Abstract
Experimental data and numerical simulations on flame acceleration, shock-flame interaction and deflagration-to-detonation transition mechanism for stoichiometric hydrogen-oxygen mixtures at reduced pressure in relatively short tubes have been analysed. It was shown that the detonation occurs as a result of multiple reflections of precursor shock wave and its interaction with the flame. Adiabatic compression and heating of unreacted gas a front of the flame together with flame surface increase due to the Richtmyer-Meshkov instability provide preconditioning of the DDT process. Several times higher pressure, temperature and reaction rate within a preconditioning zone leads to significant decrease of run-up-distance to DDT in relatively short tubes. Results of the work will provide detailed information on multiple shock - flame interactions leading to the DDT process for numerical code validations.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
- Short Tube
- Flame Acceleration (FA)
- Stoichiometric Hydrogen-oxygen Mixture
- Precursor Shock Wave
- Richtmyer-Meshkov Instability
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
A problem of run-up distance for the detonation onset and the effect of tube length on detonation transition is of the great importance from practical and theoretical points of view. Critical conditions for flame acceleration and DDT in obstructed channels were evaluated in papers [1, 2]. The run-up distance from ignition to sonic flame and then to detonation in obstacle-laden tubes was experimentally investigated in paper [3]. Such conditions significantly differ from that in smooth tubes without obstacles. There are some experimental data on the effects of tube diameter on the run-up distance to detonation for tubes without obstacles [4, 5]. The ratio of run-up distance to tube diameter was found to be in the range from 15 to 40. Such an attempt is very conservative and does not take into account the mixture reactivity, geometry, and roughness of the channel.
The influence of tube roughness, boundary layer, and mixture reactivity in terms of chemical reaction length δ and detonation cell size λ was recently investigated in papers [6, 7]. To take into account a factor of mixture reactivity, the run-up distance to DDT x D was evaluated for hydrogen–oxygen mixtures in terms of a detonation cell width as a ratio x D/λ which has to be larger than 500. For ethylene–oxygen mixtures, characteristic time for detonation onset t D normalized by chemical reaction time τ as a measure of chemical reactivity has to be of the order of 25 (t D/τ ~25). In terms of chemical reaction length, the critical ratio x D/δ is about 1500–1700. The authors [6] demonstrated that the detonation onset may occur at dimensionless distances expressed in a tube diameter x D/D in the range from 1 to 50 depending on initial pressure (changes from 8 to 0.2 bar, respectively). Reducing the reactivity by reducing the initial pressure, the run-up distance might be comparable with a tube length.
The authors [6] also analyzed an effect of boundary layer thickness and a mixture reactivity on DDT run-up distance. As they found for highly reactive mixtures with a tube diameter D > 20 · λ, the run-up distance x D/D = 1÷100 and actually does not depend on tube diameter. Only for mixtures with 10 · λ < D < 20 · λ, the ratio x D/D = 15–25 depends on tube diameter. And, again, for low reactive mixtures, the run-up distance does not depend on tube diameter. It usually relates to so-called relatively short tubes. The criteria for relatively short tube can be the ratio t D/t s ≪ 1, where t s = L/cs is the time of reflected shock passing through the tube length. In this case, the effect of precursor shock reflection on DDT can be dominant, and we may classify the tube as relatively short. Adiabatic compression of the mixture and shock–flame interaction affected the mixture preconditioning prior the DDT. Using a relatively short tube with a tube length almost equal or even less than run-up distance to detonation, the detonation can be earlier initiated after a collision or several collisions of reflected precursor shock wave with a flame front. An abrupt flame acceleration due to Richtmyer–Meshkov instability may also lead to additional reaction rate increase and then to deflagration-to-detonation process.
The objective of this work is to investigate the DDT process for stoichiometric hydrogen–oxygen mixture in relatively short tubes. To change mixture reactivity, the run-up distances for the onset of detonations were studied in a series of tests as a function of initial pressure.
Experimental Results and Discussion
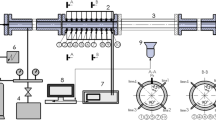
A series of experiments have been carried out in stainless steel detonation tube of 3.5–6 m long with rectangular cross section of 50 × 50 mm. Flame acceleration (FA) mechanism and triggering from subsonic to sonic flame with following detonation onset were investigated using light sensors and a high-speed camera combined with an optical Schlieren system. An optical access (l = 30 cm) to the combustion process was provided by optical section with two transparent quartz windows. The section has a variable position along the tube to capture images of the combustion process at different stages, from an ignition to steady-state detonation propagation (Fig. 1). Detailed description of the experimental facility is given in our previous paper [7].
Stoichiometric hydrogen–oxygen mixtures at pressures 100 and 200 mbar have been used as test mixtures to provide so-called short tube conditions. Table 1 gives main combustion properties of test mixtures.
Figure 2 shows a typical distance–time diagram for flame acceleration and DDT processes in a short tube. Initially, the flame accelerates as a “finger” flame pushing a precursor shock ahead the flame. It accelerates exponentially only due to enhanced side surface of “finger” flame, as written by Liberman et al. [8]. In relatively short tube, the detonation occurs after multiple shock wave reverberations because the pressure and the temperature of unburned material will reach the conditions for the flame to be accelerated fast enough for DDT. For instance, in comparison with an initial state, the pressure grows in six times, the temperature in two times, and the laminar flame speed in six times after five reflections (Fig. 3). The pressure and temperature of unreacted gas were calculated from shock wave velocity obtained by high-speed movie.
This means that shock–flame interaction and adiabatic compression of unreacted material play very important role for detonation preconditioning and DDT process in short tubes. A boundary layer and a turbulence may play not so significant role for DDT in relatively short smooth tubes filled with highly reactive mixtures.
As one can see in Fig. 4, an interaction of flame front initially propagating with a velocity v 0 = 30 m/s with reflected shock at velocity v s = 550 m/s occurred in Frame #1. Then the flame moves backward after the interaction with shock wave in Frames #2–#3. Such a shock interaction with a density gradient across the flame surface leads to dramatic flame area increase due to the Richtmyer–Meshkov instability. As a result of Richtmyer–Meshkov instability, the combustion velocity suddenly increases in ten times to 300–400 m/s. It significantly reduces the run-up distance to DDT. For instance, rough evaluation of run-up distance for initial pressure of 100 and 200 mbar based on the effect of boundary layer on DDT [6] gives the values of 4.8 m and 10.7 m, respectively. As it follows from x–t diagram (Fig. 2), the run-up distance to DDT in a short tube reduces to 1.7–2.5 m (3–4 times shorter).
1D Numerical Simulation
A series of 1D numerical simulations of hydrogen–oxygen mixture at subatmospheric pressures in a tube 12.5 m long was done to model gas dynamic effects in a short tube on DDT. In order to simplify the program and to make it more quick and flexible, the program was based on the following assumptions [9]:
-
Solution of the reactive Euler equations, i.e., neglect of molecular transportation processes such as diffusion, thermal conduction, and viscosity
-
No turbulence
-
1D geometry, i.e., neglect of real tube geometry (variable cross section) and radial gradients of concentrations, pressure, temperature, and velocity
-
Global dominant reaction for the H2/O2 combustion
-
Prescribed flame acceleration law
-
Temperature-dependent thermodynamic data for all components (H2, O2, H2O, N2)
-
First-order solution procedure, numerical cell size is 1–2 mm
-
Adiabatic assumption (no heat losses of gas to tube wall)
-
Reflecting boundary conditions at the tube ends
In particular the last assumption leads to conservative results during the pressure computation. Simulation of flame propagation was based on flame position tracking taking into account real exponential flame acceleration law in a smooth channel as a “finger flame”:
where \( k=\sigma \cdot {S}_{\mathrm{L}}/ R \) is the exponential factor depending on the expansion ratio of unburned and burned components \( \sigma ={\rho}_{\mathrm{u}}/{\rho}_{\mathrm{b}} \) and a tube radius R; u 0 = S L is the effective initial flame speed. So that with a smaller tube size R and a higher mixture reactivity defined as σ · S L, the flame accelerates faster. To approach the results of numerical simulations to real scenario, the parameters R, σ, and u 0 should be implemented into the code in advance.
For general description of the deflagration, three main parameters are necessary: the initial flame speed u 0, the flame acceleration distance x a which is a function of Eq. 1, and the maximum flame speed S max which is of the order of speed of sound in reactants. At the given DDT point x D, the flame speed is increased suddenly to the speed of sound in the burned gas c p, which can be determined from thermodynamic calculations (Table 1). This value of the flame speed corresponds to the CJ detonation. The run-up distance x D should be given to satisfy the “short tube” conditions.
The tube was virtually equipped with pressure and light sensors to register pressure signals and a flame position. Temperature profiles were used as light signals assuming that the flame radiation is proportional to the temperature. Stoichiometric hydrogen–oxygen mixture at normal temperature and subatmospheric pressure of 200 mbar was used as a test mixture. To a more efficient approach to the “short tube” conditions, stoichiometric hydrogen–oxygen was additionally diluted with nitrogen to the ratio 2H2:O2:4.5 N2.
Calculated dynamics of the flame and shock waves during the combustion process is shown in Fig. 5 together with pressure and light signals. The flame initially exponentially accelerates producing a precursor shock wave with a strength of 0.5 bar (377 K). Then it reflects pushing flame backward after the collision. After reflection from ignition end, the shock is getting stronger (0.7 bar, 415 K) and it turns the flame again forward. Such interaction repeats one more time leading to final pre-detonation pressure 1.16 bar and temperature 483 K. Then the detonation occurs with a CJ detonation pressure 15.5 bar which is five times higher than for a long tube. Finally the detonation pressure reaches the value of 47 bar after reflection which is unexpected for initial pressure of 0.2 bar and might be very dangerous from practical point of view. The run-up distance of 11 m in short tube is roughly three times shorter than 30 m evaluated for a long tube according to paper [6].
Figure 6 shows calculated pressure and temperature record at a distance of 11 m from ignition point. It is shown that in case of multiple reflections of precursor shock wave, the post-shock temperature may reach 500 K. The strength of precursor shock in terms of overpressure may increase seven times in comparison with incident precursor shock wave. These simulations demonstrate that even a limited space and a reduced pressure may lead to very strong hydrogen explosion with a maximum pressure of 100 times higher than initial subatmospheric pressure. The probability of detonation is also increased due to the reduced run-up distance. Of course, the 1D numerical model is not able to simulate the Richtmyer–Meshkov instability and effect of turbulence on flame acceleration and DDT, but even gas dynamics demonstrates strong effect of adiabatic pre-compression on flame behavior and detonation preconditioning.
Conclusions
Experimental data and numerical simulations on flame acceleration, shock–flame interaction, and deflagration-to-detonation transition mechanism for stoichiometric hydrogen–oxygen mixtures in relatively short tubes have been analyzed. It was shown that detonation occurs as a result of multiple reflections of precursor shock wave and its interaction with flame. Adiabatic compression and heating of unreacted gas a front of the flame together with flame surface increase due to Richtmyer–Meshkov instability provide preconditioning of the DDT process. Several times higher pressure, temperature, and reaction rate within a preconditioning zone lead to significant decrease of run-up distance to DDT in relatively short tubes. Results of the work will provide detailed information on mutual shock–flame interactions leading to the DDT process and for numerical code validations.
References
Dorofeev, S.B., Sidorov, V.P., Kuznetsov, M.S., Matsukov, I.D., Alekseev, V.I.: Effect of scale on the onset of detonations. Shock Waves 10, 137–149 (2000)
Dorofeev S.B., Kuznetsov M.S., Alekseev V.I., Efimenko A.A., Breitung W.: Evaluation of limits for effective flame acceleration in hydrogen mixtures. J. Loss Prev. Proc. Ind. 14/6, 583–589 (2001)
Veser, A., Breitung, W., Dorofeev, S.B.: Run-up distances to supersonic flames in obstacle-laden tubes. J. Phys. IV France 12(7), 333–340 (2002)
Schelkin, K.I., Sokolik, A.S.: The effect of chemical presentation on the initiation of the detonation wave. Soviet. Zhurn. Phys. Chem. 10, 479–485 (1937)
Bollinger, L.E., Fong, M.C., Edse, R.: Experimental measurements and theoretical analysis of detonation induction distance. Am. Rocket Soc. J. 31, 588–596 (1961)
Kuznetsov, M., Alekseev, V., Matsukov, I., Dorofeev, S.: DDT in a smooth tube filled with hydrogen–oxygen mixtures. Shock Waves 14(3), 205–215 (2005)
Kuznetsov, M., Liberman, M., Matsukov, I.: Experimental study of the preheat zone formation and deflagration to detonation transition. Comb. Sci. Tech. 182(11), 1628–1644 (2010)
Liberman, M.A., Kuznetsov, M., Ivanov, A., Matsukov, I.: Formation of the preheated zone ahead of a propagating flame and the mechanism underlying the deflagration-to-detonation transition. Phys. Lett. A 373, 501–510 (2009)
Kuznetsov, M., Lelyakin, A., Breitung, W.: Numerical simulation of radiolysis gas detonations in a BWR exhaust pipe and mechanical response of the piping to the detonation pressure loads. In: Angermann, L. (ed.) Numerical Simulations - Examples and Applications in Computational Fluid Dynamics, p. 440. InTech, Rijeka, Croatia (2010)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Kuznetsov, M., Lelyakin, A., Alekseev, V., Matsukov, I. (2017). Detonation Transition in Relatively Short Tubes. In: Ben-Dor, G., Sadot, O., Igra, O. (eds) 30th International Symposium on Shock Waves 1. Springer, Cham. https://doi.org/10.1007/978-3-319-46213-4_83
Download citation
DOI: https://doi.org/10.1007/978-3-319-46213-4_83
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46211-0
Online ISBN: 978-3-319-46213-4
eBook Packages: EngineeringEngineering (R0)