Abstract
The spinal cord (SC) is the part of the central nervous system (CNS) that is responsible for the motor, somato-sensory, and visceral innervation of the extremities, trunk, and large parts of the neck as well as all inner organs. Spinal nerves of the peripheral nervous system (PNS) serve as connections between the CNS and distal receptors and organs. And just as the SC controls many aspects of locomotion and visceral function, it also serves as an important relay station for incoming, afferent information from the periphery to central brain regions. It thus constitutes the major coordination hub for how humans unconsciously perceive their periphery and how our bodies react to this information, often involuntarily and without involvement of higher brain functions. And while the topography and cytoarchitecture of the human spinal cord is fairly well understood, the functional implications of some well-described structures remain elusive. Because of the central role the spinal cord plays in many forms of CNS impairment, a better understanding of the functional neuroanatomy of this structure is a prerequisite for addressing potential therapeutic approaches. This chapter gives an overview of spinal cord development, topography, cytoarchitecture, and functional assembly with a special focus on two aspects often compromised during spinal cord injury, namely, the control of micturition and the propriospinal neuron networks that hold great promise for the future improvement of therapies for patients suffering from spinal cord injury.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Anatomical Organization of the Spinal Cord
The spinal cord resides within the spinal canal of the vertebral column (Fig. 2.1) and, like the brain, is surrounded by cerebrospinal fluid and meninges. In the adult, the spinal cord extends from the foramen magnum to L1–L2. During development and up to 14 weeks post-conception (pc), the spinal cord covers the entire length of the embryo, with spinal nerves leaving the vertebral column through their corresponding intervertebral foramina. However, with subsequent growth and elongation of the vertebral column, a relative growth emerges (termed ascensus medullae): the vertebral column and its meninges extend faster than the actual spinal cord, so that in the adult, the caudal end of the spinal cord will eventually lie more rostral than the vertebral column. This final position explains the profoundly lengthened dorsal and ventral spinal roots, especially at the sacral end of the vertebral column (Fig. 2.1a). The distal end of the spinal cord is formed the conus medullaris (Fig. 2.1a, b), which is tethered to the bony spinal column via a terminal filum, a thread-like structure mostly composed of fibrous tissue.
The human vertebral column from (a) median-sagittal, (b) ventral, and (c) in a cross-sectional view of a cervical segment. Note the increasing shift between spinal segments and in the vertebrae. 1 posterior median sulcus, 2 dorsal horn, 3 dorsal column, 4 posterior median septum, 5 dorsal horn, 6 posterolateral column, 7 anterolateral column, 8 ventral horn, 9 posterior gray commissure, 10 central canal, 11 anterior gray commissure, 12 ventral (white) commissure, 13 anterior column, 14 anterior median fissure, 15 ventral root, 16 posterior spinal arteries, 17 posterior radicular artery, 18 lateral spinal artery, 19 anterior radicular artery, 20 anterior spinal artery (c) (adapted with permission from [79])
A longitudinal fissure along the spinal cord is termed the ventral (anterior) median fissure (Fig. 2.1c). The spinal cord’s ventral white commissure (Fig. 2.1c) forms its floor. The ventral median fissure has a corresponding counterpart at the dorsal side, the shallower posterior (dorsal) median sulcus (Fig. 2.1c). From there, the posterior median septum, which is made up of pial tissue, extends to the central gray matter. A typical anatomical feature of spinal cord cross sections is the “butterfly-shaped” form of the central gray matter, which has two distinct regions, the dorsal and ventral horns (Fig. 2.1c). Of note, only in the thoracic and upper lumbar spinal cord, a third horn is visible, the (intermedio)lateral horn. This region contains neurons that belong to the autonomic nervous system. The thoracic lateral horn specifically contains preganglionic sympathetic neurons. Across all segments, the central connection of the gray matter (anterior gray commissure and intermediate gray matter, respectively [79, 120]), surrounds the central canal, which is lined by ependymal cells and contains cerebrospinal fluid.
The human spinal cord is subdivided into a total of 31 segments: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal (outlined in Fig. 2.1a). From each segment a pair of dorsal and ventral roots originates, which are composed of individual dorsal and ventral rootlets and together exit the spinal cord at the corresponding anterolateral and posterolateral depression, respectively. This point of exit (fila radicularia) is a continuity along the length of the spinal cord. The dorsal roots contain the dorsal root (spinal) ganglia, which are located within the intervertebral foramina (Fig. 2.1b). They are composed of pseudounipolar neurons, which give rise to a single axon with a bifurcation into a central and peripheral process. At the level of dorsal root ganglia, the dorsal and ventral roots of the spinal cord unite into a short spinal nerve. Of note, the nomenclature of spinal roots differs from the spinal canal segments. A segment is defined as the section of spinal cord from which a spinal nerve pair (left and right) extends toward the periphery. Consequently, the spinal cord segments do not necessarily correspond to those of the spinal canal due to the above-described developmental ascension of the spinal cord. As a consequence, the spinal roots have to descend further down to then exit the column at the appropriate intervertebral foramen (rostral for C1–C7 roots, caudal for all others). As a result, the definitive spinal cord ends at L1 with the conus medullaris (Fig. 2.1a, b), while the remaining bundle of lumbar, sacral, and coccygeal fibers further extend as the cauda equina down to Co1 (Fig. 2.1a, b).
1.1 Meninges of the Spinal Cord
As is typical for the CNS, three layers of meninges surround the spinal cord: (1) the superficial dura mater, (2) the arachnoid mater (intermediate), and (3) the inner pia mater. The pia mater is the origin of the denticulate ligament, an elastic structure that tethers the pia and the dura mater along the entire spinal cord. It is an essential part of the supporting structure for the cord in the spinal canal and provides stability. Three presumptive spaces appear within this sheathing of the spinal cord: the epidural, subdural, and subarachnoid space. Moving from the outside in, the epidural space is external of the dura mater and contains mostly loose connective tissue, epidural adipose tissue, the spinal nerve roots, and the internal vertebral venous plexus (see Sect. 2.1.2). In its rostrocaudal orientation, the epidural space diminishes at the foramen magnum (here, the spinal dura mater merges with the endosteal dura of the cranium) and at the sacral hiatus. In the cervical and thoracic regions, the epidural space is filled with a large basivertebral vein.
Classic textbook anatomy highlights the subdural space as a true anatomical structure between the dura mater and arachnoid. However, as discussed by Watson and colleagues [120] based on data obtained by Reina, Vandenabeele, Haines, and others, this is most likely not the case [40, 94, 118]. This “space” is more likely a preparation artifact.
Finally, the subarachnoid space lies between the arachnoid and the pia mater. It contains cerebrospinal fluid and a loose network structure of collagen fibers and fibroblasts, the arachnoid trabeculae [40]. This trabecular structure connects the arachnoid to the pia mater and further contains leptomeningeal cells traversing the space and thus creating the so-called intermediate leptomeningeal layer, which closely resembles the cranial arachnoid trabecular network [76]. Overall, the subarachnoid space extends from the cranium down to the S2 vertebra. Functionally, the subarachnoidal space plays an important role for cerebrospinal fluid drainage [91], which is mostly accomplished by arachnoid villi extending into the subarachnoid space.
1.2 Vasculature of the Spinal Cord
Apart from the usual functional aspects of gas and nutrient exchange, blood flow in the CNS is fundamentally important for clearance of metabolic heat. Thus, it is only logical that during embryonic development, angiogenesis and neurogenesis go hand in hand, supporting each other also by using similar signaling pathways [102, 113]. Examples of growth factors that support both angiogenesis and neurogenesis include vascular endothelial growth factor (VEGF), members of the fibroblast growth factor (FGF) family, and brain-derived neurotrophic factor (BDNF), among others (reviewed in [93]).
1.2.1 Arteries
The main arterial supply of the spinal cord is provided by posterior (dorsal), lateral, and anterior (ventral) vessels that span the entire cord length (Fig. 2.1c, see also [120]). The anterior spinal artery is located directly at the indentation of the anterior median fissure (Fig. 2.1c). In contrast, two posterior spinal arteries are located just ventral of the dorsal root entrance to the spinal cord, and the lateral spinal arteries can be observed somewhat halfway between the dorsal and ventral roots (Fig. 2.1c). Various transverse arteries extend from this arterial transverse anastomotic circle into the spinal cord and predominantly branch in the gray matter. The origin can be variable, but usually the anterior spinal artery is a derivate of two branches of the vertebral arteries at the level of the pyramidal decussation. The posterior spinal arteries either derive directly from the vertebral artery in the neck region or stem from the posterior inferior cerebellar artery. The posterior spinal arteries also receive blood from segmental arteries (ascending cervical artery, profound cervical artery, posterior intercostal artery, and lumbar arteries). The most prominent supporting artery is the artery of Adamkiewicz, which supplies the thoracolumbar and sacral spinal cord. In most cases it originates from the abdominal aorta approximately at T9, traverses the intervertebral foramen, and eventually anastomoses with the anterior spinal artery. Radicular arteries enter the spinal canal alongside the spinal nerves in the intervertebral foramina, then branch outside the dura mater, and follow the dorsal and ventral roots, respectively, to enter the spinal cord (Fig. 2.1c). Within the gray and white matter of the spinal cord, an intricate capillary network is formed and is significantly more elaborated in the gray matter. It was discovered in the mid-1940s that this imbalance of microvascularization mostly correlates with the number and density of synapses with high levels of mitochondria, of which there are many more in the gray than white matter [103]. Furthermore, some regions also seem to contain more capillaries than others, for example, the corticospinal tract contains about twice the amount of capillaries than the cuneate fasciculus [129].
1.2.2 Veins
Along the surface of the spinal cord, an elaborate venous plexus can be observed. Similar to the arterial layout, an anterior and posterior spinal vein spans the longitudinal axis of the spinal cord but can show significant caliber variations. Both the anterior and posterior veins anastomose via a network of smaller veins that circumvent the spinal cord. Especially at the cervical and lumbar enlargements, where the majority of spinal nerves innervating the extremities exit the cord, a larger venous transverse anastomotic circle can be found.
2 Development of the Spinal Cord
2.1 The Neural Plate and Neural Tube
The first significant step during vertebrate nervous system development is the achievement of neural identity from dorsal ectodermal cells positioned along the midline of the gastrulating embryo. Subsequently, the neural plate will form and, along its two major axes (anterior-posterior and dorsal-ventral), it supports the generation of all neural cell types that will ultimately shape the mature CNS.
The CNS derives entirely from ectoderm. Its earliest derivatives include the neural plate and the neural crest. The neural plate is a somewhat oval-shaped region with elevated epithelial cells rostral to the primitive pit (Fig. 2.2a). Beginning with Carnegie stage 6 (day 18pc), the neural plate can be distinguished from the surrounding ectoderm in the anterior germinal disc and is wider there than at its caudal end. The neural crest and the embryo’s placodes develop in a band of cells directly adjacent to the neural plate. These cells are in direct contact with the surrounding epidermal ectoderm.
Embryonic development of the (a) neural plate and (b) neural tube, dorsal view. At Carnegie stage 6 (day 18pc), the neural plate can be distinguished from the surrounding ectoderm and is induced due to an inhibition of epidermis formation, which in turn is driven by signals that derive from the primitive node. Within 24 h of the first appearance of the neural plate, folds appear at its respective edges, thereby creating the neural fold along the midline. At Carnegie stage 9 (20 days pc), fusion of the opposing neural folds and subsequent formation of the neural tube occurs at the hindbrain – spinal cord junction. The two open ends after neural fold closure are the neuropores. They remain temporarily open (modified from [101, 120])
The neural plate is induced due to an inhibition of epidermis formation, which in turn is driven by signals that derive from the primitive node at the cranial end of the embryo (Fig. 2.2a), [101]. Within 24 h of the first appearance of the neural plate, folds appear at its respective edges, thereby creating the neural fold along the embryo’s midline (Fig. 2.2a, b).
Several factors contribute to the complex event of axial patterning and neurulation – the latter describing the event of embryonic folding not only along the midline but also in a dorsoventral orientation to ultimately close and form the neural tube. Cell intercalation into the embryonic midline results in a narrowing along the medial-lateral axis (convergence) and simultaneous rostrocaudal lengthening (extension) [50]. Specifically, the caudalizing event is driven by signaling molecules of the fibroblast growth factor (FGF) family, while the rostralizing event relies heavily on retinoic acid signals [56].
The other directional folding event along the dorsoventral axis is carried by bone morphogenic proteins (BMPs) for the dorsalizing orientation and sonic hedgehog (SHH) for the major ventralizing factor of this event [127]. Both factors significantly contribute to so-called hinge point (HP) formation [108]. Median and dorsolateral HPs are required for correct neural tube folding and closure, and lack of correct signaling results in the emergence of a number of neural tube closure defects along the embryo, which, depending on the position of the defect, can range from anencephaly to craniorachischisis, lumbosacral spina bifida, or spinal dysraphism and encephalocele [18, 37].
In humans, the next major time point in the development of the spinal cord is at around Carnegie stage 9 (20 days pc). Here, fusion of the opposing neural folds and subsequent formation of the neural tube occur at the hindbrain – spinal cord junction. This event requires several days to be completed and is coordinated simultaneously both at the rostral and caudal ends [82]. After closure of the neural folds, both ends temporarily remain open and are referred to as neuropores (Fig. 2.2b). When by 28 days pc both neuropores have been closed, the rostral neural tube initiates brain vesicle development, while the caudal pore is triggered to form the primitive spinal cord. This process of neural tube closure and extension of the spinal cord to S4/S5 is termed primary neurulation, with the secondary neurulation describing the event during which more caudal levels of the spinal cord are generated via connection and fusion of mesodermal cells. These cells then epithelize and subsequently merge with the rest of the tube [101].
2.2 Derivatives of the Neural Crest
Cells that emerge along the edge of the neural folds during the above-described process form what is termed the neural crest. They reside along the entire length of the neural tube, and while those that migrate along a dorsolateral pathway give rise to a number of neural cell populations later in life, those that transform into sensory ganglia are of distinct significance for spinal cord development: they are the origin of all dorsal root ganglia (DRG). Morphologically, the somata of these neurons remain in place, but the cells project a peripheral process to innervate somatic or visceral regions, while a central projection extends into the forming dorsal horn of the spinal cord. The extension of the peripheral projections heavily depends on appropriate trophic factor-mediated guidance and survival cues provided by the respective target sites. At the same time, a solid balance with repulsion events contributes to process pathfinding. The notochord, ventral spinal cord, and dermomyotome of the developing embryo have putative chemorepulsive effects on the extending peripheral DRG processes. Masuda and Shiga suggested members of the semaphorin family along with chondroitin sulfate proteoglycans be included among factors secreted by the notochord and dermomyotome, respectively, while the chemorepulsive carriers secreted by the ventral spine remain elusive [61].
2.3 Derivatives of the Alar and Basal Plates
A fundamental step during spinal cord development is the formation of the regions that will later form sensory and motor areas, respectively. A cross section through a 6-week-old human embryo is depicted in Fig. 2.3. Here, the roof and floor plates as well as a ventricular zone of the developing embryo are separated clearly. They are surrounded by neuroepithelial cells that produce a pseudostratified wall to the neural tube lumen. In this region, a number of cells with similar fates come to lie close to each other and ultimately perform cell-type-specific coupling, which depends on both chemical and bioelectrical signaling [1, 8], thus building the mantle layer between the ventricular zone and the marginal zone. The mantle layer, which resides around the primitive spinal cord, arises from multiple neuroepithelial cell divisions. These then lead to the subsequent emergence of primitive nerve cells and the accumulation of postmitotic neuroblasts beneath the external limiting membrane of the neural tube. The mantle layer will become the gray matter of the spinal cord. In turn, the marginal zone will ultimately become the white matter of the mature spinal cord.
Cell type specification and migratory paths in the human spinal cord. At around 6 weeks of age, progenitor domains and daughter cells have been specified (inserts for basal and roof plate). In the ventral spinal cord (basal plate insert, left), five progenitor pools in the ventricular zone (p0–p3, pMN) give rise to five mature neuron populations in the mantle zone (V0–V3, MN). In the dorsal spinal cord (alar plate insert, right), six progenitor domains (dp1–dp6) give rise to six early-born interneurons (dl1–dl6) and two late-born interneurons (dlLA, dlLB). Cells and processes in the center graphic depict migratory routes of neuroblasts (see text for details). 1 spinal canal, 2 roof plate, 3 alar plate, 4 basal plate, 5 floor plate, 6 matrix zone, 7 mantle zone (later gray matter), 8 marginal zone (later white matter), 9 dorsal root, 10 dorsal root ganglion, 11 ventral root, 12 spinal nerve, 13 ependymal cell (guides radial migration of neuroblasts), 14 arcuate fibers (guide tangential migration of neuroblasts), 15 motoneurons, FP floor plate, RP roof plate (adapted from [79, 133])
Within the mantle layer, two distinct, paired regions can be observed: the dorsal thickening is referred to as the alar plate (future sensory areas of the spinal cord) and the ventral thickening is the basal plate (future motor areas of the spinal cord). Notably, the cells that reside outside of the roof and floor plates will ultimately serve as dorsal and ventral white commissures that facilitate the crossing of axons in the mature spinal cord.
In terms of cellular derivatives, the alar and basal plates and their induction to produce the cell types of the spinal cord have been investigated mostly in animal models. However, it is reasonable to assume similar mechanisms for human spinal cord development, as many of the signaling molecules and guidance factors along with their gene regulation are highly conserved among vertebrate species. While the roof plate is most likely initiated by signaling from the overlying ectoderm [16, 59], the floor plate is induced by axial mesoderm [2, 72]. Once established, the floor plate has been shown to generate a SHH gradient, which in turn induces the production of distinct pools of progenitors in the neuroepithelial ventricular zone ([13, 133], Fig. 2.3 left panel). Likewise, the roof plate and its external ectoderm, by generating a BMP gradient that is also reciprocal to the floor plate’s SHH gradient, produces six progenitor domains, which in turn generate six distinct early-born and two late-born dorsal interneuron cell populations [133] (Fig. 2.3 right panel). Like in the developing brain, the majority of neuroblasts use migration paths along radial glia cells to leave the matrix zone and reach the mantle zone (Fig. 2.3). However, not all neuroblasts follow this radial migration pattern. A subset of neuroblasts has been observed to migrate tangentially. For example, axons of some early-differentiating alar plate-derived neurons extend toward the ventral spinal cord and intersect in the floor plate. These arcuate fibers build a pathway, the so-called circumferential pathway [42], which in various species serves as both a dorsal-to-ventral [54] and ventral-to-dorsal migration path for neuroblasts [87]. Thus, it provides important commissural axon corridors that are essential for correct target innervation.
2.4 Cell Type Specification of the Spinal Cord
2.4.1 Motoneurons
All motoneurons originate from a single ventral progenitor domain (Fig. 2.3, left panel), yet strikingly, their delicate specification later allows for the coordinated movement of the large variety of muscles in the human body. For this, the positional identity of motoneurons along the spinal cord’s rostral-caudal axis is key. This position and location identity is the direct result of the concerted action of multiple signaling molecules (for review see [21, 107]).
These spatiotemporal signaling events lead to different birthdates of motoneuron populations. Early-born motoneurons migrate into the basal plate while trailing a radially oriented process that has a distinct central orientation, already aiming at its target region. Next, the emerging motoneuron develops an early axonal domain, which is distinguishable from the cell’s somatodendritic domain by expression of early axon-specific markers such as scaffolding proteins for the evolving axon initial segment [53]. Finally, the axons will traverse the marginal zone and emerge from the ventral surface of the cord, thus forming the ventral roots of the spinal cord. Likewise, dendrites will branch into the newly forming neuropil of the ventral horn and gray matter.
Ventral horn neurons arise from five distinct columnar subpopulations, of which four are interneuronal and one is motoneuronal (Fig. 2.3, left panel). The latter will produce three columns of neurons: (1) lateral motoneurons positioned at the cervical and lumbosacral enlargement of the spinal cord will later innervate the musculature of the extremities, (2) medial motoneurons that can be found throughout the entire spinal cord will later innervate axial muscles, and (3) visceral motoneurons that arise from an intermediolateral column in the thoracic and cranial lumbar segments. At 8–10 weeks pc in humans, motoneurons will segregate into specific somatic motor columns, a process which depends both on genetic and epigenetic regulation [19]. Francius and Clotman most recently published a comprehensive review on motoneuron specification and diversity [34].
As already mentioned above, target-finding and long-term survival of motoneurons depends predominantly on specific guidance- and survival cues. In this context, far more motoneurons are produced than will ultimately survive and innervate target sites. Several signaling molecule families are involved in this important elimination event, for example, classic neurotrophins (BDNF, NT3, NT4), several members of the cytokine family (ciliary-derived neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), cardiotropin-1), several TGF-β family members (glial-derived neurotrophic factor (GDNF), neurturin, artemin, persephin), insulin-like growth factors IGF-I and IGF-II, and members of the fibroblast growth factor family (FGF-1, FGF-2, and FGF-5) (for review see [58, 106]).
2.4.2 Interneurons
Interneurons are born both in the areas composed of, and directly adjacent to, the dorsal roof plate as well as the ventral floor plate (Fig. 2.3). The dorsal spinal cord establishes six distinct progenitor pools (dp1–dp6), which in turn give rise to six early-born (dI1–dI6) and two late-born interneuronal subtypes (dILA and dILB) that differ by birthdate, relative dorsoventral position, and gene expression profiles [133]. Interneurons in the ventricular zone close to the floor plate emerge from the above mentioned four progenitor domains p0–p03 that result in mature interneuronal subtypes V0–V3 (Fig. 2.3, also refer to Sect. 2.5.3.1). Interestingly, many of these early subtypes have been found to develop into commissural interneurons projecting to the contralateral side of the spinal cord. In the mature CNS, these commissural interneurons are essential for left-right locomotor coordination and represent a major component of central pattern generators (CPGs) that play a fundamental role for the rhythmic, coordinated movement of limbs and trunk [117]. CPGs will be discussed in more detail in Sect. 2.5. It is important to keep in mind that while the nomenclature for interneuron subtypes may seem simple with four major ventral classes V0–V3 and six dorsal classes, respectively, recent data actually identified several subsets within each class depending on the expression of specific sets of transcription factors [35].
2.4.3 Glia
Glial subtypes of the early spinal cord include radial glia, oligodendrocytes, astrocytes, and ependymal cells. Generally, gliogenesis occurs after the brief phase of neurogenesis, and astrocytes, which develop in large parts from radial glia [6, 64], emerge before oligodendrocytes, which later are the sole source for CNS myelin. Notably, in humans, oligodendrocyte precursors first reside in the ventral ventricular zone close to the floor plate and in the direct neighborhood of a motoneuron domain [41]. In fact, data from genetic studies suggest that oligodendrocytes and motoneurons share a common ancestral progenitor [60, 109]. In contrast, ventral interneurons seem to share a common ancestor with different astrocyte subtypes that ultimately settle in various gray and white matter areas depending on their origin [97, 115]. Oligodendrocytes are sequentially produced in three distinct waves. First, ventral precursors from the motoneuron domain arise and migrate along the entire neural tube. As with motoneuron specification, the development of these cells is SHH mediated and is repressed by dorsally secreted BMPs and WNT proteins. The second wave occurs later in the fetal phase. Here, progenitors located in the dorsal spinal cord develop from dp3–dp6 interneuron domains. Finally, the third wave is characteristically initiated after birth. The origin of these cells remains elusive [70, 97].
Ependymal cells eventually come to line the central canal of the spinal cord but are absent from other spinal structures. They guide radially migrating neuroblasts during development (Fig. 2.3). Last but not least, microglia appear in the human spinal cord around 9 weeks pc and undergo major infiltration of the tissues from 16 weeks pc on [96]. They most likely colonize the developing spinal cord along with the emerging vascularization of the tissue [96].
2.5 Emergence of Ascending and Descending Spinal Tracts
The earliest time point at which discernible ascending (spinocerebellar) neurons appear in humans is 10 weeks pc [17]. In a similar time window, by 13 weeks pc, the lateral corticospinal tract in humans has reached the caudal medulla oblongata. Just 2 weeks later, the pyramidal decussation is completed (reviewed in [112]). Next, the cervical spinal cord is reached between 14 and 16 weeks pc, and then a brief stop in further progression can be observed. The corticospinal tract invades the more caudal regions of the spinal cord at a later stage of development: 17 weeks pc for the low thoracic spinal cord, 27 weeks pc for the lumbosacral spinal cord. The early contact between descending axons of the corticospinal tract and corresponding synaptic targets in the spinal cord represents an important event for proper structural and functional maturation of the spinal cord. The maturation of spinal motor centers requires neuronal activity provided by the aforementioned contact [31]. This activity-dependent pattern for correct tract formation is especially important, considering that the developmental hallmark of initial exuberant axonal growth with subsequent substantial axon loss depends largely on neuronal activity and growth factors (whose expression can also be activity dependent). Hence, descending inputs are essential for the induction of plasticity and appropriate circuit formation [110]. In fact, recent data suggest that different motor systems can interact during development to drive each other’s adult specification [123]. The authors suggest that the developing rubrospinal system is under activity-dependent regulation by the corticospinal system for establishing mature rubrospinal connections and a corresponding red nucleus motor map.
2.6 Myelination of Spinal Cord Tracts
Contrary to many mammals where myelination is part of the postnatal developmental period, first myelination of axons in the human can be observed in the early fetal spinal cord (e.g., less than 16 weeks pc shown by [78]). However, the majority of myelination cannot be seen until the second trimester. A distinct temporal order of myelinating events has been described previously. The earliest structure to myelinate is the medial longitudinal fasciculus (20 weeks pc), with the corticospinal tract being the last, still undergoing myelination at birth [121]. The same authors also described a spatiotemporal gradient of myelination, in which the three major protein classes myelin basic protein (MBP), proteolipid protein (PLP), and myelin-associated glycoprotein (MAG) appear along an anteroposterior and rostrocaudal gradient, with MBP emerging first. On a molecular level, oligodendrocyte progenitors – after migration and arrival at the correct location – undergo terminal differentiation into so-called premyelinating oligodendrocytes. This event follows a rostrocaudal gradient in the spinal cord [11]. Their terminal differentiation then includes the actual process of myelination, which requires physical contact to the axon that is to be myelinated. Again all these steps underlie intricate molecular regulation that depends on a number of developmental genes and factors (for a detailed review, see [70]).
3 Cytoarchitecture and Pathways of the Spinal Cord
3.1 Spinal Cord Gray Matter
Based on a fundamental anatomical nomenclature for the cat spinal cord gray matter first published by Rexed [95], a similar laminar cytoarchitecture of the gray matter also applies to humans [105]. An overview is provided in Table 2.1. The dorsal horn includes laminae I–VI, while laminae V–X reside in the base of the dorsal horn and the central region of the ventral horn (Fig. 2.4). However, not all laminae can be clearly distinguished from each other. Laminae I–VIII span the entire length of the spinal cord, although their size can vary significantly from one segment to the next. Of note, lamina IX cannot be understood as one section in the classical sense, but rather as motoneuron pools interspersed within laminae VII and VIII (Fig. 2.4).
Cytoarchitecture of the spinal cord gray matter. Left side: Lamina of the gray matter after the nomenclature of Rexed indicated by roman numerals (laminae I through X). Right side: 1 tract of Lissauer, 2 marginal cells (lamina I), 3 substantia gelatinosa (laminae II + III), 4 nucleus proprius (lamina IV), 5 intermediate zone, 6 intermediate zone (laminae V–VIII), 7 lateral motor column (lamina IX), 8 ground bundles (fasciculi proprii), 9 medial motor column (lamina IX), 10 ventral root (modified with permission from [79])
Lamina I (zona marginalis, substantia spongiosa) has a reticular appearance and is composed of predominantly fusiform neurons (marginal cells). It borders with the tract of Lissauer, the region, where the dorsal horn contacts the pial surface of the spinal cord. It is visible as a band of unmyelinated or sparely myelinated afferent fibers also known as the posterolateral tract and contains axons projecting toward central regions, carrying nociceptive and thermoreceptive information. Neurons in lamina I are important for nociception and thermoreception and are the principal site for termination of nociceptive afferents. They use mainly glutamate, substance P, and calcitonin gene-related peptide (CGRP) as well as other peptides as transmitters. Lamina I neurons can also respond to mechanical stimulation from Aβ-fibers [124]. Interneurons in lamina I modulate nociception and signal predominantly via opioids, γ-aminobutyric acid (GABA), and glycine.
Like lamina I, lamina II (substantia gelatinosa) is a major nociceptive transmission and computation center and principal site for termination of Aδ- and C-fibers. It is composed of a great number of smaller, densely packed neurons and many unmyelinated axons. Neurons in lamina II form mostly local connections, with terminal axon collaterals reaching from lamina III as deep as lamina V [55]. Lamina II neurons function mostly as interneurons, and an estimated third of these contain GABA and glycine [114]. Other neurotransmitters in lamina II interneurons include CGRP, substance P, and vasoactive intestinal polypeptide (VIP).
Neurons in laminae III and IV (nucleus proprius) are larger and less densely packed than in laminae I and II due to numerous myelinated axons that traverse this lamina. Again, a large portion of neurons here are interneurons, with dendrites that extend mostly rostrocaudally. Transmitter types include GABA, glycine, dopamine, and substance P, among others. Functionally, some neurons seem to respond to fine tactile information, while others respond to rather strong, coarse pressure application. In the upper cervical segments, the medial region of lamina IV builds the internal basilar nucleus, a caudal extension of the cuneate nucleus [7].
Lamina V is the site of convergence between somatic and visceral input. It can be further divided into a medial part (ascending projection neurons) and a lateral part (preganglionic neurons in the lateral horn). The latter contains large somata, intercalated with thick myelinated axon tracts. In the human, this lamina cannot be clearly separated from lamina VI [104]. Dendrites in lamina V extend mostly in a dorsoventral orientation and can reach into laminae II and VII, respectively. Neurons in lamina V receive afferent input from the skin, musculature, and visceral organs with mixed modalities (mechanosensory, nociceptive, chemoreceptive). The major neurotransmitters present are GABA, dopamine, glycine, and substance P, among others.
Lamina VI is the deepest layer of the dorsal horn. As mentioned before, this lamina is almost indistinguishable from laminae V and VII, only visible in the cervical and lumbar enlargements. Similar to lamina V, it is also divided into a medial and lateral zone. Afferent input comes from collaterals of Aα-fibers (from muscle spindles). Lamina VI also receives input from cutaneous and nociceptive fibers. The majority of lamina VI neurons are either propriospinal neurons or interneurons. The latter are more local in their projection, many of them innervating ventral horn motoneurons. Hence, the major function of lamina VI interneurons seems to be the control of reflex pathways.
Lamina VII comprises most of the intermediate zone. The cell population in lamina VII is evenly sized and spaced, and the majority of cells are premotor interneurons projecting to the large motoneurons of lamina IX. In fact, lamina VII is a motor computation hub in that descending motor pathways control spinal motoneurons by synaptic transmission via interneurons in lamina VII. It is assumed that lamina VII interneurons are typically prewired to execute all aspects of a voluntary movement [120]. The modular arrangement of these interneuron-to-motoneuron connections thus provides a very potent way for the brain to execute directed voluntary movements. Indeed, the topographical arrangement of motoneuron columns matches the distribution of interneurons in lamina VII, with those modulating proximal musculature being placed more medial, and those driving distal movements being located more laterally.
Lamina VII contains three distinct nuclei: (1) the nucleus dorsalis (Stilling-Clarke, (C8) T1–L2/L3; see Sect. 2.4), with afferent innervation from proprioceptive fibers from muscles and joints; (2) the central cervical nucleus (C1–C4), with afferent input from the head and neck musculature, the vertebral joints, and the vestibular nuclei, which all coordinate head and neck movement; and (3) the intermediomedial nucleus (T1–L3), a part of the autonomic cell columns in lamina VII (cholinergic neurons). The latter are interconnected with sympathetic cells from the intermediolateral nucleus (lateral in C8–L3). These cells are preganglionic, visceroefferent neurons of the sympathetic and parasympathetic systems with input from supraspinal centers in the hypothalamus, pons, and medulla oblongata. Importantly, these nuclei also receive afferent visceral input. Both the intermediolateral and medial nuclei are connected with each other and with the contralateral side. Also located in lamina VII (lateral part) are the parasympathetic sacral nuclei (S2–S4), whose neurons innervate the large intestine, the urethral and anal sphincter, the reproductive organs, and the urinary bladder (see Sect. 2.4).
Neurons in lamina VIII are heterogeneous in size and shape at different levels along the spinal cord and their dendrites project mostly in the dorsoventral orientation. Dorsal dendrites, while not crossing the midline, are extended toward lamina VII and the ventral gray commissure. The majority of cells are GABAergic with input from propriospinal afferents and from supraspinal, vestibular, and reticulospinal afferents. Neurons of lamina VIII have a central role in the coordination of motor activity. For example, the left-right coordination of movement seems to be controlled by lamina VIII neurons [80]. Also, the majority of neurons with projections in the long propriospinal pathways that connect the lumbar and cervical enlargements are located in lamina VII, and they play a fundamental role for the coordination of front and hind limb movement [120]. Further details are discussed in Sect. 2.5.
Lamina IX is not a true lamina, but a set of columns in laminae VII and VIII and along the edge of X. It is the major site for large α-motoneurons (innervate striate musculature) and smaller γ-motoneurons (innervate muscle spindles) in the spinal cord. They are surrounded by a specific class of interneurons, so-called Renshaw cells, which provide recurrent inhibition for motoneurons and thus build an essential negative feedback mechanism for motoneuron activity. Motoneurons in lamina IX are organized in columns, which differ in size and number depending on the segmental level. Generally, four distinct columns are separated:
-
1.
Medial group: Innervation of axial musculature; divided into the anteromedial nucleus (C1–Co1) and posteromedial nucleus (C1, T1–L2).
-
2.
Lateral group: Innervation of intercostal and peritoneal wall musculature; clear differentiation into separate nuclei in the region of the cervical and lumbar enlargements with the anterior and anterolateral nuclei (C5–C8, L2–S1), the posterolateral nucleus (C5–C8, L2–S2), and the retroposterolateral nucleus (C8–T1, S1–S3). These innervate the musculature of the extremities.
-
3.
Central group: Only visible in specific segments. From C3–C5, contains the nucleus of the phrenic nerve (innervation of the diaphragm); from C1–C5, contains the nucleus of the accessory nerve (ultimately becomes one origin of the accessory nerve after exiting the spinal cord and ascending in the subarachnoidal space through the foramen magnum).
-
4.
Onuf’s nucleus: In S1–S3, with motoneurons of the pudendal nerve, innervates the striate muscles of the urethra sphincter and rectum.
Lamina X (central gray of the spinal cord) extends along the entire spinal cord and encompasses the region around the central canal. Neurons in this lamina are smaller than those from the surrounding lamina VII and are more densely packed. Neurons of the central autonomic area are located here. Cells in lamina X receive input from C-fibers and Aδ-fibers of both somatic and visceral origin. Likewise, lamina X functions in both nociception and visceroception (including visceral pain and mechanoreception). Axons from lamina X project into laminae V, VI, and VII, among other targets in central regions of the brain.
3.2 Spinal Cord White Matter
As outlined in Sect. 2.1, the horns of the gray matter divide the surrounding white matter into three columns: a dorsal (posterior), a lateral, and a ventral (anterior). Yet boundaries between these parts are not necessarily distinct and clear. Within the white matter, ascending and descending pathways are distinguished from each other and will be discussed in the following sections (summarized in Table 2.2).
Overall, the ascending and descending tracts can be subdivided further into functional groups (Figs. 2.5 and 2.6). In the ascending orientation, we find (1) the posterior column-medial lemniscal system, which relays sensory information on vibration, proprioception, and fine touch (via the cuneate and gracile fasciculus), (2) the anterolateral system, which transmits nociceptive, thermoreceptive, and crude touch information (via the anterior and lateral spinothalamic tracts, spinoreticular tract, etc.), and (3) the cerebellar input system, which is responsible for proprioceptive sensibility of the upper and lower limbs (composed of the dorsal spinocerebellar tract, cuneocerebellar tract, and smaller tracts such as ventral and rostral spinocerebellar tracts).
Origin and position of ascending fiber tracts in the spinal cord. Exemplary sections from various spinal segments are shown. Filled circles represent ganglia/postsynaptic structures, open triangles depict presynaptic structures. 1 substantia gelatinosa, 2 central cervical nucleus, 3 motor nuclei of the ventral horn, 4 anterolateral fascicle, 5 central cervical spinocerebellar tract, 6 dorsal spinocerebellar tract, 7 cuneate fasciculus, 8 gracile fasciculus, 9 ventral spinocerebellar tract, 10 nucleus proprius of the dorsal horn, 11 intermediate zone, 12 marginal cell layer, 13 Clarke’s column, 14 spinal border cells (adapted with permission from [79])
The descending pathways are grouped into (4) the lateral motor system for the movement of contralateral limbs (via the lateral corticospinal tract, rubrospinal tract) and (5) the medial motor system, which is responsible for control of bilateral trunk muscles, head/neck positioning, balance, and other posture and gait-related movements (via the anterior corticospinal tract, the medial and lateral vestibulospinal tracts, the reticulospinal tract, and the tectospinal tract).
3.2.1 The Main Ascending (Somatosensory) Pathways
As outlined below, two major systems, the posterior column-medial lemniscal pathway and the anterolateral system, compose the ascending system and convey somatosensory information to the brain (Figs. 2.5 and 2.6). Sensory neurons, which transmit information from the periphery to the spinal cord, are classified according to their axon diameter and hence, conduction velocities. They include 1) myelinated Aα-fibers for proprioception (receptors are Golgi tendon organs and muscle spindles), 2) myelinated Aß-fibers also for proprioception (receptors are muscle spindles, Meissner’s corpuscles (superficial touch), Merkel receptors (superficial touch), Pacinian corpuscles (vibration), Ruffini endings (stretch), and hair follicle receptors (touch, vibration)), 3) scarcely myelinated Aδ-fibers for itch, nociception, and cold thermoreception (intraepidermal nerve fibers), and 4) unmyelinated C-fibers for itch, nociception, and warm thermoreception (intraepidermal nerve fibers). Somata of these sensory neurons are located in the DRG.
The Posterior Column-Medial Lemniscal Pathway
The white matter dorsal funiculus is mostly composed of the central processes of DRGs. Information about the exact location and quality of tactile sensation and information from muscle, tendon, and joint receptors are combined here. As more fibers are added in a caudorostral orientation, the pathway actually grows from sacral to cervical segments, with incoming fibers always being added to the most medial section. At high cervical levels, this organization of the already placed axons from T6 and lower is visible as a “strip,” the gracile funiculus (Figs. 2.5 and 2.6). The lateral group of axons, which was added above T6, is the cuneate fasciculus (Figs. 2.5 and 2.6). According to their relative position, the two tracts contain either mostly information from the upper extremities (cuneatus, begins in upper thoracic cord) or predominantly from the lower extremities (gracilis). None of these fibers decussate or have synapses in the spinal cord. Both terminate in their respective nuclei (cuneate and gracile nuclei) in the medulla oblongata. From there, the information is passed upstream via the medial lemniscus to be crossed on its way to the ventral posterior lateral nucleus of the thalamus (VPL).
The Anterolateral Pathways
Small-diameter Aδ-fibers and unmyelinated C-fibers, which transmit nociceptive and thermoreceptive information, enter the spinal cord also at the dorsal horns but then immediate synapse onto second-order neurons in the gray matter, mainly in laminae I and V (see Sect. 2.3.1). The fibers then decussate in the anterior (ventral) commissure to ascend in the anterolateral white matter, a process which takes 2–3 spinal segments to be completed. The anterolateral pathway (Figs. 2.5 and 2.6) is composed of three distinct tracts: (1) the spinothalamic tract conveys discriminative aspects of nociception and thermoreception (e.g., location and intensity of a peripheral stimulus) and arises mostly from lamina I. As already pointed out for the posterior column-medial lemniscal pathway, a major upstream target is the VPL of the thalamus, although the pathways terminate on different neuronal populations within the VPL. Spinothalamic projections can also target other thalamic nuclei and are thought to convey similar information as the spinoreticular tract. (2) The spinoreticular pathway is considered to be phylogenetically older and transmits other aspects of nociception (e.g., arousal and emotion). This tract is composed of C-fibers that originate from more ventral laminae in the dorsal horn (e.g., VI–VIII). (3) The spinomesencephalic tract, which arises predominantly from lamina V neurons, projects to the periaqueductal gray in the midbrain and the superior colliculus. The role of the periaqueductal gray especially for micturition is discussed in Sect. 2.4. Other smaller tracts also contribute to the anterolateral system and are summarized in Table 2.2.
The Cerebellar Input System
The spinocerebellar tracts are a major source of input for the cerebellum and ascend in four tracts: the cuneocerebellar and rostral spinocerebellar tracts (upper extremities and neck) and the dorsal and ventral spinocerebellar tracts (lower extremities). Their feedback information is composed of afferent input about limb movement (cuneocerebellar tract for the upper limb and neck, dorsal spinocerebellar tract for the lower limb) and information on spinal interneuron activity during locomotion (reflective of descending pathway activity) by the rostral spinocerebellar tract for the upper limb and the ventral spinocerebellar tract for the lower limb.
The cuneocerebellar tract is composed of proprioceptive Aα- and Aß-fibers from the upper extremities, which ascend ipsilaterally in the cuneate fasciculus, but target the external cuneate nucleus positioned just lateral to the cuneate nucleus. From there, cuneocerebellar fibers further ascend to the ipsilateral cerebellum. The anatomical equivalent to this tract in the lower extremity is the dorsal spinocerebellar tract, which has its origin in a distinct group of neurons in the nucleus dorsalis (Stilling-Clarke, Fig. 2.6 [79]). The dorsal nucleus resides as a column in the dorsomedial gray matter in the intermediate zone, extending from (C8)T1 to L2/3. Neurons in this nucleus predominantly convey information from proprioceptive Aα- and Aß-fibers. Eventually, these fibers will give rise to mossy fibers in the ipsilateral cerebellum. Information transmitted via both these tracts remains unconscious, and this allows for fast feedback about current limb movement that can then be fine-tuned by the cerebellum.
The rostral spinocerebellar tract remains elusive in humans but is most likely an upper limb equivalent of the ventral spinocerebellar tract. The latter has its origin with so-called spinal border cells that reside along the outer edge of the central gray matter and from scattered neuron populations in the intermediate zone. These axons cross over in the ventral commissure and ascend in the ventral spinocerebellar tract. Before terminating in the cerebellum, the fibers cross back to the ipsilateral side.
Other fibers that have their origin within the contralateral gray matter and cross over in the commissura alba end up in a pronounced ventral position in the anterior and lateral funiculus. These fibers include the anterolateral fasciculus and the ventral cerebellar tract, which is located more superficially.
The anterior spinocerebellar tract (Fig. 2.5) has its origin mostly in neurons at the base of the ventral horn (laminae V–VII) and ascends both ipsi- and contralaterally. Information transmitted via this tract is also proprioceptive, but in a coarser fashion than in the posterior tract, because the anterior neurons have larger receptive fields in the periphery. When these fibers reach the cerebellum, they decussate back to the ipsilateral side, with the consequence that the cerebellum actually only receives ipsilateral input. Last but not least, several smaller, ascending pathways reach the cerebellum to contribute to the cerebellar input system, namely, the spino-olivary tract, terminating in the olivary nuclear complex, and the spinovestibular tract, terminating in the vestibular nuclei.
3.2.2 The Main Descending Pathways
The descending motor pathways are divided into lateral and medial motor systems (see Table 2.2). The general layout of these systems is that upper motoneurons (located in the primary motor cortex and adjacent regions) project to lower motoneurons in the spinal cord and brain stem. These then convey motor system information to peripheral muscles.
The Lateral Motor System
The major descending tract of the lateral motor system is the corticospinal tract (CST; Fig. 2.5), which innervates lower motoneurons in the ventral horn. It is especially important for rapid, skilled movement at individual digits and joints. Its origins are predominantly pyramidal neurons in the motor cortex, whose axons (bundled in the CST) traverse through the brain stem. Just caudal to the pyramids, about 70–90 % of the axons cross over to the contralateral side and then descend as the lateral CST. The uncrossed axons establish the narrower anterior CST, which descends very medially, right next to the median anterior fissure. Before these axons reach their target motoneurons, they largely cross to the contralateral side. The anterior CST ends in the cervical cord.
The other lateral motor system tract, the rubrospinal tract, decussates still within the brain stem and is thought to influence muscle tone of distal extremities. Its exact location and function in humans remain elusive [9, 79].Given that the difference between the flexor posturing in the upper extremities in decorticate states and the extensor posturing in the upper extremities in decerebrate states corresponds to brainstem lesions above or below the red nucleus respectively, it is thought that the greatest impact of rubrospinal projections is on flexor motoneurons of the upper extremities.
The Medial Motor System
The tracts of the medial motor system include the abovementioned anterior CST, the vestibulospinal tracts (for head/neck position and balance), the reticulospinal tract (automatic posture and gait-related movements), and the tectospinal tract (presumably coordination of head and eye movement). It is important to note that the lateral and medial motor systems actually work in concert, and both have voluntary and involuntary components, which act synergistically for the majority of all human movements.
The Coeruleospinal Tract
The coeruleospinal tract is best described in rodents at this point, while relatively little is known about its precise topography and function in the human. Fibers descending from the nucleus locus coeruleus and the nucleus subcoeruleus (the coeruleospinal inhibitory pathway) travel within the lateral column and provide input to all segments of the spinal cord, terminating in the posterior and anterior horns as well as the intermediate substance. In rodents, the system has been shown to have a pivotal function in pain control and processing. It provides noradrenergic innervation of the spinal cord [92], and its activation can lead to substantial antinociception [122].
4 Visceral Pathways and the Regulation of Micturition
4.1 Visceral Efferent Pathways
Visceral organs receive input from autonomic neurons that belong to either the sympathetic (arise from T1–L2/L3) or parasympathetic (cranial nuclei and S2–S4) systems. The autonomic neurons in the spinal cord are preganglionic, while neurons in the peripheral ganglia are postganglionic. Just like somato-efferent fibers of motoneurons, preganglionic neurons leave the spinal cord via the ventral roots and are cholinergic. A peripheral ganglion can easily contain several hundred sympathetic and parasympathetic contacts, which connect to a large number of postganglionic neurons that then innervate the target organ or tissue. Somata of autonomic preganglionic neurons are located in the spinal cord intermediate zone where they cluster in two distinct nuclei, namely, the intermediolateral nucleus of the lateral horn (lamina VII, T1–L2/L3; Fig. 2.7, upper panel) and the dorsolateral intermediate gray matter (S2–S4; Fig. 2.7, lower panel). The intermediolateral nucleus connects to the intermediomedial nucleus via an intricate network of fibers and dendrites [3].
Sympathetic and parasympathetic components of spinal micturition control. In an exemplary figure of thoracic and sacral spinal cord segments, the sympathetic trunk and bladder are depicted (left side of image). Thoracic myelinated fibers reach the sympathetic trunk by first joining the ventral roots and then passing through the white communicating rami. They ascend or descend to ultimately terminate on postganglionic neurons in its ganglia or those of the prevertebral plexus (not shown). Unmyelinated postganglionic fibers and pass through the gray communicating rami to join the spinal nerves. Sacral preganglionic fibers are parasympathetic and terminate on juxta- and intramural ganglia, respectively. Alternatively, they can also terminate on the autonomous plexus of the gastrointestinal tract. The periaqueductal gray (PGA), retrofacial nucleus, raphe nuclei, and hypothalamus in turn innervate the spinal autonomous nuclei (intermediolateral and intermediomedial nuclei). The PGA receives afferent information from the sacral cord and controls micturition via the pontine micturition center (PMC). The PMC innervates preganglionic neurons that regulate the contraction of the detrusor muscle of the bladder. These PMC neurons also regulate interneurons inhibiting the motoneurons of Onuf’s nucleus, which innervate the external urethral sphincter. The retroambiguus nucleus, which receives direct input from the PGA and also projects to Onuf’s nucleus, has been omitted in this image. (adapted with permission from [79])
Preganglionic sympathetic fibers are myelinated and comprise the white communicating rami to connect to the sympathetic trunk (Fig. 2.7, left side), where they either ascend or descend, depending on their target. They ultimately terminate on postganglionic neurons located either in the trunk’s ganglia or in the abdominal prevertebral ganglia. Postganglionic fibers are unmyelinated and eventually merge with spinal nerves via the gray communicating rami (Fig. 2.7, left side). They reach their targets by forming a perivascular plexus along blood vessels.
Preganglionic neurons of the sacral cord, as outlined above, belong to the parasympathetic system. In contrast to sympathetic neurons, they terminate on intramural, or juxtamural, ganglia positioned close to their respective target organs or tissues, including the plexus of the enteric nervous system.
The spinal autonomous nuclei are innervated by several CNS structures, such as the periaqueductal gray (see Sect. 2.4.2.3), the raphe nuclei (e.g., modulation/inhibition of nociception), and, importantly, the hypothalamus (e.g., coordination of the autonomous system, endocrine regulation).
4.2 Spinal Regulation of Micturition
The control of micturition is an essential physiological process for mammalian life (detoxification, territorial demarcation, estrus signaling, etc.). In adult humans, the conscious control of micturition signals is a socially very important ability. Its partial or complete loss after spinal cord injury continues to be one of the most stressful consequences of the injury (see chapter 15). Interestingly, while voiding of the bladder is controlled by a spinal reflex, humans do have at least partial voluntary control over some parts of the micturition process (control of the external sphincter muscle), which highlights the fact that a number of cerebral regions impact the complex regulation of this basic physiological event with its major regulatory center situated in the sacral spinal cord.
Three major pathways are involved in the control of the lower urinary tract: (1) sympathetic preganglionic neurons (L1–L2 mostly; responsible for continence), (2) parasympathetic preganglionic neurons (L5/L6–S1; active during voiding), and (3) somatic motoneurons (S1–S3, Onuf’s nucleus; innervate the striate musculature of the external urethral sphincter during continence). The major connections of the micturition pathways are displayed in Fig. 2.7, and the spinal control of micturition is summarized in Table 2.3.
4.2.1 Afferent Pathways from Bladder to Spinal Cord
Timing of micturition depends on proper signaling from the urinary tract to the CNS, not only when the bladder is filled acutely but also at all other times. Mechanosensory endings in the wall of the human bladder have been described as chains of unmyelinated varicosities in the suburothelial plexus as well as in the lamina propria, where these varicosities make contact with a specific cell type, the myofibroblasts. Combined with closely associated axon terminals, myofibroblasts have been suggested to function as stretch sensors [125, 126]. The current model is that these low threshold mechanoreceptors, which relay normal filling information from the bladder to the spinal cord, connect to myelinated Aδ-fibers. C-fibers mostly convey polymodal nociceptive information to the sacral spinal cord (reviewed in [49]).
The urethra is also innervated by afferent fibers, which are activated during urine flow, presumably by triggering mild stretch sensors [51], and remain silent during normal bladder filling.
The sacral spinal cord is the most important center for the control of micturition and not only receives sensory input from the urinary tract that it relays to central regions, but it also contains the motoneurons that directly control effector muscles of the urinary tract (detrusor muscle). Nociceptive C-fibers terminate in laminae I, II, and V–VIII. From here, their information is projected to the various areas in the brain stem (e.g., the dorsomedial, lateral, and ventrolateral parts if the periaqueductal gray (PAG)). Other brain regions that receive nociceptive bladder information include the medial hypothalamus, medial preoptic area, and the thalamus. Aδ-fibers that relay bladder filling information do not innervate the same laminae as C-fibers but rather terminate in a distinct group of neurons known as Gert’s nucleus [44], located lateral of the dorsal horn, receiving input not only from the aforementioned Aδ-fibers but also from higher-order pontine micturition centers (see Sect. 2.4.2.3). Incidentally, Gert’s nucleus also contains axon collaterals and dendrites of parasympathetic preganglionic motoneurons that innervate the smooth detrusor muscle.
4.2.2 Motor Innervation of the Bladder and Its Internal and External Sphincter Muscles
Autonomic Innervation
The detrusor muscle, a three-dimensional network of smooth muscle cells that supplies the bladder wall, is innervated by parasympathetic preganglionic motoneurons located in the sacral parasympathetic nucleus (SPN). In addition, the bladder is innervated by sympathetic autonomic motoneurons located in the upper lumbar spinal cord. They reach the bladder via the hypogastric nerve (Fig. 2.7). Generally, sympathetic innervation of the bladder is believed to contribute to a decrease in bladder pressure during the filling phase so that, overall, the number of micturition events decreases [74].
Somatomotor Innervation
The external sphincter of the bladder, which is composed of striate muscle, is innervated by motoneurons from the ventromedial Onuf’s nucleus, ON [83] (Fig. 2.7). ON motoneurons act together, hence organizing the rhythmic contraction of the pelvic floor musculature to constrict not only the external sphincter of the bladder but also the anal sphincter. Interestingly, ON neurons are both somatic motoneurons in the classical sense with innervation of striate musculature under voluntary control and motoneurons that exhibit distinct autonomous function.
4.2.3 The Periaqueductal Gray and Pontine Micturition Center
As outlined in Sect. 2.4.2.1, two different fiber populations reach the sacral cord from the bladder, and their information is relayed to the brain stem and cortical regions. Neurons of Gert’s nucleus project specifically to the central part of the PAG, while nociceptive and mechanoreceptive afferents from laminae I, II, and V target the dorsomedial, lateral, and ventrolateral PAG. The latter also reach the thalamus.
The PAG is phylogenetically very old and plays a pivotal role in basic mammalian survival by establishing projections to innervate the lower brain stem. Here, the PAG controls such vital functions as control of respiration, circulation, nociception, and locomotion, among others. In the context of micturition, the PAG governs the timing of the process. While the PAG has no direct control of the sacral motoneurons, it is the direct upstream regulator of the paramedian pontine micturition center (PMC, also known as Barrington’s nucleus). The PMC is a group of neurons located in the dorsolateral pontine tegmentum [5, 46], Fig. 2.7. Experiments in cats and rats highlighted its predominant role for micturition. When stimulated, the bladder was voided [81], when lesioned, pressure-induced reflective voiding was severely impaired [5, 123]. Recent work suggests an integrative role for the PMC in coordinating the micturition reflex with forebrain activity, namely, because PMC neurons project to both spinal autonomic motoneurons innervating the pelvis and cortical neurons involved in modulating behavior, thus linking these two systems [116]. The PMC’s role in humans is highlighted by the negative effects in patients suffering from spinal cord injury. Of note, upper motoneuron impairment affects descending micturition pathways, leading to severe lower urinary tract dysfunctions (reviewed in [46]). In contrast, lower motoneuron impairment after spinal cord injury (e.g., at the conus medullaris below the sacral level) negatively impacts motoneurons and sacral parasympathetic preganglionic neurons that innervate the external urethral sphincter and bladder.
Besides the PAG, there is evidence that various other cortical or forebrain regions have afferent control over the PMC and micturition control [43, 120] Fig. 2.7, such as limbic structures (preoptic area of the hypothalamus, central nucleus of the amygdala, the lateral bed nucleus of the stria terminalis) and cortical areas (anterior cingulate cortex, insula). Their specific roles remain to be elucidated, but recently, a comprehensive body of evidence has been provided pointing to tight interactions of centers controlling anxiety, sexual behavior, and sleep with micturition control centers such as the PAG and PMC to suppress voiding and strengthen continence in situations of heightened stress and allowing for the conscious decision, when and where to micturate [44, 71].
5 The Propriospinal System and Central Pattern Generators
Per definition, propriospinal neurons (PNs) are those that reside within the confines of the spinal cord, as compared to supraspinal neurons, which are located above the cord with axons projecting to spinal regions. The majority of PNs are interneurons connecting multiple segments and playing a fundamental role in the computation of motor reflexes and sensory input. Some of their most central roles include the coordination of information flow from and to central pattern generators (CPGs) and the modulation of these forelimb and hind limb circuits to orchestrate directed movement. As outlined in Sect. 2.5.3, CPGs are networks which generate the rhythm and pattern shape of motoneuron bursts under the control of supraspinal centers and hence function as initiators and mediators of locomotion (reviewed in [29]).
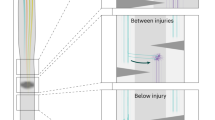
PN function is especially important in the spinal enlargements in the cervical and lumbar segments. For example, in the cervical enlargement, PNs with short axons (premotoneurons) modulate and integrate corticospinal as well as sensory information to motoneurons of upper/forelimbs, thus providing the anatomical substrate for reaching and grasping as well as more delicate hand tasks [89]. Likewise, in the lumbar enlargement, PNs control lower-/hind limb motoneurons [36]. They contribute to local spinal CPGs that control and coordinate flexor and extensor motoneurons of the shoulder/forelimb and pelvis/hind limb regions and are further connected to couple appropriate interlimb coordination to allow for rostrocaudal processing of movement initiation [48]. The latter is mediated by long-axon propriospinal projections [120]. Finally, the ventral horn gray matter is surrounded by a band of propriospinal fibers termed ground bundles (Fig. 2.4). They only descend and ascend over a few segments and play a role for fast reflex reactions.
Functional aspects other than locomotion that are modulated and coordinated by PNs include respiration and autonomic functions. Other autonomic functions are governed by groups of PNs residing in laminae II–IV of the dorsal horn and X surrounding the central canal. They are organized in ipsi- and contralateral projection circuits [62, 86] and compute nociceptive and visceroreceptive input to and from the spinal cord.
5.1 Propriospinal Projections
Generally, two PN projections are distinguished: local short-axon projections that span only a few spinal cord segments and long-axon projections which mainly serve to connect the cervical and lumbar enlargements.
Short-axon PNs typically have their somata in the gray matter, especially lamina VII, and extend their axons intra- and intersegmentally before contacting lamina IX motoneurons or other interneurons. Humans, unlike many other mammals, have a large number of direct monosynaptic corticomotoneuronal connections, which are essential for dexterous locomotion since they have a preferential impact upon motoneurons controlling distal muscles of the upper limb. Monosynaptic connections are also of critical importance for movements that require the most voluntary control (e.g., fractionated finger movement) and are a typical hallmark of human evolution. However, many “simpler” motor tasks, e.g., reaching and grasping, are most likely the result of CST neurons first contacting cervical short-axon premotoneurons, hence impacting motoneurons only indirectly [77]. This system is also referred to as the C3-C4 propriospinal system in humans [90]. At thoracic levels, short-axon PNs are located throughout the gray matter with the exception of lamina IX. These axons ascend or descend just a few segments and then form synapses in laminae III–VIII as well as with lamina X interneurons and lamina IX motoneurons, respectively [120]. Functionally, they control the activity of axial musculature involved in postural stability.
A similar system has been suggested in the lumbar spinal cord. Early data from the cat indicated that short-axon lumbosacral PNs are dispersed throughout the intermediate gray matter [98]. Accordingly, projections to motoneurons for the pelvic girdle and thigh musculature reside in laminae VII and VIII and receive input from the reticulospinal and vestibulospinal tracts that descend in the ventral spinal cord. On the other hand, short-axon projections to motoneurons for the distal hind limb musculature are located in laminae V–VII and, in some mammals, receive input from the rubrospinal and corticospinal tracts in the dorsal, dorsolateral, and lateral spinal cord [98]. This way, short-axon lumbosacral PNs can modulate descending brain stem information with the lumbar CPG for hind limb locomotion (reviewed in [47]; see Sect. 2.5.2).
Long-axon PN origins and projections are anatomically well described in nonhuman mammals and have their somata predominantly in lamina VIII and the medial lamina VII but can also be observed in laminae I, IV–VI, and X. Like short-axon projections, they can project bidirectionally, both rostrally and caudally, and terminate preferentially in laminae V–VIII. With less frequency, they are also observed terminating in lamina IX [4, 73] (reviewed in [33]). Moreover, they also show a high degree of both contra- and ipsilateral projections. In humans, the existence of these long-axon projections is predominantly based on evidence obtained from patients suffering from cervical spinal cord lesions. Several groups showed that after electrical stimulation of lower limb muscles, interlimb reflexes could be triggered in contralateral upper limb regions [14, 65]. Furthermore, several authors have suggested that forelimb-hind limb coordination as it is required for swimming, walking, or crawling is mediated by similar circuits coupled by long-axon PN projections [22, 119].
5.2 Quadruped and Biped Locomotor Propriospinal Systems
As pointed out in Sect. 2.5.1, humans have the highest number of monosynaptic descending projections with most important direct cortical-motoneuronal influence of all mammals and use especially the forelimb for both skilled hand movements and coarser movement (grasping, reaching) at the same time. It has been speculated that the gradual weakening of the propriospinal neuronal system (from e.g., cat to primate) is accompanied by a progressive strengthening of direct cortical-motoneuron projections (strongest in human), leading to the ability for skilled motor tasks with the forelimb [22, 75]. Yet at the same time, interlimb coordination in humans seems to work surprisingly similar to other quadrupedal mammals like cats [22]. Typically, quadruped locomotion requires precise spatiotemporal coordination of fore- and hind limb movement, and this is mainly accomplished by CPGs in the rostral spinal cord (forelimb) and in the caudal spinal cord (hind limb) as well as long-axon PN projections between the cervical and lumbar enlargements and propriospinal feedback control to adapt to the environment, e.g., ground asymmetries [29, 68, 128].
5.2.1 Interlimb Coordination
Human bipedal gait depends on the intricate ispi- and bilateral coordination of extensors and flexors in both legs. This coordination is under the partial influence of reticulospinal projections from the reticular formation [10] (reviewed in [22]). Furthermore, lower limbs react in a coordinated manner under physiological conditions as shown, e.g., by experiments with subjects walking on split-belt treadmills where each side runs at a different speed [30]. Here, the limbs affect each other in terms of their spatiotemporal behavior. These data suggest that the spinal cord contains networks that control individual limbs and that these networks are interconnected.
In humans, a task-dependent neuronal coupling of cervical and thoracolumbar PN networks coordinating arm and leg movements has been proposed [25] (Fig. 2.8). Supporting evidence was obtained in studies where reflex responses to tibial nerve stimulation were identified in proximal arm muscles during walking, but not during sitting or standing. Also, arm responses to tibial stimuli were not observed in subjects standing with voluntary arm swinging or subjects sitting and writing [25]. In fact, the author implies that arm swinging (associated with walking) may actually be a residual function of phylogenetically older, quadrupedal movement. This model of task-dependent activation also requires a gating mechanism that can differentiate between skilled forelimb movement (grasping, reaching) and coarser locomotion, and this function is most likely accomplished by PNs.
Task-dependent movement control during different motor tasks. (a) A strong direct corticomotoneuronal excitation is predominant during skilled hand movements (red lines). At the same time, the cervical propriospinal system is inhibited (black lines). (b) During locomotion, the brain input is predominantly mediated by interneurons. Cervical and thoracolumbar propriospinal systems become coupled and coordinate arm and leg movements (red lines) (reprinted with permission from [24])
Another significant component of human locomotion seems to be anticipatory spinal activity. Michel and colleagues showed that proximal arm muscles are involved in the acquisition and performance of a precision locomotor task when human subjects were asked to walk on a treadmill, freely moving their arms and tasked with stepping over an obstacle [67]. Presumably, this is accomplished by an anticipatory upregulation of PN circuit activity coupling between the cervical and thoracic segments. Functionally, this resulted in a more pronounced swing of the arm over the obstacle than when walking normally. The authors concluded that the spinal reflex activity actually anticipated the subsequent arm muscle activation and is an essential component of balancing the body. If less balance is required (e.g., after body stabilization), or if subjects were verbally instructed to not expect an obstacle despite being presented with one, this upregulation of reflexes was not observed [66].
5.3 Central Pattern Generators
The concept of PN networks controlling distinct movement at different segmental levels is not new. In fact, intricate PN networks have been proposed over a century ago [12]. With the advances in rodent genetics and computer modeling, new insights into CPG organization have been presented in the past decades (reviewed in [38, 99]). CPGs are composed of both excitatory and inhibitory neurons (Fig. 2.9), which interact to generate rhythm and patterns for control of muscles and are under the influence of supraspinal input. Their exact cellular composition in humans remains elusive. The current models describe CPG-initiated stepping movement as follows: after gait initiation, movement-related information (e.g., position of limbs) is conveyed to spinal and supraspinal regions. Some of this afferent feedback directly modulates CPGs to assist the phase transition during the step cycle. Thus, potential environmental requirements are included in the subsequent CPG-driven computation. However, afferent feedback is also affecting motoneurons directly by modulating various reflex pathways. Those in turn are under the control of CPGs as well. The concept of this phase-dependent modulation ensures that reflex activation of a specific set of muscles only occurs at the appropriate time in the step cycle [28].
A model of central pattern generator composition in mammalians. Schematic diagram of a two-level asymmetrical model of the locomotor CPG in mouse. This CPG controls rhythmic activity in one hemicord and consists of a rhythm generator (RG) and a pattern formation network (PF). The asymmetrical CPG has two half-centers: a flexor half-center (RG-F population, red sphere), which is intrinsically rhythmic and generates a flexor-related rhythmic activity, and a flexor half-center (green sphere) that is tonically active. Both interact via inhibitory interneuron populations (In-RG-E and In-RG-F). The PF contains interneuron populations (PF-F and PF-E, green spheres) receiving input from the corresponding RG populations. These interneuron populations also inhibit each other via inhibitory populations (In-PF-E and In-PF-F). F stands for flexor, E for extensor. See text for details (reprinted with permission from [132])
The work on human CPGs stems mostly from studies on patients with spinal cord injuries of different severity that exhibited involuntary stepping movements or lower limb alternation, the latter only in supine position and after strong cutaneous trigger [15, 26]. Interestingly, even involuntary rhythmic muscle contractions were observed in patients with completely transected cervical or thoracic spinal cords after strong lumbar cord stimulation ([69], reviewed in [29]). Most recently, Danner and colleagues presented compelling data that suggest that the human lumbar spinal cord can form burst-generating circuits, which combine to control a wide range of movements just after receiving a simple, constant, and repetitive afferent stimulus [20] (see chapter 24). Taken together with the data obtained from laboratory animals and computational models, these studies support the idea of interneuronal networks at different segments of the spinal cord that integrate peripheral stimuli with the local reflex circuitry to provoke movement. Accordingly, in spinal cord injury, the absence of peripheral input potentially leads to the degeneration of these circuits below the level of the actual lesion or might lead to a pathophysiological dominance of inhibitory signaling to the CPG. In turn, specific training or the induction of afferent input to spinal neurons within pattern generators might offer new approaches for the prevention or at least alleviation of neuronal dysfunction in spinal cord injury [23, 32]. Apart from the classic locomotion CPG, evidence has been presented for several other types of pattern generators for other functions such as ejaculation, micturition, and scratching (reviewed in [39]).
Considering the possibly high degree of phylogenetic conservation of CPGs [85], it seems plausible to assume a conserved cellular composition of CPGs between the species. At this point the generally accepted model for CPGs for locomotion in mammals is composed of several smaller circuits: (1) a separate CPG for each limb, (2) neural pathways within the spinal cord that coordinate these limb-specific CPGs, (3) supraspinal and afferent input, and (4) inhibitory and excitatory commissural interneurons (CINs) crossing the midline, which provide contralateral control (Fig. 2.9) [52, 63]. Under physiological conditions, contralateral inhibitory signals do not override ipsilateral signals to the corresponding motoneurons so that balanced gait is possible. To this end, CINs most likely also act as mediators between the left and right CPGs by coordinating phase relationships between the rhythms elicited by left and right CPGs [99], Fig. 2.9.
5.3.1 Cellular Composition of CPGs
A number of genetic studies have shed light on the identity of cells comprising CPGs in mammals (reviewed in [99]). Briefly, the following groups outlined in Sect. 2.2.4, Fig. 2.3, seem to play a fundamental role for CPG function:
-
1.
V0 neurons, which settle in the ventral cord, generate a subgroup of neurons based on the differential expression of transcription factors including V0D neurons (dorsally located), glutamatergic V0V neurons (ventrally located), and cholinergic V0C neurons. V0D and V0V neurons project contralaterally and are involved in bilateral coordination of muscle activity [88, 111].
-
2.
V1 neurons are a heterogeneous group of interneurons that project ipsilaterally. This group includes reciprocal Ia interneurons as well as recurrent Renshaw cells. Zhang and colleagues suggested that these neurons play a role for speed of locomotion [130].
-
3.
V2 neurons are uniformly ipsilateral in their projection and include a group of excitatory V2a neurons and inhibitory V2b neurons [57]. Together with V1 neurons, V2b neurons play a role for maintaining alternating flexor-extensor activation. V2a neurons in turn are suggested to deliver excitatory input to V0V commissural pathways [52].
-
4.
Excitatory V3 neurons are mainly commissural in their projections. They contribute to the maintenance of a symmetrical rhythm for locomotion [131].
How do these cells act in concert and influence flexor and extensor muscles bilaterally for locomotion? Several models have been proposed for this, and one that combines several previous models is the so-called two-level asymmetrical model of the locomotor CPG generating and controlling rhythmic activity in one hemicord [132]. A simplified version is outlined in Fig. 2.9 (from [132]). This model is based on data obtained from mouse and cat spinal cord experiments, incorporating findings from several groups. Two concepts are brought together here: a two-level CPG organization [63, 100] and an asymmetric rhythm generator with a dominant flexor half-center or a pure flexor-related rhythm generator [27]. Half-centers are defined as oscillators composed of two neurons that individually are unable to generate rhythm but produce rhythmic responses when coupled reciprocally. The function of these half-centers can have different shapes. Depending on the synaptic release, these neurons can, for example, fire in a relative phase, even synchrony, or in fact be completely antiphasic. Another mode that has been proposed for half-centers is the “escape” and “release” mode, meaning the way the “off”-neuron turns on, either by escaping or releasing inhibition. In addition, intrinsic and network properties can also alter the function of half-centers. They therefore constitute an important component of CPGs [45].
The above mentioned model published by Zhong and colleagues has two functional levels, the rhythm generator (RG) and pattern formation networks (PF, Fig. 2.9). The RG has one intrinsically rhythmic half-center and generates flexor-related rhythmic activity (RG-F population in Fig. 2.9). Bursting activity in this cell population is based on persistent, slowly inactivating sodium currents in individual neurons and excitatory synapses between these cells in the RG-F population. On the other hand, the nonrhythmic extensor half-center (RG-E population in Fig. 2.9) is tonically active and contributes to the control of the duration of the extensor phase. Also, it controls the timing of the switch to the next flexion through the inhibitory component of the In-RG-E population. In turn, this population’s activity is regulated by the rhythmic RG-F half-center via the inhibitory In-RG-F population. So in summary, the RG network, according to this model, functions as a clock and defines the locomotor frequency, drives the activity of the PF network (lower panel in Fig. 2.9), and also coordinates left and right rhythmic patterns. The latter is accomplished via CINs.
The PF network on the other hand consists of two main cell populations: PF-F (flexor) and PF-E (extensor), which initiate locomotor activity in motoneurons responsible for flexor and extensor activation, respectively. The PF-F and PF-E populations reciprocally inhibit each other via their respective interneuron pools In-PF-F- and In-PF-E (Fig. 2.9). Moreover, they coordinate the alternating activity in the flexor- and extensor-related populations of the ipsilateral locomotor network. For example, the PF-F population receives rhythmic input from the RG-F RG and in turn produces rhythmic activity for the flexor motoneurons. The PF-E population, however, obtains tonic activity from the RG-E population and is therefore rhythmically inhibited during the flexor phase during a step cycle. This is coordinated by PF-F neurons via the In-PF-F population. In turn, the PF-E population rhythmically activates extensor motoneurons and contributes to PF-F activity regulation via the inhibitory In-PF-E interneurons. This “simple” network of course is bilateral, meaning that the left and right sides of the spinal cord need to be reflected in CPG models. The model depicted in Fig. 2.9 is therefore only an ipsilateral model, omitting the CINs that connect the two sides (for a full model with bilateral organization of CPGs, refer to [132]).
Much of the concepts of CPG organization rely on animal and computational studies, and there is certainly a good chance that subpopulations of neurons with additional significant contribution are not yet known. However, the models outlined above provide a valuable basis for further studies on functional implications of CPGs and their alterations in spinal cord injury, including potential therapeutic interventions.
Conclusion
The spinal cord is a highly complex, yet rather plausibly composed anatomical region that serves as the body’s major relay station for the computation of peripheral and central information. It serves not only as a major locomotion initiator but also maintains the body’s ability to react fast to potentially dangerous external stimuli by providing the anatomical substrate for reflex reactions. To date, much information about spinal cord anatomy has been derived from various mammalian animal models, and a surprisingly large amount of this data has been verified in humans as well, suggesting that the complex functional network structure of the spinal cord drove (or is the consequence of) mammalian evolution. Our knowledge from different species, especially with regard to the development of locomotion patterns and interlimb movement coordination, may be in parts transferrable to human anatomy and physiology. Likewise, further development of experimental approaches with human spinal cord injury in mind offers a unique opportunity to address spinal dysfunction in patients.
References
Adams DS, Levin M (2013) Endogenous voltage gradients as mediators of cell-cell communication: strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res 352:95–122
Balczerski B, Zakaria S, Tucker AS, Borycki AG, Koyama E, Pacifici M, Francis-West P (2012) Distinct spatiotemporal roles of hedgehog signalling during chick and mouse cranial base and axial skeleton development. Dev Biol 371:203–214
Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE (1984) The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol 229:329–346
Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, WEINMANN O, Schwab ME (2004) The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7:269–277
Barrington FJF (1925) The effect of lesions of the hind- and mid-brain on micturition in the cat. Q J Exp Physiol 15:81–102
Barry D, Mcdermott K (2005) Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia 50:187–197
Benninghoff A, Denckhahn D (2004) Anatomy, 16th edn, vol 2. Urban and Fischer/Elsevier, Munich, Germany
Bittman KS, Panzer JA, Balice-Gordon RJ (2004) Patterns of cell-cell coupling in embryonic spinal cord studied via ballistic delivery of gap-junction-permeable dyes. J Comp Neurol 477:273–285
Blumenfeld H (2010) Neuroanatomy through clinical cases, 2nd edn. Sinauer Associates, Sunderland
Bonnet M, Gurfinkel S, Lipchits MJ, Popov KE (1976) Central programming of lower limb muscular activity in the standing man. Agressologie 17 SPECNO:35–42
Brody BA, Kinney HC, Kloman AS, Gilles FH (1987) Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol 46:283–301
Brown GT (1914) On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol 48:18–46
Butler SJ, Bronner ME (2015) From classical to current: analyzing peripheral nervous system and spinal cord lineage and fate. Dev Biol 398:135–146
Calancie B, Molano MR, Broton JG (2002) Interlimb reflexes and synaptic plasticity become evident months after human spinal cord injury. Brain 125:1150–1161
Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, Green BA (1994) Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain 117(Pt 5):1143–1159
Chizhikov VV, Millen KJ (2005) Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol 277:287–295
Clowry GJ, Moss JA, Clough RL (2005) An immunohistochemical study of the development of sensorimotor components of the early fetal human spinal cord. J Anat 207:313–324
Copp AJ, Greene ND (2013) Neural tube defects – disorders of neurulation and related embryonic processes. Wiley Interdiscip Rev Dev Biol 2:213–227
Cui D, Dougherty KJ, Machacek DW, Sawchuk M, Hochman S, Baro DJ (2006) Divergence between motoneurons: gene expression profiling provides a molecular characterization of functionally discrete somatic and autonomic motoneurons. Physiol Genomics 24:276–289
Danner SM, Hofstoetter US, Freundl B, Binder H, Mayr W, Rattay F, Minassian K (2015) Human spinal locomotor control is based on flexibly organized burst generators. Brain 138:577–588
Davis-Dusenbery BN, Williams LA, Klim JR, Eggan K (2014) How to make spinal motor neurons. Development 141:491–501
Dietz V (2002) Do human bipeds use quadrupedal coordination? Trends Neurosci 25:462–467
Dietz V (2010) Behavior of spinal neurons deprived of supraspinal input. Nat Rev Neurol 6:167–174
Dietz V (2011) Quadrupedal coordination of bipedal gait: implications for movement disorders. J Neurol 258:1406–1412
Dietz V, Fouad K, Bastiaanse CM (2001) Neuronal coordination of arm and leg movements during human locomotion. Eur J Neurosci 14:1906–1914
Dobkin BH, Harkema S, Requejo P, Edgerton VR (1995) Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J Neurol Rehabil 9:183–190
Duysens J, Pearson KG (1976) The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res 24:245–255
Duysens J, Tax AA, Trippel M, Dietz V (1992) Phase-dependent reversal of reflexly induced movements during human gait. Exp Brain Res 90:404–414
Duysens J, Van De Crommert HW (1998) Neural control of locomotion; the central pattern generator from cats to humans. Gait Posture 7:131–141
Erni T, Dietz V (2001) Obstacle avoidance during human walking: learning rate and cross-modal transfer. J Physiol 534:303–312
Eyre JA, Taylor JP, Villagra F, Smith M, Miller S (2001) Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology 57:1543–1554
Filli L, Schwab ME (2015) Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen Res 10:509–513
Flynn JR, Graham BA, Galea MP, Callister RJ (2011) The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 60:809–822
Francius C, Clotman F (2014) Generating spinal motor neuron diversity: a long quest for neuronal identity. Cell Mol Life Sci 71:813–829
Francius C, Harris A, Rucchin V, Hendricks TJ, Stam FJ, Barber M, Kurek D, Grosveld FG, Pierani A, Goulding M, Clotman F (2013) Identification of multiple subsets of ventral interneurons and differential distribution along the rostrocaudal axis of the developing spinal cord. PLoS One 8:e70325
Gerasimenko YP, Makarovskii AN, Nikitin OA (2002) Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neurosci Behav Physiol 32:417–423
Greene ND, Copp AJ (2014) Neural tube defects. Annu Rev Neurosci 37:221–242
Guertin PA (2012) Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neurol 3:183
Guertin PA, Steuer I (2009) Key central pattern generators of the spinal cord. J Neurosci Res 87:2399–2405
Haines DE, Harkey HL, Al-Mefty O (1993) The “subdural” space: a new look at an outdated concept. Neurosurgery 32:111–120
Hajihosseini M, Tham TN, Dubois-Dalcq M (1996) Origin of oligodendrocytes within the human spinal cord. J Neurosci 16:7981–7994
Holley JA, Nornes HO, Morita M (1982) Guidance of neuritic growth in the transverse plane of embryonic mouse spinal cord. J Comp Neurol 205:360–370
Holstege G (2005) Micturition and the soul. J Comp Neurol 493:15–20
Holstege G (2010) The emotional motor system and micturition control. Neurourol Urodyn 29:42–48
Hooper SL (2001) Central pattern generators. In: Encyclopedia of Life Sciences. Wiley, Weinheim, Germany
Hou S, Rabchevsky AG (2014) Autonomic consequences of spinal cord injury. Compr Physiol 4:1419–1453
Jordan LM, Schmidt BJ (2002) Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res 137:125–139
Juvin L, Simmers J, Morin D (2005) Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J Neurosci 25:6025–6035
Keast JR, Smith-Anttila CJ, Osborne PB (2015) Developing a functional urinary bladder: a neuronal context. Front Cell Dev Biol 3:53
Keller R, Shook D, Skoglund P (2008) The forces that shape embryos: physical aspects of convergent extension by cell intercalation. Phys Biol 5:015007
Kenton K, Simmons J, Fitzgerald MP, Lowenstein L, Brubaker L (2007) Urethral and bladder current perception thresholds: normative data in women. J Urol 178:189–192; discussion 192
Kiehn O (2011) Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol 21:100–109
Le Bras B, Freal A, Czarnecki A, Legendre P, Bullier E, Komada M, Brophy PJ, Davenne M, Couraud F (2014) In vivo assembly of the axon initial segment in motor neurons. Brain Struct Funct 219:1433–1450
Leber SM, Sanes JR (1995) Migratory paths of neurons and glia in the embryonic chick spinal cord. J Neurosci 15:1236–1248
Light AR, Kavookjian AM (1988) Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III–V). J Comp Neurol 267:172–189
Liu JP, Laufer E, Jessell TM (2001) Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron 32:997–1012
Lundfald L, Restrepo CE, Butt SJ, Peng CY, Droho S, Endo T, Zeilhofer HU, Sharma K, Kiehn O (2007) Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur J Neurosci 26:2989–3002
Mantilla CB, Sieck GC (2008) Trophic factor expression in phrenic motor neurons. Respir Physiol Neurobiol 164:252–262
Marklund U, Alekseenko Z, Andersson E, Falci S, Westgren M, Perlmann T, Graham A, Sundstrom E, Ericson J (2014) Detailed expression analysis of regulatory genes in the early developing human neural tube. Stem Cells Dev 23:5–15
Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, Ogawa Y, Nabeshima Y, Alvarez-Buylla A, Shimizu K, Ikenaka K (2006) Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev Biol 293:358–369
Masuda T, Shiga T (2005) Chemorepulsion and cell adhesion molecules in patterning initial trajectories of sensory axons. Neurosci Res 51:337–347
Matsushita M (1998) Ascending propriospinal afferents to area X (substantia grisea centralis) of the spinal cord in the rat. Exp Brain Res 119:356–366
Mccrea DA, Rybak IA (2008) Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57:134–146
Mcdermott KW, Barry DS, Mcmahon SS (2005) Role of radial glia in cytogenesis, patterning and boundary formation in the developing spinal cord. J Anat 207:241–250
Mcnulty PA, Burke D (2013) Self-sustained motor activity triggered by interlimb reflexes in chronic spinal cord injury, evidence of functional ascending propriospinal pathways. PLoS One 8:e72725
Michel J, Van Hedel HJ, Dietz V (2007) Facilitation of spinal reflexes assists performing but not learning an obstacle-avoidance locomotor task. Eur J Neurosci 26:1299–1306
Michel J, Van Hedel HJ, Dietz V (2008) Obstacle stepping involves spinal anticipatory activity associated with quadrupedal limb coordination. Eur J Neurosci 27:1867–1875
Miller S, Van Der Burg J, Van Der Meche F (1975) Coordination of movements of the kindlimbs and forelimbs in different forms of locomotion in normal and decerebrate cats. Brain Res 91:217–237
Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, Dimitrijevic MR (2004) Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42:401–416
Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B (2014) Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276:29–47
Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD (2007) When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317:1079–1083
Moghadam KS, Chen A, Heathcote RD (2003) Establishment of a ventral cell fate in the spinal cord. Dev Dyn 227:552–562
Molenaar I, Kuypers HG (1978) Cells of origin of propriospinal fibers and of fibers ascending to supraspinal levels. A HRP study in cat and rhesus monkey. Brain Res 152:429–450
Morrison J (1999) The activation of bladder wall afferent nerves. Exp Physiol 84:131–136
Nakajima K, Maier MA, Kirkwood PA, Lemon RN (2000) Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species. J Neurophysiol 84:698–709
Nicholas DS, Weller RO (1988) The fine anatomy of the human spinal meninges. A light and scanning electron microscopy study. J Neurosurg 69:276–282
Nicolas G, Marchand-Pauvert V, Burke D, Pierrot-Deseilligny E (2001) Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans. J Physiol 533:903–919
Niebroj-Dobosz I, Fidzianska A, Rafalowska J, Sawicka E (1980) Correlative biochemical and morphological studies of myelination in human ontogenesis. I. Myelination of the spinal cord. Acta Neuropathol 49:145–152
Nieuwenhuys R, Voogd J, Van Huijzen C (2008) The human central nervous system. Springer, Berlin/New York
Nissen UV, Mochida H, Glover JC (2005) Development of projection-specific interneurons and projection neurons in the embryonic mouse and rat spinal cord. J Comp Neurol 483:30–47
Noto H, Roppolo JR, Steers WD, De Groat WC (1989) Excitatory and inhibitory influences on bladder activity elicited by electrical stimulation in the pontine micturition center in the rat. Brain Res 492:99–115
O’rahilly R, Muller F (2002) The two sites of fusion of the neural folds and the two neuropores in the human embryo. Teratology 65:162–170
Onufrowicz B (1899) Notes on the arrangement and function of the cell groups in the sacral region of the spinal cord. J Nerv Ment Dis 26:363–369
Patestas MA, Gartner LP (2006) Textbook of neuroanatomy. Wiley-Blackwell, Malden
Pearson KG (1993) Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 16:265–297
Petko M, Antal M (2000) Propriospinal afferent and efferent connections of the lateral and medial areas of the dorsal horn (laminae I-IV) in the rat lumbar spinal cord. J Comp Neurol 422:312–325
Phelps PE, Vaughn JE (1995) Commissural fibers may guide cholinergic neuronal migration in developing rat cervical spinal cord. J Comp Neurol 355:38–50
Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM (2001) Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron 29:367–384
Pierrot-Deseilligny E (2002) Propriospinal transmission of part of the corticospinal excitation in humans. Muscle Nerve 26:155–172
Pierrot-Deseilligny E, Marchand-Pauvert V (2002) A cervical propriospinal system in man. Adv Exp Med Biol 508:273–279
Pollay M (2010) The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res 7:9
Proudfit HK, Clark FM (1991) The projections of locus coeruleus neurons to the spinal cord. Prog Brain Res 88:123–141
Raab S, Plate KH (2007) Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol 113:607–626
Reina MA, De Leon Casasola Ode L, Villanueva MC, Lopez A, Maches F, De Andres JA (2004) Ultrastructural findings in human spinal pia mater in relation to subarachnoid anesthesia. Anesth Analg 98:1479–1485, table of contents
Rexed B (1954) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100:297–379
Rezaie P, Male D (1999) Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc Res Tech 45:359–382
Rowitch DH, Kriegstein AR (2010) Developmental genetics of vertebrate glial-cell specification. Nature 468:214–222
Rustioni A, Kuypers HG, Holstege G (1971) Propriospinal projections from the ventral and lateral funiculi to the motoneurons in the lumbosacral cord of the cat. Brain Res 34:255–275
Rybak IA, Dougherty KJ, Shevtsova NA (2015) Organization of the Mammalian Locomotor CPG: review of computational model and circuit architectures based on genetically identified spinal interneurons(1,2,3). eNeuro 2, p. 1–20
Rybak IA, Stecina K, Shevtsova NA, Mccrea DA (2006) Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. J Physiol 577:641–658
Sadler TW (2005) Embryology of neural tube development. Am J Med Genet C Semin Med Genet 135C:2–8
Sawada M, Matsumoto M, Sawamoto K (2014) Vascular regulation of adult neurogenesis under physiological and pathological conditions. Front Neurosci 8:53
Scharrer E (1945) Capillaries and mitochondria in neutrophil. J Comp Neurol 83:237–243
Faull RL, Schoenen J (2004) Spinal cord: cyto- and chemoarchitecture. In: The Human Nervous System, 2nd edn. Elsevier Academic Press, Amsterdam/Boston
Schoenen J, Faull RLM (2004) Spinal cord: Cyto- abd chemoarchitecture. In: Paxinos G, Mai JK (eds) The human nervous system. Elsevier Academic Press, Amsterdam/Boston, pp 190–232
Sendtner M, Pei G, Beck M, Schweizer U, Wiese S (2000) Developmental motoneuron cell death and neurotrophic factors. Cell Tissue Res 301:71–84
Stifani N (2014) Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci 8:293
Stottmann RW, Berrong M, Matta K, Choi M, Klingensmith J (2006) The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev Biol 295:647–663
Sun T, Hafler BP, Kaing S, Kitada M, Ligon KL, Widlund HR, Yuk DI, Stiles CD, Rowitch DH (2006) Evidence for motoneuron lineage-specific regulation of Olig2 in the vertebrate neural tube. Dev Biol 292:152–164
Tahayori B, Koceja DM (2012) Activity-dependent plasticity of spinal circuits in the developing and mature spinal cord. Neural Plast 2012:964843
Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O (2013) Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500:85–88
Ten Donkelaar HJ, Lammens M, Wesseling P, Hori A, Keyser A, Rotteveel J (2004) Development and malformations of the human pyramidal tract. J Neurol 251:1429–1442
Thomas JL, Baker K, Han J, Calvo C, Nurmi H, Eichmann AC, Alitalo K (2013) Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells. Cell Mol Life Sci 70:1779–1792
Todd AJ, Sullivan AC (1990) Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol 296:496–505
Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH (2012) Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337:358–362
Valentino RJ, Wood SK, Wein AJ, Zderic SA (2011) The bladder-brain connection: putative role of corticotropin-releasing factor. Nat Rev Urol 8:19–28
Vallstedt A, Kullander K (2013) Dorsally derived spinal interneurons in locomotor circuits. Ann N Y Acad Sci 1279:32–42
Vandenabeele F, Creemers J, Lambrichts I (1996) Ultrastructure of the human spinal arachnoid mater and dura mater. J Anat 189(Pt 2):417–430
Wannier T, Bastiaanse C, Colombo G, Dietz V (2001) Arm to leg coordination in humans during walking, creeping and swimming activities. Exp Brain Res 141:375–379
Watson C, Paxinos G, Kayalioglu G (2009) The spinal cord. Academic Press, London, UK
Weidenheim KM, Bodhireddy SR, Rashbaum WK, Lyman WD (1996) Temporal and spatial expression of major myelin proteins in the human fetal spinal cord during the second trimester. J Neuropathol Exp Neurol 55:734–745
West WL, Yeomans DC, Proudfit HK (1993) The function of noradrenergic neurons in mediating antinociception induced by electrical stimulation of the locus coeruleus in two different sources of Sprague–Dawley rats. Brain Res 626:127–135
Williams PT, Martin JH (2015) Motor cortex activity organizes the developing rubrospinal system. J Neurosci 35:13363–13374
Willis WD, Coggeshall RE (1991) Sensory mechanisms of the spinal cord. Plenum Press, New York
Wiseman OJ, Brady CM, Hussain IF, Dasgupta P, Watt H, Fowler CJ, Landon DN (2002) The ultrastructure of bladder lamina propria nerves in healthy subjects and patients with detrusor hyperreflexia. J Urol 168:2040–2045
Wiseman OJ, Fowler CJ, Landon DN (2003) The role of the human bladder lamina propria myofibroblast. BJU Int 91:89–93
Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ (2002) Sonic hedgehog and the molecular regulation of mouse neural tube closure. Development 129:2507–2517
Zehr EP, Stein RB (1999) What functions do reflexes serve during human locomotion? Prog Neurobiol 58:185–205
Zeman W, Innes JRM (1963) Craigie’s neuroanatomy of the rat. Academic, New York
Zhang J, Lanuza GM, Britz O, Wang Z, Siembab VC, Zhang Y, Velasquez T, Alvarez FJ, Frank E, Goulding M (2014) V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron 82:138–150
Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M (2008) V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60:84–96
Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM (2012) Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol 590:4735–4759
Zhuang B, Sockanathan S (2006) Dorsal-ventral patterning: a view from the top. Curr Opin Neurobiol 16:20–24
Acknowledgments
The authors would like to thank Christian Schultz for helpful comments on the manuscript and Volker Dietz and Ilya Rybak for the kind permission to reprint images.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Engelhardt, M., Sobotzik, JM. (2017). Functional Neuroanatomy of the Spinal Cord. In: Weidner, N., Rupp, R., Tansey, K. (eds) Neurological Aspects of Spinal Cord Injury. Springer, Cham. https://doi.org/10.1007/978-3-319-46293-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-46293-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46291-2
Online ISBN: 978-3-319-46293-6
eBook Packages: MedicineMedicine (R0)