Abstract
Alveolar epithelial injury is a feature of ARDS. In this chapter, we will address strategies to analyze alveolar epithelial cell function in lung injury and repair processes. These include an overview of cell isolation procedures for murine and human alveolar epithelial type II cells and examples for phenotypic characterization of ATII cells in the context of lung injury. Moreover, we will discuss functional in vitro assays to evaluate differentiation and regeneration potential of alveolar epithelial cells.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Acute and chronic lung diseases constitute a significant health burden worldwide and a better and deeper understanding of the mechanisms that initiate and drive disease progression [1–3]. Alveolar epithelial injury represents a hallmark of acute lung injury (ALI) as well as chronic lung diseases such as idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD) [1, 4, 5]. In the healthy adult lung, alveolar epithelial type I (ATI) and alveolar epithelial type II (ATII) cells are the main cell types that form the alveolar epithelium and establish the alveolar epithelial barrier [6]. ATI cells represent large, thin squamous epithelial cells that cover an enormous surface area (95 % of the alveolus) and are in close vicinity to the underlying capillary endothelium to facilitate gas exchange [7–9]. ATII cells, however, display a cuboidal shape and one of their main functions is the production, storage and release of surfactant. Surfactant consists of an intricate combination of proteins and lipids which lines the alveolar epithelium, lowers the surface tension in the lung and plays an important role in host defense mechanisms [10, 11]. Both ATI and ATII cells participate in ion transport in the lung and contribute to the fluid balance within the alveolus [7, 12, 13]. In ALI, the alveolar epithelial barrier, formed by ATI and ATII cells as well as endothelial cells of the alveolar capillary, represents the first point of injury. Disruption of the barrier structure with subsequent accumulation of protein-rich edema fluid in the alveolar air spaces is a main feature of ALI [14–16]. Tight junctions (TJ) localizing to the cell–cell junctions connecting alveolar epithelial cells are essential for normal epithelial barrier function [17]. ATII cells are a critical cell population driving repair in the alveolar epithelium [18]. ATII cells are able to proliferate, self-renew and serve as a progenitor cell population for ATI cells in injury and repair processes induced by a variety of different triggers. Thus, ATII cells are considered one of the important epithelial stem cell populations in the adult distal lung [19–22]. Restoration of the normal epithelial barrier requires the spreading and migration of cells in close proximity to the injury to cover the denuded basement membrane. This is followed by migration and proliferation of progenitor cells to compensate for the cellular loss. Finally differentiation processes have to be initiated to restore a functional epithelium [14, 23, 24]. However, the loss of reparative function of ATII cells and a shift towards pro-fibrotic functions has been described for ALI as well as for IPF [25–29]. The elucidation of mechanisms driving alveolar epithelial cell responses in a beneficial versus a potentially detrimental direction during lung injury and repair is therefore of prime interest and the development of novel models and methods to study those mechanisms is of utmost importance.
Methods of Alveolar Epithelial Type II Cell Isolation
A prerequisite for analyzing alveolar epithelial type II cell characteristics and functional properties in vitro is the isolation of a pure population of the respective primary cell type. This requires the identification of specific ATII cell markers within the tissue together with morphology and localization. Several markers expressed in adult ATII cells have been described over the past decades. These include the surfactant proteins A, B, C, and D (SPA, SPB, SPC, and SPD), of which SPC has been reported to be specific for ATII cells [30]. ATII specific expression of ATP-binding cassette sub-family A member 3 (ABCA3), a membrane component of lamellar bodies [31], in which surfactant proteins are stored [32], has been reported. In addition, lysosome-associated membrane glycoprotein 3 (LAMP3) [33] and pepsinogen C [34] have been proposed as ATII markers. Immunization strategies by Boylan [35] and Gonzalez [36] used ATII cells as immunogens to generate monoclonal antibodies for ATII cell surface proteins for rat and human. The monoclonal antibody MMC4 recognized a novel antigen on the apical surface of rat ATII cells, but also bound to rat club cells [35]. An antibody generated against human ATII cells recognized a protein of 280- to 300-kDa on the apical plasma membrane, which was termed HTII-280 and, by analysis of its biochemical characteristics, represents an integral membrane protein [36].
Two main isolation strategies have been widely used to isolate ATII cells from rodent and human tissue. Both strategies share a common procedure of enzymatic dissociation of lung tissue to obtain a single cell suspension of the lung. Enzymes most frequently used include porcine elastase, dispase, or collagenase as well as different combinations thereof [37–40]. In case of the murine lung, enzymes are directly instilled into the parenchyma via the cannulated trachea [38]. In case of human tissue, direct instillation into the alveolar region via a bronchus can only be applied for closed lung segments [28, 39]. Alternatively, minced human distal lung tissue can be subjected to enzymatic digestion [40]. Following digestion, alveolar tissue is dissected from the lager airways and minced mechanically. After sequential filtration of cells through nylon meshes of different pore sizes ranging from 100 to 10 µm to obtain a single cell suspension [38, 39], ATII cells can be isolated via positive or negative selection or a combinatorial approach making use of positive as well as negative selection markers.
Several different separation methods can be applied subsequently using different marker combinations. The most commonly used methods for depletion of specific subset of cells include the use of antibody coated cell culture plates, where cells expressing the respective markers adhere to the plate and non-adherent cells are collected [41–43], or using antibodies coupled to magnetic beads [38, 44, 45], and similarly the non-bound cells are collected.
Antibodies directed against CD45, CD14, and CD16/32 for hematopoietic lineages such macrophages, neutrophils and lymphocytes are commonly used [21, 27, 38, 43, 45–47] for negative selection. Species-specific IgG antibodies binding with their Fc domain to the Fcγ-receptors on the cell surface of phagocytes, B-lymphocytes, natural killer cells and dendritic cells are also widely used to eliminate these cell populations from the preparation [41–43, 45, 48–50]. Depletion of CD31 positive endothelial cells is often included in different protocols [44]. Furthermore, a fluorescence activated cell sorting (FACS) based approach can be utilized by using fluorescently labeled antibodies and subsequent sorting of cells, displaying no positive signal for any of the utilized markers [51]. Isolation protocols applied for human ATII cells frequently contain previous enrichment of ATII cells for the downstream depletion strategy by subjecting the crude singe cell suspension after enzymatic digestion to a discontinuous Percoll density gradient (1.04–1.09 g/ml). After centrifugation ATII cells and macrophages will be found in the same layer of the Percoll gradient. The cells in this layer can be isolated and subsequently a depletion of alveolar macrophages can be performed [41, 42, 45].
Positive selection strategies are mainly applied for the isolation of lineage labeled ATII cells in murine mouse models by FACS. For this strategy SPC-driver lines are used, which express GFP or EGFP under the control of the SPC promoter [7, 52–54]. In this context, it has been shown that transgenic mice exhibiting GFP expression under the control of the human SPC promoter in the murine system display GFP expression in only a subset of ATII cells or additionally in bronchiolar epithelial cells [55, 56]. However, the use of murine SPC promoters was reported to generate a higher specificity of labeling ATII cells [52, 53] although the efficiency of labeling was also described to be dependent on the age of mice [52] and isolated ATII cell displayed some heterogeneity as cells with bronchiolar and alveolar epithelial gene signatures were detected under three-dimensional culture conditions [52]. The positive selection of human ATII cells by FACS using an antibody against the previously described HTII-280 membrane protein has been initially described by Gonzalez and colleagues [36].
Combined negative and positive selection strategies are applied using different combinations of negative depletion for of a variety of cell types, and are listed in Table 6.1. These markers include markers for cells from hematopoietic lineages [22, 28, 40, 57–60], and ATI cells (T1α positive cells) [40] and a positive selection for general epithelial markers such as EpCAM [22, 40, 57, 59–61] and E-cadherin [28, 58] or a lysosomal marker such as the fluorescent dye LysoTracker [59], labeling acidic organelles within live cells.
Overall, the different ATII cell isolation strategies result in some variability of cell purity (between 80 and 99 %). The choice of the appropriate isolation method is widely discussed in particular with respect to the utilization for the isolation of ATII cells from different models of lung disease or the subsequent usage of cells for different downstream applications (direct analysis versus culture for functional assays). When choosing which methods to apply, several points need to be taken under consideration. In general, the use of positive selection markers might result in higher cell purity—and better characterized cell (sub)populations, however, changes in the expression pattern of single specific markers used for selection might be altered in different disease models, which in turn changes the population analyzed.
Alveolar Epithelial Type II Cell Analysis
Obtaining insight into molecular mechanisms of alveolar epithelial injury and repair is of prime interest to identified potential targets for therapeutic intervention needed for the treatment of various lung diseases, including ARDS.
Alveolar Epithelial Type II Cell Culture
Analyzing freshly isolated ATII cells from rodent injury models or human diseased tissue using microarray technology is a powerful tool to determine disease related altered phenotypes in lung injury and repair [27, 40]. However, for functional analysis, the culture of primary ATII cells is of utmost importance. Challenged by the fact that ATII cells possess the intrinsic properties to differentiate into ATI cells when placed into normal cell culture, a wide range of culture conditions and media compositions for ATII cells are described. A careful selection of culture conditions is crucial to obtain meaningful results. Depending on the applied assay, culture vessels as well as the presence of specific media supplements might influence experimental outcomes. Plating fresh ATII cells on plastic dishes induces the gradual loss of ATII cell characteristic [62, 63]. Coating cell culture dishes with extracellular matrix (ECM) components such as fibronectin, collagen or laminin or a combination thereof, will lead to differences in the dynamics of trans-differentiation processes. Additionally, culturing of ATII cells on trans-well filter inserts has been described to result in stabilized monolayers of ATII cells and allow cultures at the air liquid interphase [61]. Furthermore, the supplementation of commonly used cell culture media (e.g., DMEM or DMEM/F12) with KGF [64–67] and glucocorticoids in the combination with cAMP [68, 69] has been described to promote ATII cell phenotype in culture.
Alveolar Epithelial Type II Cell Proliferation
Due to their role as progenitor cells, the proliferative capacity of ATII cells is a critical feature in lung injury and repair processes within the lung. Thus, the assessment of proliferative behavior of this cell population is one of the most assessed cell characteristics. For the determination of in vitro proliferation capacity several different methods can be applied. Determination of gene and protein expression of genes related to cell cycle progression such as Ki67, Ccng1, and Ccng2 [27], are widely used to compare proliferative capacities of injured versus non-injured ATII cells and furthermore their response to different stimuli. The analysis of protein expression of proliferation markers Ki67 [27, 70], PCNA [71, 72] and phosphorylated histone H3 [27, 73] in cells in vitro as well as in in vivo models by immunofluorescence/immunohistochemistry represents a complementary approach. Direct functional assays for the detection of proliferating cells include the use of metabolic activity assays such as the WST-1 assay [74, 75] where a tetrazolium salt is converted in a colored formazan by endogenous dehydrogenates displaying a proportional relationship to cell number. Furthermore, the incorporation of bromodeoxyuridine (BrdU) [21] or [3H]thymidine [27] into the DNA of proliferating cells represents the gold standard for determining proliferation. Usage of several of these techniques provided insight in the reprogrammed phenotype and aberrant proliferative capacity of fibrotic ATII cells and the observation that targeting this phenotype attenuates pulmonary fibrosis in different models [27, 70].
Alveolar Epithelial Type II Cell Apoptosis
Besides changes in the proliferative behavior of injured ATII cells, the presence of apoptosis is another important parameter when analyzing injury and repair processes in the lung epithelium. The most commonly applied strategies are the analysis of caspase activity [76, 77] and the caspase mediated cleavage of endogenous substrates such as PARP [77], a crucial step in the apoptotic process. Furthermore, early apoptotic changes such as the flip of phosphatidylserine from the inside to the outside of the cell plasma membrane is used as a surrogate marker for apoptosis and detected by Annexin V binding and further analysis by flow cytometry [77]. TdT-mediated dUTP-biotin nick end labeling (TUNEL), a method to detect DNA fragmentation occurring in apoptotic cells, can be used for in vitro studies as well as for the detection of apoptotic cells in tissues of in vivo models of lung injury [70, 78]. The use of TUNEL staining provided data on the presence of increased numbers of apoptotic alveolar epithelial cells in fibrotic mouse models, as well as IPF tissue [70, 78, 79].
Epithelial to Mesenchymal Transition (EMT) of ATII Cells
Besides the described imbalance of proliferation and apoptosis in models of epithelial injury, the occurrence of EMT is widely discussed in the context of attempted alveolar repair processes. In vitro studies of EMT of cultured epithelial cells regularly use the cytokine TGF-β1 for EMT induction, which has been demonstrated in several organs including the lung [80–82]. Monitoring of decreased expression of epithelial marker genes such as E-Cadherin, cytokeratin and TJ-proteins is performed for the characterization of epithelial integrity on gene expression level as well as on protein level. Moreover, analysis should include the expression of EMT transcription factors, such as Snail, Slug, Zeb, or Twist as well as mesenchymal markers. Several mesenchymal markers, including αSMA, Calponin, and ECM related proteins such as collagen1, fibronectin and vimentin are used to describe the gain of mesenchymal cell characteristics [27, 80, 83, 84]. Importantly, co-expression of epithelial and mesenchymal markers by immunofluorescence/immunohistochemistry staining should be analyzed and has been demonstrated in human tissue of different lung diseases [28, 85, 86]. These descriptive investigations should be further complemented with functional cell assays, such as cell migration, which is a prominent feature of EMT.
For studying the in vivo relevance of findings generated by in vitro cultures, lineage tracing animals can be used to determine the cell fate in the context of lung injury. These studies utilize different transgene mouse strains, which express traceable markers under the control of the surfactant protein C promotor [19, 86, 87].
Alveolar Epithelial Trans-Differentiation
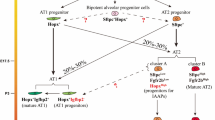
ATII cells expressing surfactant proteins are able to self-renew and trans-differentiate into ATI cells [20, 22, 88–90]. Depending on the type of injury applied, other epithelial cell populations, negative for SPC, can contribute to the attenuation of lung injury [58, 91]. Therefore, understanding how ATII cells differentiate into an ATI cell phenotype is under intense investigation including respective gene expression signatures as well as the morphological conversion from a cuboidal to a squamous cell shape. Early studies described the trans-differentiation of ATII cells into ATI-like cells in primary culture. These observations were based on the gradual loss of gene and protein expression of surfactant proteins as well as the loss of lamellar bodies, investigated by the use of electron microscopy [92]. Furthermore, the gain of features of ATI cells such as a flattened cell morphology and the expression of ATI cell-associated markers T1α (podoplanin) [93–96], aquaporin 5 (AQP5) [64, 97], receptor for advanced glycosylation end products (RAGE), and caveolin [98–100] were described. An overview of ATII and ATI cell specific markers is displayed in Table 6.2. Applying freshly isolated ATII cells to standard cell culture conditions is now widely used to mimic differentiation and repair mechanisms to investigate molecular cues in response to lung injury. The model has been utilized to study ATII cell trans-differentiation potential in various species including rat, mouse and human. Monitoring of epithelial cell identity and trans-differentiation is mainly achieved by gene and protein expression analysis of the respective markers in combination with microscopic evaluation of cell morphology.
Utilizing the spontaneous trans-differentiation of primary ATII cells in culture shed light into molecular programs that regulate this process and identified essential developmental pathways, such as the Wnt/β-catenin pathway [47, 60, 96, 103, 104] as well as TGF-β and BMP signaling [46, 105] to be involved.
However, it has to be taken into account that the model of ATII to ATI cell trans-differentiation in vitro does not fully resemble the processes occurring in vivo, as the specific trigger of injury has been shown to modulate a differential response of ATII and other progenitor cell populations. Furthermore, data indicate that the expression profile of freshly isolated ATI cells does not completely concur with the profile of ATI-like cells derived from trans-differentiation models in vitro [106].
To overcome the limitations of 2D cell culture models for studying ATII to ATI trans-differentiation and the regenerative potential of the ATII cell progenitor pool, the establishment of new 3D culture methods has been expedited extensively [22, 107], comparably to strategies for the generation of 3D organoids from colon, small intestine, and stomach [108]. For the purpose of mimicking the 3D microenvironment of the lung alveolus, primary ATII cells are seeded as a single cell suspension in an ECM mixture which is secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells (Matrigel), [22, 109]. Studies utilizing the co-culture of ATII cells with other cell populations such as fibroblasts or endothelial cells in matrigel describe the formation of lung organoids which display cuboidal ATII cells expressing SPC on the outer layer of the organoid. The organoid lumen, however, is lined by thin, squamous epithelial cells expressing markers of differentiated ATI cells such as AQP5 and T1α, indicating a self-renewal as well as a trans-differentiation capacity of ATII cells in this setting, and therefore representing an advanced model for studying mechanisms involved in this process in a more in vivo-related fashion [22, 101].
Short Summary
Alveolar epithelial cells play a crucial role in lung injury and repair processes in response to different stimuli and in the context of various lung diseases. A careful characterization of specific disease related alveolar epithelial phenotypes using comprehensive approaches and improved culturing methodologies will lead to important insights into novel therapeutic strategies targeting lung injury and repair.
Abbreviations
- α-SMA:
-
Alpha smooth muscle actin
- ALI:
-
Acute lung injury
- ABCA3:
-
ATP-binding cassette sub-family A member 3
- AQP5:
-
Aquaporin 5
- ATI:
-
Alveolar epithelial type I cell
- ATII:
-
Alveolar epithelial type II cell
- BMP:
-
Bone morphogenetic protein
- BrdU:
-
Bromodeoxyuridine
- COPD:
-
Chronic obstructive pulmonary disease
- ECAD:
-
E-cadherin, epithelial cadherin
- ECM:
-
Extracellular matrix
- EGFP:
-
Enhanced green fluorescent protein
- EMT:
-
Epithelial-to-mesenchymal transition
- EpCAM:
-
Epithelial cell adhesion molecule
- GFP:
-
Green fluorescent protein
- FACS:
-
Fluorescence Activated Cell Sorting
- IPF:
-
Idiopathic pulmonary fibrosis
- KGF:
-
Keratinocyte growth factor
- LAMP3:
-
Lysosome-associated membrane glycoprotein 3
- PARP:
-
Poly (ADP-ribose) polymerase
- RAGE:
-
Receptor for advanced glycosylation end products
- SPA:
-
Surfactant protein A
- SPB:
-
Surfactant protein B
- SPC:
-
Surfactant protein C
- SPD:
-
Surfactant protein D
- TGF-β:
-
Transforming growth factor beta
- T1α:
-
Podoplanin
- TJ:
-
Tight junctions
- TJP1:
-
Tight junction protein 1
- TUNEL:
-
TdT-mediated dUTP-biotin nick end labeling
- WNT:
-
Wingless-type MMTV integration site family member
References
Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615 (Epub 2013/02/13).
Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380(9842):680–8 (Epub 2012/08/21).
Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71–86 (Epub 2014/02/11).
Thorley AJ, Tetley TD. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int J Chronic Obstr Pulm Dis. 2007;2(4):409–28 (Epub 2008/02/14).
Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3(4):364–72 (Epub 2006/06/02).
Dobbs LG, Johnson MD. Alveolar epithelial transport in the adult lung. Respir Physiol Neurobiol. 2007;159(3):283–300 (Epub 2007/08/11).
Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. The great big alveolar TI cell: evolving concepts and paradigms. Cellular Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2010;25(1):55–62 (Epub 2010/01/08).
Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126(2):332–7 (Epub 1982/08/01).
Weibel ER. On the tricks alveolar epithelial cells play to make a good lung. Am J Respir Crit Care Med. 2015;191(5):504–13 (Epub 2015/02/28).
Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2(1):33–46 (Epub 2001/11/01).
Mulugeta S, Beers MF. Surfactant protein C: its unique properties and emerging immunomodulatory role in the lung. Microbes and infection/Institut Pasteur. 2006;8(8):2317–23 (Epub 2006/06/20).
Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol. 2009;71:403–23 (Epub 2008/10/04).
Kim KJ, Malik AB. Protein transport across the lung epithelial barrier. Am J Physiol Lung Cell Mol Physiol. 2003;284(2):L247–59 (Epub 2003/01/21).
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Eng J Med. 2000;342(18):1334–49 (Epub 2000/05/04).
Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27(2):304–12 (Epub 1999/03/13).
Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243–52 (Epub 2010/01/16).
Koval M. Tight junctions, but not too tight: fine control of lung permeability by claudins. Am J Physiol Lung Cell Mol Physiol. 2009;297(2):L217–8 (Epub 2009/06/16).
Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123–38 (Epub 2014/08/12).
Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108(52):E1475–83 (Epub 2011/11/30).
Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507(7491):190–4 (Epub 2014/02/07).
Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, Natarajan V, et al. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med. 2011;208(7):1473–84 (Epub 2011/06/29).
Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Investig. 2013;123(7):3025–36 (Epub 2013/08/08).
Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L715–31 (Epub 2010/04/07).
Zahm JM, Kaplan H, Herard AL, Doriot F, Pierrot D, Somelette P, et al. Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskelet. 1997;37(1):33–43 (Epub 1997/01/01).
Bitterman PB. Pathogenesis of fibrosis in acute lung injury. Am J Med. 1992;92(6A):39S–43S (Epub 1992/06/22).
Sapru A, Flori H, Quasney MW, Dahmer MK. Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2015;16(5 Suppl 1):S6–22 (Epub 2015/06/03).
Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Investig. 2009;119(4):772–87 (Epub 2009/03/17).
Marmai C, Sutherland RE, Kim KK, Dolganov GM, Fang X, Kim SS, et al. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301(1):L71–8 (Epub 2011/04/19).
Aumiller V, Balsara N, Wilhelm J, Gunther A, Königshoff M. WNT/β-catenin signaling induces IL-1β expression by alveolar epithelial cells in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2013;49(1):96–104 (Epub 2013/03/26).
Kuroki Y, Voelker DR. Pulmonary surfactant proteins. J Biol Chem. 1994;269(42):25943–6 (Epub 1994/10/21).
Yamano G, Funahashi H, Kawanami O, Zhao LX, Ban N, Uchida Y, et al. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508(2):221–5 (Epub 2001/11/24).
Schmitz G, Muller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J Lipid Res. 1991;32(10):1539–70 (Epub 1991/10/01).
Salaun B, de Saint-Vis B, Pacheco N, Pacheco Y, Riesler A, Isaac S, et al. CD208/dendritic cell-lysosomal associated membrane protein is a marker of normal and transformed type II pneumocytes. Am J Pathol. 2004;164(3):861–71 (Epub 2004/02/26).
Foster C, Aktar A, Kopf D, Zhang P, Guttentag S. Pepsinogen C: a type 2 cell-specific protease. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L382–7 (Epub 2003/10/28).
Boylan GM, Pryde JG, Dobbs LG, McElroy MC. Identification of a novel antigen on the apical surface of rat alveolar epithelial type II and Clara cells. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1318–26 (Epub 2001/05/15).
Gonzalez RF, Allen L, Gonzales L, Ballard PL, Dobbs LG. HTII-280, a biomarker specific to the apical plasma membrane of human lung alveolar type II cells. J Histochem Cytochem Off J Histochem Soc. 2010;58(10):891–901 (Epub 2010/06/23).
Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986;134(1):141–5 (Epub 1986/07/01).
Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14(4):309–15 (Epub 1996/04/01).
Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, et al. Transforming growth factor-β1 decreases expression of the epithelial sodium channel αENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem. 2003;278(45):43939–50 (Epub 2003/08/22).
Fujino N, Kubo H, Ota C, Suzuki T, Suzuki S, Yamada M, et al. A novel method for isolating individual cellular components from the adult human distal lung. Am J Respir Cell Mol Biol. 2012;46(4):422–30 (Epub 2011/10/29).
Ballard PL, Lee JW, Fang X, Chapin C, Allen L, Segal MR, et al. Regulated gene expression in cultured type II cells of adult human lung. Am J Physiol Lung Cell Mol Physiol. 2010;299(1):L36–50 (Epub 2010/04/13).
Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, et al. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(2):L242–9 (Epub 2005/09/07).
Chen J, Chen Z, Narasaraju T, Jin N, Liu L. Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Laboratory investigation; a journal of technical methods and pathology. 2004;84(6):727–35 (Epub 2004/04/13).
Unkel B, Hoegner K, Clausen BE, Lewe-Schlosser P, Bodner J, Gattenloehner S, et al. Alveolar epithelial cells orchestrate DC function in murine viral pneumonia. J Clin Investig. 2012;122(10):3652–64 (Epub 2012/09/22).
Mao P, Wu S, Li J, Fu W, He W, Liu X, et al. Human alveolar epithelial type II cells in primary culture. Physiol Rep. 2015;3(2) (Epub 2015/02/14).
Zhao L, Yee M, O’Reilly MA. Transdifferentiation of alveolar epithelial type II to type I cells is controlled by opposing TGF-beta and BMP signaling. Am J Physiol Lung Cell Mol Physiol. 2013;305(6):L409–18 (Epub 2013/07/09).
Mutze K, Vierkotten S, Milosevic J, Eickelberg O, Königshoff M. Enolase 1 (ENO1) and protein disulfide-isomerase associated 3 (PDIA3) regulate Wnt/beta-catenin-driven trans-differentiation of murine alveolar epithelial cells. Dis Models Mech. 2015;8(8):877–90 (Epub 2015/06/03).
Borok Z, Danto SI, Lubman RL, Cao Y, Williams MC, Crandall ED. Modulation of t1α expression with alveolar epithelial cell phenotype in vitro. Am J Physiol. 1998;275(1 Pt 1):L155–64 (Epub 1998/08/05).
Zhou B, Zhong Q, Minoo P, Li C, Ann DK, Frenkel B, et al. Foxp2 inhibits Nkx2.1-mediated transcription of SP-C via interactions with the Nkx2.1 homeodomain. Am J Respir Cell Mol Biol. 2008;38(6):750–8 (Epub 2008/02/02).
Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, et al. TGF-β is a critical mediator of acute lung injury. J Clin Investig. 2001;107(12):1537–44 (Epub 2001/06/20).
Gereke M, Autengruber A, Grobe L, Jeron A, Bruder D, Stegemann-Koniszewski S. Flow cytometric isolation of primary murine type II alveolar epithelial cells for functional and molecular studies. J Vis Exp JoVE. 2012;(70) (Epub 2013/01/05).
Lee JH, Kim J, Gludish D, Roach RR, Saunders AH, Barrios J, et al. Surfactant protein-C chromatin-bound green fluorescence protein reporter mice reveal heterogeneity of surfactant protein C-expressing lung cells. Am J Respir Cell Mol Biol. 2013;48(3):288–98 (Epub 2012/12/04).
Vanderbilt JN, Gonzalez RF, Allen L, Gillespie A, Leaffer D, Dean WB, et al. High-efficiency type II cell-enhanced green fluorescent protein expression facilitates cellular identification, tracking, and isolation. Am J Respir Cell Mol Biol. 2015;53(1):14–21 (Epub 2015/02/19).
Lo B, Hansen S, Evans K, Heath JK, Wright JR. Alveolar epithelial type II cells induce T cell tolerance to specific antigen. J Immunol. 2008;180(2):881–8 (Epub 2008/01/08).
Roper JM, Staversky RJ, Finkelstein JN, Keng PC, O’Reilly MA. Identification and isolation of mouse type II cells on the basis of intrinsic expression of enhanced green fluorescent protein. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L691–700 (Epub 2003/05/13).
Teisanu RM, Chen H, Matsumoto K, McQualter JL, Potts E, Foster WM, et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2011;44(6):794–803 (Epub 2010/07/27).
Messier EM, Mason RJ, Kosmider B. Efficient and rapid isolation and purification of mouse alveolar type II epithelial cells. Exp Lung Res. 2012;38(7):363–73 (Epub 2012/08/15).
Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Investig. 2011;121(7):2855–62 (Epub 2011/06/28).
Van der Velden JL, Bertoncello I, McQualter JL. LysoTracker is a marker of differentiated alveolar type II cells. Respir Res. 2013;14:123 (Epub 2013/11/13).
Marconett CN, Zhou B, Rieger ME, Selamat SA, Dubourd M, Fang X, et al. Integrated transcriptomic and epigenomic analysis of primary human lung epithelial cell differentiation. PLoS Genetics. 2013;9(6):e1003513 (Epub 2013/07/03).
Daum N, Kuehn A, Hein S, Schaefer UF, Huwer H, Lehr CM. Isolation, cultivation, and application of human alveolar epithelial cells. Methods Mol Biol. 2012;806:31–42 Epub 2011/11/08.
Wang J, Wang S, Manzer R, McConville G, Mason RJ. Ozone induces oxidative stress in rat alveolar type II and type I-like cells. Free Radic Biol Med. 2006;40(11):1914–28 (Epub 2006/05/24).
Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta. 1985;846(1):155–66 (Epub 1985/07/30).
Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, et al. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol. 1998;18(4):554–61 (Epub 1998/05/02).
Fehrenbach H, Kasper M, Tschernig T, Pan T, Schuh D, Shannon JM, et al. Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cells in vivo is resolved by differentiation into type I cells and by apoptosis. Eur Respir J. 1999;14(3):534–44 (Epub 1999/10/30).
Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1146–58 (Epub 2000/11/15).
Isakson BE, Lubman RL, Seedorf GJ, Boitano S. Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am J Physiol Cell Physiol. 2001;281(4):C1291–9 (Epub 2001/09/08).
Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, et al. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol. 2007;36(6):661–8 (Epub 2007/01/27).
Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L940–51 (Epub 2002/10/12).
Weng T, Poth JM, Karmouty-Quintana H, Garcia-Morales LJ, Melicoff E, Luo F, et al. Hypoxia-induced deoxycytidine kinase contributes to epithelial proliferation in pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190(12):1402–12 (Epub 2014/10/31).
Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174(8):886–93 (Epub 2006/08/05).
Tickner J, Fan LM, Du J, Meijles D, Li JM. Nox2-derived ROS in PPARgamma signaling and cell-cycle progression of lung alveolar epithelial cells. Free Radic Biol Med. 2011;51(3):763–72 (Epub 2011/06/15).
Ochieng JK, Schilders K, Kool H, Boerema-De Munck A, Buscop-Van Kempen M, Gontan C, et al. Sox2 regulates the emergence of lung basal cells by directly activating the transcription of Trp63. Am J Respir Cell Mol Biol. 2014;51(2):311–22 (Epub 2014/03/29).
Falfan-Valencia R, Camarena A, Juarez A, Becerril C, Montano M, Cisneros J, et al. Major histocompatibility complex and alveolar epithelial apoptosis in idiopathic pulmonary fibrosis. Hum Genet. 2005;118(2):235–44 (Epub 2005/09/01).
Ballweg K, Mutze K, Königshoff M, Eickelberg O, Meiners S. Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2014;307(11):L895–907 (Epub 2014/10/19).
Mercer PF, Woodcock HV, Eley JD, Plate M, Sulikowski MG, Durrenberger PF, et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax. 2016 (Epub 2016/04/23).
Pagano A, Pitteloud C, Reverdin C, Metrailler-Ruchonnet I, Donati Y. Barazzone Argiroffo C. Poly(ADP-ribose)polymerase activation mediates lung epithelial cell death in vitro but is not essential in hyperoxia-induced lung injury. Am J Respir Cell Mol Biol. 2005;33(6):555–64 (Epub 2005/09/10).
Tanjore H, Degryse AL, Crossno PF, Xu XC, McConaha ME, Jones BR, et al. beta-catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med. 2013;187(6):630–9 (Epub 2013/01/12).
Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. Eur Respir J. 2008;32(6):1631–8 (Epub 2008/12/02).
Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69(8):760–5 (Epub 2013/12/18).
Moustakas A, Heldin CH. Mechanisms of TGFbeta-induced epithelial-mesenchymal transition. J Clin Med. 2016;5(7) (Epub 2016/07/02).
Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21–45 (Epub 2016/07/02).
Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-β1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166(5):1321–32 (Epub 2005/04/28).
Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005;6:56 (Epub 2005/06/11).
Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc. 2004;1(2):93–8 (Epub 2005/08/23).
Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103(35):13180–5 (Epub 2006/08/23).
Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180(7):657–65 (Epub 2009/06/27).
Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Investig J Tech Methods Pathol. 1974;30(1):35–42 (Epub 1974/01/01).
Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22(1):142–50 (Epub 1975/02/01).
Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509(7500):371–5 (Epub 2014/04/18).
Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–5 (Epub 2014/12/24).
Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258(4 Pt 1):L134–47 (Epub 1990/04/01).
Rishi AK, Joyce-Brady M, Fisher J, Dobbs LG, Floros J, VanderSpek J, et al. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol. 1995;167(1):294–306 (Epub 1995/01/01).
Williams MC, Cao Y, Hinds A, Rishi AK, Wetterwald A. T1 alpha protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus, and ciliary epithelia of adult rats. Am J Respir Cell Mol Biol. 1996;14(6):577–85 (Epub 1996/06/01).
Dobbs LG, Williams MC, Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta. 1988;970(2):146–56 (Epub 1988/06/30).
Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, et al. β-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem. 2010;285(5):3157–67 (Epub 2009/11/26).
Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol. 1997;273(5 Pt 1):C1549–61 (Epub 1997/12/31).
Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449–52 (Epub 2001/08/11).
Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276(41):38121–38 (Epub 2001/07/18).
Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, et al. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res. 2003;311(1):31–45 (Epub 2002/12/17).
Jain R, Barkauskas CE, Takeda N, Bowie EJ, Aghajanian H, Wang Q, et al. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nature Commun. 2015;6:6727 (Epub 2015/04/14).
Shirasawa M, Fujiwara N, Hirabayashi S, Ohno H, Iida J, Makita K, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells Devot Mol Cell Mech. 2004;9(2):165–74 (Epub 2004/03/11).
Wang Y, Huang C, Reddy Chintagari N, Bhaskaran M, Weng T, Guo Y, et al. miR-375 regulates rat alveolar epithelial cell trans-differentiation by inhibiting Wnt/β-catenin pathway. Nucleic Acids Res. 2013;41(6):3833–44 (Epub 2013/02/12).
Ghosh MC, Gorantla V, Makena PS, Luellen C, Sinclair SE, Schwingshackl A, et al. Insulin-like growth factor-I stimulates differentiation of ATII cells to ATI-like cells through activation of Wnt5a. Am J Physiol Lung Cell Mol Physiol. 2013;305(3):L222–8 (Epub 2013/05/28).
Bhaskaran M, Kolliputi N, Wang Y, Gou D, Chintagari NR, Liu L. Trans-differentiation of alveolar epithelial type II cells to type I cells involves autocrine signaling by transforming growth factor β1 through the Smad pathway. J Biol Chem. 2007;282(6):3968–76 (Epub 2006/12/13).
Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol. 2005;288(1):L179–89 (Epub 2004/09/28).
Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156(3):440–55 (Epub 2014/02/04).
Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586–97 (Epub 2016/06/18).
Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–86 (Epub 2005/06/25).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Mutze, K., Königshoff, M. (2017). Analysis of Epithelial Injury and Repair. In: Schnapp, L., Feghali-Bostwick, C. (eds) Acute Lung Injury and Repair. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-46527-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-46527-2_6
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-46525-8
Online ISBN: 978-3-319-46527-2
eBook Packages: MedicineMedicine (R0)