Abstract
Ischemic heart disease is the most frequent causes of death worldwide. The urgent need for new therapies fostered the development of strategies that focus on the recovery of the cardiac structure and function. Initial investigations of cell-based therapies provided evidence of beneficial outcomes such as improved heart function and reduced remodelling. Although key players and pathways have not yet been identified; the repair or regenerative capacity of cardiac cell-based therapies may be mediated primarily via the secretion of cyto-protective factors that stimulate revascularization, increase the recruitment of host progenitor cells, and trigger in situ repair mechanisms. Poor retention of the administered cells for the treatment of the damaged myocardium represents a major issue. Therefore, in the past decade, the strategies for cell delivery have taken an important turn; new approaches using combinations of cells, biomaterials, and/or growth factors and cytokines have been flourishing. Myocardial tissue engineering for ischemic heart diseases had become the promising new strategy and supplanted the intra myocardial or intracoronary injection of cells. We present the different stem cells and engineered tissues developed to treat myocardial infarction.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Since the end of the nineteenth century, cardiovascular diseases (CVDs) have become and remained the N°1 serial killer. Among CVDs, coronary artery disease (CAD) represents the main etiology. The acute coronary ischemia induces myocardial infarction (MI) that progressively develops toward a chronic phase and severe heart failure. During the last decades, the identification and early treatment of risk factors, aggressive medical and interventional strategies allowed to significantly impact cardiovascular mortality after acute MI. Despite tremendous progress in diagnosis, prevention, and treatments, the chronic phase and the associated alarming progression of heart failure emphases the need for new therapies. Currently, no curative treatment exists for patients that survived acute MI; consequently, these patients face an excess risk of further cardiovascular events. Current therapeutic modalities including medical (life-style, drugs, psychology, etc.) and interventional procedures (percutaneous or surgical coronary revascularization, ventricular assistance, and implantable cardiac defibrillators with or without resynchronization therapy) intend to lower cardiovascular mortality and delay the progression of chronic heart failure by reducing the left ventricular remodelling. These therapies efficiently improve the clinical outcome and the quality of life of patients suffering acute MI. Nevertheless, despite these significant technological advances, the morbidity and mortality due to the progression of heart failure is still growing (Gjesdal et al. 2011). Therefore, new therapeutic options that will stop the progression of the disease to heart failure and foster cardiac regeneration are eagerly awaited.
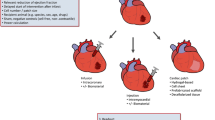
Following myocardial injury, the recently identified regenerative capacity of the myocardium appears rapidly overloaded and clearly insufficient to repair the damaged muscle. Heart function is initially maintained due to a compensatory mechanism that involves hypertrophy of the myocardium. When this physiological remodelling is overwhelmed, a decompensating phase results in left ventricle dilatation, thinning of the wall, remodelling of the mitral annulus, and appearance of heart failure. The possibility to stop the progression of the physio-pathological remodelling and/or to stimulate in situ repair mechanism has gained increasing interest during the last two decades. Reparative cell-based therapy has then emerged to become a clinical reality (Tongers et al. 2011). Initially, pre-clinical investigations provided compelling evidence of the beneficial effects of stem cell transplantation, including heart function recovery, decrease in infarct size and ventricle dilation, and increase in vascular density. Clinical application had rapidly followed. Bone marrow-derived cells , such as mesenchymal stem cells (MSC), circulating progenitors, adipose tissue-derived stem cells, resident cardiac stem cells (CSCs), and skeletal muscle cells, have been injected into patients with acute and chronic MI. Clinical trials revealed beneficial outcome, but pointed out important drawbacks that impair treatment efficacy. Concerns have been raised on low ability of the injured myocardium to permit cell retention and survival. Indeed, the damaged myocardium represents a rather hostile environment, due to hypoxia as well as intense immunologic and inflammatory activities. Improved cell retention and optimal delivery approaches have then been actively investigated. An interesting solution relies on the concomitant delivery of cells and exogenous matrix into the myocardium or the epicardial implantation of an in vitro engineered tissue. These promising alternatives have been shown to improve cell retention (Hamdi et al. 2011; Karam et al. 2012). Furthermore, the engineered tissues allow the creation of an adequate microenvironment favorable for (1) an improved cell survival, (2) the development of the contractile function resulting from the development of an engineered construct that mimic the myocardial structure, (3) a potential electrical pacing and/or coupling with the host myocardium, and (4) the packing of cytokines and growth factors involved in the paracrine related cardiac regeneration mechanism (Hwang and Kloner 2010). The emerging field of cardiac reparative tissue-based therapy offers unprecedented opportunities. Early attempt dealing with injection of isolated cells within the myocardium or via intracoronary delivery has nowadays been substituted with engineered tissues-based therapy with the exception of the injection of reprogrammed cells (Steppich et al. 2016).
Constant progress in biomaterials processes has fostered researchers’ focus to develop advanced cardiac biografts . Alternatively, engineered tissues were developed without matrix and composed of a stack of cell monolayers, named cell sheets obtained with thermoresponsive cell culture dishes (Bel et al. 2010; Miyagawa et al. 2010).
A large majority of the actual engineered tissue-based therapies aim at replacing, repairing, or regenerating the damaged myocardium. Nevertheless, the ultimate goal of creating an entire beating heart has recently moved forward: Guyette et al. decellularized human heart that was used as a functional matrix to create de novo beating organ following recellularization with induced pluripotent stem cells (iPSCs) (Guyette et al. 2016).
2 Cells
Different types of cells are being evaluated for their capacity of promoting cardiac repair and cardiac regeneration after myocardial infarction. In the past, cardiomyocytes (CM) of neonatal or fetal origins have been initially studied to validate the proof of concept of engineered tissue-based cardiac therapy. Nowadays, focuses have been redirected on clinically relevant cell types including CM derived from pluripotent stem cells (embryonic stem cells (ESCs), iPSCs) and adult stem cells (MSCs, skeletal myoblasts (SMs), adipose-derived stem cells, and CSCs).
2.1 Pluripotent Stem Cells Derived-Cardiomyocytes
The main advantage of using pluripotent stem cells , such as ESCs and iPSCs , relies on their high capacity for self-renewal and the possibility to direct their differentiation into functional CM. Their potential to replace the lost CM and directly contribute to the host myocardium contraction were demonstrated with human embryonic stem cell-derived cardiomyocytes (hESC-CMs) transplanted in a guinea pig model; transplanted cells developed electric coupling with intact native myocardium. Nevertheless, the electrical integration was shown to be heterogeneous in injured hearts (Shiba et al. 2012). Furthermore, Chong et al. provided an evidence of cellengraftment, remuscularization, and electromechanical synchronization two to seven weeks following injection of one billion hESC-CMs into the hearts of primates with MI injury (Chong et al. 2014).
Besides the electromechanical coupling of CM derived from pluripotent stem cells, the paracrine regulation for neovascularization in particular has also been explored. Indeed, the transplantation of non-CM derived cells , such as human iPSC-derived ECs (hiPSC-ECs) and smooth muscle cells (hiPSC-SMCs) into ischemic porcine myocardial tissue, contributed to improvements of the perfusion, wall stress, and cardiac performance (Gu et al. 2012; Xiong et al. 2011).
2.1.1 Pluripotent Stem Cell Derived-Cardiomyocytes Associated with Matrices
The proof of concept of feasibility and importance of pluripotent stem cells in cardiac regeneration has been followed by investigation associated cell and various matrices. When transplanted into rodent hearts with chronic MI, ESC-CM injected with chitosan hydrogel repopulated ischemic and necrotic regions of dysfunctional myocardium, improved contractile performance resulting in improved cardiac function and cell survival (Lu et al. 2009). In addition, fibrin scaffold loaded with human ESC-derived cardiac progenitors improved contractility and attenuated remodelling up to 4 months in a chronic immune-deficient rat model. Nevertheless, the authors reported no sustained cell engraftment (Bellamy et al. 2015). However, when fibrin patches were loaded with insulin growth factor (IGF)-encapsulated microspheres and three types of human iPSC-derived cells (CMs, endothelial cells, and smooth muscle cells), cell integration was improved in the porcine infarcted myocardium after 4 weeks in comparison to scaffold-free cell transplantation. Main outcomes were an improvement in ventricular function, a reduction in the infarct size and apoptosis, an increase in angiogenesis and a normalised myocardial metabolism. No reverse effects such as arrhythmias were recorded (Ye et al. 2014).
Investigations reporting the implantation of iPSC-derived cardiomyocytes (iPSC-CM) organized in cell sheets in a chronic MI rodent model confirmed an increase of the cardiac function, neovascularization, engraftment of the cells, and a short-term survival. Remodelling and fibrosis were also reduced (Chang et al. 2014; Higuchi et al. 2015; Kawamura et al. 2012; Masumoto et al. 2014; Matsuo et al. 2015). Interstingly, Masumoto et al. (2012) provide evidence of the paracrine effect of cell sheets composed of purified CM, EC and mural cell derived from mouse iPCs. Improvement of the systolic function and neovascularisation in a rat MI model were sustained after the loss of the implanted cells. This effect was mediated by the presence of VEGF secreted by iPCS derived CM.
Engraftment of the cell sheet has been further improved when gelatin hydrogel microspheres were inserted between each cell sheet composed of iPSC-CM, endothelial cells, and vascular mural cells. Functional capillary network was improved and therefore allowed to increase the number of cell sheets stacked (Matsuo et al. 2015).
Moreover, an upscaling study established the feasibility of implanting iPSC-CM cell sheets in a large animal model and confirmed cardiac function improvement (Kawamura et al. 2012).
Although teratocarcinoma formation is a major concerned when implanting pluripotent stem cells derived CM, improved protocol for differentiation as well as cell purification may reduce the risk (Masumoto and Yamashita, 2016). Interestingly, Kawamura et al. (Kawamura 2016) provided evidence that the growth of malignant tumors from induced pluripotent stem cell-derived cardiac tissue constructs was blocked by host immune response.
The proof of concept of the safety of transplanting ESC-CM was followed by the recent launch of a clinical trial phase I. ESC-CMs (Isl-1+ and SSEA-1+ cells) associated with a fibrin scaffold were delivered to the infarct area of a first patient suffering from severe heart failure. After 3 months, the patient showed MI symptoms improvement and a new-onset contractility was observed by echocardiography. There have been no complications such as arrhythmias, tumor formation, or immunosuppression-related adverse events (Menasché et al. 2015).
2.2 Adult Stem Cells and Progenitors
Adult stem cells, found in many tissues , including the heart, are undifferentiated cells that can renew themselves and are capable of differentiating into specialized cell types. These cells are limited in their differentiation potential and are located in niches.
2.2.1 Mesenchymal Stem Cells
Mesenchymal Stem Cells also named Mesenchymal Stromal Cells are currently the most frequently used adult stem cell in regenerative medicine. Their therapeutic potential relies on several properties. They can be easily isolated from bone marrow, cord blood, peripheral blood, and fat tissue and largely amplified. They can differentiate into various lineages including adipocytes, chondrocytes, and osteoblasts. However, their differentiation into CM has been largely controversial and stays marginal. Of particular interest, MSCs exhibit immune regulatory activities both in vitro and in vivo, which are mediated by complex mechanisms that inhibit the function of different immune cell subpopulations of the innate and adaptive immunity. Finally, paracrine regulation mediated via MSC-secreted factors may contribute to the myocardial repair and have been investigated; identified factors include vascular endothelial growth factor (VEGF), hepatocyte growth factor, stromal cell-derived factor-1a, interleukin-6, macrophage inhibitory factor, and monocyte chemoattractant protein-1.
MCSs have also been associated with different scaffolds. Seeded in polymeric scaffolds of alginate and chitosan, MCSs maintain their proliferation and paracrine activity (Ceccaldi et al. 2014). Silk fibroin/hyaluronic acid MSCs patches reduced apoptosis, significantly promoted neovascularization and stimulated the secretions of various paracrine factors such as VEGF (Chi et al. 2012). Modified MSCs with insulin-like growth factor-1 (IGF-1) gene , loaded into a fibrin patch, were transplanted into a porcine model of MI and showed beneficial outcomes (Li et al. 2015).
2.2.2 Skeletal Myoblasts
They originate from satellite cells, which reside beneath the muscle fiber basal lamina . These myogenic precursor cells present several advantages for cell-based therapy including the possibility to expand them in vitro due to their proliferative capacity and their tolerance for a prolonged ischemia. Nevertheless, direct injection of myoblast in MI has raised several concerns such as the poor engraftment of injected cells and the risk of an arrhythmia when injected cell is not functionally coupled with the host myocardium (Narita et al. 2013). These shortcomings were overcome when SM, organized in cell sheets were implanted at the surface of the injured heart. Using acute and chronic MI rodent models , enhanced cell survival and improved cardiac function have been confirmed. Numerous investigations provided evidence of reduced infarct size, decreased fibrosis, attenuated cardiomyocyte hypertrophy, and increased neovascular formation (Narita et al. 2013; Pätilä et al. 2015; Shudo et al. 2014; Tano et al. 2016). Sheet of elastin secreting SM improved the long-term cardiac function and reduced the left ventricle end-diastolic dimensions and remodelling (Uchinaka et al. 2012).
The safety of SM sheet implantation and the absence of arrhythmia have been established in a porcine MI model (Terajima et al. 2014). Furthermore, SM sheet improved both systolic and diastolic function of severely damaged canine heart, especially by controlling the collagen I/III balance (Shirasaka et al. 2016). Following pre-clinical and safety evaluation, investigations on myoblast implanted as a stack of cell sheets have reached clinical phase II (Imamura et al. 2016). LV ejection fraction and heart failure symptoms improved significantly in the treated group, during the 6-month follow-up (p < 0.05) (Imamura et al. 2016).
2.2.3 Adipose-Derived Stem Cells
Adipose tissue includes a heterogeneous mixture of MSCs, hematopoietic stem cells, and endothelial progenitor cells. The major clinical advantage of this type of cells is their availability. Investigations reporting the implantation of adipose-derived stem cells organized in cell sheets in acute and chronic MI, confirmed an increased survival and engraftment. In addition, the secretion of a wide array of angiogenic and anti-apoptotic factors have been demonstrated and induced beneficial effects on perfusion and function in myocardial infarction models (Ishida et al. 2015; Hamdi et al. 2011; Yeh et al. 2014). The cardioprotective factors including HGF and VEGF contributed to the attenuation of the infarct size, inflammation, and left ventricular remodelling (Imanishi et al. 2011).
The implantation of adipose-derived stem cells sheet in a porcine model of chronic heart failure improved the left ventricular ejection fraction (Ishida et al. 2015).
An overexpression of VEGF in adipose-derived stem cell sheets promoted cell survival under hypoxia in vitro. When evaluated in a rabbit MI model, the authors observed a reduction in infarct size, an improved cardiac function, the suppression of fibrosis and an enhanced blood vessel formation (Yeh et al. 2014).
Adiposite-derived mesenchymal stem cells embedded in platelet-rich fibrin scaffold demonstrated superior effects compared to a direct implantation, improved left ventricular performance, and reduced left ventricular remodelling (Chen et al. 2015; Sun et al. 2014).
2.2.4 Cardiac Stem Cells
Cardiosphere-derived cells (CDCs ) or cardiac progenitor cells (CPCs) can be isolated from postnatal heart . Their ability to self-renew and differentiate in CM have driven investigations for cardiac repair (Hosoyama et al. 2015). Beside direct injection of the CSC the regenerative capacity of engineered tissues composed of CSC and CDC sheets has been also studied. Their implantation in rodent MI models confirmed a reduced accumulation of interstitial fibrosis and an improved cardiac function (Alshammary et al. 2013; Hosoyama et al. 2015). Therapeutic efficacy was increased using hypoxic preconditioning cell sheets that augmented the angiogenesis and reducing the fibrosis (Hosoyama et al. 2015).
When delivered with poly(l-lactic acid) (PLLA) scaffold, CSC and VEGF showed modest effects on angiogenesis and cardiomyogenesis in the acutely infarcted hearts. The authors reported a reduction in cardiac remodelling and enhanced global cardiac function (Chung et al. 2015).
3 Matrices: A Multifaceted Substrate for the Cells
The development of fully organized and potentially contractile tissues for myocardium replacement request the selection of biomaterials that allow cell guidance and differentiation, mimic cardiac mechanical properties, and t permit electromechanical integration. Elaborating cardiac biografts have led to numerous progresses in biomaterials. INvestigations focussed on the modulation of their mechanic-electrical properties, the optimization of the cell–matrix interactions and the promotion of the long-term survival and vascularization after the implanted biograft.
The first challenge is the fine-tuning of the mechanical properties. In general, the e-modulus of solid matrix-based biografts stays markedly higher compared with the myocardial one. The maximal passive young moduli of the heart ventricle vary from 40 to 200 kPa depending on the axial strain (Hu et al. 2003). A stiff biomaterial would prompt a girdling effect and consequently limit the LV dilation. But, the rigidity of the scaffold may also hinder optimal heart contractility. When assessing the potential girdling effect of the PCL fibrous scaffold, Guex et al, found that the epicardial implantation of the matrix alone failed to prevent the progressive heart function decrease in a rat model of MI. Only when the e-spun fibers of PCL were seeded with MSC, a stabilization in heart function was recorded 4 weeks post implantation (Guex et al. 2014a).
The cell response to biomaterials properties is of paramount for the development of functional tissue. The substrate stiffness and/or the topography influence the cell differentiation and proliferation (Curtis and Russell 2011; Guex et al. 2012, 2013a; Valles et al. 2015) as well as the regulation of collagen and fibronectin deposit (Flück et al. 2003). Proteins complex such as focal adhesion sites and integrins provide the connections for mechanochemical transduction to signalling cascades and downstream the cellular adaptation. For example, Marsano et al. (2012) demonstrated that modulating the elasticity of poly(glycerol sebacate) matrices positively correlated with the contractile function of engineered cardiac constructs.
In parallel, the creation of semi-conductive matrices is rapidly evolving for excitable tissues. Indeed, the cardiac interstitial matrices play an important role in the propagation of the signal within the interconnecting ventricular myocyte layers (Coghlan et al. 2006). Consequently, new electroactive scaffolds have been developed. As examples, gold nanowire impregnated alginate scaffolds permitted electrical conductance and promoted CM connectivity through an increase in connexin 43 expression (Dvir et al. 2012). Electroactive polymer or eGel composed of electroactive nanoparticles greatly promoted excitable biografts development (Wang et al. 2016).
Finally, functionalization of the matrices and in particular the chemical surface modification of the scaffold and the addition of various molecules are necessary to respond to the complexity of muscle regeneration. Different approaches have been investigated. For examples Guex et al used a plasma coating process to enrichment with oxygen an electrospun polycaprolactone (PCL) fibrous matrix (Guex et al. 2012). This process modified the physical properties of the matrix and resulted in higher hydrophilicity and stability. The functionalized matrix presented a suitable environment for cell growth. It was demonstrated that oxygen enrichment significantly favored adhesion, orientation, and differentiation of SMs as well as bone marrow MSCs (Guex et al. 2012. Guex et al. 2014a). MSC adhered and spread on the matrix, producing a homogeneous cell layer. In addition, oxygen surface enrichment allowed the culture of ESC derived-cardiomyocytes, the cells maintained their cardiomyogenic phenotype with striated α-actinin mature sarcomeric structures and spontaneously beating areas, indicating of a mature differentiation into cardiomyocytes (Guex et al. 2013b).
Furthermore, active molecules (such as VEGF, IGF-1, FGF-2, thymosin β4, HGF, and FSLT1) have been added to the scaffolds in order to modify the microenvironment of the implanted cells, to stimulate cells survival and to promote stem cells recruitment as well as angiogenesis. This approach often provided a local delivery of the molecule and foster cardiac repair (Hwang and Kloner 2010; Segers and Lee 2011; Giraud et al. 2012; Guex et al. 2014b). In particular, collagen patches were dehydrated by compression and loaded with recombinant active molecules such as FSTL1 or conditioned medium. When suture on the surface of infarcted myocardium in mice, the authors documented a remarkably cardiac function recovery with a treatment-induced complete fractional shortening level reestablishment after 3 months. Interestingly, following implantation, FSTL1 loaded collagen patch promoted CM proliferation within the artificial matrix (Wei et al. 2015).
3.1 Solid Matrix: The Example of Electrospun Fibers
Solid matrices can be porous or fibrous with versatile pore size, fiber/pore diameters, and topographies. Processing matrices with electrospinning had become a primordial technique to engineered substrates for cardiac tissue . Electrospinning produces submicron fibers using an electrostatically driven jet of a polymer solution or a melt. A polymer solution is forced through a syringe needle where a high voltage (5–60 kV) is applied. The e-spun fibers are collected on a ground support and form of non-woven mats. E-spun matrices mimic the size and fibrous pattern of biologic extracellular matrices found in tissues. A large number of biomaterials composed of single or blended polymers have been electrospun (Zhao et al. 2015), and assessed for cardiac tissue. The versatility of this technique relies on the possibility to control the fiber composition, diameters and their alignment. Furtheremore, the simplicity of its implementation has contributed to the success of this technique. In addition, more sophisticated fibers have been developed such as a core/shell, electro-conductive, or functionalised fibers .
The cells are generally seeded on the surface of the e-spun scaffold and form a monolayer. A major drawback of this approach is the limited number of cells that can infiltrate the scaffold; efforts have been undertaken to control and increase the size of the pore of the fibrous matrices and improve the seeding method in order to develop more effective scaffold. As an alternative, cell electrospinning allowing 3D deposition of both cells and fibers have recently provide successful engineered cardiac like tissue with neonatal cardiomyocytes (Ehler and Jayasinghe 2014).
Interestingly, electrospinning allows incorporation of bioactive agents (proteins, enzymes, silver, etc.). The addition of cytokines or growth factor into the fiber may contribute to improve the cell survival, stem cell homing, or the vascularization of the patch. For example, fibronectin-immobilized PCL e-spun nanofibers allowed survival of umbilical-cord-blood-derived MSC in a rat MI model, induced cardiac function partial recovery, and reduced infarct (Kang et al. 2014). Interestingly, the authors provided evidence that fibronectin immobilization at the surface of the scaffold induced changes in the expression levels of genes involved in the paracrine regulation of cardiac repair including stem cell homing, angiogenesis and protection against apoptosis , inflammation, and fibrosis.
3.2 Fibrin as a Noteworthy Choice for Hydrogel Type Matrices
Hydrogels present implantable and injectable form s. The injectable hydrogel presents a liquid phase that becomes solid under temperature/pH changes or mix of two components and can be directly applied in combination of cells on the surface or within the myocardium. Hydrogels allow the control of the physical and chemical microenvironment of implanted cells and would determine their functionality (Li and Guan 2011). The hydrogel would modulate the reparative pathways involved in cell survival and gene expression after myocardial injury (Dobaczewski et al. 2010).
Among them, gel type scaffold such as fibrin formed from thrombin and fibrinogen has recently gained increasing interest for cardiac application (Roura et al. 2016). Other formulation of fibrin such as micro-particles has enlarged the options for fibrin-based cell and active molecules delivery. Fibrin is a natural matrix that is initially involved in physiological repair mechanisms. Following tissue injury, fibrinogen and plasma fibronectin extravagated from the vascular network provide a fibrin-based provisional matrix that plays a scaffolding role in inflammation regulation.
For example, fibrin patch-based transplantation of hESC-derived vascular cells in an MI porcine model allowed significant engraftment of cells and increased neovascularization and improved LV contractile function (Xiong et al. 2011). Fibrin has recently been associated with a large selection of cells such as endothelial progenitors (Atluri et al. 2014), ESC derived cardiac progenitors (Bellamy et al. 2015), MSC derived from bone marrow (Li et al. 2015), umbilical cord blood (Roura et al. 2015), or adipocyte tissue (Chen et al. 2015; Sun et al. 2014). Although, fibrin or plasma rich fibrin-based cardiac constructs have been successfully investigated in small and large animal model for cardiac salvage (Barsotti et al. 2011), clinical trials have not yet been initiated. Nevertheless, to date, optimal condition for cardiac biografts implantation remains undefined. The rapidly biodegradable fibrin may improve cell survival; however, the timing for the paracrine regulation is unknown, questions such as the time of implantation and the required minimal duration of cell survival stay open.
On the other hand, controlled delivery of bioactive molecules within fibrin has gained increasing interest. Recently, Ye et al. demonstrated that fibrin combined with encapsulated IGF-1 enhanced engraftment of induced pluripotent stem cell-derived cardiovascular cell populations injected into the injured myocardium of swine (Ye et al. 2014).
4 Conclusion
Experimental investigations of engineered cardiac tissue have provided a real enthusiasm. However, only a few clinical trials with engineered tissue have been so far initiated. Nevertheless, the numerous challenges such as the optimal timing for graft delivery, the minimal duration for cell survival necessary to initiate paracrine regulation of cardiac repair, and the identification of the mechanism of action should be carefully investigated to avoid the skepticism raised in the past from clinical trials using cell injection.
References
Alshammary S, Fukushima S, Miyagawa S et al (2013) Impact of cardiac stem cell sheet transplantation on myocardial infarction. Surg Today 43:970–976
Atluri P, Miller JS, Emery RJ, Hung G, Trubelja A, Cohen JE, Lloyd K1, Han J1, Gaffey AC, MacArthur JW, Chen CS, Woo YJ (2014) Tissue-engineered, hydrogel-based endothelial progenitor cell therapy robustly revascularizes ischemic myocardium and preserves ventricular function. J Thorac Cardiovasc Surg 148(3):1090–1097; discussion 1097–1098. doi:10.1016/j.jtcvs.2014.06.038. Epub 2014 Jun 28
Barsotti MC, Felice F, Balbarini A, Di Stefano R (2011) Fibrin as a scaffold for cardiac tissue engineering. Biotechnol Appl Biochem 58(5):301–310
Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L et al (2010) Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation 122(11 Suppl 1)
Bellamy V, Vanneaux V, Bel A et al (2015) Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J Hear Lung Transplant 34:1198–1207
Ceccaldi C, Bushkalova R, Alfarano C et al (2014) Evaluation of polyelectrolyte complex-based scaffolds for mesenchymal stem cell therapy in cardiac ischemia treatment. Acta Biomater 10:901–911
Chang D, Wen Z, Wang Y et al (2014) Ultrastructural features of ischemic tissue following application of a bio-membrane based progenitor cardiomyocyte patch for myocardial infarction repair. PLoS One 9:1–8
Chen YL, Sun CK, Tsai TH et al (2015) Adipose-derived mesenchymal stem cells embedded in platelet-rich fibrin scaffolds promote angiogenesis, preserve heart function, and reduce left ventricular remodeling in rat acute myocardial infarction. Am J Transl Res 7:781–803
Chi NH, Yang MC, Chung TW et al (2012) Cardiac repair achieved by bone marrow mesenchymal stem cells/silk fibroin/hyaluronic acid patches in a rat of myocardial infarction model. Biomaterials 33:5541–5551
Chong JJH, Yang X, Don CW et al (2014) Human embryonic stem cell-derived cardiomyocytes regenerate non-human primate hearts james. Nature 510:273–277
Chung HJ, Kim JT, Kim HJ et al (2015) Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. J Control Release 205:218–230
Coghlan HC, Coghlan AR, Buckberg GD, Cox JL (2006) “The electrical spiral of the heart”: its role in the helical continuum. The hypothesis of the anisotropic conducting matrix. Eur J Cardio Thoracic Surg 29(Suppl 1):S178–S187
Curtis MW, Russell B (2011) Micromechanical regulation in cardiac myocytes and fibroblasts: implications for tissue remodeling. Pflugers Arch 462(1):105–117
Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG (2010) The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48(3):504–511
Dvir T, Timko BP, Brigham MD, Naik SR, Sandeep S, Levy O et al (2012) Nanowired three dimensional cardiac patches. Nat Nanotechnol 6(11):720–725
Ehler E, Jayasinghe SN (2014) Cell electrospinning cardiac patches for tissue engineering the heart. Analyst 139(18):4449–4452
Flück M, Giraud MN, Tunç V, Chiquet M (2003) Tensile stress-dependent collagen XII and fibronectin production by fibroblasts requires separate pathways. Biochim Biophys Acta 1593(2–3):239–248
Giraud MN, Guex AG, Tevaearai HT (2012) Cell therapies for heart function recovery: focus on myocardial tissue engineering and nanotechnologies. Cardiol Res Pract 2012:971614
Gjesdal O, Bluemke DA, Lima JA (2011) Cardiac remodeling at the population level--risk factors, screening, and outcomes. Nat Rev Cardiol 8(12):673–685
Gu M, Nguyen PK, Lee AS et al (2012) Microfluidic single cell analysis show porcine induced pluripotent stem cell-derived endothelial cells improve myocardial function by paracrine activation mingxia. Circ Res 111:882–893
Guex AG, Kocher FM, Fortunato G, Körner E, Hegemann D, Carrel TP et al (2012) Fine-tuning of substrate architecture and surface chemistry promotes muscle tissue development. Acta Biomater 8(4):1481–1489
Guex AG, Birrer DL, Fortunato G, Tevaearai HT, Giraud M-N (2013a) Anisotropically oriented electrospun matrices with an imprinted periodic micropattern: a new scaffold for engineered muscle constructs. Biomed Mater 8(2):021001
Guex AG, Romano F, Marcu IC, Tevaearai HT, Ullrich ND, Giraud M-N (2013b) Culture of Cardiogenic Stem Cells on PCL -Scaffolds: Towards the Creation of Beating Tissue Constructs. IASTED Conference Proceedings, Vol. 791
Guex AG, Frobert A, Valentin J, Fortunato G, Hegemann D, Cook S et al (2014a) Plasma-functionalized electrospun matrix for biograft development and cardiac function stabilization. Acta Biomater 10(7):2996–3006
Guex AG, Hegemann D, Giraud MN, Tevaearai HT, Popa AM, Rossi RM et al (2014b) Covalent immobilisation of VEGF on plasma-coated electrospun scaffolds for tissue engineering applications. Colloids Surf B Biointerfaces 123:724–733
Guyette JP, Charest J, Mills RW, Jank B, Moser PT, Gilpin SE et al (2016) Bioengineering human myocardium on native extracellular matrix. Circ Res 118(1):56–72
Hamdi H, Planat-Benard V, Bel A et al (2011) Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections. Cardiovasc Res 91:483–491
Higuchi T, Miyagawa S, Pearson JT et al (2015) Functional and electrical integration of induced pluripotent stem cell-derived cardiomyocytes in a myocardial infarction rat heart. Cell Transplant 24:2479–2489
Hosoyama T, Samura M, Kudo T et al (2015) Cardiosphere-derived cell sheet primed with hypoxia improves left ventricular function of chronically infarcted heart. Am J Transl Res 7:2738–2751
Hu Z, Metaxas D, Axel L (2003) In vivo strain and stress estimation of the heart left and right ventricles from MRI images. Med Image Anal 7(4):435–444
Hwang H, Kloner RA (2010) Improving regenerating potential of the heart after myocardial infarction: factor-based approach. Life Sci 86(13–14):461–472
Imamura T, Kinugawa K, Sakata Y et al (2016) Improved clinical course of autologous skeletal myoblast sheet (TCD-51073) transplantation when compared to a propensity score-matched cardiac resynchronization therapy population. J Artif Organs 19:80–86
Imanishi Y, Miyagawa S, Maeda N et al (2011) Induced adipocyte cell-sheet ameliorates cardiac dysfunction in a mouse myocardial infarction model: a novel drug delivery system for heart failure. Circulation 124:10–17
Ishida O, Hagino I, Nagaya N et al (2015) Adipose-derived stem cell sheet transplantation therapy in a porcine model of chronic heart failure. Transl Res 165:631–639
Kang B-J, Kim H, Lee SK, Kim J, Shen Y, Jung S et al (2014) Umbilical cord blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized PCL nanofiber improve the cardiac function. Acta Biomater 10:3007–3017
Karam JP, Muscari C, Montero-Menei CN (2012) Combining adult stem cells and polymeric devices for tissue engineering in infarcted myocardium. Biomaterials 33(23):5683–5695
Kawamura M, Miyagawa S, Miki K et al (2012) Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126:29–37
Kawamura A, Miyagawa S, Fukushima S et al (2016) Teratocarcinomas arising from allogeneic induced pluripotent stem cell-derived cardiac tissue constructs provoked host immune rejection in mice. Sci Rep 6:1–13
Li Z, Guan J (2011) Hydrogels for cardiac tissue engineering. Polym (Basel) 3(2):740–761
Li J, Zhu K, Yang S et al (2015) Fibrin patch-based insulin-like growth factor-1 gene-modified stem cell transplantation repairs ischemic myocardium. Exp Biol Med 240:585–592
Lu W-N, Lü S-H, Wang H-B et al (2009) Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng Part A 15:1437–1447
Marsano A, Maidhof R, Wan LQ, Wang Y (2012) NIH Public Access 26(5):1382–1390
Masumoto H, Yamashita JK (2016) Exploiting human iPS cell-derived cardiovascular cell populations toward cardiac regenerative therapy. Stem Cell Transl Invest 3:e1226
Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, Marui A, Shimizu T, Ikeda T, Okano T et al (2012) Pluripotent stem cell-engineered cell sheets re-assembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells 30:1196–1205
Masumoto H, Ikuno T, Takeda M et al (2014) Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep 4:1–7
Matsuo T, Masumoto H, Tajima S et al (2015) Efficient long-term survival of cell grafts after myocardial infarction with thick viable cardiac tissue entirely from pluripotent stem cells. Sci Rep 5:1–14
Menasché P, Vanneaux V, Hagege A et al (2015) Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 36:2011–2017
Miyagawa S, Saito A, Sakaguchi T, Yoshikawa Y, Yamauchi T, Imanishi Y et al (2010) Impaired myocardium regeneration with skeletal cell sheets--a preclinical trial for tissue-engineered regeneration therapy. Transplantation 90(4):364–372
Narita T, Shintani Y, Ikebe C et al (2013) The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mol Ther 21:860–867
Pätilä T, Miyagawa S, Imanishi Y et al (2015) Comparison of arrhythmogenicity and proinflammatory activity induced by intramyocardial or epicardial myoblast sheet delivery in a rat model of ischemic heart failure. PLoS One 10:1–15
Roura S, Soler-Botija C, Bagó JR, Llucià-Valldeperas A, Férnandez MA, Gálvez-Montón C, Prat-Vidal C, Perea-Gil I, Blanco J, Bayes-Genis A (2015) Postinfarction functional recovery driven by a three-dimensional engineered fibrin patch composed of human umbilical cord blood-derived mesenchymal stem cells. Stem Cells Transl Med 4(8):956–966
Roura S, Gálvez-Montón C, Bayes-Genis A (2016) Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. J Tissue Eng Regen Med. doi:10.1002/term.2129 [Epub ahead of print]
Segers VF, Lee RT (2011) Biomaterials to enhance stem cell function in the heart. Circ Res 109(8):910–922
Shiba Y, Fernandes S, Zhu W et al (2012) hESC-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489:322–325
Shirasaka T, Miyagawa S, Fukushima S et al (2016) Skeletal myoblast cell sheet implantation ameliorates both systolic and diastolic cardiac performance in canine dilated cardiomyopathy model. Transplantation 100:295–302
Shudo Y, Miyagawa S, Ohkura H et al (2014) Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng Part A 20:728–739
Steppich B1, Hadamitzky M, Ibrahim T, Groha P, Schunkert H, Laugwitz KL, Kastrati A, Ott I (2016) Regenerate vital myocardium by vigorous activation of bone marrow stem cells (REVIVAL-2) study investigators. Stem cell mobilisation by granulocyte-colony stimulating factor in patients with acute myocardial infarction. Long-term results of the REVIVAL-2 trial. Thromb Haemost 115(4):864–868
Sun CK, Zhen YY, Leu S et al (2014) Direct implantation versus platelet-rich fibrin-embedded adipose-derived mesenchymal stem cells in treating rat acute myocardial infarction. Int J Cardiol 173:410–423
Tano N, Kaneko M, Ichihara Y et al (2016) Allogeneic mesenchymal stromal cells transplanted onto the heart surface achieve therapeutic myocardial repair despite immunologic. J Am Heart Assoc 5:1–14
Terajima Y, Shimizu T, Tsuruyama S, Sekine H (2014) Autologous skeletal myoblast sheet therapy for porcine myocardial infarction without increasing risk of arrhythmia. Cell Med 6:99–109
Tongers J, Losordo DW, Landmesser U (2011) Stem and progenitor cell-based therapy in ischaemic heart disease: Promise, uncertainties, and challenges. Eur Heart J 32(10):1197–1206
Uchinaka A, Kawaguchi N, Hamada Y et al (2012) Transplantation of elastin-secreting myoblast sheets improves cardiac function in infarcted rat heart. Mol Cell Biochem 368:203–214
Valles G, Bensiamar F, Crespo L, Arruebo M, Vilaboa N, Saldaña L (2015) Topographical cues regulate the crosstalk between MSCs and macrophages. Biomaterials 37:124–133
Wang Q, Wang Q, Teng W (2016) Injectable, degradable, electroactive nanocomposite hydrogels containing conductive polymer nanoparticles for biomedical applications. Int J Nanomed 11:131–144
Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S et al (2015) Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525(7570):479–485
Xiong Q, Hill KL, Li Q et al (2011) A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells 29:367–375
Ye L, Chang Y-H, Xiong Q et al (2014) Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cell populations. Cell Stem Cell 15:750–761
Yeh TS, Dean Fang YH, Lu CH et al (2014) Baculovirus-transduced, VEGF-expressing adipose-derived stem cell sheet for the treatment of myocardium infarction. Biomaterials 35:174–184
Zhao G, Zhang X, Lu TJ, Xu F (2015) Recent advances in electrospun nanofibrous scaffolds for cardiac tissue engineering. Adv Funct Mater 25(36):5726–5738
Acknowledgement
This work was supported by the Swiss National Foundation [SNF 310030-149986] and the University of Fribourg.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Giraud, MN., Borrego, I. (2017). Myocardial Tissue Engineering: A 5 Year—Update. In: Pham, P. (eds) Liver, Lung and Heart Regeneration. Stem Cells in Clinical Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-46693-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-46693-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46692-7
Online ISBN: 978-3-319-46693-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)