Abstract
Research conducted over the last decades indicates a necessity of having larger number of EMG sensors in order to extract sufficient information needed for natural control of upper limb prosthetics. Various studies have addressed this issue, though clinical transition and evaluation of such systems on a larger pool of patients is still missing. We propose a specifically designed system which allows users to perform clinically relevant tests in an unobstructed way while handling dexterous prosthesis. Eight electrodes were embedded into customized sockets along with the controllers driving an algorithm recently tested in laboratory conditions that allows simultaneous manipulation of four out of seven prosthetic functions. The fully self-contained system was evaluated on seven amputees conducting the Southampton Hand Assessment Procedure. The scores achieved were compared to those obtained using their own commercial devices. The study shows the necessary steps to validate novel control algorithms in a clinically meaningful context.
This work was supported by the Christian Doppler Research Foundation of the Austrian Federal Ministry of Science, Research and Economy and by the European Research Council Advanced Grant DEMOVE (contract #267888).
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
- Linear Discriminant Analysis
- Wrist Flexor
- Prosthetic Hand
- Limb Prosthetic
- Linear Discriminant Analysis Classifier
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Various research conducted in the last decades indicated a necessity for introducing additional EMG sensors in order to provide upper limb prosthetic users with more versatile and natural control [1, 2]. Namely, the currently available solutions are almost strictly depending on just a few electrodes and a cumbersome control paradigm [3, 4]. On the other hand, more advanced systems have seemed to fail in reaching the clinical testing stage. Reasons for this are numerous, but they mostly boil down to the issue of robustness.
Transferring myoelectric solutions into systems that can be tested in an acceptable manner through different clinical scenarios requires production of sockets which are capable of hosting the majority of the hardware. Even once the transfer is made, the system is facing challenges such as extensive sweat production, electrode misplacement, uneven loading of the socket and stump volume changes [5].
Here, we present a socket-ready implementation of a previously laboratory tested system for simultaneous and proportional control of the dexterous prosthetic hand [6]. The outcomes of a clinical evaluation are presented and discussed along with the challenges that were encountered during the testing and fitting process.
2 Methods
2.1 Subjects
Total of seven transradial amputees (6 male, 1 female, aged 35.14 ± 10.11) agreed to participate in the study after reading and signing consent forms approved by local ethics board of Medical University of Vienna (Ethics Commission number: 1044/2015). All participants are active myoelectric users, though have no previous experience with advanced control systems.
2.2 Hardware and Control Algorithm
All seven subjects were fitted with custom sockets by a certified prosthetist. Each socket was able to host eight Otto Bock raw signal electrodes 13E200 = 50AC, through a predefined gap positioned over the most muscular circumference of the forearm. Individual electrodes were placed into the 3D printed casings which enabled their quick equidistant placement using a single rubber band. Tip of the thermoplast based liner provided the support for lamination rings needed for prosthetic attachment and was enclosed in cellacast, the fiberglass free plaster.
The prosthesis that was used throughout the experiment was Ottobock Michelangelo hand with wrist flexor and rotation units. If the length of the stump prevented battery pack and the Axon®bus system to be tucked into the socket, these components were simply attached on the outside of the shaft.
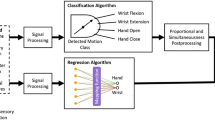
Communication with the computer which was running the control algorithm was done via Bluetooth. In this way full flexibility and control over the implemented algorithm which allowed proportional activation of the wrist flexor and rotation units (linear regression) combined with sequential, proportional hand function (linear discriminant analysis classifier) [6] was available without compromising comfort of the user during testing.
2.3 Clinical Testing
In order to make a clinically relevant evaluation of patient performance using the implemented system Southampton Hand Assessment Procedure (SHAP) [7] was conducted. This test includes a variety of tasks ranging from more abstract ones to those replicating the activities of daily living. The overall end score can range from 0 representing no useful hand function at all, to 100 which is equivalent to a fully functional able bodied hand. Subjects were given two opportunities in performing each task and the execution time out was set to 100 s.
2.4 Experiment Protocol
Each subject was invited to come three times. During the first visit prosthetists conducted all the necessary measurements for building up the socket, and participants were once again briefed on what is expected from them throughout the experiment. In the second session the new socket was tested and modifications were made if needed. Additionally, subjects were asked to perform the SHAP test using their own myoelectric device in order to acquire the “baseline” score. In the final session, the training data needed for the control of the prosthetic device was collected in a standard myoelectric pattern recognition based manner, as in previously conducted study [8]. After a short brake the participant was invited to test the device and get familiar and comfortable with its capabilities and a final SHAP test was conducted.
3 Results
All subjects were successfully fitted with the prosthesis and were able to follow the experiment. No participant reported any problems with the fitting during the third, advanced session. The only notable complaints were regarding the overall weight of the system and its poor weight distribution in cases of users with longer stumps.
The results of SHAP testing are presented in Table 1.
Baseline score across all subjects was 62.00 ± 10.65 and in the final session patients achieved the average score of 42.14 ± 8.93. Closest score using the advanced prosthesis to the baseline one was achieved by subject S6 with 11 points difference, while the greatest discrepancy was observed in S3, 36 points. Average SHAP score difference between the two systems was 19.86 ± 8.05.
4 Conclusions and Discussion
In this study we have implemented an eight channels based advanced control system, which is able of delivering simultaneous and proportional control over 3.5 degrees of freedom, into a custom fitted wearable socket. The purpose was to conduct a clinically viable evaluation of the solution in order to identify its potential for real world applications.
Based on the feedback received from the participants the terminal load of the prosthetic fitting was regarded problematic and a more balanced weight distribution should be aimed at, especially in patients with longer stumps. The fact that all participants managed to conduct the entire experiment using the proposed system, even though somewhat lower scores were achieved, indicates the potential of this approach of becoming a candidate for a proper clinical study. Though, it also points out a necessity for conducting a longitudinal evaluation which would eliminate the effects of learning and accommodation of the patient to an entirely new system and control paradigm. In addition, inclusion of motion analysis could indicate possible changes in compensatory movements [9].
References
Hudgins, B., Parker, P., Scott, R.N.: A new strategy for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 40(1), 82–94 (1993)
Jiang, N., Vujaklija, I., Rehbaum, H., Graimann, B., Farina, D.: Is accurate mapping of EMG signals on kinematics needed for precise online myoelectric control? IEEE Trans. Neural Syst. Rehabil. Eng. 22(3), 549–558 (2014)
Scott, R.N., Parker, P.A.: Myoelectric prostheses: state of the art. J. Med. Eng. Technol. 12(4), 143–151 (1988)
Oskoei, M.A., Hu, H.: Myoelectric control systems-a survey. Biomed. Signal Process. Control 2(4), 275–294 (2007)
Hargrove, L., Englehart, K., Hudgins, B.: A training strategy to reduce classification degradation due to electrode displacements in pattern recognition based myoelectric control. Biomed. Signal Process. Control 3(2), 175–180 (2008)
Amsuess, S., Vujaklija, I., Gobel, P., Roche, A., Graimann, B., Aszmann, O., Farina, D.: Context-dependent upper limb prosthesis control for natural and robust use. IEEE Trans. Neural Syst. Rehabil. Eng., 1 (2015)
Light, C.M., Chappell, P.H., Kyberd, P.J.: Establishing a standardized clinical assessment tool of pathologic and prosthetic hand function: normative data, reliability, and validity. Arch. Phys. Med. Rehabil. 83(6), 776–783 (2002)
Roche, A.D., Vujaklija, I., Amsuess, S., Sturma, A., Göbel, P., Farina, D., Aszmann, O.C.: Structured rehabilitation training for improved multifunctional prosthetic control : a case study. J. Vis. Exp. (2015)
Montagnani, F., Controzzi, M., Cipriani, C.: Exploiting arm posture synergies in activities of daily living to control the wrist rotation in upper limb prostheses: a feasibility study. In: Proceedings of Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, vol. 2015, pp. 2462–2465, November 2015
Acknowledgments
The authors would like to extend their gratitude to Mr. Hans Opel and his team of prosthetists at Otto Bock Healthcare Products GmbH for manufacturing and fitting all the patients with custom made sockets.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Vujaklija, I., Amsuess, S., Roche, A.D., Farina, D., Aszmann, O.C. (2017). Clinical Evaluation of a Socket-Ready Naturally Controlled Multichannel Upper Limb Prosthetic System. In: González-Vargas, J., Ibáñez, J., Contreras-Vidal, J., van der Kooij, H., Pons, J. (eds) Wearable Robotics: Challenges and Trends. Biosystems & Biorobotics, vol 16. Springer, Cham. https://doi.org/10.1007/978-3-319-46532-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-46532-6_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46531-9
Online ISBN: 978-3-319-46532-6
eBook Packages: EngineeringEngineering (R0)