Abstract
Current shock tube combustion experiments generally assume that the test environment behind a reflected shock wave is quiescent and that ignition processes progress uniformly over the entire test volume. However, various past investigations, including those based on schlieren data and sidewall imaging [1, 2], have observed nonuniform ignition in certain test regimes. Here, we use both conventional diagnostics (pressure, emission, and laser absorption) and a high-speed chemiluminescent imaging system to investigate the ignition behavior of n-heptane/oxygen/argon in shock tubes at long test times (greater than 2 ms), in an attempt to map the boundary of uniform and nonuniform ignition behavior in one of our shock tubes.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Introduction
Current shock tube combustion experiments generally assume that the test environment behind a reflected shock wave is quiescent and that ignition processes progress uniformly over the entire test volume. However, various past investigations, including those based on schlieren data and sidewall imaging [1, 2], have observed nonuniform ignition in certain test regimes. Here, we use both conventional diagnostics (pressure, emission, and laser absorption) and a high-speed chemiluminescent imaging system to investigate the ignition behavior of n-heptane/oxygen/argon in shock tubes at long test times (greater than 2 ms), in an attempt to map the boundary of uniform and nonuniform ignition behavior in one of our shock tubes.
Ignition delay times from separate low-pressure n-heptane/O2/argon experiments conducted in our laboratory are shown in Fig. 1. Note the relatively good agreement between the model of Mehl et al. [3] and experiment at the highest (above 1100 K) and lowest (700 K) temperatures for the second-stage (primary) ignition delay time. However, modeling and experiment vary significantly and the data exhibits larger scatter, over the intermediate temperature regime from 700 to 1100 K for second-stage ignition. Note that this temperature window includes the negative-temperature-coefficient (NTC) regime, and the observed second-stage ignition delay times at temperatures above about 850 K have similar values to those seen in the first-stage ignition below 800 K. Also, we note that the Mehl et al. model has been very successful in modeling NTC ignition behavior at higher pressures (12–55 atm). Thus it is worth considering whether the differences between the model and experiment in Fig. 1 are due to deficiencies in the reaction mechanism describing the oxidation of n-heptane at this low pressure or because the assumptions about uniform zero-dimensional chemistry in the test volume are not justified.
Low-pressure ignition delay time measurements in n-heptane/O2/Ar mixtures. Blue symbols and lines, second-stage ignition; red symbols and lines, first-stage ignition. Solid lines: best fit to data. Dashed lines: constant-pressure modeling using the Mehl et al. (2011) mechanism [3]. Ignition delay times in this study were based on times of complete fuel removal or OH* emission
Because of these significant and consistent differences between model and experiment, n-heptane oxidation experiments at low pressures appear to represent an opportunity to investigate combustion behavior where nonuniform ignition processes are very likely. Measurements of ignition delay time combined with high-speed imaging of the test gas volume should provide evidence of combustion nonuniformity if and when it exists and permit a mapping of the boundary of uniform and nonuniform ignition behavior in this particular shock tube facility. Furthermore, these diagnostics should enable study of strategies for ameliorating these nonideal effects and expanding the boundary of uniform behavior.
High-Speed Imaging Measurements
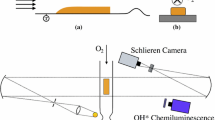
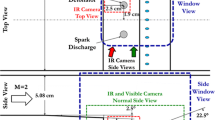
A schematic of the experimental setup including the imaging arrangement through the end wall of the shock tube is shown in Fig. 2. Images of OH* were acquired with a Phantom v710 CMOS camera that provides grayscale images using a 12 kHz acquisition rate, with a typical resolution of 200 μm per pixel. This light from the shock tube was intensified using a LaVision UV Intensifier High Speed IRO. OH* emission and sidewall pressure were used to determine ignition delay time in the conventional manner. In situ fuel concentration time-history measurement was used to accurately determine initial fuel loading and time histories.
Constrained Reaction Volume Shock Tube
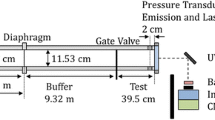
Measurements were performed in the Stanford University with 10 cm ID long-test-time shock tube. The acquisition of the long-test-time data was made possible by extending the shock tube driver and using tailored driver gas mixtures, and uniform pressure and temperature conditions were achieved by using driver inserts [4]. This arrangement allowed the reliable achievement of 50+ ms of test time.
The shock tube was operated using the constrained reaction volume (CRV) strategy [5]. This strategy provided a short test volume adjacent to the end wall followed by a long nonreactive buffer gas section extending back to the diaphragm. The two zones of the shock tube-driven section could be filled separately by closing the gate valve near the end wall. The arrangement reduced the chance of remote ignition and allowed near-constant pressure operation (until the very rapid energy release at ignition).
Results and Discussion
Figures 3 and 4 exhibit images of two different CRV reflected shock experiments. The frames have been artificially colored to assist the viewer in observing details of the image, and times of each image are indicated. In addition, the figures include the corresponding time-dependent graphs of sidewall pressure (P) acquired using a Kistler™ PZT, OH emission (OH*) using a Si photodiode and UG-5 Schott glass filter, relative fuel concentration (HeNe) using 3.39 μm IR laser absorption, and beam steering across the tube diameter (Schlieren) using 0.628 μm visible laser extinction. All diagnostics were located 2 cm from the shock tube end wall. The horizontal red line in the images is the beam path for the HeNe and Schlieren lasers; the blue lines outline the observation volume for OH emission.
The higher-temperature example (1166 K), Fig. 3, experiences sharp detonation-like ignition, as indicated by the rapid increase of the pressure and OH* emission graphs, at 1.28 ms. Previous to that there is evidence of burning, limited to the regions around the circumference, but no sign of emission in the core flow. Until the ignition event at 1.28 ms, the pressure is effectively constant, and the burning around the circumference does not appear to affect the measured core ignition delay time. At 1.28 ms, there appears to be effectively uniform homogenous ignition throughout the test volume. Hence this time can be taken as a correct value for the ignition delay time at the reflected shock conditions.
As indicated in the HeNe trace, fuel is consumed slowly over the entire ignition time, until 1.28 ms when there is a rapid final removal of fuel. The laser beams traversing the shock tube diameter experience very little steering/beam schlieren from density variations until the ignition event.
The lower-temperature (986 K) case, Fig. 4, by contrast, does not experience a rapid ignition event. The pressure remains near constant, as expected for CRV-fill experiments. The OH emission rises over a long time interval of 3 ms (5–8 ms) even though the stoichiometry is the same as that of the Fig. 3 experiment. Here, the ignition event is dominated by early burning in regions growing from the circumference, as revealed by the chemiluminescence images. An ignition delay time using the conventional definition of extrapolating the fastest rise of the OH* emission signal to the baseline would give a value of approximately 6 ms, but clearly this does not accurately reflect the ignition delay for homogeneous ignition. An ignition delay time cannot properly be derived from the sidewall pressure signal.
Some of the fuel appears to be abruptly consumed at 5.96 ms; this is consistent with the growing combustion region crossing the HeNe laser beam. The Schlieren signal also exhibits large variations with time consistent with nonuniform burning.
A comparison of both these two examples reveals early evidence of ignition near the circumference. By cleaning the shock tube between shock wave experiments, only a limited number of hot spots are caused from dust or diaphragm particles. The relatively uniform distribution of the ignition hot spots around the circumference in both experiments suggests that this ignition process is related to the boundary layer rather than to particle hot spots.
In the higher-temperature example, the core ignition rapidly dominates the test volume before the boundary layer flame can expand significantly into the core. In the lower-temperature example, the core ignition shows no evidence of occurring before the flame from the boundary layer covers the greater portion of the test gas volume.
In both examples, at the lower temperatures expected in the boundary layer, first-stage ignition can occur at relatively short times, as evident in Fig. 1. The NTC ignition chemistry is very pressure sensitive, approaching a pressure scaling for ignition of P−1.6 in the case of n-heptane [6], and very temperature sensitive. Thus, early ignition in the boundary layer for fuels, such as n-heptane with strong NTC character, is difficult to avoid. However, efforts to mitigate this behavior, e.g., using lower fuel concentrations and different stoichiometries, have shown favorable results.
Conclusion
It is critically important to identify the combustion regimes that occur in long-test-time ignition delay time experiments. Current detailed reaction mechanism modeling assumes in many cases that these processes are zero dimensional, and as is evident in this study, this is not accurate under some test conditions. A facility-dependent test-condition map is thus needed to identify the regimes where more detailed gasdynamic/combustion modeling is needed for quantitative combustion kinetics experiments. Work on the development of this operational map is in progress for the current shock tube facility.
References
Heufer, K.A., Olivier, H., Medvedev, S.P., Khomik, S.V.: Optical investigation of shock induced ignition of different biofuels. In: 23rd International Colloquium on the Dynamics of Explosions and Reactive Systems (ICDERS) at Irvine, California, USA, 2011
Lee, C., Vranckx, S., Heufer, K.A., Khomik, S., Uygun, Y., Olivier, H., Fernandes, R.X.: On the chemical kinetics of ethanol oxidation: shock tube, rapid compression machine and detailed modeling study. Zeitschrift für Physikalische Chemie 226, 1–28 (2012)
Mehl, M., Pitz, W.J., Westbrook, C.K., Curran, H.J.: Kinetic modeling of gasoline Surrogate components and mixtures under engine conditions. Proc. Combust. Inst. 33, 193–200 (2011)
Hanson, R.K., Davidson, D.F.: Recent advances in laser absorption and shock tube methods for studies of combustion chemistry. Prog. Energy Combust. Sci. 44, 103–114 (2014)
Hanson, R.K., Pang, G.A., Chakraborty, S., Ren, W., Wang, S., Davidson, D.F.: Constrained reaction volume approach for studying chemical kinetics behind reflected shock waves. Combust. Flame 160, 1550–1558 (2013)
Gauthier, B.M., Davidson, D.F., Hanson, R.K.: Shock tube determination of ignition delay times in full-blend and surrogate fuel mixtures. Combust Flame 139, 300–311 (2004)
Acknowledgments
This material is based upon work supported by, or in part by, the US Army Research Laboratory and the US Army Research Office under contract/grant number W911NF1310206.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Davidson, D.F. et al. (2017). Rapid Chemiluminescent Imaging Behind Reflected Shock Waves. In: Ben-Dor, G., Sadot, O., Igra, O. (eds) 30th International Symposium on Shock Waves 1. Springer, Cham. https://doi.org/10.1007/978-3-319-46213-4_52
Download citation
DOI: https://doi.org/10.1007/978-3-319-46213-4_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46211-0
Online ISBN: 978-3-319-46213-4
eBook Packages: EngineeringEngineering (R0)