Abstract
Despite the upsurge of publications on ischemic preconditioning in recent years, the concept of preconditioning an organ with ischemia is not new. In 1986, Murry et al. demonstrated short periods of regional ischemia and reperfusion resulting in protection against necrosis to a later longer period of ischemia in the canine myocardium [1]. In reperfusion injury following a brief period of ischemia, tissues begin to adapt to anaerobic metabolism. Restoration of blood flow can lead to an oxygen supply that exceeds tissue requirements, the activation of macrophages, and the generation of reactive oxygen species [2]. This can ultimately result in endothelial injury and further release of pro-inflammatory cytokines [3]. Ischemic preconditioning occurs when a tissue undergoes brief periods of ischemia to later protect against longer ischemic events and reperfusion injury.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
15.1 Introduction
Despite the upsurge of publications on ischemic preconditioning in recent years, the concept of preconditioning an organ with ischemia is not new. In 1986, Murry et al. demonstrated short periods of regional ischemia and reperfusion resulting in protection against necrosis to a later longer period of ischemia in the canine myocardium [1]. In reperfusion injury following a brief period of ischemia, tissues begin to adapt to anaerobic metabolism. Restoration of blood flow can lead to an oxygen supply that exceeds tissue requirements, the activation of macrophages, and the generation of reactive oxygen species [2]. This can ultimately result in endothelial injury and further release of pro-inflammatory cytokines [3]. Ischemic preconditioning occurs when a tissue undergoes brief periods of ischemia to later protect against longer ischemic events and reperfusion injury.
The protection conferred by brief episodes of ischemia and reperfusion to a later more sustained episode of ischemia occurs in organs other than the heart, such as the kidneys and the brain. In 1985, Zager et al. reported that rats exposed to 15 min of bilateral renal artery occlusion had improved renal function when compared to a control group of rats after exposure to a second ischemic insult 30 min later [4]. In mice Joo et al. performed right nephrectomies and ischemic preconditioning by 5-min episodes of left-sided renal ischemia followed by reperfusion [5]. When the mice were later subjected to a more prolonged ischemic event, serum creatinine levels in the mice that underwent ischemic preconditioning were significantly lower when compared to a control group of mice who had just received unilateral nephrectomy [5]. Kitagawa and colleagues introduced the concept of “ischemic tolerance” in the brain when they introduced cerebral ischemia in gerbils by occluding both common carotid arteries [6]. Two-minute ischemic treatments performed daily for 2 days leading up to a 5-min cerebral ischemic period provided protection against neuronal death [6].
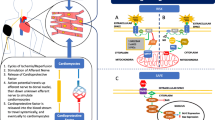
Remote ischemic preconditioning (RIPC) was first described in the literature in 1993 with an experiment in dogs where occlusion of the circumflex artery protected the myocardium supplied by the left anterior descending artery (LAD) [7]. When infarct sizes of the LAD were evaluated after 1 h of sustained LAD occlusion by triphenyltetrazolium staining, the infarct size of the preconditioned group was significantly less than the control group [7]. Since that time, numerous studies have been published on the clinical use of RIPC whereby a brief ischemic insult is provided to one area of the body to induce protection to a longer ischemic insult at a remote site. This chapter will review the most commonly discussed mechanisms for RIPC as well as the more recent clinical studies done using RIPC as they pertain to reducing morbidity and mortality in the perioperative period [8, 9].
15.2 Proposed Mechanisms of Remote Ischemic Preconditioning
15.2.1 Humoral Mechanism
The process by which RIPC occurs is complex and not fully understood. There have been numerous proposed mechanisms in the literature. The hypothesis that the RIPC event is triggered by a humoral mediator has been investigated. Dickson et al. provides evidence of the involvement of humoral mediators for eliciting RIPC by showing that a rabbit could be preconditioned by transfer of coronary effluent [10]. Effluent was collected during normal perfusion from donor hearts and during ischemia-reperfusion from donor preconditioned hearts. The effluent was then transferred to acceptor control and acceptor preconditioned hearts. All hearts were subject to 40 min of ischemia [10]. The resulting mean infarct size was smaller in the donor and acceptor preconditioned hearts [10]. There was an increase in adenosine and norepinephrine in the effluent from the preconditioned animals [10]. These results support the release of a hormonal trigger signal that is given off from the preconditioned myocardium and that when delivered to an acceptor heart evokes a cardioprotective effect. Some of the common mediators that have been studied include adenosine, catecholamine, bradykinin, and opioids [11–14].
In a recent review, Zarbock and Kellum discuss that kidney protection with RIPC occurs through the release of damage-associated molecular patterns (DAMPs) [15]. Increased levels of high-mobility group box 1 (HMGB-1), a prototypical DAMP, after RIPC were associated with a lower risk of AKI in an investigation discussed in more detail later in this chapter (OR 0.75, CI 0.35–0.94, p = 0.03) [16]. It is possible that DAMPs released from an initial location of ischemia-reperfusion travel to a target organ. In this case DAMPs may be filtered by the kidney and, through pattern-recognition receptors in the proximal tubular epithelia, signal renal protective mechanisms [15].
15.2.2 Neural Pathway
The potential for a neural pathway of communication to a target organ has been shown. Pretreatment with hexamethonium, a ganglion blocker, negated remote cardioprotection in rats receiving 15 min of mesenteric artery occlusion [17]. In humans endothelial injury caused by arm ischemia and reperfusion was measured with a reduction in flow-mediated dilation. The protective effect of RIPC prior to injury was reduced with the infusion of trimetaphan, another ganglion blocker [18]. In rabbits, vagal nerve ligation and atropine administration negated RIPC-induced reduction in myocardial infarct size [19].
15.2.3 The Final Common Event
The final common event in the protection induced by RIPC most commonly cited in the literature involves intracellular kinases acting on the mitochondria causing a closure of the mitochondrial transition pore, preventing the influx of ions [20]. Three main pathways acting on the mitochondrion have been proposed: (i) the reperfusion injury salvage pathway [21], (ii) the cyclic guanosine monophosphate/CGMP-dependent protein kinase pathway [22], and (iii) the survivor activating factor enhancement pathway [23]. The potassium-dependent adenosine triphosphate (ATP) channel blocker glibenclamide was shown to block the benefit of RIPC indicating that the protection may depend on potassium-dependent ATP channel activation [24]. Thus, it is proposed that in RIPC the potassium-dependent ATP channel is activated, leading to closure of the mitochondrial transition pore, reducing mitochondrial permeability in a target organ, and slowing the rate of ATP depletion [25].
15.3 Clinical Studies on Remote Ischemic Preconditioning
The majority of clinical studies describe the application of a blood pressure to the arm or leg to induce RIPC. Generally the cuff is inflated to 200 mmHg or 50 mmHg greater than the systolic atrial pressure and then deflated. This procedure is then repeated three to five times. The majority of clinical studies using RIPC have been done on the cardiothoracic patient population prior to cardiopulmonary bypass. Most studies report the effect of cardiac biomarkers in patients who receive RIPC when compared to a control group of patients [26–39]. For example, one of the initial studies to demonstrate the effect of RIPC on troponin T levels randomized 57 adult patients prior to coronary bypass grafting to receive RIPC through the use of timed arm blood pressure cuff inflations or to a control group [27]. When troponin T was measured prior to surgery and at time points after surgery, RIPC decreased the total area under the curve of troponin T by 43 % when compared to controls [27]. In 37 children undergoing congenital heart defect repair, Cheung et al. reported lower troponin I levels, airway resistance, and postoperative need for ionotropic medications for patients who received preoperative RIPC when compared to children who did not receive RIPC [26]. Regarding cardiac outcomes in both children and adults in the perioperative period, there have been discrepant findings with some studies showing a benefit to RIPC [26–29, 33] and other showing no benefit [31, 34, 36–39].
Additionally, the effect of RIPC on kidney outcomes has been studied in both the adult and pediatric cardiac and vascular surgery populations. The association of surgical procedures and AKI has been consistently shown [40–42]. When 82 adult patients were randomized to receive abdominal aortic aneurysm repair with either RIPC by intermittent cross clamping of the common iliac artery for 10 min followed by 10 min of reperfusion or no RIPC prior to surgery, RIPC was found to reduce the incidence of myocardial injury by 27 % and renal impairment by 23 % [43]. When AKI was defined as a rise in serum creatinine ≥0.3 mg/dL or ≥50 % within 48 h after cardiac surgery where cardiopulmonary bypass was expected, a 27 % absolute risk reduction in AKI was found when comparing a randomized group of patients who received RIPC to those who received no intervention prior to surgery [44]. However, there have been investigations that have not reported a protective effect of RIPC for AKI [45–48].
Given the differences in study results, it may be that different patient characteristics make an individual more or less likely to respond to RIPC. For example, it may be those patients at a greater risk for AKI that will be more likely show a beneficial effect of the intervention. In a recent study, 240 adult patients at very high risk for AKI (Cleveland Clinic Foundation scores ≥6 [49]) undergoing cardiac surgery were randomized to RIPC with upper arm blood pressure cuff inflation compared to a control group [16]. There was a 15 % absolute risk reduction (95 % CI 2.56–27.44 %, p = 0.02) for those who received RIPC when compared to those who did not [16]. A unique feature of this study was the use of urinary biomarkers of AKI, tissue inhibitor of metalloproteinase-2 (TIMP-2), and insulin-like growth factor-binding protein 7 (IGFBP7), which increased in the majority of patients who are receiving RIPC [16]. Furthermore, in those who experienced an increase in TIMP-2 and IGFBP7 after RIPC and prior to cardiopulmonary bypass, the incidence of AKI was reduced when compared to those who did not [16]. Also, higher levels of HMGB-1 after RIPC, discussed earlier in this chapter, were associated with a reduction in AKI [16].
The use of RIPC for neurologic as well was pulmonary protection prior to surgical procedures has been explored. Patients undergoing elective carotid endarterectomy (CEA) were randomized to receive either RIPC with 10 min of lower limb ischemia followed by reperfusion or no RIPC prior to CEA [50]. There were less saccadic latency deteriorations in the patients who received RIPC; however, this did not reach statistical significance (32 % versus 53 %, p = 0.11) [50]. Patients undergoing elective thoracic pulmonary resection (N = 216) were randomized to either RIPC or a sham procedure [51]. Compared to the control group, the patients who received RIPC had a significantly increased PaO2/FiO2 at 30 and 60 min after one-lung ventilation, 30 min after lung reexpansion, and 6 h after surgery [51].
15.4 The Future of Remote Ischemic Preconditioning for Improving Surgical Outcomes
Over 15 clinical trials were published in 2015 on the clinical use of RIPC. The ease of administration of the RIPC procedure and lack of adverse events reported in clinical trials are likely contributing factors to the continued interest in this intervention. However, RIPC is not used in routine perioperative care. The differences between study results as discussed above make it difficult to identify the patients that may benefit from the intervention. The use of biomarkers to predict RIPC response shows great promise for this purpose.
There is a need to standardize the RIPC procedure. The timing of placement of the blood pressure cuff, location of the blood pressure cuff, and duration of cuff inflation/deflation varies between studies. Also, future studies controlling for medication administration around the time of the RIPC procedure are important. Medication exposure has been discussed as a potential reason for a lack of RIPC benefit in two recently published large multicenter trials. Mehbohm et al. randomly assigned 1,403 patients undergoing cardiopulmonary bypass from 14 centers to four 5-min cycles of RIPC or sham-RIPC [38]. No differences were seen in mortality, stroke, or stage 2–3 AKI [38]. Hausenloy and colleagues using 30 centers randomized 1,612 patients undergoing cardiopulmonary bypass to RIPC or sham-RIPC as well and also found no difference in their combined primary endpoint of nonfatal myocardial infarction, death from cardiovascular causes, coronary revascularization, or stroke when evaluated 12 months after randomization [39]. Propofol was used in the perioperative period in the majority of patients in both studies [38, 39]. Propofol as well as certain inhaled anesthetics have been thought to affect the RIPC response [52–54].
Conclusion
Given that surgical procedures are often associated with a predicted ischemic insult to an organ, there is great potential benefit for the use of RIPC in the perioperative period. Future studies comparing differing blood pressure cuff positions and RIPC timing may help to standardize a preconditioning protocol. Investigations stratifying patients by risk factors and comorbid conditions are warranted. Additionally, studies exploring the use of biomarkers as a method to predict which surgical patients may ultimately benefit from the routine clinical use of RIPC are needed.
References
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Carden DL, Granger DN (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190:255–266
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121–1135
Zager RA, Jurkowitz MS, Merola AJ (1985) Responses of the normal rat kidney to sequential ischemic events. Am J Physiol 249:F148–F159
Joo JD, Kim M, D’Agati VD, Lee HT (2006) Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol 17:3115–3123
Kitagawa K, Matsumoto M, Tagaya M et al (1990) ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res 528:21–24
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P (1993) Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899
Landoni G, Rodseth RN, Santini F et al (2012) Randomized evidence for reduction in perioperative mortality. J Cardiovasc Anesth 26:764–772
Landoni G, Pisano A, Lomivorotov V et al (2016) Randomized evidence for reduction of perioperative mortality: an updated consensus process. J Cardiothorac Vasc Anesth pii: S1053-0770(16)30281–6
Dickson EW, Lorbar M, Porcaro WA et al (1999) Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol 277:H2451–H2457
Hu S, Dong H, Zhang H et al (2012) Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res 1459:81–90
Bankwala Z, Hale SL, Kloner RA (1994) Alpha-adrenoceptor stimulation with exogenous norepinephrine or release of endogenous catecholamines mimics ischemic preconditioning. Circulation 90:1023–1028
Schoemaker RG, van Heijningen CL (2000) Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol 278:H1571–H1576
Tomai F, Crea F, Gaspardone A et al (1994) Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. Circulation 90:700–705
Zarbock A, Kellum JA (2015) Remote ischemic preconditioning and protection of the kidney-A novel therapeutic option. Crit Care Med 44(3):607–16
Zarbock A, Schmidt C, Van Aken H et al (2015) Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 313:2133–2141
Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD (1996) Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94:2193–2200
Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ (2005) Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 46:450–456
Donato M, Buchholz B, Rodriguez M et al (2013) Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol 98:425–434
Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM (2002) Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res 55:534–543
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61:448–460
Burley DS, Ferdinandy P, Baxter GF (2007) Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol 152:855–869
Lecour S (2009) Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol 47:32–40
Kristiansen SB, Henning O, Kharbanda RK et al (2005) Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol 288:H1252–H1256
Loukogeorgakis SP, Williams R, Panagiotidou AT et al (2007) Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation 116:1386–1395
Cheung MM, Kharbanda RK, Konstantinov IE et al (2006) Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47:2277–2282
Hausenloy DJ, Mwamure PK, Venugopal V et al (2007) Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 370:575–579
Venugopal V, Hausenloy DJ, Ludman A et al (2009) Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart 95:1567–1571
Thielmann M, Kottenberg E, Boengler K et al (2010) Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 105:657–664
Luo W, Zhu M, Huang R, Zhang Y (2011) A comparison of cardiac post-conditioning and remote pre-conditioning in paediatric cardiac surgery. Cardiol Young 21:266–270
Rahman IA, Mascaro JG, Steeds RP et al (2010) Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation 122:S53–S59
Zhou W, Zeng D, Chen R, Liu J, Yang G, Liu P, Zhou X (2010) Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol 31:22–29
Hong DM, Jeon Y, Lee CS et al (2012) Effects of remote ischemic preconditioning with postconditioning in patients undergoing off-pump coronary artery bypass surgery – randomized controlled trial. Circ J 76:884–890
Lee JH, Park YH, Byon HJ, Kim HS, Kim CS, Kim JT (2012) Effect of remote ischaemic preconditioning on ischaemic-reperfusion injury in pulmonary hypertensive infants receiving ventricular septal defect repair. Br J Anaesth 108:223–228
Jones BO, Pepe S, Sheeran FL et al (2013) Remote ischemic preconditioning in cyanosed neonates undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg 146:1334–1340
Pavione MA, Carmona F, de Castro M, Carlotti AP (2012) Late remote ischemic preconditioning in children undergoing cardiopulmonary bypass: a randomized controlled trial. J Thorac Cardiovasc Surg 144:178–183
Young PJ, Dalley P, Garden A et al (2012) A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic Res Cardiol 107:256
Meybohm P, Bein B, Brosteanu O et al (2015) A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 373:1397–1407
Hausenloy DJ, Candilio L, Evans R et al (2015) Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 373:1408–1417
Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML (2009) Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med 37:2552–2558
Chertow GM, Lazarus JM, Christiansen CL et al (1997) Preoperative renal risk stratification. Circulation 95:878–884
Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP (2005) Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int 67:1112–1119
Ali ZA, Callaghan CJ, Lim E et al (2007) Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 116:I98–I105
Zimmerman RF, Ezeanuna PU, Kane JC et al (2011) Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int 80:861–867
Choi YS, Shim JK, Kim JC (2011) Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: a randomized controlled trial. J Thorac Cardiovasc Surg 142:148–154
Pedersen KR, Ravn HB, Povlsen JV, Schmidt MR, Erlandsen EJ, Hjortdal VE (2012) Failure of remote ischemic preconditioning to reduce the risk of postoperative acute kidney injury in children undergoing operation for complex congenital heart disease: a randomized single-center study. J Thorac Cardiovasc Surg 143:576–583
Murphy N, Vijayan A, Frohlich S et al (2014) Remote ischemic preconditioning does not affect the incidence of acute kidney injury after elective abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth 28:1285–1292
Gallagher SM, Jones DA, Kapur A et al (2015) Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney Int 87:473–481
Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP (2005) A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16:162–168
Walsh SR, Nouraei SA, Tang TY, Sadat U, Carpenter RH, Gaunt ME (2010) Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. Vasc Endovascular Surg 44:434–439
Li C, Xu M, Wu Y, Li YS, Huang WQ, Liu KX (2014) Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: a randomized controlled study. Anesthesiology 121:249–259
Landoni G, Biondi-Zoccai GG, Zangrillo A et al (2007) Desflurane and sevoflurane in cardiac surgery: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth 21:502–511
Yu CH, Beattie WS (2006) The effects of volatile anesthetics on cardiac ischemic complications and mortality in CABG: a meta-analysis. Can J Anaesth 53:906–918
Kottenberg E, Thielmann M, Bergmann L et al (2012) Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol – a clinical trial. Acta Anaesthesiol Scand 56:30–38
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Fuhrman, D.Y., Kellum, J.A. (2017). Reducing Mortality in the Perioperative Period: Remote Ischemic Preconditioning. In: Landoni, G., Ruggeri, L., Zangrillo, A. (eds) Reducing Mortality in the Perioperative Period. Springer, Cham. https://doi.org/10.1007/978-3-319-46696-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-46696-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46695-8
Online ISBN: 978-3-319-46696-5
eBook Packages: MedicineMedicine (R0)