 60–90 % of the time with either treatment and often can either discontinue or reduce the use of rescue treatments (i.e., platelet transfusion, steroids, IVIG, etc.) and/or discontinue or reduce the dose of concomitant medications (e.g., prednisone) after starting the thrombopoietic agent. Patients usually reduce bleeding in proportion to their platelet count increase and, overall, at least some of them have an improvement in their level of energy and/or HRQoL. Some of the latter may experience less bleeding and therefore less fear of bleeding but, in addition, thrombocytopenia has reversible organic effects on energy levels and vitality that have been amply demonstrated in a number of studies (McMillan et al. 2008).
Responses with TPO receptor agonists in adults (platelets >50 × 109/L): Results from two randomized controlled trials (Kuter et al. 2010; Cheng et al. 2011). Description of two of the five large randomized controlled trials with these agents in adults with ITP (there are three large randomized trials in children with ITP)
Long-term results from both treatments have been collected and published or presented. The overall thromboembolic event rate was approximately 6 % for both agents over 6–8 years of treatment (Kuter et al. 2013; Saleh et al. 2013). There is still debate over the exact cause of the thromboembolisms. They appear to be a combination of the underlying disease, predisposition of certain individuals who have been “protected” by their thrombocytopenia, and the availability of an increased number of younger platelets. Bone marrow reticulin fibrosis, which raised considerable concern early on, seems to occur infrequently and does not cause any major issues (Ghanima et al. 2014). In addition, in patients who have discontinued treatment, the reticulin fibrosis has regressed in almost all cases (Ghanima et al. 2014). With eltrombopag, it seems that approximately 3 % of patients are unable to take it because of increases in transaminases and/or bilirubin, but most people with increased transaminases who discontinue eltrombopag can successfully resume it (Saleh et al. 2013). If eltrombopag can indeed cause cataracts, it appears to be a very small effect. Determination of a specific rate has been confounded by the many people with ITP who take steroids for prolonged periods of time, who are elderly, who may have smoked cigarettes, and who potentially have other risk factors for cataracts. There does not appear to be in ITP per se any induction of malignancy. If, however, a patient has myelodysplastic syndrome instead of ITP, there could be induction of blasts, which usually regress with discontinuation of medication (Bryan et al. 2010).
For both therapies, there are patients who, after a variable duration of treatment, can successfully discontinue a TPO agent and yet maintain an adequate count. What fraction of patients this is, how long they have had ITP, and how long they were on medication remains unknown. There is a weak tendency for patients who have been able to discontinue medication not to have been on TPO agents for a long time and not to have had ITP for a long time. Furthermore, even in adults, there can be spontaneous improvement over a number of years. This continues to be studied without clear resolution at this time, although one could hazard an estimate of 20–40 % improvement within 3 years of starting thrombopoietic agents (Fig. 11).
There is also the question of why remission should occur. Possibly, it occurs as a result of spontaneous improvement over time and the TPO agent is merely supporting the platelet count until “spontaneous remission or improvement” does occur. Alternatively, thrombopoietic agents may not be as immunologically inert as originally thought. This is consistent with platelets being part of the innate immune response (see Slaba and Kubes 2017). At least one study has showed the induction of Tregs, not just by number but also by function (Bao et al. 2010).
Figure 12 outlines one possible schema for an approach to ITP. Clearly, the great majority of the decisions in this setting are not informed by randomized controlled trials. However, an unequivocal issue here is not to overly prolong the use of steroids. If there is no early remission, the side effects of prolonged use of steroids outweigh their benefit. IVIG as a rescue medicine (i.e., to bring the count up quickly) is a reasonable early approach but has relatively little curative effect, although there may be a small amount with repeated use as demonstrated in children with ITP. In persistent ITP, MMF has been widely used. Other treatments are TPO agents or rituximab. The latter is more likely to work with dexamethasone and if used closer to diagnosis rather than further from it. Whether the benefit of adding three cycles of dexamethasone to rituximab outweighs the side effects is not clear. However, at this time, for women within 1 year of diagnosis, especially those who are early persistent, it seems very useful (Chapin et al. 2016). Other agents for chronic patients, in addition to splenectomy, include danazol, dapsone, azathioprine, and possibly even hydroxychloroquine if there are lupus-like features (Provan et al. 2010).
Pediatric ITP
Children with ITP are thought to have the “same” disease as adults. This may be most true for children with very persistent and chronic disease in which no spontaneous improvement occurs and standard treatments are sometimes ineffective. However, for most children with ITP, the ITP resolves spontaneously within 3–6 months. It is thought that many of these cases reflect “virally induced” ITP with antibodies to an infective agent that cross-reacts with platelets. As the infection clears and antibody titers fall, the platelet count returns to normal. For these patients, the major debate is whom to treat and with what. Some physicians prefer treatment when patients have very low platelet counts, whereas others prefer to “watch and wait” if there is no major ongoing bleeding.
Reduced quality of life is probably overlooked more often in this population than in adults because children do not express it as well as adults. It may be expressed as increased irritability, poor performance, or greater fatigue.
Serious bleeding is primarily intracranial hemorrage (ICH), which is estimated to occur in approximately 1 in 200–500 cases (Psaila et al. 2009). It may occur at presentation or at any time during the course of the disease. It is slightly over-represented in those with chronic disease.
Treatment effects are similar to those seen in adults. The major issue is that the patient can improve at any time up to the end of the first year so there is less need than with adults to provide a “definitive” therapy. Splenectomy is avoided whenever possible and is ideally reserved for those who have had ITP for more than 1 year, are older than 5 years, have very low platelet counts, bleeding, and are not doing well with other therapies. The effects of rituximab are similar to those seen in adults, with younger children not doing so well and adolescent girls within 1 year of ITP diagnosis responding best when rituximab is combined with dexamethasone (Chapin et al. 2016). TPO agents have recently been studied in children, with one small and three large randomized controlled studies completed (Bussel et al. 2011, 2015a, b; Tarantino et al. 2016; Grainger et al. 2015). All studies have shown good efficacy and good safety and resulted in eltrombopag being licensed for children with ITP down to the age of 1 by the FDA in the USA and by the EMA in Europe. Similar licensure is expected for romiplostim in the near future. More data demonstrating long-term safety and persistent efficacy is available for romiplostim than for eltrombopag at this time (Bussel et al. 2015a, b). One retrospective study has investigated bone marrow and not seen problems (e.g., high grades of fibrosis or abnormal cells) (Ramaswamy et al. 2014). An ongoing romiplostim study is prospectively exploring marrow findings.
Other treatments (e.g., immunosuppressive agents such as 6-MP and azathioprin) have also been studied in single-arm trials (Grace et al. 2012). In the 1980s, long-term IVIG was also tried but caused post-infusion headaches and was cumbersome because of the time needed for infusion. Similarly, intravenous anti-D was tried (Andrew et al. 1992) but its use declined with the discovery of serious intravascular hemolysis occurring for 1 in 1115 infusions (Gaines 2000). This was carefully reviewed after a “black box” warning was issued by the FDA (Despotovic et al. 2012).
Overall, the good news is that ITP in children mostly resolves spontaneously and the rate of serious bleeding is rare. The bad news is that it is not currently possible to predict the advent of chronic disease or of serious bleeding. Furthermore, chronic disease in children is not easier to treat than chronic disease in adults.
Conclusion
In summary, this is a very exciting time for ITP as newer treatments are better studied and additional treatments continue to be developed. Furthermore, other considerations are also being taken into account (e.g., HRQoL), but this is just one example of the “whole patient” approach. Molecular studies may allow separating patients into different groups and directing treatment choices; unfortunately this is still not possible.
We now know that, although there are many factors that influence megakaryocyte development and the regulation of platelet production, the role of TPO is key. It remains to be clarified whether aging platelets are cleared in the liver and stimulate synthesis of additional TPO, and what other factors are important and operative in this system to up- or downregulate endogenous TPO production. The more the autoimmune response is studied, the more complex it seems. The good news is that there are more targets for intervention; the bad news is that it is impossible to test them all. Although antibody-mediated platelet destruction (and impaired platelet production) is certainly central to the pathogenesis of ITP, the role of cell-mediated immunity has been studied on many levels and it seems that autoreactive T cells drive the disease in more difficult or more chronic ITP patients. Even though TPO receptor agonists are not the “be all and end all,” if a patient responds to them they usually provide a good quality of life even if patients do not improve enough to be able to discontinue treatment. Because cure is the goal, perhaps additional rituximab combinations or better prediction of splenectomy outcome will allow us to take care of at least a sizeable fraction of our patients.
Take-Home Message Boxes
The diagnosis and management of ITP is still a work in progress. Although novel treatments have improved the management strategy in ITP, many questions remain to be answered and some patients continue to be refractory to treatment. A better understanding of the pathology of ITP, biomarkers to direct treatment and new therapeutics for refractory patients is needed.
References
Andrew M, Blanchette VS, Adams M, Ali K, Barnard D, Chan KW, DeVeber LB, Esseltine D, Israels S, Korbrinsky N et al (1992) A multicenter study of the treatment of childhood chronic idiopathic thrombocytopenic purpura with anti-D. J Pediatr 120(4 Pt 1):522–527
Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, Yazdanbakhsh K (2010) Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 116:4639–4645
Boyle S, White RH, Brunson A, Wun T (2013) Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood 121:4782–4790
Bryan J, Jabbour E, Prescott H, Kantarjian H (2010) Thrombocytopenia in patients with myelodysplastic syndromes. Semin Hematol 47(3):274–280
Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser JS, Wiznitzer I, Kelly R, Chen CF, Nichol JL (2006) AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med 355(16):1672–1681 Erratum in: N Engl J Med. 2006 Nov 9;355(19):2054
Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, Arning M, Provan D, Jenkins JM (2007 Nov 29) Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 357(22):2237–2247
Bussel JB, Buchanan GR, Nugent DJ, Gnarra DJ, Bomgaars LR, Blanchette VS, Wang YM, Nie K, Jun S (2011) A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood 118:28–36
Bussel JB, de Miguel PG, Despotovic JM, Grainger JD, Sevilla J, Blanchette VS, Krishnamurti L, Connor P, David M, Boayue KB, Matthews DC, Lambert MP, Marcello LM, Iyengar M, Chan GW, Chagin KD, Theodore D, Bailey CK, Bakshi KK (2015a) Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol 2:e315–e325
Bussel JB, Hsieh L, Buchanan GR, Stine K, Kalpatthi R, Gnarra DJ, Ho RH, Nie K, Eisen M (2015b) Long-term use of the thrombopoietin-mimetic romiplostim in children with severe chronic immune thrombocytopenia (ITP). Pediatr Blood Cancer 62:208–213
Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, Nugent DJ (2003) Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood 102(3):887–895
Chapin J, Lee S, Zhang H, Zehnder JL, Bussel JB (2016) Gender and duration of disease differentiate responses to rituximab-dexamethasone therapy in adults with Immune Thrombocytopenia (ITP). Am J Hematol 91(9):907–911
Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, Arning M, Stone NL, Bussel JB (2011) Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet 377:393–402
Cines DB, Blanchette VS (2002 Mar 28) Immune thrombocytopenic purpura. N Engl J Med 346(13):995–1008
Cohen YC, Djulbegovic B, Shamai-Lubovitz O, Mozes B (2000) The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med 160:1630–1638
Cooper N, Stasi R, Cunningham-Rundles S, Feuerstein M, Leonard J, Amadori S, Bussel JB (2004) The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenia purpura. Br J Haematol 125(2):232–239
Corbacioglu S, Bux J, König A, Gabrilove JL, Welte K, Bussel JB (2000) Serum granulocyte colony-stimulating factor levels are not increased in patients with autoimmune neutropenia of infancy. J Pediatr 137(1):96–99
Cortelazzo S, Finazzi G, Buelli M, Molteni A, Viero P, Barbui T (1991) High risk of severe bleeding in aged patients with chronic idiopathic thrombocytopenic purpura. Blood 77(1):31–33
Despotovic JM, Lambert MP, Herman JH, Gernsheimer TB, McCrae KR, Tarantino MD, Bussel JB (2012) RhIG for the treatment of immune thrombocytopenia: consensus and controversy (CME). Transfusion 52:1126–1136, quiz 1125
Gaines AR (2000) Acute onset hemoglobinemia and/or hemoglobinuria and sequelae following Rh(o)(D) immune globulin intravenous administration in immune thrombocytopenic purpura patients. Blood 95:2523–2529
Ghanima W, Godeau B, Cines DB, Bussel JB (2012) How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second-line treatment. Blood 120(5):960–969
Ghanima W, Geyer JT, Lee CS, Boiocchi L, Imahiyerobo AA, Orazi A, Bussel JB (2014) Bone marrow fibrosis in 66 patients with immune thrombocytopenia treated with thrombopoietin-receptor agonists: a single-center, long-term follow-up. Haematologica 99:937–944
Grace RF, Bennett CM, Ritchey AK, Jeng M, Thornburg CD, Lambert MP, Neier M, Recht M, Kumar M, Blanchette V, Klaassen RJ, Buchanan GR, Kurth MH, Nugent DJ, Thompson AA, Stine K, Kalish LA, Neufeld EJ (2012) Response to steroids predicts response to rituximab in pediatric chronic immune thrombocytopenia. Pediatr Blood Cancer 58:221–225
Grainger JD, Locatelli F, Chotsampancharoen T, Donyush E, Pongtanakul B, Komvilaisak P, Sosothikul D, Drelichman G, Sirachainan N, Holzhauer S, Lebedev V, Lemons R, Pospisilova D, Ramenghi U, Bussel JB, Bakshi KK, Iyengar M, Chan GW, Chagin KD, Theodore D, Marcello LM, Bailey CK (2015) Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet 386:1649–1658
Grozovsky R, Giannini S, Falet H, Hoffmeister KM (2015) Regulating billions of blood platelets: glycans and beyond. Blood 126:1877–1884
Gudbrandsdottir S, Birgens HS, Frederiksen H, Jensen BA, Jensen MK, Kjeldsen L, Klausen TW, Larsen H, Mourits-Andersen HT, Nielsen CH, Nielsen OJ, Plesner T, Pulczynski S, Rasmussen IH, Rønnov-Jessen D, Hasselbalch HC (2013) Rituximab and dexamethasone vs. dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood 121:1976–1981
Harrington WJ, Minnich V, Hollingsworth JW, Moore CV (1951) Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med 38(1):1–10
Italiano JE Jr (2017) Megakaryocyte development and platelet production. In: Gresele P et al (eds) Platelets in thrombotic and non-thrombotic disorders. Springer, Cham, pp 39–53
Kaushansky K (1998) Thrombopoietin. N Engl J Med 339(11):746–754
Khellaf M, Viallard JF, Hamidou M, Cheze S, Roudot-Thoraval F, Lefrere F, Fain O, Audia S, Abgrall JF, Michot JM, Dauriac C, Lefort S, Gyan E, Niault M, Durand JM, Languille L, Boutboul D, Bierling P, Michel M, Godeau B (2013) A retrospective pilot evaluation of switching thrombopoietic receptor-agonists in immune thrombocytopenia. Haematologica 98(6):881–887
Kojouri K, Vesely SK, Terrell DR, George JN (2004) Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood 104:2623–2634
Kuter DJ, Beeler DL, Rosenberg RD (1994) The purification of megapoietin: a physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci U S A 91(23):11104–11108
Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X, Berger DP (2010) Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med 363:1889–1899
Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, Viallard JF, Macik G, Rummel M, Nie K, Jun S (2013) Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol 161(3):411–423
Li S, Wang L, Zhao C, Li L, Peng J, Hou M (2007) CD8+ T cells suppress autologous megakaryocyte apoptosis in idiopathic thrombocytopenic purpura. Br J Haematol 139:605–611
McMillan R, Wang L, Tomer A, Nichol J, Pistillo J (2004) Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood 103:1364–1369
McMillan R, Bussel JB, George JN, Lalla D, Nichol JL (2008) Self-reported health-related quality of life in adults with chronic immune thrombocytopenic purpura. Am J Hematol 83(2):150–154
Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA, American Society of Hematology (2011) The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 117(16):4190–207
Olsson B, Andersson PO, Jernås M, Jacobsson S, Carlsson B, Carlsson LM, Wadenvik H (2003) T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med 9:1123–1124
Patel VL, Mahévas M, Lee SY, Stasi R, Cunningham-Rundles S et al (2012) Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood 119:5989–5995
Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A (2001) Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 97:2549–2554
Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB et al (2010) International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 115:168–186
Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB (2009) Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood 114:4777–4783
Ramaswamy K, Hsieh L, Leven E, Thompson MV, Nugent D, Bussel JB (2014) Thrombopoietic agents for the treatment of persistent and chronic immune thrombocytopenia in children. J Pediatr 165(3):600–605
Rørholt M, Ghanima W, Farkas D, Toft Sørensen H, Nørgaard M (2016) Long-term risk of cardiovascular events following splenectomy—a Danish population-based cohort study. Haematologica 101(S1):334
Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, Brainsky A (2013) Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia (ITP): results of the long-term, open-label EXTEND study. Blood 121(3):537–545
Semple JW, Freedman J (1991) Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood 78:2619–2625
Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, Freedman J (1996) Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood 87:4245–4254
Semple JW, Provan D (2012) The immunopathogenesis of immune thrombocytopenia: T cells still take center-stage. Curr Opin Hematol 19:357–362
Shulman NR, Marder VJ, Weinrach RS (1965) Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura. Physiologic, serologic and isotopic studies. Ann N Y Acad Sci 124:499–542
Slaba I, Kubes P (2017) Platelets and immunity. In: Gresele P et al (eds) Platelets in thrombotic and non-thrombotic disorders. Springer, Cham, pp 489–512
Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S (2007) Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood 110(8):2924–2930
Tarantino MD, Bussel JB, Blanchette VS, Despotovic J, Bennett C, Raj A, Williams B, Beam D, Morales J, Rose MJ, Carpenter N, Nie K, Eisen M (2016) Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet 388(10039):45–54
Taylor A, Neave L, Solanki S, Westwood JP, Terrinonive I, McGuckin S, Kothari J, Cooper N, Stasi R, Scully M (2015) Mycophenolate mofetil therapy for severe immune thrombocytopenia. Br J Haematol 171(4):625–630
Thomsen RW, Schoonen WM, Farkas DK, Riis A, Fryzek JP, Sørensen HT (2010) Risk of venous thromboembolism in splenectomized patients compared with the general population and appendectomized patients: a 10-year nationwide cohort study. J Thromb Haemost 8:1413–1416
Wei Y, Ji XB, Wang YW, Wang JX, Yang EQ, Wang ZC, Sang YQ, Bi ZM, Ren CA, Zhou F, Liu GQ, Peng J, Hou M (2016) High-dose dexamethasone vs. prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood 127(3):296–302
Zaja F, Baccarani M, Mazza P, Bocchia M, Gugliotta L, Zaccaria A, Vianelli N, Defina M, Tieghi A, Amadori S, Campagna S, Ferrara F, Angelucci E, Usala E, Cantoni S, Visani G, Fornaro A, Rizzi R, De Stefano V, Casulli F, Battista ML, Isola M, Soldano F, Gamba E, Fanin R (2010) Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood 115(14):2755–2762
Acknowledgments
James Bussel has received research funding from Amgen, Cangene, GSK, Genzyme, InG of America, Immunomedics, Ligand, Eisai, Shionogi, and Sysmex; has participated in advisory boards for Amgen, GSK, Ligand, Shionogi, and Eisai; and had a 1-day consultation with Portola. G.C. is on the speakers' bureau and receives honoraria from GSK
Conflict of interest
Nichola Cooper receives salary and research funding from National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) Imperial College Healthcare NHS Trust and research support from the UK ITP patient support association, The National Organization for Rare Diseases (NORD), and Pfizer and has received honoraria for speaking at educational events and consultancy work from Amgen, GSK and Novartis.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Cooper, N., Bussel, J. (2017). Immune Thrombocytopenia: Where Are We Now?. In: Gresele, P., Kleiman, N., Lopez, J., Page, C. (eds) Platelets in Thrombotic and Non-Thrombotic Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-47462-5_50
Download citation
DOI: https://doi.org/10.1007/978-3-319-47462-5_50
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47460-1
Online ISBN: 978-3-319-47462-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)