Abstract
The platelet cytoskeleton maintains the discoid shape of the resting platelet and mediates a rapid shape change in response to external stimuli at sites of vascular injury. At rest, specific cytoskeletal structures and molecules maintain the unique architecture of the platelet actin cytoskeleton, connect it to the plasma membrane, and prevent actin monomer assembly onto actin filaments. Platelet activation stimulates the interactions of cytoplasmic signals with actin regulatory proteins to initiate and amplify actin filament growth necessary for platelet shape change. This chapter reviews the cellular mechanisms and proteins maintaining the morphology of resting and activated platelets, focusing on the actin cytoskeleton and exciting recent mRNA and protein profiling studies, clinical observations, and mouse models.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Following blood vessel injury and disruption of the vascular endothelium, platelets interact avidly with exposed elements of the basement membrane and rapidly change from resting discs to active forms, first by rounding and then by extending finger-like filopodia and spreading thin sheet-like lamellipodia. This shape change reaction is further amplified by soluble factors released by activated platelets. Shape change is orchestrated by the platelet cytoskeleton, which has therefore two main functions: (1) to maintain the biconvex discoid shape of the resting platelet and (2) to mediate a rapid shape change in response to external stimuli at sites of vascular injury. The platelet cytoskeleton is composed of actin and tubulin polymers and associated cytoskeletal proteins, which compose a large fraction of the platelet proteome. Mutations and single nucleotide polymorphisms (SNPs) in genes encoding cytoskeletal proteins often lead to altered platelet counts and mean platelet volume. Table 1 lists the major cytoskeletal proteins regulating the resting and activated platelet cytoskeleton, based on recent mRNA and protein profiling studies, clinical observations, and mouse models, while the major actin regulatory proteins regulating the platelet actin assembly reaction are listed in Table 2.
The Cytoskeleton of the Resting Platelet
Actin
Actin is by far the most abundant protein in platelets, where it represents 15–20 % of the total cellular protein mass. Platelet actin is evenly distributed between β-actin and γ-actin, also called cytoplasmic actins 1 and 2, respectively (Rowley et al. 2011; Burkhart et al. 2012) (Table 1). In resting platelets, about 40 % of total actin is assembled into dynamic polymers or filaments (F-actin) that can be reversibly assembled from monomeric or globular actin (G-actin). The distribution of actin in filaments reaches 80 % in activated platelets.
Actin filaments are polarized structures, first recognized by electron microscopy by the way filaments can be decorated with the myosin head domain, which binds periodically along the filament length to define barbed and pointed ends. This polarity is reflected in different rates of monomer addition to the two ends. The barbed end of the filament is the preferred end of actin filament assembly in vitro, as it has high affinity for G-actin and elongates at a rate approximately ten times faster than the pointed end. Barbed ends are the only ends contributing to actin assembly in cells, particularly in platelets. When assembled, actin filaments can be cross-linked or bundled into higher-order structures or fragmented into smaller pieces. A large number of platelet cytoskeletal-associated proteins control these dynamic processes.
The Membrane Cytoskeleton
The morphology of the resting platelet is maintained by a membrane cytoskeleton, a thick protein meshwork composed principally of spectrin, adducin, actin filaments, and actin-associated proteins laminating the cytoplasmic side of the platelet plasma membrane (Fig. 1) (Fox and Boyles 1988; Hartwig and DeSisto 1991). Analogies have been made with the well-characterized spectrin-based erythrocyte membrane cytoskeleton (Lux 2016). Platelet spectrin is composed of both erythroid and non-erythroid α/β-spectrin heterodimers forming 200-nm long tetramers assembled head-to-head. Assembly of spectrin into tetramers is required for platelet production by megakaryocytes (Patel-Hett et al. 2011). Contrary to erythrocytes, where spectrin is connected to short actin oligomers capped by adducin at their barbed end and tropomodulin at their pointed end (Lux 2016), platelet spectrin strands interconnect at the barbed ends of the actin filament network originating from the cytoplasm (Fox and Boyles 1988; Hartwig and DeSisto 1991). Therefore, the dense platelet spectrin lattice and its associated actin filaments assemble into a continuous ultrastructure.
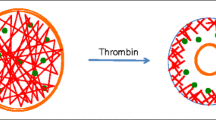
Organization of the resting platelet cytoskeleton. Spectrin strands laminate the cytoplasmic surface of the plasma membrane and interconnect using barbed ends of actin filaments. Adducin binding to the actin filament barbed end targets spectrin. Actin filaments are cross-linked by FlnA, which provides the major membrane–cytoskeletal connection linking the actin cytoskeleton to the cytoplasmic tail of the GPIbα subunit of the VWF receptor complex GPIb-IX-V
Capture of actin filament barbed ends by spectrin requires adducin, which forms a high-affinity ternary complex with spectrin and actin (Fig. 1) (Gilligan et al. 2002; Barkalow et al. 2003). Platelet express both erythroid α-adducin (encoded by human ADD1, mouse Add1) and non-erythroid γ-adducin (human ADD3, mouse Add3). Add1-null mice lack both α- and γ-adducin in platelets and have slightly elevated platelet counts with reduced mean platelet volume (Robledo et al. 2008), indicating that α-adducin regulates both the expression of γ-adducin and the morphology of the resting platelet membrane cytoskeleton. By contrast, the role of γ-adducin appears redundant, as Add3-null mice have normal platelet counts and morphology and express α-adducin normally in platelets (Robledo et al. 2008).
Platelets express several other cytoskeletal proteins previously characterized in the erythrocyte membrane cytoskeleton. These include the pointed-end capping protein tropomodulin and tropomyosin, which binds along the side of the short actin oligomers of the erythrocyte membrane cytoskeleton, where it is believed to control their length (Lux 2016). Megakaryocytes and platelets principally express tropomodulin 3 (human TMOD3, mouse Tmod3), deficiency of which is embryonic lethal in mice (Sui et al. 2014). Tmod3-null embryos develop thrombocytopenia with large platelets, due to impaired platelet production by megakaryocytes, which show increased actin assembly and actin redistribution from the cortical membrane cytoskeleton to the cytoplasm (Sui et al. 2015). In humans, SNPs in TPM1 and TPM4 encoding tropomyosin 1 and 4 have been associated with altered platelet counts and mean platelet volume (Soranzo et al. 2009; Gieger et al. 2011; Qayyum et al. 2012). However, further functional studies are required to determine the role of tropomyosin 1 and 4 in megakaryocytes and platelets.
Dematin is an actin-bundling protein of the erythrocyte membrane skeleton abundantly expressed in platelets (Wieschhaus et al. 2012). Dematin consists of a C-terminal headpiece and an N-terminal tail that both contain F-actin binding sites. Dematin binds to spectrin and enhances its binding to actin filaments. Platelets lacking the dematin headpiece exhibit defects in calcium mobilization in response to multiple agonists, associated with concomitant inhibition of platelet aggregation and granule secretion, unveiling dematin as a regulator of internal calcium mobilization affecting multiple platelet cytoskeletal and signaling functions.
Cross-Linking and Bundling Proteins
Actin filaments in the resting platelet are organized into a rigid cytoplasmic scaffold by filamin A (FlnA; previously known as ABP-280), which cross-links actin filaments at a distinctive 90° angle, and by α-actinins, which tend to align actin filaments in parallel bundles.
FlnA (human FLNA, mouse Flna) is a large antiparallel homodimer that cross-links actin filaments, tethers membrane glycoproteins, and serves as a scaffold for signaling intermediates (Falet 2013). FlnA contains an N-terminal tandem calponin-homology actin-binding domain followed by 24 immunoglobulin-like repeats of 90–100 amino acid residues, the C-terminal of which mediates dimerization (Nakamura et al. 2007; Seo et al. 2009). Two calpain-sensitive hinges separate FlnA repeats into an elongated and linear rod 1 (repeats 1–15) that can bind to actin filaments independently of the actin-binding domain, a compact rod 2 (repeats 16–23), and the self-association domain (repeat 24) (Nakamura et al. 2007). Rod 2 contains most of the binding sites for FlnA partners, e.g., the von Willebrand factor (VWF) receptor subunit GPIbα (repeat 17) and β-integrins (repeat 21) (Nakamura et al. 2006; Kiema et al. 2006).
FlnA traverses the pores of the spectrin-based membrane cytoskeleton to link the VWF receptor GPIb-IX, specifically the GPIbα subunit (Cranmer et al. 2005, 2011; Nakamura et al. 2006), to the underlying actin filaments (Fig. 1). The FlnA–GPIbα linkage initiates in mature megakaryocytes and is constitutively maintained during the platelet lifespan (Begonja et al. 2011). It plays a critical role in maintaining the platelet discoid shape, as has been established in studies of Bernard–Soulier syndrome (BSS) platelets lacking the VWF receptor subunits GPIbα, GPIbβ, or GPIX, or in platelets lacking functional FlnA, all of which are abnormally large in size, are fragile, and circulate poorly. FlnA also interacts with the β-subunits of integrins, thereby competing with talin 1 (Kiema et al. 2006), an essential activator of integrin αIIbβ3 in activated platelets (Petrich et al. 2007; Nieswandt et al. 2007). FlnA binding to β-integrin subunits is not constitutive, but regulated by mechanotransduction (Ehrlicher et al. 2011), the significance of which for platelet production and function remains to be investigated.
The gene encoding FlnA is X-linked in both humans and mice. FLNA mutations causing early truncation of FlnA are typically embryonic lethal in males, due to cardiovascular defects and hemorrhage, and lead to the brain malformation disorder, periventricular heterotopia (PH) in females (Feng and Walsh 2004). Missense mutations cause otopalatodigital (OPD) syndrome spectrum disorders, primarily affecting skeletal development. Thrombocytopenia, bleeding tendency, and giant platelets have been associated with FLNA mutations and multiple FlnA degradation products have been observed in platelets of female carriers (Nurden et al. 2011; Berrou et al. 2013; Li et al. 2015). However, the distribution of mutant platelets is not easily detectable and analysis of their hemostatic function is cumbersome, due to the mosaicity of the platelet population and the high proportion of normal platelets in female carriers.
In mice, Flna deficiency is embryonic lethal in hemizygous males, due to pericardiac and visceral hemorrhage, severe cardiac structural defects, and aberrant vascular patterning (Hart et al. 2006; Feng et al. 2006). Heterozygous female mice carrier for Flna deficiency have mild thrombocytopenia, and more than 95 % of their platelets contain FlnA, which can be differentiated from Flna-null platelets by intracellular flow cytometry (Falet et al. 2010). Mice specifically lacking FlnA in the megakaryocyte/platelet lineage develop severe thrombocytopenia, giant platelets, and increased tail bleeding time, due to severely impaired platelet hemostatic functions (Falet et al. 2010). As expected, GPIbα is not linked to the actin cytoskeleton in Flna-null megakaryocytes and platelets, where its surface expression is reduced and altered (Falet et al. 2010; Begonja et al. 2011). Flna-null megakaryocytes prematurely release large and fragile platelets that undergo microvesiculation and are cleared rapidly from the circulation by macrophages (Begonja et al. 2011). These observations corroborate earlier studies showing that cleavage of FlnA by the protease calpain causes microvesiculation in aggregating platelets or during platelet storage (Robey et al. 1979; Basse et al. 1994). Thus, the FlnA–GPIbα linkage not only regulates platelet size but also maintains the mechanical stability of the platelet plasma membrane. Flna-null megakaryocytes have impaired demarcation membrane system (DMS) formation, which is likely due to FlnA interaction with the endocytic membrane-binding and membrane-deforming Fes/CIP4 homology Bin/amphiphysin/Rvs (F-BAR) protein PACSIN2, an internal component of the initiating DMS (Begonja et al. 2015). Expression of the FlnA paralog FlnB has been reported in megakaryocytes and platelets (Falet et al. 2010; Kanaji et al. 2012). However, FlnB does not appear to compensate for loss of FlnA.
The role of α-actinins in the actin organization of resting platelets is less well defined. α-Actinins belong to the spectrin gene superfamily, including α/β-spectrins and dystrophins. α-Actinins are antiparallel homodimers composed of an N-terminal tandem calponin-homology actin-binding domain, four spectrin repeats, and a C-terminal calmodulin-like domain. Platelets primarily express α-actinins 1 and 4. α-Actinin 1 is an abundant actin filament bundling protein often associated with adhesion sites, where it binds to the cytoplasmic tail of β1-integrins, suggesting that α-actinin 1 links platelet actin filaments to the membrane, particularly at adhesion sites. Mutations in ACTN1 encoding α-actinin 1 have been associated with mild thrombocytopenia, large platelets, and low risk for bleeding (Kunishima et al. 2013; Gueguen et al. 2013; Bottega et al. 2015; Yasutomi et al. 2015). These mutations in the α-actinin 1 actin-binding domain enhance association with actin filaments (Murphy et al. 2016). Mouse fetal liver cell-derived megakaryocytes transfected with α-actinin 1 variants display altered proplatelet formation and size (Kunishima et al. 2013).
Focal Adhesion and Membrane-Associated Proteins
Focal adhesions are integrin-associated actin-rich structures that enable cells to adhere to the extracellular matrix and at which protein complexes involved in signal transduction assemble. Platelet activation leads to a conformational change of integrin αIIbβ3 to promote fibrinogen binding and platelet aggregation. Several cytoskeletal proteins modulate integrin αIIbβ3 activation, including talin 1 (human TLN1, mouse Tln1) and kindlin 3 (human FERMT3, mouse Fermt3). Talin 1 is composed of an N-terminal head domain binding to β-integrin subunits, followed by an elongated C-terminal actin-binding rod domain. Talin 1 binding to β3 is required for αIIbβ3 activation in platelets, as Tln1-null platelets have impaired agonist-mediated platelet aggregation (Petrich et al. 2007; Nieswandt et al. 2007). Mice specifically lacking talin 1 in platelets exhibit prolonged tail bleeding time and pathological gastrointestinal bleeding, similar to mice lacking the integrin β3 (Hodivala-Dilke et al. 1999). FERMT3 mutations cause leukocyte adhesion deficiency (LAD), which is characterized by impaired activation of the β2-integrin in leukocytes and β3 in platelets (Shattil et al. 2010; van de Vijver et al. 2012). Fermt3-null platelets exhibit defective integrin αIIbβ3 activation and impaired platelet aggregation (Moser et al. 2008). However, kindlins are unable to activate αIIbβ3 in the absence of talin 1 head domain (Ma et al. 2008; Harburger et al. 2009), suggesting that kindlins co-activate αIIbβ3 in synergy with talin 1.
Other notable platelet focal adhesion proteins include vinculin, zyxin, and paxillin. Vinculin (human VCL, mouse Vcl) links integrins to the actin cytoskeleton by virtue of binding of its N-terminal globular head domain to talin 1 and α-actinins, while its C-terminal tail region binds actin filaments, paxillin, and membrane phospholipids. Vinculin has been implicated in the transmission of mechanical forces from the extracellular matrix to the cytoskeleton of migrating cells (Bailly 2003). However, Vcl-null platelets display normal agonist-induced integrin αIIbβ3 activation, aggregation, spreading, and actin polymerization and organization and adhere normally to immobilized fibrinogen or collagen under both static and flow conditions (Mitsios et al. 2010), indicating that platelet vinculin is not required for the traditional functions of αIIbβ3 or the actin cytoskeleton. Both zyxin and paxillin contain lin-11/Islet-1/mec-3 (LIM) domains composed of zinc fingers that mediate protein–protein interactions and serve as targeting motif for focal adhesions. Similar to vinculin, the role of platelet zyxin and paxillin appears redundant based on the generation of mutant mice (Hoffman et al. 2003; Sakata et al. 2014).
Yeast two-hybrid system screening has identified skelemin (also called myomesin 1) as another cytoskeletal protein interacting with the integrin β3 cytoplasmic tail (Reddy et al. 1998). Skelemin is a member of a superfamily of cytoskeletal proteins that regulate the three-dimensional organization of myosin filaments in skeletal muscle cells. Skelemin does not associate with αIIbβ3 in resting platelets but is recruited to αIIbβ3 during cell adhesion (Podolnikova et al. 2009). Biochemical and structural studies have shown that the skelemin-binding domain on β3 is cryptic but becomes exposed as a result of αIIbβ3 binding to immobilized ligands (Podolnikova et al. 2009; Gorbatyuk et al. 2014). These studies illuminate a potential link between αIIbβ3 and actomyosin filaments, which may serve as a basis for generating the mechanical forces necessary for cell migration and remodeling.
Moesin is a member of the ezrin/radixin/moesin (ERM) family of proteins, which localize in cell extensions like filopodia and function as cross-linkers between the plasma membrane and actin filaments. Moesin associates with the tips of filopodia in spread platelets and associates with platelet/endothelial cell adhesion molecule 1 (PECAM-1) in lysates from thrombin-stimulated, but not resting platelets (Nakamura et al. 1995; Gamulescu et al. 2003), suggesting that moesin may play a role in platelet adhesion, linking PECAM-1 with the actin cytoskeleton. Mice lacking moesin develop normally and are fertile, with no obvious histological abnormalities. Particularly, targeted moesin deletion does not affect platelet aggregation in response to stimulation (Doi et al. 1999).
Contractile Proteins
Myosin-mediated contractile forces are critical for maintaining the integrity of a hemostatic plug at wound sites independently of thrombin and fibrin generation. The principal myosin isoform in platelets is non-muscle myosin heavy chain (NMMHC)-IIA (human MYH9, mouse Myh9). NMMHC-IIA is a parallel homodimer, whose two subunits are composed of an N-terminal head domain that binds actin filaments, a neck region, and a C-terminal coiled-coil rod domain holding the two chains together. A myosin regulatory light chain of 20 kDa (LC20, also called MRLC1) and an essential light chain of 17 kDa (LC17) bind each NMMHC-IIA subunit in the neck region, between the head and the tail. The functional properties of NMMHC-IIA are controlled by phosphorylation of both its heavy and light chains. Phosphorylated myosin assembles into filaments that are essential for its interaction with actin to form a contractile unit whose function is analogous to that of actomyosin filaments in smooth muscle cells. Phosphorylation of LC20 by myosin light chain kinase (MLCK), RhoA-associated kinase (ROCK), or p21-activated kinase 1 (PAK1) increases its actin-stimulated ATPase activity and is required for NMMHC-IIA to move along actin filaments, thereby providing contractile force. LC17 is believed to contribute to the structural stability of NMMHC-IIA along with LC20. NMMHC-IIA is homogeneously distributed in resting platelets (Painter and Ginsberg 1984). Following activation, NMMHC-IIA localizes in the platelet center and contributes to the initiation of platelet shape change, platelet internal contraction, granule exocytosis, and centralization of GPIb-IX on the platelet surface (Kovacsovics and Hartwig 1996; Johnson et al. 2007).
MYH9 mutations cause MYH9-related disease (MYH9-RD), previously classified as May–Hegglin anomaly or Epstein, Fechtner, or Sebastian syndromes and characterized by macrothrombocytopenia, mild bleeding tendency, and leukocyte inclusions, with or without loss of hearing, cataract, or nephritis (Favier and Raslova 2015). The mechanisms of thrombocytopenia underlying MYH9 mutations are linked to defects in proplatelet formation by megakaryocytes, which show decreased branching and increased tip size in cultures from patient progenitors (Pecci et al. 2010; Chen et al. 2013). In mice, NMMHC-IIA is required to form the megakaryocyte DMS and to maintain the megakaryocyte shape through internal tension and anchorage to the extracellular matrix (Eckly et al. 2009). Mice specifically lacking NMMHC-IIA in megakaryocytes develop severe macrothrombocytopenia. However, Myh9 deletion in mouse megakaryocytes results in enhanced proplatelet formation (Chen et al. 2007; Leon et al. 2007; Eckly et al. 2009), suggesting that large Myh9-null platelets are prematurely released from the bone marrow and cleared rapidly from the circulation.
MYH10-encoded NMMHC-IIB is expressed in immature megakaryocytes, where it specifically localizes in the contractile ring (Lordier et al. 2012). NMMHC-IIB expression is silenced by the hematopoietic Runt-related transcription factor 1 (RUNX1), a requirement for megakaryocyte ploidization and maturation. Anomalous NMMHC-IIB expression in platelets has been proposed as a tool to identify inherited platelet disorders and myeloid neoplasms with abnormalities in RUNX1 and associated proteins (Antony-Debre et al. 2012). Mutations in RUNX1 have also been associated with reduced platelet expression of MYL9-encoded LC20 (Sun et al. 2007; Jalagadugula et al. 2010).
The Marginal Band
Microtubules contribute to the maintenance of the discoid shape of the resting platelet (Sadoul 2015). Microtubules are highly dynamic tubular polymers formed by the polymerization of a dimer of α- and β-tubulin. Approximately 40 % of tubulin in platelets is assembled into a specialized microtubule coil, called the marginal band, which lies at the periphery of resting platelets, below the plasma membrane. Many proteins bind microtubules, including the motor proteins kinesin and dynein, and other proteins important for regulating microtubule dynamics.
The marginal band is maintained in its resting state by both tubulin acetylation and antagonistic microtubule motors (Sadoul 2015). During platelet activation, microtubules undergo major reorganization, thereby contributing to the shape change of activated platelets. A dramatic tubulin deacetylation mediated by the cytoplasmic histone deacetylase 6 (HDAC6) occurs (Sadoul et al. 2012; Aslan et al. 2013). Dynein slides microtubules apart, resulting in marginal band extension and further coiling. New microtubule polymerization within the coiled marginal band leads to the formation of a smaller microtubule ring, in concerted action with NMMHC-IIA-mediated tension (Diagouraga et al. 2014). Microtubules are then reacetylated in spread platelets, and the capacity of HDAC6 to prevent tubulin hyperacetylation influences the speed of platelet spreading.
Microtubules play an essential role in proplatelet formation by megakaryocytes (Italiano et al. 1999; Patel et al. 2005; Bender et al. 2015b). The predominant β-tubulin isoform in platelets and megakaryocytes is the hematopoietic β1-tubulin (human TUBB1, mouse Tubb1). Early studies have shown that Tubb1-null mice develop thrombocytopenia due to defective proplatelet production by megakaryocytes (Schwer et al. 2001). Circulating mouse Tubb1-null platelets have normal platelet size, but develop spherocytosis, lacking their characteristic discoid shape, and have defective marginal bands with reduced microtubule coilings and frequent kinks and breaks (Italiano et al. 2003). Following platelet activation, the disorganized microtubules in Tubb1-null platelets fail to condense into central rings and instead are dispersed in short bundles and linear arrays. More recently, an SNP in TUBB1 has been associated with altered mean platelet volume in humans (Gieger et al. 2011), and TUBB1 mutations have been associated with congenital macrothrombocytopenia due to impaired proplatelet formation by megakaryocytes (Kunishima et al. 2009, 2014; Stachele et al. 2015). The discrepancies between mouse and human phenotypes relative to platelet size and morphology are likely due to compensation by different β-tubulin isoforms.
The Cytoskeleton of the Activated Platelet
Actin Filament Assembly
Following receptor-mediated activation, platelet shape change proceeds through two recognizable steps. First, platelets convert from disc into an irregular or round shape. With further surface contact, platelets extend finger-like filopodia and spread thin sheet-like lamellipodia, as evidenced by electron microscopy (Fig. 2). Temporal and spatial actin filament assembly by specific cytoskeletal proteins orchestrates these morphological changes (Fig. 3 and Table 2) (Hartwig 1992).
Cytoskeleton of the activated platelet. Platelet activation is accompanied by a striking reorganization of the actin cytoskeleton. Platelets generate finger-like projections called filopodia (f) composed of long bundled actin filaments that derive from the cytoskeletal center. Platelets spread using thin sheet-like extensions called lamellipodia (lm) that are densely filled with a three-dimensional network of newly assembled short actin filaments
Regulation of platelet actin assembly. In resting platelets, CapZ caps actin filament barbed ends, and thymosin β4 sequesters ATP-bound actin monomers, thereby maintaining the large actin pool that drives actin filament assembly. Activation leads to rise of intracellular calcium and the activation of gelsolin. Gelsolin severs cortical actin filaments and caps the barbed ends, thereby inducing platelet rounding. PIP2 is robustly generated at the cytoplasmic surface of the platelet plasma membrane and inactivates gelsolin, thereby leading to the initiation of ATP-bound actin assembly onto exposed barbed ends and actin filament branching by the Arp2/3 complex. Actin filaments are subsequently buffered by CapZ, which recaps the barbed ends and terminates the assembly reaction. Subsequent actin filament treadmilling is stimulated by cofilin 1
In vitro, G-actin reversibly polymerizes into F-actin in three sequential phases defined as nucleation, elongation, and steady state. Nucleation is marked by a lag period in which G-actin slowly aggregates into short, unstable oligomers. Once a stable nucleus of three or four actin subunits is formed, it rapidly elongates into a filament by the addition of G-actin to its ends. In cells, actin monomers incorporated into filaments are bound to ATP. As filaments elongate, the bound ATP is slowly hydrolyzed to ADP. As a result of this hydrolysis, only actin filament barbed ends are composed of ATP-bound actin, and most of the filament consists of ADP-bound actin.
Actin Monomer-Sequestering Proteins and Actin Filament Capping Proteins
The G-actin–F-actin equilibrium of the resting platelet is principally maintained by two mechanisms that prohibit actin monomer addition to actin filaments (Fig. 3). First, actin subunits not incorporated into filaments complex with the actin monomer-sequestering proteins thymosin β4 and profilin 1. Second, virtually all actin filament barbed ends are constitutively capped by the high-affinity actin filament capping protein CapZ (Barkalow et al. 1996). Therefore, actin assembly in platelets occurs when actin monomers release from thymosin β4 and profilin 1 and add to uncapped actin filament barbed ends. Despite these control mechanisms, actin filaments are believed to dynamically turnover in resting platelets.
Thymosin β4 (human TMSB4X, mouse Tmsb4x) and profilin 1 (human PFN1, mouse Pfn1) are two of the most abundant proteins in platelets. Thymosin β4 forms a 1:1 complex with G-actin, in levels sufficient to account for essentially all sequestered actin monomers in platelets (Weber et al. 1992; Nachmias 1993). It has higher affinity for ATP-bound actin than for ADP-bound actin, suggesting that platelet thymosin β4 sequesters primarily ATP-bound actin monomers ready to assemble into filaments. The proportion of G-actin bound to thymosin β4 decreases following platelet activation (Nachmias et al. 1993). Tmsb4x deletion leads to partial embryonic lethality due to hemorrhage (Rossdeutsch et al. 2012). It is likely that other thymosins such as thymosin β10 compensate for thymosin β4 loss in mice reaching adulthood. Megakaryocyte-specific Pfn1 deletion in mice results in mild thrombocytopenia with small platelets due to premature release of platelets into the bone marrow and accelerated platelet clearance (Bender et al. 2014). However, actin assembly is minimally affected by profilin 1 deletion. Instead, Pfn1-null platelets contain hyper-stable and misarranged microtubules, revealing an unexpected novel function of profilin 1 as a regulator of microtubule organization.
CapZ (also called capping protein) is a key cellular component regulating actin filament assembly and organization, as it caps the barbed ends of actin filaments, preventing addition and loss of actin monomers. CapZ is a heterodimer composed of α- and β-subunits (Yamashita et al. 2003). The distribution of α-subunits varies among cell types, with platelets expressing both α1 and α2 (Nachmias et al. 1996; Hart et al. 1997). In platelets, the amount of CapZ bound to actin is proportional to that of F-actin (Barkalow et al. 1996). The kinetics of CapZ association with actin closely follows the kinetics of actin assembly and binding reaches a maximum when actin assembly ceases. The observations show that CapZ acts primarily as an actin assembly buffer to maintain actin filament barbed ends capped and inaccessible at rest and to terminate actin assembly in activated platelets by capturing exposed barbed ends. Because CapZ maintains most barbed ends capped in resting platelets (Barkalow et al. 1996), either filament ends must be uncapped, or barbed end nucleation sites must be generated de novo to trigger actin filament assembly. Both mechanisms contribute to actin filament assembly in activated platelets.
The Gelsolin Family
The actin filament severing and capping protein gelsolin (human GSN, mouse Gsn) is responsible for most of the actin assembly occurring during platelet activation (Witke et al. 1995; Barkalow et al. 1996). Gelsolin is one of the best-characterized molecules in terms of its effects on actin in vitro. It is activated to bind actin filaments in the presence of micromolar concentration of calcium. Association with actin begins with gelsolin binding to the side of an actin filament, followed by interdigitation into the filament to sever it. After severing, gelsolin remains on the newly formed actin filament barbed end until membrane phosphoinositides, particularly phosphatidylinositol 4,5-bisphosphate (PIP2), mediate its dissociation (Janmey and Stossel 1987).
In resting platelets, the cytoplasmic calcium level is low, and gelsolin is not associated with actin. Following platelet activation, cytoplasmic calcium rises within seconds and activates gelsolin to bind and sever long cortical actin filaments into short ones (Fig. 2) (Lind et al. 1987). Gelsolin severing destabilizes the highly ordered and constrained structure formed between the membrane cytoskeleton and actin filaments cross-linked by FlnA, initiating platelet rounding, before the actin assembly reaction begins. Subsequently, the small guanosine triphosphatase (GTPase) Rac1 is activated and stimulates the synthesis of membrane PIP2 that mediate the dissociation of gelsolin from actin filament barbed ends, thereby promoting actin assembly (Hartwig et al. 1995; Azim et al. 2000; McCarty et al. 2005; Aslan and McCarty 2013). These new ATP-bound actin filaments are preferred for Arp2/3 complex-mediated actin nucleation and branching (Fig. 3) (Falet et al. 2002b). Platelets from Gsn-null mice have a severe reduction in their capacity to generate actin filament barbed ends, assemble actin, and spread following platelet activation (Witke et al. 1995; Barkalow et al. 1996; Falet et al. 2000, 2002b; Hoffmeister et al. 2001). Gsn-null mice have prolonged bleeding time (Witke et al. 1995), but normal platelet counts and size, indicating that gelsolin is dispensable for platelet production and survival. Gelsolin-mediated actin filament severing can be blocked by vasodilator-stimulated phosphoprotein (VASP) binding to the sides of actin filaments. VASP is a major substrate of protein kinase A in platelets, where its phosphorylation correlates with reduced activation. Consistently, mouse platelets lacking VASP have slightly increased adhesion and spreading (Hauser et al. 1999; Massberg et al. 2004).
Platelets express at least two other actin severing and capping proteins of the gelsolin family: flightless 1 (human FLII, mouse Flii) and supervillin (human SVIL, mouse Svil). In platelets, flightless 1 is present in a complex with leucine-rich repeat (in FLII) interacting protein 1 (LRRFIP1), an SNP of which has been associated with altered platelet functions (Goodall et al. 2010). An SNP in SVIL has been associated with low supervillin expression in platelets and increased platelet thrombus formation in the shear-dependent platelet function analyzer (PFA)-100 (Edelstein et al. 2012). Consistent with these observations, Svil-null mouse platelets exhibit enhanced platelet thrombus formation at high shear stress, indicating that supervillin plays an inhibitory role in platelet adhesion and arterial thrombosis. The specific molecular details of flightless 1 and supervillin function in platelet actin assembly and remodeling have not yet been elucidated.
The ADF/Cofilin Family
Actin-depolymerizing factor (ADF)/cofilin is a family of actin-binding proteins that regulate both assembly and disassembly of actin filaments. Two distinct mechanisms have been proposed: severing and depolymerization. First, ADF/cofilin proteins can sever actin filaments to create barbed ends without capping, thereby promoting actin assembly. Second, ADF/cofilin proteins can depolymerize actin filaments at their pointed ends, thereby accelerating actin filament turnover in cells, a process called treadmilling (Carlier et al. 1997). ADF/cofilin proteins are primarily regulated by phosphorylation of serine 3 (Ser3), which inhibits their function.
Platelets express primarily non-muscle cofilin 1 (human CFL1, mouse Cfl1) and ADF (also called destrin; human DSTN, mouse Dstn). Cofilin 1 is phosphorylated on Ser3 in resting platelets and, therefore, is inactive (Davidson and Haslam 1994; Falet et al. 2005; Pandey et al. 2006). Following platelet activation, cofilin 1 is dephosphorylated and incorporates into the actin cytoskeleton, shortly after the peak of actin assembly occurs (Falet et al. 2005). Ser3 dephosphorylation and activation are maintained by αIIbβ3-mediated outside-in signals and are transient and reversible in the absence of integrin αIIbβ3 engagement, such as in platelets isolated from a patient with Glanzmann thrombasthenia expressing low integrin αIIbβ3 levels (Falet et al. 2005). The observations indicate that cofilin 1 is not essential for the initial polymerization of actin filaments that follows platelet activation, but for actin filament turnover mediated by outside-in signals (Fig. 3).
Cofilin 1-mediated actin filament turnover plays a critical role in the late stages of platelet production by megakaryocytes and in the proper sizing of platelets in the periphery, as mice specifically lacking cofilin 1 in the megakaryocyte lineage develop mild thrombocytopenia with large platelets due to defective platelet production (Bender et al. 2010). Macrothrombocytopenia is severe in mice expressing a germ line mutation in the cofilin 1 partner WD40 repeat-containing protein 1 (WDR1), the vertebrate homolog of actin-interacting protein 1 (AIP1) (Kile et al. 2007). Actin filament assembly and platelet spreading on immobilized fibrinogen are profoundly impaired in the absence of cofilin 1 (Bender et al. 2010). The observations show that cofilin 1 is essential for the maintenance of G-actin–F-actin equilibrium in resting platelets and for agonist-induced actin assembly. By contrast, Dstn-null mice lacking ADF have normal platelet counts, size, and actin assembly reaction following activation (Bender et al. 2010).
Actin Nucleation by the Arp2/3 Complex
The Arp2/3 complex plays a major role in the regulation of actin assembly in cells, as it nucleates actin filaments and localizes to the leading edge of crawling cells (Pollard and Borisy 2003). The Arp2/3 complex also initiates the actin-based motility of intracellular pathogenic bacteria such as Shigella flexneri and Listeria monocytogenes. The Arp2/3 complex is composed of two actin-related proteins, Arp2 and Arp3, and five additional subunits. Arp2 and Arp3 closely resemble the structure of G-actin and serve as nucleation sites for new actin filaments. In vitro, the Arp2/3 complex binds to the side of existing actin filaments to initiate the growth of a new filament at a 70° angle. These observations suggest that the Arp2/3 complex is responsible for generating a branched meshwork of actin filaments and de novo actin filament nucleation at the cell cortex that leads to cell movement. Binding of Arp2/3 complex to ATP-bound actin, as found at uncapped actin filament barbed ends, is preferred (Ichetovkin et al. 2002; Falet et al. 2002b). Therefore, the Arp2/3 complex does not explicitly initiate actin nucleation de novo, but rather amplifies actin filament barbed ends generated by other means, such as gelsolin- or cofilin-mediated severing, thereby exponentially doubling the number of actin filament barbed ends at each branching.
The Arp2/3 complex contributes to the burst of actin assembly that follows platelet activation, associates with the actin cytoskeleton, and redistributes rapidly and uniformly to the lamellar edge of spread platelets (Falet et al. 2002a, b). It is unlikely that the Arp2/3 complex associates to the tip of platelet filopodia, as has been described in one study (Li et al. 2002), because actin filaments in filopodia are bundled, not branched. The Arp2/3 complex contribution to actin filament nucleation in platelets importantly requires free barbed ends generated by gelsolin-mediated severing, capping, and uncapping (Fig. 2) (Falet et al. 2002b). The Arp2/3 complex clusters in marginal actin filament clumps in Gsn-null platelets and fibroblasts, consistent with few uncapped ATP-bound actin-containing barbed ends generated in the absence of gelsolin, placing gelsolin function upstream of Arp2/3 complex nucleation in these cells (Falet et al. 2002b).
The Arp2/3 complex is also recruited to sites of clathrin-mediated endocytosis in megakaryocytes and is responsible for the accumulation of clathrin-coated vesicles and F-actin clusters in megakaryocytes lacking the large GTPase dynamin 2 (DNM2) (unpublished observations). DNM2 normally mediates the fission of endocytic vesicles from the plasma membrane (Ferguson and De Camilli 2012). Mutations in DNM2 have been associated with mild thrombocytopenia and neutropenia in patients with Charcot–Marie–Tooth disease (Züchner et al. 2005). Mice specifically lacking DNM2 in the megakaryocyte lineage develop severe macrothrombocytopenia, megakaryocyte hyperplasia, myelofibrosis, extramedullary hematopoiesis, and severe and rapid splenomegaly due to impaired endocytosis in megakaryocytes and platelets (Bender et al. 2015a).
WASp Family and Actin Nucleation-Promoting Factors
Activation of the Arp2/3 complex requires actin nucleation-promoting factors (NPFs), such as WASp family proteins, which integrate signals leading to actin assembly (Pollard and Borisy 2003). WASp (human WAS, mouse Was) is the protein mutated in Wiskott–Aldrich syndrome (WAS), a rare X-linked recessive disorder characterized by severe immunodeficiency, eczema, and thrombocytopenia. The disease affects most non-erythroid hematopoietic lineages, including lymphocytes, monocytes, neutrophils, and platelets, which are among the most severely affected cells. X-linked thrombocytopenia (XLT) is a milder form of WAS, in which platelets are primarily affected. Platelets from WAS and XLT patients are abnormally small, and circulating platelet counts can be 10 % of normal or lower. The defect is likely due to increased platelet clearance, as WASp deficiency in megakaryocytes induces premature proplatelet formation and platelet production in the bone marrow compartment (Haddad et al. 1999; Sabri et al. 2006).
In platelets, WASp is present in a constitutive 1:1 complex with WASp-interacting protein (WIP; human WIPF1, mouse Wipf1) (Falet et al. 2009), and WAS mutation hotspots map to the WIP-binding region in WASp and disrupt the WIP–WASp complex interaction (Luthi et al. 2003). A homozygous mutation in WIPF1 has been reported in one patient to cause recurrent infections, eczema, and severe thrombocytopenia, all features of WAS (Lanzi et al. 2012). Cells isolated from the patient have undetectable WASp, but normal WAS sequence and mRNA levels. Wipf1-null mouse platelets lack WASp, and WIP expression is reduced in Was-null mouse platelets, indicating that both proteins are required for the stability of each subunit of the complex (Falet et al. 2009). Was-null and Wipf1-null mice have mild thrombocytopenia, and their platelets have normal size (Snapper et al. 1998; Zhang et al. 1999; Falet et al. 2009).
The role of the WIP–WASp complex in platelet shape change and actin assembly is unclear, as platelets lacking either WASp or WIP do not show any defect in actin assembly and shape change (Gross et al. 1999; Rengan et al. 2000; Falet et al. 2009), and the Arp2/3 complex redistributes normally to the actin cytoskeleton and to the edge of lamellipodia in the absence of WASp (Falet et al. 2002a). These observations suggest that platelets differ from the other hematopoietic cells affected by WASp deficiency, raising two fundamental questions. First, what is the role of WASp in platelets? Second, what is the identity of the NPFs activating the Arp2/3 complex in platelets? Recent studies have shown that human and mouse platelets lacking WASp contain abnormally organized and hyper-stable microtubules, suggesting an unexpected role of WASp as a regulator of microtubule organization (Bender et al. 2014). WASp also controls the delivery of platelet transforming growth factor-β1 (TGF-β1) (Kim et al. 2013) and regulates the formation of specific platelet actin structures, called actin nodules, which likely play a role in the early stages of platelet spreading (Calaminus et al. 2008; Poulter et al. 2015).
Platelets express other WASp family proteins and NPFs unrelated to WASp, which could theoretically activate the Arp2/3 complex. These include the ubiquitous WASp paralog neural WASp (N-WASP), WASp family verprolin homologs 1 (WAVE1) and 2 (WAVE2), cortactin, and hematopoietic lineage cell-specific protein 1 (HS1). An SNP in WASL encoding N-WASP has been associated with altered platelet counts (Soranzo et al. 2009; Gieger et al. 2011). However, the role of N-WASP in platelet production and function, particularly in Arp2/3 complex activation, is unclear. N-WASP can be detected in platelet lysates by immunoblot analysis (Shcherbina et al. 2001; Falet et al. 2002a), but its expression appears to be low because platelet lysates fail to initiate the Arp2/3 complex-dependent motility of bacteria expressing Shigella flexneri IcsA, which normally recruits and requires N-WASP in host cells (Egile et al. 1999).
WAVE1 (human WASF1, mouse Wasf1) and WAVE2 (human WASF2, mouse Wasf2) localize at the edge of lamellipodia and at the tips of filopodia in spread platelets (Kashiwagi et al. 2005). WAVE2 deficiency is lethal by embryonic day 12.5 in mice. Wasf2-null mouse megakaryocytes differentiated from embryonic stem cells are severely impaired in terminal differentiation, spreading onto fibrinogen and platelet production (Eto et al. 2007). By contrast, WAVE1 does not appear to be required for platelet production in vivo, as Wasf1-null mice have normal platelet counts (Eto et al. 2007), but is critical for platelet spreading and cytoskeletal reorganization downstream of the collagen receptor GPVI (Calaminus et al. 2007). Interestingly, Wasf1-null mouse platelets respond normally to stimulation by G-protein coupled receptors, indicating that platelet WAVE1 plays a signaling role in the GPVI signaling cascade, rather than a cytoskeletal role. Early studies have shown that cortactin redistributes to the cortex of spread platelets (Ozawa et al. 1995), similar to the Arp2/3 complex. However, cortactin and its hematopoietic specific paralog HS1 appear to be dispensable for platelet production, spreading, and lamellipodia formation in mice (Thomas et al. 2007; Schnoor et al. 2011).
Based on these observations, N-WASP and WAVE2 appear to be the best candidates for further investigation in their contribution to Arp2/3 complex activation in platelet production and function. N-WASP is believed to activate Arp2/3 complex-mediated actin nucleation activity to induce filopodia formation downstream of the small GTPase Cdc42, whereas WAVE2 orchestrates lamellae spreading downstream of Rac1 (Aslan and McCarty 2013). Rac1 deletion in platelets leads to impaired lamellipodia spreading, as well as to altered responses to GPVI stimulation due to defective phospholipase C-γ2 (PLC-γ2) activation (McCarty et al. 2005; Pleines et al. 2009, 2013). By contrast, platelets lacking Cdc42 display normal filopodia formation, but enhanced secretion and increased aggregation in response to agonist stimulation (Pleines et al. 2010). Platelet-specific Cdc42 deletion also leads to thrombocytopenia, due to impaired DMS formation and platelet production by megakaryocytes (Pleines et al. 2013).
Actin Nucleation by Formins
Formins are another group of proteins regulating actin nucleation (Goode and Eck 2007). Formins are involved in various cellular functions such as cell polarity, motility, and cytokinesis. At cellular levels, formins are required for assembly of stress fibers, cytoplasmic actin networks used for vesicle transport, cytokinetic actin rings, and phagocytic cups. Formins are multidomain proteins that interact with diverse signaling molecules and cytoskeletal proteins. Formins associate with the fast-growing barbed end of actin filaments via their signature formin homology 2 (FH2) domain. Formins initiate filament assembly and remain persistently attached to the barbed end without dissociating, enabling rapid insertion of actin subunits while protecting the end from capping proteins. Formins generate single actin filaments that are typically oriented orthogonally to membranes. Formins have also been shown to directly regulate microtubule dynamics (Chesarone et al. 2010).
Fifteen formin isoforms are present in humans, classified in seven subgroups. The most predominantly expressed diaphanous-related formins are autoinhibited through intramolecular interactions and appear to be activated by the small GTPase RhoA and additional factors. Other classes of formins lack the autoinhibitory and/or RhoA-binding domains and thus are likely to be controlled by alternative mechanisms. Three formins have been characterized biochemically in platelets: diaphanous homolog 1 (DIAPH1; human DIAPH1, mouse Diaph1), disheveled-associated activator of morphogenesis 1 (DAAM1; human DAAM1, mouse Daam1), and FH1/FH2 domain-containing protein 1 (FHOD1; human FHOD1, mouse Fhod1) (Higashi et al. 2008; Thomas et al. 2011). Based on profiling studies, platelets also appear to abundantly express inverted formin 2 (INF2; human INF2, mouse Inf2). However, its role in platelet formation and function has not yet been investigated.
DIAPH1 and DAAM1 purified from platelet extracts assemble actin in the presence of RhoA, suggesting that they contribute to actin dynamics in activated platelets downstream of RhoA (Higashi et al. 2008). A gain-of-function mutation in DIAPH1 has been associated with dominant macrothrombocytopenia (Stritt et al. 2016). DIAPH1 knockdown increases proplatelet formation by CD34+ cell-derived human megakaryocytes by decreasing their F-actin content, but increasing tubulin polymerization and stability (Pan et al. 2014). Inversely, DIAPH1 overexpression in megakaryocytes increases stress fiber formation. Interestingly, analysis of Diaph1-null mice reveals no alteration in platelet counts or platelet hemostatic function in response to stimulation, including spreading and clot retraction (Thomas et al. 2011), although mice lacking platelet RhoA develop macrothrombocytopenia (Pleines et al. 2012). Diaph1-null mice develop age-dependent myeloproliferative/myelodysplastic phenotypes, including splenomegaly, fibrotic and hypercellular bone marrow, and extramedullary hematopoiesis in both spleen and liver, suggesting that DIAPH1 acts as a tumor suppressor (Peng et al. 2007). FHOD1 undergoes rapid phosphorylation downstream of ROCK in activated platelets and may therefore be involved in the formation of stress fibers (Thomas et al. 2011). Further studies are required to establish the physiological role of formins in platelet actin dynamics.
Conclusion
The platelet cytoskeleton has two main functions: (1) maintain the discoid shape of the resting platelet and (2) mediate a rapid shape change in response to external stimuli at sites of vascular injury. At rest, specific cytoskeletal structures, such as the spectrin-based membrane cytoskeleton and cytoplasmic actin filaments cross-linked by FlnA or bundled by α-actinins, maintain the unique architecture of the platelet actin cytoskeleton and connect it to the plasma membrane. Actin monomer-sequestering proteins, such as thymosin β4, and actin filament capping proteins, such as CapZ, prevent the assembly of actin monomers onto the barbed ends of the actin filaments composing the resting cytoskeleton. Platelet activation leads to a rise in intracellular calcium and synthesis of membrane PIP2 to stimulate the activities of actin regulatory proteins, such as gelsolin and the Arp2/3 complex. The interactions of these cytoplasmic signals and molecules initiate and amplify actin filament growth necessary for platelet shape change.
References
Antony-Debre I, Bluteau D, Itzykson R, Baccini V, Renneville A, Boehlen F, Morabito M, Droin N, Deswarte C, Chang Y, Leverger G, Solary E, Vainchenker W, Favier R, Raslova H (2012) MYH10 protein expression in platelets as a biomarker of RUNX1 and FLI1 alterations. Blood 120(13):2719–2722
Aslan JE, McCarty OJ (2013) Rho GTPases in platelet function. J Thromb Haemost 11(1):35–46
Aslan JE, Phillips KG, Healy LD, Itakura A, Pang J, McCarty OJ (2013) Histone deacetylase 6-mediated deacetylation of α-tubulin coordinates cytoskeletal and signaling events during platelet activation. Am J Physiol Cell Physiol 305(12):C1230–C1239
Azim AC, Barkalow K, Chou J, Hartwig JH (2000) Activation of the small GTPases, rac and cdc42, after ligation of the platelet PAR-1 receptor. Blood 95(3):959–964
Bailly M (2003) Connecting cell adhesion to the actin polymerization machinery: vinculin as the missing link? Trends Cell Biol 13(4):163–165
Barkalow K, Witke W, Kwiatkowski DJ, Hartwig JH (1996) Coordinated regulation of platelet actin filament barbed ends by gelsolin and capping protein. J Cell Biol 134(2):389–399
Barkalow KL, Italiano JE Jr, Chou DE, Matsuoka Y, Bennett V, Hartwig JH (2003) Alpha-adducin dissociates from F-actin and spectrin during platelet activation. J Cell Biol 161(3):557–570
Basse F, Gaffet P, Bienvenue A (1994) Correlation between inhibition of cytoskeleton proteolysis and anti-vesiculation effect of calpeptin during A23187-induced activation of human platelets: are vesicles shed by filopod fragmentation? Biochim Biophys Acta 1190(2):217–224
Begonja AJ, Hoffmeister KM, Hartwig JH, Falet H (2011) FlnA-null megakaryocytes prematurely release large and fragile platelets that circulate poorly. Blood 118(8):2285–2295
Begonja AJ, Pluthero FG, Suphamungmee W, Giannini S, Christensen H, Leung R, Lo RW, Nakamura F, Lehman W, Plomann M, Hoffmeister KM, Kahr WHA, Hartwig JH, Falet H (2015) FlnA binding to PACSIN2 F-BAR domain regulates membrane tubulation in megakaryocytes and platelets. Blood 126(1):80–88
Bender M, Eckly A, Hartwig JH, Elvers M, Pleines I, Gupta S, Krohne G, Jeanclos E, Gohla A, Gurniak C, Gachet C, Witke W, Nieswandt B (2010) ADF/n-cofilin-dependent actin turnover determines platelet formation and sizing. Blood 116(10):1767–1775
Bender M, Stritt S, Nurden P, van Eeuwijk JM, Zieger B, Kentouche K, Schulze H, Morbach H, Stegner D, Heinze K, Dutting S, Gupta S, Witke W, Falet H, Fischer A, Hartwig JH, Nieswandt B (2014) Megakaryocyte-specific Profilin 1-deficiency alters microtubule stability and causes a Wiskott-Aldrich syndrome-like platelet defect. Nat Commun 5:4746
Bender M, Giannini S, Grozovsky R, Jonsson T, Christensen H, Pluthero FG, Ko A, Mullally A, Kahr WH, Hoffmeister KM, Falet H (2015a) Dynamin 2-dependent endocytosis is required for normal megakaryocyte development in mice. Blood 125(6):1014–1024
Bender M, Thon JN, Ehrlicher AJ, Wu S, Mazutis L, Deschmann E, Sola-Visner M, Italiano JE, Hartwig JH (2015b) Microtubule sliding drives proplatelet elongation and is dependent on cytoplasmic dynein. Blood 125(5):860–868
Berrou E, Adam F, Lebret M, Fergelot P, Kauskot A, Coupry I, Jandrot-Perrus M, Nurden A, Favier R, Rosa JP, Goizet C, Nurden P, Bryckaert M (2013) Heterogeneity of platelet functional alterations in patients with filamin a mutations. Arterioscler Thromb Vasc Biol 33(1):e11–e18
Bottega R, Marconi C, Faleschini M, Baj G, Cagioni C, Pecci A, Pippucci T, Ramenghi U, Pardini S, Ngu L, Baronci C, Kunishima S, Balduini CL, Seri M, Savoia A, Noris P (2015) ACTN1-related thrombocytopenia: identification of novel families for phenotypic characterization. Blood 125(5):869–872
Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, Geiger J, Sickmann A, Zahedi RP (2012) The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 120(15):e73–e82
Calaminus SD, McCarty OJ, Auger JM, Pearce AC, Insall RH, Watson SP, Machesky LM (2007) A major role for Scar/WAVE-1 downstream of GPVI in platelets. J Thromb Haemost 5(3):535–541
Calaminus SD, Thomas S, McCarty OJ, Machesky LM, Watson SP (2008) Identification of a novel, actin-rich structure, the actin nodule, in the early stages of platelet spreading. J Thromb Haemost 6(11):1944–1952
Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D (1997) Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol 136(6):1307–1322
Chen Z, Naveiras O, Balduini A, Mammoto A, Conti MA, Adelstein RS, Ingber D, Daley GQ, Shivdasani RA (2007) The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood 110(1):171–179
Chen Y, Boukour S, Milloud R, Favier R, Saposnik B, Schlegel N, Nurden A, Raslova H, Vainchenker W, Balland M, Nurden P, Debili N (2013) The abnormal proplatelet formation in MYH9-related macrothrombocytopenia results from an increased actomyosin contractility and is rescued by myosin IIA inhibition. J Thromb Haemost 11(12):2163–2175
Chesarone MA, DuPage AG, Goode BL (2010) Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol 11(1):62–74
Cranmer SL, Pikovski I, Mangin P, Thompson PE, Domagala T, Frazzetto M, Salem HH, Jackson SP (2005) Identification of a unique filamin A binding region within the cytoplasmic domain of glycoprotein Ibalpha. Biochem J 387(Pt 3):849–858
Cranmer SL, Ashworth KJ, Yao Y, Berndt MC, Ruggeri ZM, Andrews RK, Jackson SP (2011) High shear-dependent loss of membrane integrity and defective platelet adhesion following disruption of the GPIbalpha-filamin interaction. Blood 117(9):2718–2727
Davidson MM, Haslam RJ (1994) Dephosphorylation of cofilin in stimulated platelets: roles for a GTP-binding protein and Ca2+. Biochem J 301(Pt 1):41–47
Diagouraga B, Grichine A, Fertin A, Wang J, Khochbin S, Sadoul K (2014) Motor-driven marginal band coiling promotes cell shape change during platelet activation. J Cell Biol 204(2):177–185
Doi Y, Itoh M, Yonemura S, Ishihara S, Takano H, Noda T, Tsukita S (1999) Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J Biol Chem 274(4):2315–2321
Eckly A, Strassel C, Freund M, Cazenave JP, Lanza F, Gachet C, Leon C (2009) Abnormal megakaryocyte morphology and proplatelet formation in mice with megakaryocyte-restricted MYH9 inactivation. Blood 113(14):3182–3189
Edelstein LC, Luna EJ, Gibson IB, Bray M, Jin Y, Kondkar A, Nagalla S, Hadjout-Rabi N, Smith TC, Covarrubias D, Jones SN, Ahmad F, Stolla M, Kong X, Fang Z, Bergmeier W, Shaw C, Leal SM, Bray PF (2012) Human genome-wide association and mouse knockout approaches identify platelet supervillin as an inhibitor of thrombus formation under shear stress. Circulation 125(22):2762–2771
Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF (1999) Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol 146(6):1319–1332
Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP (2011) Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature 478(7368):260–263
Eto K, Nishikii H, Ogaeri T, Suetsugu S, Kamiya A, Kobayashi T, Yamazaki D, Oda A, Takenawa T, Nakauchi H (2007) The WAVE2/Abi1 complex differentially regulates megakaryocyte development and spreading: implications for platelet biogenesis and spreading machinery. Blood 110(10):3637–3647
Falet H (2013) New insights into the versatile roles of platelet FlnA. Platelets 24(1):1–5
Falet H, Barkalow KL, Pivniouk VI, Barnes MJ, Geha RS, Hartwig JH (2000) Roles of SLP-76, phosphoinositide 3-kinase, and gelsolin in the platelet shape changes initiated by the collagen receptor GPVI/FcR gamma-chain complex. Blood 96(12):3786–3792
Falet H, Hoffmeister KM, Neujahr R, Hartwig JH (2002a) Normal Arp2/3 complex activation in platelets lacking WASp. Blood 100(6):2113–2122
Falet H, Hoffmeister KM, Neujahr R, Italiano JE Jr, Stossel TP, Southwick FS, Hartwig JH (2002b) Importance of free actin filament barbed ends for Arp2/3 complex function in platelets and fibroblasts. Proc Natl Acad Sci U S A 99(26):16782–16787
Falet H, Chang G, Brohard-Bohn B, Rendu F, Hartwig JH (2005) Integrin alpha(IIb)beta3 signals lead cofilin to accelerate platelet actin dynamics. Am J Physiol Cell Physiol 289(4):C819–C825
Falet H, Marchetti MP, Hoffmeister KM, Massaad MJ, Geha RS, Hartwig JH (2009) Platelet-associated IgAs and impaired GPVI responses in platelets lacking WIP. Blood 114(21):4729–4737
Falet H, Pollitt AY, Begonja AJ, Weber SE, Duerschmied D, Wagner DD, Watson SP, Hartwig JH (2010) A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med 207(9):1967–1979
Favier R, Raslova H (2015) Progress in understanding the diagnosis and molecular genetics of macrothrombocytopenias. Br J Haematol 170(5):626–639
Feng Y, Walsh CA (2004) The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol 6(11):1034–1038
Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, Kwiatkowski DJ, Walsh CA (2006) Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A 103(52):19836–19841
Ferguson SM, De Camilli P (2012) Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13(2):75–88
Fox JE, Boyles JK (1988) Structure and function of the platelet membrane skeleton. Soc Gen Physiol Ser 43:111–123
Gamulescu MA, Seifert K, Tingart M, Falet H, Hoffmeister KM (2003) Platelet moesin interacts with PECAM-1 (CD31). Platelets 14(4):211–217
Gieger C, Radhakrishnan A, Cvejic A, Tang W, Porcu E, Pistis G, Serbanovic-Canic J, Elling U, Goodall AH, Labrune Y, Lopez LM, Magi R, Meacham S, Okada Y, Pirastu N, Sorice R, Teumer A, Voss K, Zhang W, Ramirez-Solis R, Bis JC, Ellinghaus D, Gogele M, Hottenga JJ, Langenberg C, Kovacs P, O’Reilly PF, Shin SY, Esko T, Hartiala J, Kanoni S, Murgia F, Parsa A, Stephens J, van der Harst P, Ellen van der Schoot C, Allayee H, Attwood A, Balkau B, Bastardot F, Basu S, Baumeister SE, Biino G, Bomba L, Bonnefond A, Cambien F, Chambers JC, Cucca F, D’Adamo P, Davies G, de Boer RA, de Geus EJ, Doring A, Elliott P, Erdmann J, Evans DM, Falchi M, Feng W, Folsom AR, Frazer IH, Gibson QD, Glazer NL, Hammond C, Hartikainen AL, Heckbert SR, Hengstenberg C, Hersch M, Illig T, Loos RJ, Jolley J, Tee Khaw K, Kuhnel B, Kyrtsonis MC, Lagou V, Lloyd-Jones H, Lumley T, Mangino M, Maschio A, Mateo Leach I, McKnight B, Memari Y, Mitchell BD, Montgomery GW, Nakamura Y, Nauck M, Navis G, Nothlings U, Nolte IM, Porteous DJ, Pouta A, Pramstaller PP, Pullat J, Ring SM, Rotter JI, Ruggiero D, Ruokonen A, Sala C, Samani NJ, Sambrook J, Schlessinger D, Schreiber S, Schunkert H, Scott J, Smith NL, Snieder H, Starr JM, Stumvoll M, Takahashi A, Tang WH, Taylor K, Tenesa A, Lay Thein S, Tonjes A, Uda M, Ulivi S, van Veldhuisen DJ, Visscher PM, Volker U, Wichmann HE, Wiggins KL, Willemsen G, Yang TP, Hua Zhao J, Zitting P, Bradley JR, Dedoussis GV, Gasparini P, Hazen SL, Metspalu A, Pirastu M, Shuldiner AR, Joost van Pelt L, Zwaginga JJ, Boomsma DI, Deary IJ, Franke A, Froguel P, Ganesh SK, Jarvelin MR, Martin NG, Meisinger C, Psaty BM, Spector TD, Wareham NJ, Akkerman JW, Ciullo M, Deloukas P, Greinacher A, Jupe S, Kamatani N, Khadake J, Kooner JS, Penninger J, Prokopenko I, Stemple D, Toniolo D, Wernisch L, Sanna S, Hicks AA, Rendon A, Ferreira MA, Ouwehand WH, Soranzo N (2011) New gene functions in megakaryopoiesis and platelet formation. Nature 480(7376):201–208
Gilligan DM, Sarid R, Weese J (2002) Adducin in platelets: activation-induced phosphorylation by PKC and proteolysis by calpain. Blood 99(7):2418–2426
Goodall AH, Burns P, Salles I, Macaulay IC, Jones CI, Ardissino D, de Bono B, Bray SL, Deckmyn H, Dudbridge F, Fitzgerald DJ, Garner SF, Gusnanto A, Koch K, Langford C, O’Connor MN, Rice CM, Stemple D, Stephens J, Trip MD, Zwaginga JJ, Samani NJ, Watkins NA, Maguire PB, Ouwehand WH, Bloodomics C (2010) Transcription profiling in human platelets reveals LRRFIP1 as a novel protein regulating platelet function. Blood 116(22):4646–4656
Goode BL, Eck MJ (2007) Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem 76:593–627
Gorbatyuk V, Nguyen K, Podolnikova NP, Deshmukh L, Lin X, Ugarova TP, Vinogradova O (2014) Skelemin association with alphaIIbbeta3 integrin: a structural model. Biochemistry 53(43):6766–6775
Gross BS, Wilde JI, Quek L, Chapel H, Nelson DL, Watson SP (1999) Regulation and function of WASp in platelets by the collagen receptor, glycoprotein VI. Blood 94(12):4166–4176
Gueguen P, Rouault K, Chen JM, Raguenes O, Fichou Y, Hardy E, Gobin E, Pan-Petesch B, Kerbiriou M, Trouve P, Marcorelles P, Abgrall JF, Le Marechal C, Ferec C (2013) A missense mutation in the alpha-actinin 1 gene (ACTN1) is the cause of autosomal dominant macrothrombocytopenia in a large French family. PLoS One 8(9):e74728
Haddad E, Cramer E, Riviere C, Rameau P, Louache F, Guichard J, Nelson DL, Fischer A, Vainchenker W, Debili N (1999) The thrombocytopenia of Wiskott Aldrich syndrome is not related to a defect in proplatelet formation. Blood 94(2):509–518
Harburger DS, Bouaouina M, Calderwood DA (2009) Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem 284(17):11485–11497
Hart MC, Korshunova YO, Cooper JA (1997) Vertebrates have conserved capping protein alpha isoforms with specific expression patterns. Cell Motil Cytoskeleton 38(2):120–132
Hart AW, Morgan JE, Schneider J, West K, McKie L, Bhattacharya S, Jackson IJ, Cross SH (2006) Cardiac malformations and midline skeletal defects in mice lacking filamin A. Hum Mol Genet 15(16):2457–2467
Hartwig JH (1992) Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol 118(6):1421–1442
Hartwig JH, DeSisto M (1991) The cytoskeleton of the resting human blood platelet: structure of the membrane skeleton and its attachment to actin filaments. J Cell Biol 112(3):407–425
Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP (1995) Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell 82(4):643–653
Hauser W, Knobeloch KP, Eigenthaler M, Gambaryan S, Krenn V, Geiger J, Glazova M, Rohde E, Horak I, Walter U, Zimmer M (1999) Megakaryocyte hyperplasia and enhanced agonist-induced platelet activation in vasodilator-stimulated phosphoprotein knockout mice. Proc Natl Acad Sci U S A 96(14):8120–8125
Higashi T, Ikeda T, Shirakawa R, Kondo H, Kawato M, Horiguchi M, Okuda T, Okawa K, Fukai S, Nureki O, Kita T, Horiuchi H (2008) Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets. J Biol Chem 283(13):8746–8755
Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO (1999) Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 103(2):229–238
Hoffman LM, Nix DA, Benson B, Boot-Hanford R, Gustafsson E, Jamora C, Menzies AS, Goh KL, Jensen CC, Gertler FB, Fuchs E, Fassler R, Beckerle MC (2003) Targeted disruption of the murine zyxin gene. Mol Cell Biol 23(1):70–79
Hoffmeister KM, Falet H, Toker A, Barkalow KL, Stossel TP, Hartwig JH (2001) Mechanisms of cold-induced platelet actin assembly. J Biol Chem 276(27):24751–24759
Ichetovkin I, Grant W, Condeelis J (2002) Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr Biol 12(1):79–84
Italiano JE Jr, Lecine P, Shivdasani RA, Hartwig JH (1999) Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol 147(6):1299–1312
Italiano JE Jr, Bergmeier W, Tiwari S, Falet H, Hartwig JH, Hoffmeister KM, Andre P, Wagner DD, Shivdasani RA (2003) Mechanisms and implications of platelet discoid shape. Blood 101(12):4789–4796
Jalagadugula G, Mao G, Kaur G, Goldfinger LE, Dhanasekaran DN, Rao AK (2010) Regulation of platelet myosin light chain (MYL9) by RUNX1: implications for thrombocytopenia and platelet dysfunction in RUNX1 haplodeficiency. Blood 116(26):6037–6045
Janmey PA, Stossel TP (1987) Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature 325(6102):362–364
Johnson GJ, Leis LA, Krumwiede MD, White JG (2007) The critical role of myosin IIA in platelet internal contraction. J Thromb Haemost 5(7):1516–1529
Kanaji T, Ware J, Okamura T, Newman PJ (2012) GPIbalpha regulates platelet size by controlling the subcellular localization of filamin. Blood 119(12):2906–2913
Kashiwagi H, Shiraga M, Kato H, Honda S, Sako M, Kurata Y, Kanakura Y, Tomiyama Y (2005) Expression and subcellular localization of WAVE isoforms in the megakaryocyte/platelet lineage. J Thromb Haemost 3(2):361–368
Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, Campbell ID, Ylanne J, Calderwood DA (2006) The molecular basis of filamin binding to integrins and competition with talin. Mol Cell 21(3):337–347
Kile BT, Panopoulos AD, Stirzaker RA, Hacking DF, Tahtamouni LH, Willson TA, Mielke LA, Henley KJ, Zhang JG, Wicks IP, Stevenson WS, Nurden P, Watowich SS, Justice MJ (2007) Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia. Blood 110(7):2371–2380
Kim H, Falet H, Hoffmeister KM, Hartwig JH (2013) Wiskott-Aldrich syndrome protein (WASp) controls the delivery of platelet transforming growth factor-beta1. J Biol Chem 288(48):34352–34363
Kovacsovics TJ, Hartwig JH (1996) Thrombin-induced GPIb-IX centralization on the platelet surface requires actin assembly and myosin II activation. Blood 87(2):618–629
Kunishima S, Kobayashi R, Itoh TJ, Hamaguchi M, Saito H (2009) Mutation of the beta1-tubulin gene associated with congenital macrothrombocytopenia affecting microtubule assembly. Blood 113(2):458–461
Kunishima S, Okuno Y, Yoshida K, Shiraishi Y, Sanada M, Muramatsu H, Chiba K, Tanaka H, Miyazaki K, Sakai M, Ohtake M, Kobayashi R, Iguchi A, Niimi G, Otsu M, Takahashi Y, Miyano S, Saito H, Kojima S, Ogawa S (2013) ACTN1 mutations cause congenital macrothrombocytopenia. Am J Hum Genet 92(3):431–438
Kunishima S, Nishimura S, Suzuki H, Imaizumi M, Saito H (2014) TUBB1 mutation disrupting microtubule assembly impairs proplatelet formation and results in congenital macrothrombocytopenia. Eur J Haematol 92(4):276–282
Lanzi G, Moratto D, Vairo D, Masneri S, Delmonte O, Paganini T, Parolini S, Tabellini G, Mazza C, Savoldi G, Montin D, Martino S, Tovo P, Pessach IM, Massaad MJ, Ramesh N, Porta F, Plebani A, Notarangelo LD, Geha RS, Giliani S (2012) A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. J Exp Med 209(1):29–34
Leon C, Eckly A, Hechler B, Aleil B, Freund M, Ravanat C, Jourdain M, Nonne C, Weber J, Tiedt R, Gratacap MP, Severin S, Cazenave JP, Lanza F, Skoda R, Gachet C (2007) Megakaryocyte-restricted MYH9 inactivation dramatically affects hemostasis while preserving platelet aggregation and secretion. Blood 110(9):3183–3191
Li Z, Kim ES, Bearer EL (2002) Arp2/3 complex is required for actin polymerization during platelet shape change. Blood 99(12):4466–4474
Li J, Dai K, Wang Z, Cao L, Bai X, Ruan C (2015) Platelet functional alterations in a Bernard-Soulier syndrome patient with filamin A mutation. J Hematol Oncol 8:79
Lind SE, Janmey PA, Chaponnier C, Herbert TJ, Stossel TP (1987) Reversible binding of actin to gelsolin and profilin in human platelet extracts. J Cell Biol 105(2):833–842
Lordier L, Bluteau D, Jalil A, Legrand C, Pan J, Rameau P, Jouni D, Bluteau O, Mercher T, Leon C, Gachet C, Debili N, Vainchenker W, Raslova H, Chang Y (2012) RUNX1-induced silencing of non-muscle myosin heavy chain IIB contributes to megakaryocyte polyploidization. Nat Commun 3:717
Luthi JN, Gandhi MJ, Drachman JG (2003) X-linked thrombocytopenia caused by a mutation in the Wiskott-Aldrich syndrome (WAS) gene that disrupts interaction with the WAS protein (WASP)-interacting protein (WIP). Exp Hematol 31(2):150–158
Lux SE (2016) Anatomy of the red cell membrane skeleton: unanswered questions. Blood 127(2):187–199
Ma YQ, Qin J, Wu C, Plow EF (2008) Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol 181(3):439–446
Massberg S, Gruner S, Konrad I, Garcia Arguinzonis MI, Eigenthaler M, Hemler K, Kersting J, Schulz C, Muller I, Besta F, Nieswandt B, Heinzmann U, Walter U, Gawaz M (2004) Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)-deficient mice. Blood 103(1):136–142
McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP (2005) Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem 280(47):39474–39484
Mitsios JV, Prevost N, Kasirer-Friede A, Gutierrez E, Groisman A, Abrams CS, Wang Y, Litvinov RI, Zemljic-Harpf A, Ross RS, Shattil SJ (2010) What is vinculin needed for in platelets? J Thromb Haemost 8(10):2294–2304
Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R (2008) Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med 14(3):325–330
Murphy AC, Lindsay AJ, McCaffrey MW, Djinovic-Carugo K, Young PW (2016) Congenital macrothrombocytopenia-linked mutations in the actin-binding domain of alpha-actinin-1 enhance F-actin association. FEBS Lett 590:685–695
Nachmias VT (1993) Small actin-binding proteins: the beta-thymosin family. Curr Opin Cell Biol 5(1):56–62
Nachmias VT, Cassimeris L, Golla R, Safer D (1993) Thymosin beta 4 (T beta 4) in activated platelets. Eur J Cell Biol 61(2):314–320
Nachmias VT, Golla R, Casella JF, Barron-Casella E (1996) Cap Z, a calcium insensitive capping protein in resting and activated platelets. FEBS Lett 378(3):258–262
Nakamura F, Amieva MR, Furthmayr H (1995) Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem 270(52):31377–31385
Nakamura F, Pudas R, Heikkinen O, Permi P, Kilpelainen I, Munday AD, Hartwig JH, Stossel TP, Ylanne J (2006) The structure of the GPIb-filamin A complex. Blood 107(5):1925–1932
Nakamura F, Osborn TM, Hartemink CA, Hartwig JH, Stossel TP (2007) Structural basis of filamin A functions. J Cell Biol 179(5):1011–1025
Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D, Fassler R (2007) Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med 204(13):3113–3118
Nurden P, Debili N, Coupry I, Bryckaert M, Youlyouz-Marfak I, Sole G, Pons AC, Berrou E, Adam F, Kauskot A, Lamaziere JM, Rameau P, Fergelot P, Rooryck C, Cailley D, Arveiler B, Lacombe D, Vainchenker W, Nurden A, Goizet C (2011) Thrombocytopenia resulting from mutations in filamin A can be expressed as an isolated syndrome. Blood 118(22):5928–5937
Ozawa K, Kashiwada K, Takahashi M, Sobue K (1995) Translocation of cortactin (p80/85) to the actin-based cytoskeleton during thrombin receptor-mediated platelet activation. Exp Cell Res 221(1):197–204
Painter RG, Ginsberg MH (1984) Centripetal myosin redistribution in thrombin-stimulated platelets. Relationship to platelet Factor 4 secretion. Exp Cell Res 155(1):198–212
Pan J, Lordier L, Meyran D, Rameau P, Lecluse Y, Kitchen-Goosen S, Badirou I, Mokrani H, Narumiya S, Alberts AS, Vainchenker W, Chang Y (2014) The formin DIAPH1 (mDia1) regulates megakaryocyte proplatelet formation by remodeling the actin and microtubule cytoskeletons. Blood 124(26):3967–3977
Pandey D, Goyal P, Bamburg JR, Siess W (2006) Regulation of LIM-kinase 1 and cofilin in thrombin-stimulated platelets. Blood 107(2):575–583
Patel SR, Richardson JL, Schulze H, Kahle E, Galjart N, Drabek K, Shivdasani RA, Hartwig JH, Italiano JE Jr (2005) Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood 106(13):4076–4085
Patel-Hett S, Wang H, Begonja AJ, Thon JN, Alden EC, Wandersee NJ, An X, Mohandas N, Hartwig JH, Italiano JE Jr (2011) The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation. Blood 118(6):1641–1652
Pecci A, Panza E, De Rocco D, Pujol-Moix N, Girotto G, Podda L, Paparo C, Bozzi V, Pastore A, Balduini CL, Seri M, Savoia A (2010) MYH9 related disease: four novel mutations of the tail domain of myosin-9 correlating with a mild clinical phenotype. Eur J Haematol 84(4):291–297
Peng J, Kitchen SM, West RA, Sigler R, Eisenmann KM, Alberts AS (2007) Myeloproliferative defects following targeting of the Drf1 gene encoding the mammalian diaphanous related formin mDia1. Cancer Res 67(16):7565–7571
Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, Ginsberg MH (2007) Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med 204(13):3103–3111
Pleines I, Elvers M, Strehl A, Pozgajova M, Varga-Szabo D, May F, Chrostek-Grashoff A, Brakebusch C, Nieswandt B (2009) Rac1 is essential for phospholipase C-gamma2 activation in platelets. Pflugers Arch 457(5):1173–1185
Pleines I, Eckly A, Elvers M, Hagedorn I, Eliautou S, Bender M, Wu X, Lanza F, Gachet C, Brakebusch C, Nieswandt B (2010) Multiple alterations of platelet functions dominated by increased secretion in mice lacking Cdc42 in platelets. Blood 115(16):3364–3373
Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C, Nieswandt B (2012) Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 119(4):1054–1063
Pleines I, Dutting S, Cherpokova D, Eckly A, Meyer I, Morowski M, Krohne G, Schulze H, Gachet C, Debili N, Brakebusch C, Nieswandt B (2013) Defective tubulin organization and proplatelet formation in murine megakaryocytes lacking Rac1 and Cdc42. Blood 122(18):3178–3187
Podolnikova NP, O’Toole TE, Haas TA, Lam SC, Fox JE, Ugarova TP (2009) Adhesion-induced unclasping of cytoplasmic tails of integrin alpha(IIb)beta3. Biochemistry 48(3):617–629
Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112(4):453–465
Poulter NS, Pollitt AY, Davies A, Malinova D, Nash GB, Hannon MJ, Pikramenou Z, Rappoport JZ, Hartwig JH, Owen DM, Thrasher AJ, Watson SP, Thomas SG (2015) Platelet actin nodules are podosome-like structures dependent on Wiskott-Aldrich syndrome protein and ARP2/3 complex. Nat Commun 6:7254
Qayyum R, Snively BM, Ziv E, Nalls MA, Liu Y, Tang W, Yanek LR, Lange L, Evans MK, Ganesh S, Austin MA, Lettre G, Becker DM, Zonderman AB, Singleton AB, Harris TB, Mohler ER, Logsdon BA, Kooperberg C, Folsom AR, Wilson JG, Becker LC, Reiner AP (2012) A meta-analysis and genome-wide association study of platelet count and mean platelet volume in African Americans. PLoS Genet 8(3):e1002491
Reddy KB, Gascard P, Price MG, Negrescu EV, Fox JE (1998) Identification of an interaction between the m-band protein skelemin and beta-integrin subunits. Colocalization of a skelemin-like protein with beta1- and beta3-integrins in non-muscle cells. J Biol Chem 273(52):35039–35047
Rengan R, Ochs HD, Sweet LI, Keil ML, Gunning WT, Lachant NA, Boxer LA, Omann GM (2000) Actin cytoskeletal function is spared, but apoptosis is increased, in WAS patient hematopoietic cells. Blood 95(4):1283–1292
Robey FA, Freitag CM, Jamieson GA (1979) Disappearance of actin binding protein from human blood platelets during storage. FEBS Lett 102(2):257–260
Robledo RF, Ciciotte SL, Gwynn B, Sahr KE, Gilligan DM, Mohandas N, Peters LL (2008) Targeted deletion of alpha-adducin results in absent beta- and gamma-adducin, compensated hemolytic anemia, and lethal hydrocephalus in mice. Blood 112(10):4298–4307
Rossdeutsch A, Smart N, Dube KN, Turner M, Riley PR (2012) Essential role for thymosin beta4 in regulating vascular smooth muscle cell development and vessel wall stability. Circ Res 111(4):e89–e102
Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS (2011) Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 118(14):e101–e111
Sabri S, Foudi A, Boukour S, Franc B, Charrier S, Jandrot-Perrus M, Farndale RW, Jalil A, Blundell MP, Cramer EM, Louache F, Debili N, Thrasher AJ, Vainchenker W (2006) Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood 108(1):134–140
Sadoul K (2015) New explanations for old observations: marginal band coiling during platelet activation. J Thromb Haemost 13(3):333–346
Sadoul K, Wang J, Diagouraga B, Vitte AL, Buchou T, Rossini T, Polack B, Xi X, Matthias P, Khochbin S (2012) HDAC6 controls the kinetics of platelet activation. Blood 120(20):4215–4218
Sakata A, Ohmori T, Nishimura S, Suzuki H, Madoiwa S, Mimuro J, Kario K, Sakata Y (2014) Paxillin is an intrinsic negative regulator of platelet activation in mice. Thromb J 12(1):1
Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D (2011) Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med 208(8):1721–1735
Schwer HD, Lecine P, Tiwari S, Italiano JE Jr, Hartwig JH, Shivdasani RA (2001) A lineage-restricted and divergent beta-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr Biol 11(8):579–586
Seo MD, Seok SH, Im H, Kwon AR, Lee SJ, Kim HR, Cho Y, Park D, Lee BJ (2009) Crystal structure of the dimerization domain of human filamin A. Proteins 75(1):258–263
Shattil SJ, Kim C, Ginsberg MH (2010) The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 11(4):288–300
Shcherbina A, Miki H, Kenney DM, Rosen FS, Takenawa T, Remold-O’Donnell E (2001) WASP and N-WASP in human platelets differ in sensitivity to protease calpain. Blood 98(10):2988–2991
Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, Kwan SP, Ferrini R, Davidson L, Bhan AK, Alt FW (1998) Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 9(1):81–91
Soranzo N, Spector TD, Mangino M, Kuhnel B, Rendon A, Teumer A, Willenborg C, Wright B, Chen L, Li M, Salo P, Voight BF, Burns P, Laskowski RA, Xue Y, Menzel S, Altshuler D, Bradley JR, Bumpstead S, Burnett MS, Devaney J, Doring A, Elosua R, Epstein SE, Erber W, Falchi M, Garner SF, Ghori MJ, Goodall AH, Gwilliam R, Hakonarson HH, Hall AS, Hammond N, Hengstenberg C, Illig T, Konig IR, Knouff CW, McPherson R, Melander O, Mooser V, Nauck M, Nieminen MS, O’Donnell CJ, Peltonen L, Potter SC, Prokisch H, Rader DJ, Rice CM, Roberts R, Salomaa V, Sambrook J, Schreiber S, Schunkert H, Schwartz SM, Serbanovic-Canic J, Sinisalo J, Siscovick DS, Stark K, Surakka I, Stephens J, Thompson JR, Volker U, Volzke H, Watkins NA, Wells GA, Wichmann HE, Van Heel DA, Tyler-Smith C, Thein SL, Kathiresan S, Perola M, Reilly MP, Stewart AF, Erdmann J, Samani NJ, Meisinger C, Greinacher A, Deloukas P, Ouwehand WH, Gieger C (2009) A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet 41(11):1182–1190
Stachele J, Bakchoul T, Najm J, Felbor U, Knofler R (2015) Congenital macrothrombocytopenia associated with a combination of functional polymorphisms in the TUBB1 gene. Hamostaseologie 35(Suppl 1):S18–S21
Stritt S, Nurden P, Turro E, Greene D, Jansen SB, Westbury SK, Petersen R, Astle WJ, Marlin S, Bariana TK, Kostadima M, Lentaigne C, Maiwald S, Papadia S, Kelly AM, Stephens JC, Penkett CJ, Ashford S, Tuna S, Austin S, Bakchoul T, Collins P, Favier R, Lambert MP, Mathias M, Millar CM, Mapeta R, Perry DJ, Schulman S, Simeoni I, Thys C, Consortium BB, Gomez K, Erber WN, Stirrups K, Rendon A, Bradley JR, van Geet C, Raymond FL, Laffan MA, Nurden AT, Nieswandt B, Richardson S, Freson K, Ouwehand WH, Mumford AD (2016) A gain-of-function variant in DIAPH1 causes dominant macrothrombocytopenia and hearing loss. Blood 127(23):2903–2914
Sui Z, Nowak RB, Bacconi A, Kim NE, Liu H, Li J, Wickrema A, An XL, Fowler VM (2014) Tropomodulin3-null mice are embryonic lethal with anemia due to impaired erythroid terminal differentiation in the fetal liver. Blood 123(5):758–767
Sui Z, Nowak RB, Sanada C, Halene S, Krause DS, Fowler VM (2015) Regulation of actin polymerization by tropomodulin-3 controls megakaryocyte actin organization and platelet biogenesis. Blood 126(4):520–530
Sun L, Gorospe JR, Hoffman EP, Rao AK (2007) Decreased platelet expression of myosin regulatory light chain polypeptide (MYL9) and other genes with platelet dysfunction and CBFA2/RUNX1 mutation: insights from platelet expression profiling. J Thromb Haemost 5(1):146–154
Thomas SG, Calaminus SD, Auger JM, Watson SP, Machesky LM (2007) Studies on the actin-binding protein HS1 in platelets. BMC Cell Biol 8:46
Thomas SG, Calaminus SD, Machesky LM, Alberts AS, Watson SP (2011) G-protein coupled and ITAM receptor regulation of the formin FHOD1 through Rho kinase in platelets. J Thromb Haemost 9(8):1648–1651
van de Vijver E, Maddalena A, Sanal O, Holland SM, Uzel G, Madkaikar M, de Boer M, van Leeuwen K, Koker MY, Parvaneh N, Fischer A, Law SK, Klein N, Tezcan FI, Unal E, Patiroglu T, Belohradsky BH, Schwartz K, Somech R, Kuijpers TW, Roos D (2012) Hematologically important mutations: leukocyte adhesion deficiency (first update). Blood Cells Mol Dis 48(1):53–61
Weber A, Nachmias VT, Pennise CR, Pring M, Safer D (1992) Interaction of thymosin beta 4 with muscle and platelet actin: implications for actin sequestration in resting platelets. Biochemistry 31(27):6179–6185
Wieschhaus AJ, Le Breton GC, Chishti AH (2012) Headpiece domain of dematin regulates calcium mobilization and signaling in platelets. J Biol Chem 287(49):41218–41231
Witke W, Sharpe AH, Hartwig JH, Azuma T, Stossel TP, Kwiatkowski DJ (1995) Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell 81(1):41–51
Yamashita A, Maeda K, Maeda Y (2003) Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J 22(7):1529–1538
Yasutomi M, Kunishima S, Okazaki S, Tanizawa A, Tsuchida S, Ohshima Y (2015) ACTN1 rod domain mutation associated with congenital macrothrombocytopenia. Ann Hematol 95:141–144
Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, McGavin M, Kozieradzki I, dos Santos AO, Nagy A, Grinstein S, Penninger JM, Siminovitch KA (1999) Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med 190(9):1329–1342
Züchner S, Noureddine M, Kennerson M, Verhoeven K, Claeys K, De Jonghe P, Merory J, Oliveira SA, Speer MC, Stenger JE, Walizada G, Zhu D, Pericak-Vance MA, Nicholson G, Timmerman V, Vance JM (2005) Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet 37(3):289–294
Acknowledgments
Supported by bridge grant awards from the Brigham Research Institute and the American Society of Hematology and National Institutes of Health, National Heart, Lung, and Blood Institute R01 grant HL126743. I thank Dr. John Hartwig for providing the electron microscopy image in Fig. 2.
Take-Home Messages
The platelet cytoskeleton: (1) maintains the discoid shape of the resting platelet and (2) mediates a rapid shape change in response to external stimuli at sites of vascular injury.
In resting platelets: (1) the spectrin-based membrane cytoskeleton and cytoplasmic actin filaments cross-linked by FlnA or bundled by α-actinins maintain the unique architecture of the platelet actin cytoskeleton and connect it to the plasma membrane and (2) actin monomer-sequestering proteins such as thymosin β4 and actin filament capping proteins such as CapZ prevent the assembly of actin monomers onto the barbed ends of the actin filaments composing the resting cytoskeleton.
In activated platelets: a rise in intracellular calcium and synthesis of membrane PIP2 stimulate the activities of actin regulatory proteins, such as gelsolin and the Arp2/3 complex, to initiate and amplify actin filament growth necessary for platelet shape change.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Falet, H. (2017). Anatomy of the Platelet Cytoskeleton. In: Gresele, P., Kleiman, N., Lopez, J., Page, C. (eds) Platelets in Thrombotic and Non-Thrombotic Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-47462-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-47462-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47460-1
Online ISBN: 978-3-319-47462-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)