Abstract
Phlebotomine sand flies, which are biological vectors of Leishmania spp., are represented by around 400 species in the Old World and more than 600 species in the Americas. The vector sand fly species generally belong to the Phlebotomus genus in the Old World and the Lutzomyia genus in the New World. They are yellowish, long legged hairy insects and active after sunset until sunrise. Sand flies can transmit Leishmania parasites as well as some group of viruses called Phleboviruses and a bacterium, Bartonella bacilliformis. Visceral leishmaniasis (VL, Kala-azar) caused by Leishmania donovani is an important health problem in the Indian subcontinent including Bangladesh and Phlebotomus argentipes is a proven vector species of Leishmania donovani. In Bangladesh, a total of 13 sand fly species (3 Phlebotomus, 10 Sergentomyia spp.) were recorded so far. All studies showed the dominancy of P. argentipes especially in endemic areas for VL. In this chapter, besides P. argentipes and its biological and ecological features, other species constituting sand fly fauna and their geographical distribution in Bangladesh are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 General Information on Sand Flies
Sand flies are classified under the class Insecta, ordo Diptera, subordo Nematocera, family Psychodidae and sub-family Phlebotominae. The Phlebotominae sub-family consists of 6 genera: Phlebotomus, Sergentomyia, and Chinius in the Old World and Lutzomyia, Brumptomyia, and Warileya in the New World. They have medical and veterinary importance because they are biological vectors of the various Leishmania species that cause pathogenicity with different clinical presentations in humans and mammalian species. Vector Phlebotomine sand flies are represented by more than 40 species in the Old World and 30 species in the Americas. Besides Leishmania, sand flies can also transmit viruses (Phleboviruses) and bacterium (Bartonella bacilliformis). The vector sand fly species generally belong to the Phlebotomus genus in the Old World and the Lutzomyia genus in the New World [1].
Sand flies, which are smaller than other Diptera, are a light brown, yellowish color, long legged, and their entire body is hairy including the wings; the wings are in a “V” shape during rest, and because their mesonotum is longer, they appear hunchbacked. Sand flies are holometabol Diptera and complete their life cycle in humid soil with organic material. Four different phases occur in their life cycle: egg, larva (4 stages), pupa, and adult.
Sand flies rest in dark hidden locations, such as houses, barns, basements, tree holes, wall cracks, and rodent nests during the daytime and become active during the night. In addition, during this period, they may perform certain activities depending on the light, temperature , humidity, and wind density. Feeding in the form of biting starts immediately before dark and continues the whole night, increasing shortly before sunrise and continuing until the sun rises completely. Females of many species are predominantly exophagic (biting outdoors) and exophilic (resting outdoors during maturation of eggs) and cannot be controlled by spraying internal walls of habitations with insecticide. In contrast, species that are endophilic (resting indoors during maturation of eggs) can be attacked in this way.
They may be especially active in hot nights during the summer season until the sun rises and as the temperature decreases they will have decreased activity during the night. Though the humidity level that they need to survive varies depending on the species, the ideal humidity level is higher than 50 %. Adult females live for 3–4 weeks and males for 2 weeks; despite this, the ratio of males in the population is usually higher, presumably to increase mating possibilities. The ideal temperature , during which the adult sand flies are most active, is between 25 and 28 °C, though in some species, it may be lower. Although the adults are very sensitive to the cold, 4th stage larvae are more resistant against it and can survive the winter season under 5–10 cm of soil.
Characteristically, they fly in a “zigzag” pattern for even short distances and their maximum flying distance is 1 km. They travel by frequently landing and they can climb higher each time to reach the top floors of buildings. Females can perceive a human from under 10 m in distance.
Males of P. argentipes wait on the host for females coming to feed and then court the female for mating. Males of other species like P. ariasi may never be seen on the host but mate after the female has engorged [1]. Pairing generally happens in the air or on hosts and the female sand fly dies 8–10 days after pairing and feeding on blood; a female can deposit about 50–100 eggs. Larval development very much depends on the temperature/humidity and larvae hatch from the egg depending on the environmental temperature after 5–20 days. The fourth stage of larvae transform to pupae and become adults within 6–15 days. The cycle is completed in total within 40–50 days. In general, in the Mediterranean region the first generation is observed in May, the second in July, and the third in September.
It is known that the time from a blood meal to oviposition varies with the species and the ambient temperature . The number of eggs deposited depends on the size of the blood meal taken and may be as many as 200. There are 2 groups of females: (i) gonotrophically concordant (the ovaries develop as a single blood meal is digested, and the female does not feed a second time during the oviposition cycle, e.g. P. perniciosus, P. ariasi); (ii) gonotrophically discordant (no relation between digestion of a blood meal and the development of eggs, and more than 1 blood-meal may be taken during a single oviposition cycle, e.g. P. argentipes , P. papatasi). Two blood meals in different stages of digestion are commonly seen in the second group of females [1].
Male sand flies feed on plant juice only due to shapes of their mouthparts, but the females may feed on blood from cold- and hot-blooded hosts or on plant juice. Sugar meals serve as energy sources for sand flies and are important in the development of parasites within the gut. Some species suck blood only from the animals (zoophilic), some from both animals and humans (zoo-anthropophilic), and some only from humans (anthropophilic).
Sand flies may be caught with various methods in nature either alive (with CDC light trap, mouth aspirator, etc.) or dead (such as with oil paper), and the species may be determined after proper dissection/preparation. A stomach dissection may be performed to determine the presence of parasites or may be used for many other purposes after DNA/RNA isolation. Catch effectiveness is always influenced by environmental conditions, including wind, temperature , rain, relative humidity, atmospheric pressure, and moonlight. Capture with a mouth aspirator proved to be most reliable method for obtaining blood-fed and gravid P. argentipes specimens in Bangladesh .
Identification of the species caught can be done morphologically using available written keys, drawings, and photos. For the species belonging to the Phlebotomus genus, many, but not all, males can be identified by their morphology alone, but it is often more difficult to identify females, some of which are proven vectors. In last decade, DNA analysis techniques are helpful for identifying morphologically indistinguishable specimens of related species. The biology of each species of sand fly is unique and complex, covering all aspects of reproduction, feeding, dispersal, and other activities that have a direct bearing on the epidemiology of leishmaniasis and vector control [1].
1.1 Determining Sand Fly Species as Certain Disease Vectors
Sand flies have been classified as specific (restrictive) or permissive vectors. The sand fly species that support the development of multiple Leishmania species are called “permissive vectors” (e.g. P. argentipes , P. pernicious) while the sand fly species that do not or poorly support the development of different Leishmania species are called “specific vectors” (e.g. P. sergenti, P. papatasi) [1, 2]. The criteria for determining a sand fly species as a vector were first described by Dr. R. Killick-Kendrick and later were modified [3–5]. The last 2 criteria, shown next, are important for the vectors in a specific focus, like endemic areas of Bangladesh .
-
The criteria for incrimination [5]
-
Promastigotes should be isolated from female sand fly specimens more than once
-
Infective metacyclic forms should be seen in the anterior midgut and on the stomodeal valve of naturally infected female flies
-
The species should be attracted to and bite humans and any reservoir hosts
-
A strong ecological association needs to be shown between fly, humans, and any reservoir host
-
Experimental transmission needs to be shown in the laboratory model
-
Mathematical modeling demonstrates that the vector is essential for maintaining transmission with or without the involvement of other vectors
-
Mathematical modeling based on a planned control program demonstrates that disease incidence significantly decreases following a significant decrease in the biting density of the specific vector
2 Sand Flies in Bangladesh
Visceral leishmaniasis (VL) caused by Leishmania donovani is an important health problem in the Indian subcontinent including Bangladesh . VL is known as an anthroponotic disease and patients with post kala-azar dermal leishmaniasis (PKDL ) are a significant reservoir for anthroponotic transmission of VL [1]. The sustainable application of the integrated kala-azar control program is crucial for decreasing the morbidity and mortality of the disease. For increasing the efficacy of the vector component of the control program sand fly fauna should be studied in the endemic areas.
Sand fly studies in the Indian subcontinent started with Sinton [6] and Lewis who published a detailed identification key for Phlebotomus and Sergentomyia species, as well as information on their distribution in the oriental region [7]. This key is still useful and can be used for morphological identification.
After the publication of Lewis’s key, several studies were carried out in Bangladesh in different areas of the country that recorded a total of 13 sand fly species (3 Phlebotomus, 10 Sergentomyia spp.), although there is 1 questionable, S. theodori, and 1 unidentified Sergentomyia species in Bangladesh [8–11] (Table 17.1). In all studies related to sand fly fauna in Bangladesh, P. argentipes was found to be the dominant species among the members of the Phlebotomus genus. P. papatasi and P. sergenti are reported rarely from VL endemic and non-endemic areas (Fig. 17.1).
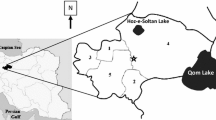
In Bangladesh , the Mymensingh district is the most highly endemic area for VL and therefore recent VL control programs, including sand fly studies, are focused on this district. In a recent study in Mymensingh, of 726 sand flies, 562 were caught from endemic areas, of which 413 were P. argentipes. It is emphasized that sand fly density as a whole differed significantly between endemic and non-endemic areas (P = 0.004). Similarly P. argentipes numbers were significantly higher (P = 0.008) in endemic areas compared with that seen in non-endemic areas [10] (Fig. 17.2). The micro-environmental and micro-climatic factors need to be investigated to better explain the reasons behind these observations. Mapping of endemic and non-endemic locations in parallel in Mymensingh and other highly endemic areas is also suggested.
Map of Mymensingh district showing sampling areas and absence/presence of P. argentipes (modified from [10])
In an ongoing study, similar results were obtained in VL endemic areas of Mymensingh; P. argentipes was the dominant species (>94 %). In 2012, a difference in the population size of sand flies between endemic and non-endemic areas was also observed. The population size was bigger in the endemic areas than that seen in the non-endemic areas. There might be some micro or nano environmental factors contributing to both sand fly abundance and kala-azar endemicity in the Mymensingh district (unpublished data).
The endophilic and peridomestic species Phlebotomus argentipes is the only known vector for L. donovani in the Indian subcontinent . This species is dominant in all endemic districts in Bangladesh and is accepted as a vector species but no infected fly has been detected so far. A unique published study was carried out in the Mymensingh highly endemic district for Kala-azar, and 376 P. argentipes were individually analyzed for Leishmania by PCR targeting Leishmania minicircle DNA. In addition to this, 679 Sergentomyia specimens were also included in the study, but none of the 1055 sand fly specimens were found positive in this study. It can be speculated that the infection rate is less than 1/376 among P. argentipes females in the district [11]. In 2015, in the Pabna district, around 50 P. argentipes females were dissected by direct microscopical method but no positive fly was found (unpublished data). Therefore, it will be needed to (i) detect Leishmania parasites by microscopical dissection and then PCR methods to incriminate the species as vector, (ii) discover the infection rate in sand flies, (iii) identify and compare Leishmania parasites isolated from humans and vectors, and (iv) perform blood-meal analysis also from Sergentomyia specimens for detecting their host preference.
Visceral leishmaniasis caused by L. donovani in the Indian subcontinent is exclusively anthroponotic in contrast to zoonotic VL caused by L. infantum in the New World and Mediterranean Basin where the animal reservoir, dog, is involved [1]. The vector sand fly species, P. argentipes, normally zoophilic on cattle, is also strongly attracted to man, making it anthropophilic and zoophilic [12]. It is reported that P. argentipes prefers animal bait 7 times more than human bait in a similar situation [13]. In a recent study performed in Trishal, Mymensingh, anti-Leishmania antibodies were detected in cattle sera by ELISA and direct agglutination test (DAT) but no Leishmania DNA was detected by nested PCR (Ln PCR) and a recently developed molecular technique, loop-mediated isothermal amplification (LAMP ). The authors emphasized that the anti-Leishmania antibodies detected by serological techniques might be the result of exposure to the Leishmania parasite by the bite of infected sand fly despite no infection developing [14]. For better knowledge about VL epidemiology in this area, studies related to the host preference of P. argentipes should be performed in a larger number of cattle or other domestic animals.
Information about the daily and seasonal activity of the endophilic vector species, P. argentipes, is also important for control programs, mainly for the timing of indoor residual spraying (IRS) application. In the Indian subcontinent , the transmission of L. donovani occurs during 2 periods: a pre-winter peak in September-November and a post-winter peak in March-May. During the monsoon season from June to September, the numbers of sand flies are low. It is also reported that P. argentipes lives in and around houses and biting occurs at night, mainly between 21:00 and 01:00, peaking at 23:00–24:00 [13]. The infection risk elevates during the peak of the season along with daily sand fly activity. But, no study thus far has been carried out in Bangladesh to study sand fly activities. Regular, preferably monthly, sand fly collection can be done in areas where patients with VL are reported.
2.1 Phlebotomus Argentipes, Annandale and Brunetti: The Vector of Visceral Leishmaniasis in Bangladesh
Phlebotomus argentipes is taxonomically classified under the subgenus Euphlebotomus Theodor and its morphological features were described together with other Oriental phlebotomine sand flies by Lewis in 1978 [7] (Table 17.2) (Figs. 17.3 and 17.4).
The studies carried out so far, support the anthropophagic behavior and vectorial competence of Phlebotomus argentipes [15, 16]. This species can be found in Asia from Iran to Western Malaysia and Indonesia and shows some geographical variations in its morphology and feeding behavior [12, 17, 18]. The ascoids on the fourth antennal segment of females in Northeastern India are shorter than those seen on the specimens in Sri Lanka [12, 19, 20]. In the studies performed in India, the presence of 2 different local P. argentipes populations with 2 different morphological features was attributed to the different vectorial capacities of these 2 morphospecies [16, 21].
The ecological parameters affecting P. argentipes population size and Leishmania transmission were studied in 2 villages of West Bengal, India [22]. In this study, they found that the abundance of the species increased during and after monsoon, and decreased during winter and summer, probably because of the prolonged duration of the gonotrophic cycle. The researchers also assessed that 8 ecological parameters (soil temperature and moisture, rainfall, air temperature, relative humidity, soil pH, soil organic carbon, and windspeed) can affect the sand fly abundance in a particular area.
P. argentipes breeds in moist organic soil at the junction of the floor and walls of cattle sheds and earthen houses [23, 24] Soil temperature and moisture may contribute to the survival and development of the immature stages of this species. Low moisture and temperature causes drying of organic material and prolongation of the life cycle , respectively. These ecological variables are highly effective on sand fly abundance and should be taken into account while implementing vector control measures.
The ecological situation is also important for the application of vector control managements, especially for indoor and outdoor residual spraying. The success of the application very much depends on the application season and time in endemic areas. Ecological data are also useful in predicting P. argentipes abundance and assessing the risk of increased Leishmania transmission.
-
In Bangladesh , the following information needs to be collected:
-
The composition and population density of P. argentipes and other sand fly species, in addition to mapping their distribution and resting habits in endemic areas,
-
Infection rate of P. argentipes: search for Leishmania parasite by microscopical dissection and/or PCR methods,
-
Identification of Leishmania parasites: comparison of Leishmania donovani isolates from humans and vectors
-
Other possible vectors: blood-meal analysis for Sergentomyia specimens
-
The seasonal and nocturnal activities of P. argentipes were studied throughout a year by monthly collections in India where the climatic and environmental conditions are similar to those in Bangladesh . In this study, it was found that the numbers of P. argentipes collected varied according to season, being significantly higher during the summer than that seen during the rainy season or winter, as expected. Related to seasonal activity, the authors concluded that the numbers of P. argentipes on humans and bovine bait in Bahapur village, Bihar, India showed 2 peaks before and after the rainy season [13].
The nocturnal activity findings showed that their night activity starts around 19:00 and peaks between 22:00 and 24:00 h in the summer season (between February and May) when most of the parasite transmission occurs [13]. The other studies on nocturnal activity of this species showed that landing on human bait peaked between 21:00 and 03:00 in West Bengal [25] while it was observed between 21:00 and 01:00 h in Southern India [26].
Both issues, seasonality and nocturnal activity, have not yet been studied in the endemic areas of Bangladesh. Because micro/nano environmental/ecological /climate factors can affect the daily and nocturnal behavior of the vector species, a detailed field study needs to be performed in at least the 2 distinct endemic areas of Bangladesh. In the Indian subcontinent , P. argentipes is known to be endophilic, but this behavior of P. argentipes should also be confirmed in Bangladesh to help the IRS part of the national control program.
References
WHO. “Control of the leishmaniasis,” Report of a meeting of the WHO expert committee on the control of Leishmaniases, World Health Organization, Geneva, Switzerland. WHO Technical Report Series No: 949; 2010.
Volf P, Myskova J. Sand flies and Leishmania: specific versus permissive vectors. Trends Parasitol. 2007;23:91–2.
Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: a review. Med Vet Entomol. 1990;4:1–24.
Ready P. Should sandfly taxonomy predict vectorial and ecological traits? J Vector Ecol. 2011;36:S17–32.
Ready P. Biology of phlebotomine sand flies as vectors of disease agents. Ann Rev Entomol. 2013;58:227–50.
Sinton JA. Notes on some Indian species of the genus Phlebotomus. Part XXX. Diagnostic table for the females of the species recorded from India. Indian J Med Res. 1932;20:55–75.
Lewis DJ. The Phlebotomine sandflies (Diptera: Psychodidae) of the oriental region. Bull Brit Mus (Nat Hist). 1978;37:217–343.
Hossain MI, Khan SA, Ameen M. Phlebotomine sandflies of Bangladesh: recent surveys (short communication). Med Vet Entomol. 1993;7:99–101.
Salahuddin M, Ameen M, Hossain M. Faunistic survey of sandflies in six districts of Bangladesh. Bangladesh J Zool. 2000;28:109–18.
Alam MS, Wagatsuma Y, Mondal D, Khanum H, Haque R. Relationship between sand fly fauna and kala-azar endemicity in Bangladesh. Acta Trop. 2009;112:23–5.
Alam MS, Kato H, Fukushige M, Wagatsuma Y, Itoh M. Application of RFLP-PCR-based identification for sand fly surveillance in an area endemic for Kala-Azar in Mymensingh, Bangladesh. J Parasitol Res. 2012;2012:467821. doi:10.1155/2012/467821.
Lewis DJ. Phlebotomine sandflies (Diptera: Pscychodidae) from the oriental region. Syst Entomol. 1987;12:163–80.
Dinesh DS, Ranjan A, Palit A, Kishore K, Kar SK. Seasonal and nocturnal landing/biting behaviour of Phlebotomus argentipes (Diptera: Psychodidae). Ann Trop Med Parasitol. 2001;95:197–202.
Alam MS, Ghoch D, Khan MG, Islam MF, Mondal D, Itoh M, et al. Survey of domestic cattle for anti-Leishmania antibodies and Leishmania DNA in a visceral leishmaniasis endemic area of Bangladesh. BMC Vet Res. 2011;7:27. doi:10.1186/1746-6148-7-27.
Lane RP, Pile MM, Amerasinghe FP. Anthropophagy and aggregation behaviour of the sandfly Phlebotomus argentipes in Sri Lanka. Med Vet Entomol. 1990;4:79–88.
Surendran SN, Kajatheepan A, Hawkes NJ, Ramasamy R. First report on the presence of morphospecies A and B of Phlebotomus argentipes sensu lato (Diptera: Psychodidae) in Sri Lanka—implication for leishmaniasis transmission. J Vector Borne Dis. 2005;42:155–8.
Illango K. Morphological characteristics of antennal flagellum and its sensilla chaetica with character displacement in sand fly Phlebotomus argentipes Annandale and Brunetti sensu lato (Diptera: Psychodidae). J Biol Sci. 2000;25:163–72.
Dinesh DS, Kishore K, Singh VP, Bhattacharya SK. Morphological variations in Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae). J Commun Dis. 2005;37:35–8.
Lewis DJ, Killick-Kendrick R. Some phlebotomid sand flies and other Diptera of Malaysia and Sri Lanka. Trans R Soc Trop Med Hyg. 1973;67:4–5.
Ilango K. A taxonomic reassessment of the Phlebotomus argentipes species complex (Diptera: Psychodidae: Phlebotominae. J Med Entomol. 2010;47:1–15.
Lane RP, Rahman SJ. Variation in the ascoids of the sandfly Phlebotomus argentipes in a population from Patna, northern India. J Com Dis. 1980;124:216–8.
Ghosh K, Mukhopadhyay J, Desai MM, Senroy S, Bhattacharya A. Population ecology of Phlebotomus argentipes (Diptera: Psychodidae) in West Bengal, India. J Med Entomol. 1999;36:588–94.
Singh R, Lal S, Saxena VK. Breeding ecology of visceral leishmaniasis vector sandfly in Bihar state of India. Acta Trop. 2008;107:117–20.
Bern C, Courtenay O, Alvar J. Of cattle, sand flies and men: a systematic review of risk factor analyses for south Asian visceral leishmaniasis and implications for elimination. PLOS Neglected Dis. 2010;4:e599. doi:10.1371/journal.pntd.0000599.
Hati AK, Ghosh KK, Das S, De N, Sur S. A longitudinal study on Phlebotomus argentipes/man contact in a village in West Bengal. Document WHO/VBC/81.801. Geneva: World Health Organization; 1981.
Rahman SJ, Menon PKM, Rajgopal R, Mathur KK. Behaviour of Phlebotomus argentipes in foothills of Nilgiris (Tamilnadu), South India. J Comm Dis. 1986;18:35–44.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing
About this chapter
Cite this chapter
Özbel, Y., Sanjoba, C., Matsumoto, Y. (2016). Geographical Distribution and Ecological Aspect of Sand Fly Species in Bangladesh. In: Noiri, E., Jha, T. (eds) Kala Azar in South Asia. Springer, Cham. https://doi.org/10.1007/978-3-319-47101-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-47101-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43611-1
Online ISBN: 978-3-319-47101-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)