Abstract
Due to rapid increase in population and explosive evolution of life standards, there is tremendous increase in solid waste generation in the last few decades. Furthermore, most of the countries are going to be industrialized; hence more amount of energy will be required in upcoming decades. Today’s more than 85% of the world demanded energy is supplied by fossil fuels. Fossil fuels are finite source of energy and therefore it is necessary to find out other alternatives for energy generation. Improper management of solid waste (MSW, waste biomass, etc.) is responsible for climate change, water and soil and local air pollution. These wastes have a high value with respect to energy recovery. The energy generation from the biological waste materials has been identified as alternative to the fossil fuels due to it’s dual benefit of resource generation and waste minimization. Anaerobic conversion of solid waste biomass is a matured technology for environmental protection and waste management. The end products are biogas (a mixture of methane and carbon dioxide), which is a useful, renewable energy source and organic manure slurry which can be used as fertilizer for agricultural purposes. Anaerobic digestion is a simple process, used to convert organic material (from a wide range of solid waste) into methane. This paper is mainly focused on the anaerobic digestion of solid waste biomass to produce methane, technologies related to pre-treatment of feed materials and post treatment of product gas to enrich the methane composition and the value addition of product fractions are also discussed.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
A remarkable rise in solid waste generation has been observed in the last few decades owing to explosive growth of population with comfortable life standards. Sustainable management of this huge quantity of solid waste has become one of the biggest challenges for mankind [1]. Unplanned dumping or dumping solid waste in engineered landfills is a common practice today, specifically in developing countries. With increasing population, land availability is decreasing day by day and such unsustainable methods of waste disposal not only occupy and deplete the land resources but also lead to anaesthetic and unpleasant environment. Another challenge faced by the whole world today is exponential increase in energy demand against its production and availability [2, 3]. At present around 88% requirement of electrical and thermal energy is met by non-renewable sources [4]. It is well known that these non-renewable resources of energy are going to exist for a finite time, therefore number of researches are going on to explore new renewable sources and to establish such technologies which can utilize existing renewable sources to its optimum extent.
There are different methods available to treat the various solid wastes. For biodegradable waste also, there are number of treatment methods available such as aerobic composting, anaerobic digestion, anaerobic fermentation, pyrolysis, incineration with or without energy recovery, etc. [5]. Out of these methods, anaerobic digestion is one of the most promising technologies to address both of the above mentioned issues. In this method, putrescible wastes are biologically degraded in absence of oxygen with the help of anaerobic bacteria in order to produce compost and biogas [6]. Biogas produced from this process majorly consists of methane (CH4), carbon dioxide (CO2) with some impurities of moisture, hydrogen sulfide (H2S), ammonia (NH3), siloxane, particulate matter, etc. Biogas can be converted to energy directly by combustion or it can be cleaned and enriched in terms of methane to increase its calorific value and to make it as a clean fuel [7].
2 Process of Waste Degradation and Biogas Generation
Degradation of putrescible fraction of the waste in an anaerobic digester takes place in four stages, namely: hydrolysis by hydrolysing bacteria, acidogenesis by acidifying bacteria, acetogenesis by acetogenic bacteria and methanogenesis by methanogenic bacteria [4, 7].
2.1 Hydrolysis
This is considered to be the first stage of anaerobic digestion in which hydrolyzing bacteria transforms complex compounds such as protein, carbohydrates and fats into simpler compounds such as amino acids, monosaccharides, soluble fatty acids and other monomers [4]. Hydrolysis can also be considered as pre-treatment of solid waste for further reactions such as acidogenesis. This step is considered to be the rate limiting step of whole process of anaerobic digestion [7, 8]. Various kinds of enzymes such as proteases, lipases and cellulases are also involved in the process of degradation of proteins, lipids or fats, and cellulose, respectively. Rate of this process depends on various parameters such as pH of the medium, enzyme production, particle size, etc.
2.2 Acidogenesis
Products of hydrolysis such as fatty acids, amino acids, sugars and other water soluble chemical substances are then undergo acidogenesis where monomers are converted into volatile fatty acids (acetic acid, butyric acid, propionic acid, formic acid), aldehydes, alcohols and gases (carbon dioxide and hydrogen). Anaerobic microorganisms use amino acids and peptides as source of energy which is derived from decomposition of proteins [4].
2.3 Acetogenesis
The acetates and hydrogen to be used by methanogenic bacteria in methanogenesis phase are produced in this phase by the acetate bacteria. It is stated in literature that the microorganisms which carry out this process, gets affected by toxic effects of hydrogen produced in this phase. Therefore a syntropy exist between acetate bacteria (producing hydrogen gas) and autotrophic methane bacteria (require hydrogen to produce methane gas). Acetogenesis is considered to be a very important phase of the process of biogas generation, as the methane produced from the acetate reduction accounts for 70% (approximately) of total methane generated in the process [4].
2.4 Methanogenesis
In this phase, products of previous phase (acetic acid, hydrogen, carbon disulphide and methanol, etc.) are converted into methane by methanogenic bacteria. Heterotrophic methane bacteria are responsible for conversion of acetic acid into methane whereas autotrophic methane bacteria are responsible for CO2 reduction into methane [4].
3 Feedstock for Anaerobic Digestion
There are variety of feedstocks which has shown the potential to generate biogas by anaerobic digestion such as waste from the dairy, food and feed industries, sludge from municipal wastewater treatment plants, wastes from slaughterhouses, kitchen (food) waste, garden waste, animal waste, crop waste from agriculture, etc. The amount and the composition of the biogas produced depend on the operating conditions as well as the type of substrates used. While selecting feedstock for anaerobic digestion it should be well ensured that it is able to fulfil all nutritional requirements of microorganisms. Usually organic material fed to the digester is of varying composition, therefore the composition and quantity of biogas produced also varies. Organic material fed to the digester majorly composed of carbohydrates, fat and protein. All these components provide different quantity of biogas with different composition when undergo anaerobic digestion. With support of previous researches, Schnurer [9] has reported that carbohydrates produce 0.38 m3 of biogas/kg VS with ratio of CH4:CO2 reaching approximately 50:50. Similarly fats are reported to produce 1 m3 of biogas/kg VS with 70:30 ratio of CH4:CO2, and Proteins are reported to produce 0.53 m3 of biogas/kg VS with CH4:CO2 ratio approximately 60:40 where VS stands for volatile solids. These data can be used for theoretical calculation of biogas production but there are several factors which affects the process and thus amount of biogas produced. Schnurer [9] has also reported approximate methane yield (m3 CH4/ton VS) by various substrate such as for food waste it varies from 400 to 600, for slaughterhouse waste it is 700 approximately, from sugar beets it ranges from 300 to 800 and for municipal sludge it shows in the range of 160–350, etc. To get better results, codigestion of different (mixed) substrate should be done instead of a single type of waste.
Results can be further improved by pre-treatment of substrate [10, 11]. Type of pre-treatment depends on type of waste; certain pre-treatment such as size reduction can be used to increase the efficiency of process when size of the substrate is bigger. There are other pre-treatments also such as chemical treatment of cellulosic material can be done to break its crystalline structure and increase the rate of degradation. Pre-treatment of saturated fats with heat is also in practice to increase their digestibility. Other Pre-treatment methods such as chemical solubilisation, thermo-chemical liquidization, wet oxidation and mechanical disruption are also used to increase the decomposition of VS, destruction of pathogens and production of methane [1].
4 Parameters Influencing the Process
The quantity and composition of biogas produced through digestion process largely depends on the operating parameters such as pH, temperature, loading rate, retention time, moisture content, degree of digestion, mixing and C/N ratio [9, 12].
-
(i)
pH: pH of the system varies during different stages of anaerobic digestion, such as during acidogenesis, pH of the system falls in acidic category whereas methanogenic activities requires pH to be in the range of 6.5–7. Therefore it is very much necessary to regulate and maintain the pH at desired range at various stages in the entire digestion process.
-
(ii)
Temperature: All the physical and chemical processes are very sensitive to temperature. The optimum temperature or temperature range is therefore very necessary for a process/reaction to occur for maximum product yield. The progress of anaerobic digestion is strongly influenced by the activities of various microorganisms. It is reported in [12] that the optimum temperatures for psychrophilic microorganisms is 10 °C, for mesophilic microorganisms this range is 20–45 °C and for thermophilic microorganisms it is even greater than 50 °C.
-
(iii)
Loading rate: Loading rate in a digester defines food availability for microorganisms. A very high loading rate can create an imbalance in system, as much of substrate will remain undecomposed because microorganisms will not be able to decompose all substrate provided.
-
(iv)
Retention time: Retention time is very much dependent on feedstock. Complex compound such as fibre and cellulosic material need more time for hydrolyses to get converted into simpler compound, thus overall retention time increases, on the other hand, easily degradable feedstock such as sugar, does not require hydrolysis therefore retention time decreases. Usually it varies from 10 to 30 days. In colder atmosphere retention time may go as high as 100 days.
-
(v)
Degree of digestion: For same retention time degree of digestion is usually much more for easily degradable material such as sugar as comparative to complex material such as fibre. More retention time is an indicator of high degree of digestion.
-
(vi)
Mixing: Mixing is the essential part of an anaerobic digester. A gentle mixing serves number of purposes in an anaerobic digester such as:
-
It provides uniform temperature throughout the process.
-
It facilitates contact between the nutrients, substrate and microorganisms.
-
Material accumulation at the bottom of digestion tank is also prevented by proper mixing and thus risk of foaming is also reduced.
-
Transfer of hydrogen between microorganisms responsible to carry out anaerobic oxidation and methane producers is also facilitated by mixing.
-
Gentle mixing helps in aggregates formation and thus helps in preventing the washing out of methane producers.
-
Uneven loading in the digestion tank can also be avoided by continuous mixing which avoids sedimentation and thus utilizes the existing digestion tank volume.
-
-
(vii)
C/N ratio: The substrate act as a source of energy for microorganisms, it must be able to meet the nutritional requirements (C and N) of the microorganisms. Microorganisms consume carbon (C) as their energy sources and nitrogen (N) as to build new cells. Therefore, It is important that the C/N ratio neither should be too low (too much nitrogen relative to carbon) nor should be too high (too much carbon relative to nitrogen). Too low C/N ratio means that carbon will get exhausted very fast as compared to nitrogen. This ultimately will lead to ammonia formation (undesirable because of ammonia inhibition). Too high C/N ratio means that significant amount of carbon remains undecomposed which ultimately leads to poor performance of digester [12].
5 Enrichment of Biogas
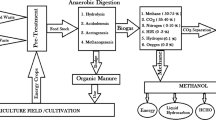
As discussed earlier, Biogas produced from anaerobic digestion mainly consists of methane (CH4) and carbon dioxide (CO2). The types of substrate used, fermentation technology and collection method can all affect the production and composition of raw biogas. Besides CH4 and CO2, raw biogas also contains some trace amounts of ammonia (NH3), hydrogen sulphide (H2S), hydrogen (H2), oxygen (O2), nitrogen (N2), water vapour, carbon monoxide (CO), and Siloxanes [13, 14]. Raw biogas contains about 50–65% CH4, 30–45% CO2 and traces of other impurities. The biogas gas having 50% of methane has the heating value of 21 MJ/m3, while upgraded biogas with 100% methane has a heating value of 33.41 MJ/m3, which indicates that upgraded biogas be utilized as a renewable energy source in combined heat and power plants, as a vehicle fuel, or as a substitute for natural gas [13,14,15,16]. The overall process for production of upgraded biogas (bio-methane) can be easily understood with the help of Fig. 1.
6 Effect of Impurities
Presence of impurities in biogas (as mentioned in previous section) not only reduces the heating value but can also cause corrosion and mechanical wear of the equipment. CO2 in biogas reduces the heating value. It takes up space when biogas is compressed and stored in cylinder. It can cause freezing problems at valves and metering points where the compressed gas undergoes expansion during engine running. It also causes corrosion (low concentrated carbonic acid) if the gas is wet. It damages the alkali fuel if biogas is used as a fuel in internal combustion engine [15].
The traces of H2S which is hazardous in nature, it produces H2SO4 which corrodes the internals of pipes, fittings, etc. It emits sulphur dioxide after burning [13, 15]. Water vapour in the biogas will lead corrosion in equipment and piping systems, this water vapours may condense and responsible for damage in instruments used in the plants. It may also lead the risk of freezing of piping system and nozzles [15, 16].
The presence of nitrogen is also lower the heating value of biogas. Traces of dust can block nozzles and fuel cells [13, 15, 16]. Siloxanes are compounds containing a silicon-oxygen bond. While burning siloxanes, silicon oxide, a white powder, is formed which can create a problem in gas engines. It acts like an abrasive and can damage engines [15, 16].
7 Bio Gas Cleaning and Upgrading Technologies
Biogas cleaning and upgrading can be referred to as biogas enrichment. Biogas cleaning is removal of corrosive products, mainly H2S, H2O, and particles while the upgrading involves removal of CO2 to increase heating value of the biogas [15, 17]. The technology used for the CO2 separation, can also remove other acidic gases, H2S and trace of nitrogen from biogas. Nevertheless, pre separation of some of these components like H2S, if present in higher amount, is necessary before the up-gradation step since these components can cause operational problems in upgrading process [17].
7.1 Biogas Cleaning
7.1.1 Removal of Hydrogen Sulphide
-
a.
Sulphide precipitation
Hydrogen sulphide is formed during microbiological reduction of sulphur containing compounds (sulphates, peptides, amino acids). The hydrogen sulphide removal can be start from the digesting tank with help of various metal salts. When Fe2+ or Fe3+ ions are added in the forms of FeCl2, FeCl3, or FeSO4, to the digester, the sulphur content of the substrate is precipitated in the form of insoluble iron sulphide. This method is primarily used for high sulphur content in the biogas [13, 14, 16]. Several technologies are also available to remove H2S from the biogas like adsorption, absorption and biological scrubbing.
-
b.
Adsorption on metal oxides
H2S can be removed by the selective adsorption onto the metal oxides like iron oxide, zinc oxide, copper oxide or on the activated carbon. During the adsorption of H2S on the metal oxides, sulphur is adhered as metal sulphide and water vapour is released [14,15,16]. The adsorption on activated carbon is usually carried out with the addition of small amount of oxygen, this oxygen helps to oxidise H2S into sulphur that binds to the surface. The rate of reaction can be enhanced by the doping or impregnating the pores of the activated carbon with potassium iodide, potassium carbonate or zinc oxide [15, 16]. Although, zinc oxide doping is expensive, it is still most preferable due to its ability to remove H2S to less than 1 ppm in biogas [16]. This technique is useful when either H2S content is very low in the raw biogas or the technology is used for final desulphurisation [14].
-
c.
Biological desulphurisation
The H2S can also be removed through oxidation by chemoautotrophic microorganisms. Most of the sulphide oxidising micro-organisms belong to the family of Thiobacillus or sulfolobus. For the microbiological oxidation of sulphide it is essential to add stoichiometric amounts of oxygen to the biogas. The degradation can occur inside the digester and can be facilitated by immobilizing the microorganisms occurring naturally in the digestate [14, 16, 18]. Depending on the temperature, reaction time, amount and place of the air/oxygen added, the hydrogen sulphide concentration can be reduced to less than 150 ppm [18]. This method seems to be a cost effective and environment friendly solution, since it can proceed at lower temperatures and pressures, and with limited or no reagent consumption [14, 19].
-
d.
Chemical-oxidative scrubbing
Chemical absorption is also one of the ways to remove H2S, as H2S is acidic in nature; it can easily be absorbed in the alkali solutions. Absorption in caustic solutions is one of the oldest methods for gas desulphurization. Nowadays NaOH is used as caustic and by controlling the pH selective separation may occur. Iron-chelated solution catalysed by ferric ethylenediamine tetraacetic acid (Fe/EDTA) has also been used for removal of hydrogen sulphide from raw biogas [20]. Chemical absorption of H2S in the iron chelated solutions offers a high efficiency and selectivity with low consumption of chemical [15, 21]. The conversion of hydrogen sulphide into elemental sulphur is catalysed by Fe/EDTA, The elemental sulphur is then easily removed from the slurry by sedimentation or by filtration operations. The entire process can be performed at ambient temperature [15].
7.1.2 Removal of Water
Water vapour in raw biogas can be removed by condensation achieved by either increasing pressure or by reducing the temperature. It can also be removed by the adsorption in which raw gas is passed over silicon dioxide or activated carbon packed bed [16, 22].
7.1.3 Removal of Siloxanes
There are several methods available for siloxanes removal from bio gas. Adsorption on the adsorbent (like activated carbon, activated alumina, silica gel, etc.) is one of the well-established technologies for siloxanes removal. Other existing methods include gas-liquid absorption and separation by refrigeration and condensation. Recently, membrane separation and biological degradation have been tested for siloxanes removal [15, 16]. Siloxanes can also be separated during the H2S removal.
7.1.4 Removal of Ammonia
Ammonia is formed during the degradation of proteins. Its concentration depends on the type of digested substrate. The concentration of ammonia in biogas is usually very low and no separate system is required for its removal. It can be removed during gas drying in the process of biogas up-gradation [15, 16].
7.1.5 Removal of Particulates
Presence of particulate in biogas can cause wear in gas and turbine engines. Particulates can be removed by passing biogas over mechanical filters [15].
7.2 Biogas Upgrading Techniques
A number of different methods available for biogas up-gradation to bio-methane are discussed in this section.
7.2.1 Absorption
In this method, separation is achieved based on the difference in the solubility of the gaseous components in an absorbent. During the up-gradation, raw biogas is contacted in counter current manner with absorbent over a packed bed filled with suitable packing material. Packing materials is used to increase the interfacial contact area and therefore increases the rate of absorption [13,14,15,16].
7.2.1.1 Water Scrubbing
In water scrubbing, water is used as absorbing solvent. The solubility of methane is much lower than the solubility of carbon dioxide in water especially at lower temperature. Literature reveals that the solubility of carbon dioxide is approximately 26 times higher than that of methane at 25 °C [15, 22, 23]. In packed column carbon dioxide is absorbed in the water while the gas stream gets enriched with methane. In principle, H2S can also be removed along with CO2, Since the H2S is more soluble than CO2 in water. As H2S is poisonous and dissolved H2S is responsible for corrosion problems. Therefore, H2S separation is necessary before the up-gradation of biogas. CH4 purity can be achieved to 80–99% by water scrubbing (depending upon the presence of non-condensable gases) [13, 15].
7.2.1.2 Physical Absorption
In principle, physical absorption is very similar to the water scrubbing. In place of water, organic solvent such as methanol, polyethylene glycol-dimethyl ether (PEG-DME), rectisol, selexol, etc. are used as absorbents [13,14,15, 24]. CO2 is more soluble in these organic solvents than in water. Hence, less scrubbing liquid circulation and smaller apparatuses are required for the same capacity [14].
7.2.1.3 Chemical Absorption
Chemical absorption process is accomplished with a reaction occurs between CO2 and a suitable chemical absorbent or a mixture of absorbents. Chemical absorption is more advantageous over physical and water scrubbing in its capacity to absorb more CO2 [25]. Chemical absorbents such as monoethanolamine (MEA), diethanolamine (DEA), methyldiethanolamine (MDEA), and di-2-propanolamine (DIPA) are commonly used. Other absorbents such as, diglycolamine (DGA), 2-(2-aminoethylamino) ethanol (AEE), 2-amino 2-methyl 1-propanol (AMP), N-2-aminoethyl 1,3-propanediamine (AEPDNH2), triethanolamine (TEA), triethylene tetra amine (TETA), piperazine (PZ), glucosamine (GA), NaOH, NH3, K2CO3, KOH, Na2CO3, etc. were also tested for CO2 removal, however their individual limitations make them less suitable for large scale operation. Nowadays concept of blending, in which two or more absorbents mixed in varying concentrations are used. A blend of MDEA and PZ are used industrially for biogas up-gradation. Chemical absorption with amines solution is a well established and mature technology, complete CO2 removal is achievable by this [15, 25, 26].
7.2.2 Pressure Swing Adsorption
Selective retention of a solute molecule on a solid surface is known as adsorption. Significant intermolecular forces between gases (including CO2) and the solid surfaces are mainly responsible for selective separation. Depending on the temperature, partial pressure, surface force and adsorbent pore sizes, single or multiple layers of gases can be adsorbed [24]. In pressure swing adsorption (PSA), carbon dioxide is separated from the biogas by adsorption on a surface under elevated pressure. The adsorbing material, usually activated carbon or zeolite, is regenerated by a sequential decrease in pressure. During the CO2 adsorption, H2S is also adsorbed irreversibly and thus H2S considered toxic to PSA. Therefore, H2S removal is necessary prior to PSA. Methane purity about 96–98% can be achieved by PSA [13, 15, 16].
7.2.3 Membrane Technology
Membranes are consists of semi-permeable barriers that separate specific component(s) from gas stream by various mechanisms (solution/diffusion, adsorption/diffusion, molecular sieve and ionic transport). In biogas upgrading, the carbon dioxide is diffused through membrane while methane is retained on it. Commercially feasible membranes for biogas upgrading are made of polymeric materials like cellulose acetate, polycarbonate, polyetherimide, polysulfone, polyimide or polydimethylsiloxane [14, 15, 23, 24]. The presence of H2S, leads softening/plasticization of membrane and thus it limits the use of membrane. Hence, H2S should be removed before up-gradation of biogas by membrane [27].
7.2.4 Cryogenic Techniques
This process involves compression and cooling of the gas mixtures in several stages to induce phase change. The cryogenic technique is based on the difference in the condensing temperature of various components contained in a biogas stream. The separation of gas components is achieved through condensation and distillation [13, 15]. The process starts with compression of raw biogas to 17–26 bar and then cooling to −26 ◦C for removal of H2S, SO2, and siloxane [15]. Further cooling of biogas reaches a temperature where CO2 gas is liquefied and separated by several condensers. The water content in biogas should be minimal in order to avoid freezing in the cooling units by dry ice or unacceptably high rise in pressure drop during operation. Therefore, water traces from the bio gas need to be pre-separated. In view of these limitations and high cost of refrigeration, this process can be used only for special circumstances as an adjunct to other processes [13, 24].
7.2.5 Hydrate Based Separation
Gas hydrate is a promising way to separate gas component(s) from a gas mixture (biogas). The difference in hydrate formation tendency of various species at specified conditions is main responsible to separate a gas component. The basic mechanism is the selective partition of the target component between the hydrate phase and the gaseous phase [13, 24]. This technology has been successfully used to remove CO2 from contaminated natural gas, for a CH4 to CO2 ratio of 3:1 and the concentration of CO2 can be reduced to 16%. However, the amount of associated CH4 removed with CO2 is still relatively high [13].
7.2.6 Biological Separation
Recently, the enzymatic based CO2 separation has also been used to enrich biogas. This is based on the naturally occurring reactions of CO2 in living organism. Carbonic anhydrases (CA) rapidly and selectively catalyse the hydration of CO2 to bicarbonate. This selective movement of CO2 in to the liquid phase is helpful for biogas enrichment. Currently, these enzymes are also being used with some chemical solvents for CO2 absorption. It is reported that absorption of CO2 in alkanolamines can be catalysed very fast at ambient condition using small amount of enzymes (such as carbonic anhydrate). Addition of CA enzyme to solution enhances the CO2 dissolution and forms carbonic acid, the production of carbonic acid adds value to this process. The rate of CO2 dissolution in water in this technology is limited by aqueous CO2 hydration and CO2 carrying capacity is limited by buffering capacity. Apart from this, production of enzymes can be costly due the difficulties of cell culture and enzyme purification and extraction from cellular materials [24, 28,29,30].
8 Conclusion
The biogas produced from putrescible fraction of waste has the potential to be used as fuel. The digestion process is mainly completed in four different stages as hydrolysis, acidogenesis, acetogenesis and methanogenesis by activity of specific microorganisms in each of these steps. The amount and composition of raw biogas is strongly dependent on the type of substrate, pre-treatment method, process and operating parameters. The temperature and pH are the two key parameters which affect the yield of biogas. The presence of impurities on raw biogas limits its use as vehicular fuel. The CO2 is responsible for low heating while other traces of impurities are responsible for operational issues. Among the all available technologies, amine based chemical absorption for upgradation of biogas is well established and mature technology. Through this technology, complete CO2 and H2S removal can be achieved.
References
Marin J, Kennedy KJ, Eskicioglu C (2010) Effect of microwave irradiation on anaerobic degradability of model kitchen waste. Waste Manag (in press)
Masebinu SO, Aboyade A, Muzenda E (2014) Enrichment of biogas for use as vehicular fuel: a review of the upgrading techniques. Int J Res Chem Metall Civil Eng (IJRCMCE) 1(1):2349–1450
Haladova D, Cundr O, Pecen J (2011) Selection of optimal anaerobic digestion technology for family sized farm use—case study of southwest Madagascar. Agricultura tropica et subtropica 44(3)
Zieminski K, Frac M (2012) Methane fermentation process as anaerobic digestion of biomass: transformations, stages and microorganisms. Afr J Biotechnol 11(18):4127–4139
Tabasova A, Kropac J, Kermes V, Nemet A, Stehlík P (2012) Waste-to-energy technologies: impact on environment. Energy 44:146–155
Priadi C, Wulandari D, Rahmatika W, Moersidik SS (2014) Biogas production in the anaerobic digestion of paper sludge. APCBEE Procedia 9:65–69
Griffin LP (2012) Anaerobic digestion of organic wastes: the impact of operating conditions on hydrolysis efficiency and microbial community composition. A thesis, Colorado State University
Trzcinski AP, Stuckey DC (2012) Determination of the hydrolysis constant in the biochemical methane potential test of municipal solid waste. Environ Eng Sci 29:848–854
Schnurer A, Jarvis A (2010) Microbiological handbook for biogas plants. Swed Waste Manag U 2009:03
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Lo KV, Liao PH (1986) Methane production from fermentation of winery waste. Biomass 9:19–27
Muzenda E (2014) Bio-methane generation from organic waste: a review. In: Proceedings of the world congress on engineering and computer science (WCECS)
Sun Q, Li H, Yan J, Liu L, Yu Z, Yu X (2015) Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew Sustain Energy Rev 51:521–532
Intelligent Energy—Europe Programme (2012) Biogas to biomethane technology review. www.thvt.at
Masebinu SO, Aboyade A, Muzenda E (2014) Enrichment of biogas for use as vehicular fuel: a review of the upgrading techniques. Int J Res Chem Metall Civil Eng (IJRCMCE) 1(1). ISSN 2349-1442, EISSN 2349-1450
Petersson A, Wellinger A (2009) Biogas upgrading technologies developments and innovation. IEA Bioenergy
Persson M (2003) Evaluation of upgrading techniques for biogas. Swedish Gas Center-Report, 142. ISSN 1102-7371. ISRN SGC-R-142-SE
Ramos I, Pérez R, Reinoso M, Torio R, Fdz-Polanco M (2014) Microaerobic digestion of sewage sludge on an industrial-pilot scale: the efficiency of biogas desulphurisation under different configurations and the impact of O2 on the microbial communities. Bioresour Technol 164:338–346
Syed M, Soreanu G, Faletta P, Béland M (2006) Removal of hydrogen sulfide from gas streams using biological processes—a review. Canadian Biosyst Eng 48:2.1–2.14
Horikawa MS, Rossi F, Gimenes ML, Costa CMM, da Silva MGC (2004) Chemical absorption of H2S for biogas purification. Braz J Chem Eng 21(03):415–422
Reijenga JC, Bini L, Maassen JI, Van Meel PA, De Hullu J, Shazad S, Vaessen JM (2008) Comparing different biogas upgrading techniques. Interim Report, Eindhoven University of Technology
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York
Bauer F, Hulteberg C, Persson T, Tamm D (2013) Biogas upgrading—review of commercial technologies. Svenskt Gastekniskt Center (SGC) AB, Malmö, Sweden
Mondal MK, Balsora HK, Varshney P (2012) Progress and trends in CO2 capture/separation technologies: a review. Energy 46:431–441
Nie H, Jiang H, Chong D, Wu Q, Xu C, Zhou H (2013) Comparison of water scrubbing and propylene carbonate absorption for biogas upgrading process. Energy Fuels 27:3239–3245
Prakash VD, Vijaykumar MV (2006) Quickly design CO2-amine absorber. Indian J Chem Technol 13:47–52
Cnop T, Dortmundt D, Schott M (2007) Continued development of gas separation membrane for highly sour service. UOP LLC, Illinios, USA
William CF, Sarah EB, Carlos AV, Joshuah KS, Jane PB, Joe HS Jr, Roger DA (2009) Evaluation of a carbonic anhydrase mimic for industrial carbon capture. Environ Sci Technol 47:10049–10055
Figueroa JD, Fout T, Plasynski S, McIlvried H, Srivastava RD (2008) Review advances in CO2 capture technology the U.S. department of energy’s carbon sequestration program. Int J Greenhouse Gas Control 2:9–20
Dilmore R, Griffith C, Liu Z, Soong Y, Hedges SW, Koepsel R (2009) Carbonic anhydrase-facilitated CO2 absorption with polyacrylamide buffering bead capture. Int J Greenhouse Gas Control 3:401–410
Acknowledgements
Authors are thankful to Shroff S.R. Rotary Institute of Chemical Technology, Ankleshwar (Gujrat) and IIT (BHU), Varanasi (UP), India for providing all necessary facilities to undertake the work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Balsora, H.K., Gautam, P., Mondal, M.K. (2017). Enrichment of Biogas from Biodegradable Solid Waste—A Review. In: Suresh, S., Kumar, A., Shukla, A., Singh, R., Krishna, C. (eds) Biofuels and Bioenergy (BICE2016). Springer Proceedings in Energy. Springer, Cham. https://doi.org/10.1007/978-3-319-47257-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-47257-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47255-3
Online ISBN: 978-3-319-47257-7
eBook Packages: EnergyEnergy (R0)