Abstract
Current understanding of the adhesion system of geckos is the culmination of efforts by investigators throughout the biological and applied sciences. Continuing research in this area promises dividends in areas such as biomechanics, evolution, ecology, adhesive technology, and robotics. We describe here the key topics involved in the gecko adhesion system: the notable properties, the underlying physical principles, and the parameters that govern system performance. Additionally, we highlight the most important unresolved issues and propose productive directions related to gecko adhesion research and bioinspired engineering.
The designers of the future will have smarter adhesives that do considerably more than just stick. (Fakley 2001)
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
Gecko toe pads are sticky because they feature an extraordinary hierarchy of structure that functions as a smart adhesive. Gecko toe pads (Russell 1975) operate under perhaps the most severe conditions of any adhesive application. Geckos are capable of attaching and detaching their adhesive toes in milliseconds (Autumn et al. 2006a) while running with seeming reckless abandon on vertical and inverted surfaces, a challenge no conventional adhesive is capable of meeting. Structurally, the adhesive on gecko toes differs dramatically from that of conventional adhesives. Conventional pressure-sensitive adhesives (PSAs) such as those used in adhesive tapes are fabricated from materials that are sufficiently soft and sticky to flow and make intimate and continuous surface contact (Pocius 2012). Because they are soft and sticky, PSAs also tend to degrade, foul, self-adhere, and attach accidentally to inappropriate surfaces. Gecko toes typically bear a series of scansors covered with uniform microarrays of hairlike setae formed from β-keratin (Wainwright et al. 1982; Russell 1986; Alibardi 2003; Rizzo et al. 2006), a material order of magnitude stiffer than those used to fabricate PSAs. Each seta branches to form a nanoarray of hundreds of spatular structures; these structures make ultimate contact with the surface.
Functionally, the properties of gecko setae are as extraordinary as their structure: the gecko adhesive (1) is directional, (2) attaches strongly with minimal preload, (3) detaches quickly and easily, (4) sticks to nearly every material, (5) exhibits rate-dependent adhesion, (6) does not stay dirty or (7) self-adhere, and (8) is nonsticky by default. While some of the principles underlying these eight functional properties are now well understood, much more research will be necessary to fully map out the parameters of this complex system.
Over two millennia ago, Aristotle (1910) commented on the ability of the gecko to “run up and down a tree in any way, even with the head downwards.” How geckos adhere has attracted substantial and sustained scientific scrutiny (Cartier 1872, 1874a; Gadow 1901; Weitlaner 1902; Schmidt 1904; Hora 1924; Dellit 1934; Mahendra 1941; Maderson 1964; Ruibal and Ernst 1965; Hiller 1968, 1969, 1976; Gennaro 1969; Russell 1975, 1986; Williams and Peterson 1982; Stork 1983; Schleich and Kästle 1986; Irschick et al. 1996; Autumn et al. 2000, 2002b; Autumn and Peattie 2002; Arzt et al. 2003; Huber et al. 2005a; Hansen and Autumn 2005). The unusual hairlike microstructure of gecko toe pads has been recognized for well over a century (Cartier 1872, 1874a, b; Braun 1878). Setal branches were discovered using light microscopy (Schmidt 1904), but the discovery of multiple split ends (Altevogt 1954) and spatular nanostructure (Ruibal and Ernst 1965) at the tip of each seta was made only after the development of electron microscopy.
A single seta of the tokay gecko (Gekko gecko) is approximately 110 μm in length and 4.2 μm in diameter (Ruibal and Ernst 1965; Russell 1975; Williams and Peterson 1982) (Fig. 11.1). Setae are similarly oriented and uniformly distributed on the scansors. Setae branch a number of times at the tips into 100–1000 terminal structures (Ruibal and Ernst 1965; Schleich and Kästle 1986; Rizzo et al. 2006) known as spatulae. A single spatula consists of a stalk with a thin, roughly triangular end whose apex connects the spatula to its stalk. Spatulae are approximately 0.2 μm long with a similar width at the tip (Ruibal and Ernst 1965; Williams and Peterson 1982).
Structural hierarchy of the gecko adhesive system; (a) ventral view of a tokay gecko climbing a vertical glass surface; (b) ventral view of the foot of a tokay gecko, showing a mesoscale array of seta-bearing scansors (adhesive lamellae) (images a and b by 1 Mark Moffett); (c) microscale array of setae are arranged in a nearly grid-like pattern on the ventral surface of each scansor. In this scanning electron micrograph, each diamond-shaped structure is the branched end of a group of four setae clustered together in tetrad; (d) micrograph of a single gecko seta assembled from a montage of five Cryo-SEM images (image by S. Gorb and K. Autumn). Note individual keratin fibrils comprising the setal shaft; (e) nanoscale array of hundreds of spatular tips of a single gecko seta; (f) synthetic spatulae fabricated from polyimide using nanomolding (Campolo et al. 2003)
While the tokay is currently the best studied of any adhesive gecko species, there are over a thousand species of gecko (Han et al. 2004), encompassing an impressive range of morphological variation at the spatula, seta, scansor, and toe levels (Maderson 1964; Ruibal and Ernst 1965; Russell 1975, 1981, 1986; Peterson and Williams 1981; Williams and Peterson 1982; Stork 1983; Schleich and Kästle 1986; Russell and Bauer 1988, 1990a, b; Röll 1995; Irschick et al. 1996; Autumn et al. 2002a; Arzt et al. 2003; Peattie and Full 2007). Setae have even evolved on the tails of some gecko species (Bauer 1998). Remarkably, setae have evolved convergently in iguanian lizards of the genus Anolis (Braun 1878; Ruibal and Ernst 1965; Peterson and Williams 1981) and in scincid lizards of the genus Prasinohaema (Williams and Peterson 1982; Irschick et al. 1996).
This chapter aims broadly at identifying the known properties of the gecko adhesive system, possible underlying principles, and quantitative parameters that affect system function. However, much of what is known is based on studies of a single species—the tokay gecko—and the degree of variation in function among species remains an open question that should be kept in mind.
11.2 Adhesive Properties of Gecko Setae
Two front feet of a tokay gecko can withstand 20.1 N of force parallel to the surface across 227 mm2 of toe-pad area (Irschick et al. 1996). The foot of a tokay bears approximately 3600 tetrads of setae per mm2; this gives a corresponding setal density ρ = 14,400 setae per mm2 (Schleich and Kästle 1986). Consequently, a single seta should produce an average force of 6.2 μN and an average shear stress of 0.090 N/mm2 (0.9 atm). However, single setae have proved both much less sticky and much more sticky than predicted by whole-animal measurements under varying experimental conditions, implying that attachment and detachment in gecko setae are mechanically controlled (Autumn et al. 2000).
A similar analysis can be performed using the clinging force and morphology data from other gecko species; these results do not indicate many universal scaling principles relating to setal density and animal-level clinging force across species. A phylogenetic analysis indicates that there is no apparent relationship between setal density and the body mass of the animal (Peattie and Full 2007). Other recent experiments (Hagey et al. 2014) indicate that, while the setal arrays of different species operate by similar mechanics, the exact connection between the morphological details and the performance is unclear.
11.2.1 Properties (1) and (2): Anisotropic Attachment and High Adhesion Coefficient (μ′)
Using a newly developed microelectromechanical system (MEMS) force sensor (Chui et al. 1998), Autumn et al. (2000) measured the adhesive and shear force of a single isolated gecko seta. The angle of the setal shaft was particularly important in achieving an adhesive bond. Strong attachment occurred when using proper orientation and a motion based on the dynamics of gecko legs during climbing (based on force-plate data; Fig. 11.2) (Autumn et al. 2006b). A small normal preload force yielded a shear force of ≈ 40 μN, six times the force predicted by whole-animal measurements (Irschick et al. 1996). The small normal preload force, combined with a 5-μm proximal lateral/shear displacement, yielded a very large shear force of 200 μN, 32 times the force predicted by whole-animal measurements of Irschick et al. and 100 times the frictional force measured with the seta oriented with spatulae facing away from the surface. The preload and drag steps were also necessary to initiate significant adhesion in isolated gecko setae, consistent with the load dependence and directionality of adhesion observed at the whole-animal scale by Haase (1900) and Dellit (1934). The ratio of preload to pull off force is the adhesion coefficient (μ′), which represents the strength of adhesion as a function of the preload (Bhushan 2013). In isolated tokay gecko setae, a 2.5-μN preload yielded adhesion between 20 μN (Autumn et al. 2000) and 40 μN (Autumn et al. 2002b), and thus μ′ lies between 8 and 16.
Single-leg ground reaction forces in running geckos (Hemidactylus garnotii): (a) during level running, geckos’ front legs produce deceleratory ground reaction forces while their hind legs produce acceleratory forces (Autumn et al. 2006b). All legs push away from the body, producing ground reaction forces aimed through the joints toward the center of mass, minimizing joint moments, and possibly stabilizing the animal as it runs. Circles with dots represent positive ground reaction forces normal to the surface. During level running these represent the forces that support the animal’s weight; (b) during vertical climbing, geckos have similar kinematics, but alter dramatically their kinetics in comparison to level running (Chen et al. 2006). While climbing, all legs accelerate the body up the wall, and all legs pull in toward the center of mass, engaging the adhesive setae and claws. Front limbs pull away from the surface and hind limbs push into the surface, producing a torque that tips the head toward the wall, counteracting the tendency of the head to pitch back as it climbs
11.2.1.1 Large Safety Factor for Adhesion and Friction?
All 6.5 million (Schleich and Kästle 1986; Irschick et al. 1996) setae of a 50-g tokay gecko attached maximally could theoretically generate 1300 N (133 kg force) of shear force—enough to support the weight of two humans. This suggests that a gecko need only attach 3 % of its setae to generate the greatest forces measured in the whole animal (≈20 N) (Irschick et al. 1996). Only less than 0.04 % of a gecko’s setae attached maximally are needed to support its weight (0.49 N) on a wall. At first glance, gecko feet seem to be enormously overbuilt; they have a safety factor of at least (20 N/0.49 N) – 1 = 41 (or 4100 %). However, it is unlikely that all setae are able to achieve the optimal orientation simultaneously. The proportion of spatulae attached may be greatly reduced on rough surfaces, particularly those with roughness on the same scale as spatulae or setae (Persson and Gorb 2003). On dusty or exfoliating surfaces, attachment to a well-anchored substrate will not be possible for every seta, perhaps even rendering the scansor surfaces useless (Delaugerre et al. 2015). Geckos may use a significant portion of their safety margin while withstanding high winds during tropical storms, resisting predator attack, or recovering adhesion after a fall (Hecht 1952; Vinson and Vinson 1969; Russell 1976; Autumn and Peattie 2002; Pianka and Sweet 2005).
Geckos have been observed to recover from a fall by reattaching their toes to leaves or branches as they plummet (Vitt and Zani 1997; Pianka and Sweet 2005). A simple calculation suggests that recovery from a fall may require a large proportion of a gecko’s safety margin in adhesion or friction. Consider a 50-g gecko falling from rest. If the gecko attaches a foot to a vertical surface after it has fallen 10 cm (neglecting air resistance), it will be moving at 1.4 m/s. If the foot is able to produce 5 N of friction, the gecko will be able to come to a stop in 15 ms after sliding 1.1 cm. In this theoretical example, recovering from a fall of the very modest distance of 10 cm would require 50 % of the shear capacity of one foot based on whole-animal measurements (Irschick et al. 1996) but still less than 4 % of the theoretical maximum shear stress calculated for single setae (Autumn and Peattie 2002).
11.2.2 Property (3): Low Detachment Force
The surprisingly large forces generated by single setae raised the question of how geckos manage to detach their feet in just 15 ms with no measurable detachment forces (Autumn et al. 2006b). Autumn et al. (2000) discovered that simply increasing the angle (α) that the setal shaft makes with the substrate to 30° causes detachment. As α increases, sliding stops and stress increases at the trailing edge of the seta, causing fracture of the seta-substrate bonds (Autumn et al. 2000) and returning the seta to the unloaded default state. This scenario is supported by models of setae as cantilever beams (Sitti and Fearing 2003; Gao et al. 2005; Spolenak et al. 2005a) and by finite element method (FEM) modeling of the seta (Gao et al. 2005). FEM simulation of the single-seta pull off experiment in Autumn et al. (2000) revealed more than an order of magnitude decrease in adhesive force as α increased from 30° to 90°. The FEM simulation also identified a transition from sliding to peeling that occurs at α = 30°, consistent with cantilever beam-based models (Sitti and Fearing 2003) and empirical observations that setae slide at α < 30° but detach at α > 30° (Autumn et al. 2000).
Later measurements (Autumn et al. 2006a) indicate that setal arrays obey a tribological law called “frictional adhesion.” In frictional adhesion, adhesive force depends on the shear/friction forces established; a decrease in shear loading decreases adhesion, and an increase in shear force increases adhesion. This relationship is consistent with a critical release angle in gecko toes, isolated setal arrays, and single setae and indicates why attachment is initiated proximally along the axis of the toe (Haase 1900) and why geckos only stick in the presence of lateral forces (Hora 1924).
This principle of directional or anisotropic adhesion is an important feature of the climbing system. The gecko adhesive can be thought of as the first known “programmable adhesive.” Preload and drag steps turn on and modulate stickiness, while increasing the shaft/array/toe angle to 30° turns off stickiness. There are many physical explanations for the directional adhesion effect that involve a variety of geometrical and material parameters across a number of length scales (see Sect. 11.2.5.5) (Gao et al. 2005; Tian et al. 2006; Chen et al. 2009; Sauer 2009; Yao and Gao 2006; Hu and Greaney 2014).
11.2.3 Integration of Body and Leg Dynamics with Setal Attachment and Detachment
How attachment and detachment of millions of setae during locomotion are integrated with the function of the scansor, toe, foot, leg, and body remains a topic of interest and ongoing research (Russell 1986; Autumn and Peattie 2002; Autumn et al. 2005; Gao et al. 2005). Since gecko setae require a preload in the normal axis for adhesion, large forces could potentially be associated with attachment of the foot. The tremendous adhesive capacity of gecko setae suggests that large forces could also occur during detachment. In fact, no measurable ground reaction forces were associated with either attachment or detachment during vertical climbing on a force plate of the house gecko Hemidactylus garnotii (Autumn et al. 2006b), indicating that these actions are either mechanically decoupled from the center of mass in this species or result in forces so small as to be undetectable. Russell (2002) suggested that in the tokay gecko, the perpendicular-preload and 5-μm drag requirements (Autumn et al. 2000; Autumn and Peattie 2002) are controlled by hydrostatic pressure in the highly derived blood sinuses and lateral digital tendon system, respectively. However, control of inflation and deflation of the sinuses remains to be demonstrated. This mechanism would not be available to those species that lack blood sinuses.
Setal preload and drag could also be a consequence of force development during the stride (Fig. 11.2). The force necessary to bend even thousands of setae into an adhesive orientation is probably quite small (at most 10 mN) (Autumn and Peattie 2002) and possibly below the sensitivity of the force plate used (Autumn et al. 2006b). Another possibility is that during attachment, which is a reversal of the peeling process of toe detachment, the required motion is decoupled from the center of mass. The gecko’s foot approaches the substrate without pressing into it and reapplies the adhesive by unrolling its toes like a new year’s party favor. This process is called digital hyperextension (Russell 1975, 2002; Xu et al. 2015). In this case, setal preload forces would be spread out over time and would likely be far below the 1-mN sensitivity of force plates used in measurements of whole-body and single-leg dynamics of small animals (Biewener and Full 1992). Peeling may reduce detachment and attachment forces, but may limit speed during vertical climbing. If toe peeling and uncurling in climbing geckos require some minimum time, then speed cannot be increased by reducing contact time as is typical in level running. Hemidactylus garnotii increased velocity by increasing stride length (Autumn et al. 2006b), and attachment and detachment occupied a constant value of approximately 20 ms.
The experiments of Gravish et al. (2008) indicate that certain detachment pathways are more energetically conservative than others. The release of stored elastic energy in the attached setal array helps drive the detachment process, and detachment at optimal foot-withdrawal angles apparently occurs spontaneously, meaning that little external force needs to be applied. This mechanical effect likely contributes significantly to the efficiency of locomotion. It is also worth noting that, after attachment, a gecko can maintain adhesion with little effort. In fact, deceased geckos continue to cling to the last surface that they attached themselves to (Stewart and Higham 2014).
11.2.4 Molecular Mechanism of Gecko Adhesion
While setal structures of many gecko species are well documented, a complete understanding of what makes them adhere has been more elusive. At the turn of the twentieth century, Haase (1900) noted that attachment is load dependent and only occurs in one direction: proximally along the axis of the toe. Haase was also the first to suggest that geckos stick by intermolecular forces (“adhesion”), noting that under this hypothesis the attractive force should increase as the space between the feet and the substrate decreases. However, at least seven possible mechanisms for gecko adhesion have been discussed over the past 185 years.
11.2.4.1 Unsupported Mechanisms: Glue, Suction, Electrostatics, Microinterlocking, and Friction
Since geckos lack glandular tissue on their toes, sticky secretions similar to those used by insects (Dewitz 1882) were ruled out early in the study of gecko adhesion (Wagler 1830; Cartier 1872; Simmermacher 1884). The hypothesis that the toe pads acted as suction cups was first proposed by Wagler (1830) (who classified geckos as amphibians). The hypothesis that individual setae act as miniature suction cups was first under debate in the insect adhesion literature (Blackwall 1845; Hepworth 1854). Dewitz (1882) argued against suction as an explanation for gecko adhesion, but Simmermacher (1884) considered suction to be the most likely mechanism. However, there are no data to support suction as an adhesive mechanism, and the adhesion experiments carried out in a vacuum by Dellit (1934) suggest that suction is not involved. Furthermore, measurements of ≈ 9 atm of adhesive stress (Autumn et al. 2002b) strongly contradict the suction hypothesis, since the adhesive stress produced by suction is limited by the ambient atmospheric pressure. Despite substantial evidence against it, the suction hypothesis has been surprisingly tenacious in the popular literature (e.g., Gennaro 1969). Electrostatic attraction (Schmidt 1904) was another hypothesized mechanism for adhesion in gecko setae. Experiments using X-ray bombardment (Dellit 1934) eliminated electrostatic attraction as a necessary mechanism for setal adhesion since geckos could still adhere to metal in ionized air. However, electrostatic effects could enhance adhesion even if another mechanism is operating (Maderson 1964).
Setae are recurved such that their tips point proximally, leading Dellit (1934) to hypothesize that setae act as micro- or nanoscale hooks, catching on surface irregularities (microinterlocking). Mahendra (1941) suggested that setae were analogous to the crampons of a climber’s boot. The microinterlocking hypothesis was challenged by the ability of geckos to adhere while inverted on polished glass. A mechanism like this could play a secondary role under some conditions, but the presence of large adhesive forces on a molecularly smooth SiO2 MEMS semiconductor surfaces (Autumn et al. 2000) demonstrates that surface irregularities are not necessary for adhesion. The friction hypothesis (Hora 1924) can be dismissed since, by definition, friction only acts in shear (Bhushan 2013) and therefore cannot in itself explain the adhesive capabilities of geckos on inverted surfaces.
11.2.4.2 Potential Intermolecular Mechanisms: Van der Waals and Capillary Forces
Using the greatly enhanced resolution of electron microscopy, Ruibal and Ernst (1965) described the spatular structures at the setal tips. They concluded that spatulae were unlikely to function like the spikes on climbing boots and postulated that the spatulae lie flat against the substrate while the seta is engaged. It was clear to them that these flattened tips increased the realized contact area, and they concluded that gecko adhesion was the result of molecular interactions, not mechanical interlocking.
The turning point in the study of gecko adhesion came with a series of experiments (Hiller 1968, 1969) that suggested that the surface energy of the substrate, rather than its texture, determined the strength of attachment. By providing evidence that intermolecular forces were responsible, Hiller paved the way for the application of modern methods of surface science (Israelachvili 2011) in studies of gecko adhesion (Autumn et al. 2000, 2002b; Autumn and Peattie 2002; Huber et al. 2005a; Hansen and Autumn 2005). Hiller (1968, 1969, 1976) showed that shear force was correlated with the water droplet contact angle of the surface, and thus with the surface energy of the substrate, providing the first direct evidence that intermolecular forces are responsible for attachment in geckos.
Intermolecular capillary forces are the principal mechanism of adhesion in many insects (Gillett and Wigglesworth 1932; Edwards and Tarkanian 1970; Lee et al. 1986; Lees and Hardie 1988; Brainerd 1994), frogs (Emerson and Diehl 1980; Green 1981; Hanna et al. 1991), and even mammals (feathertail gliders) (Rosenberg and Rose 1999). Unlike many insects, geckos lack glands on the surface of their feet (Cartier 1874a; Bellairs 1970). However, this does not preclude the role of capillary adhesion (von Wittich 1854, quoted directly in Simmermacher 1884; Baier et al. 1968; Israelachvili 2011; Stork 1980) since layers of water molecules are commonly present on hydrophilic surfaces at ambient humidity and can cause strong attraction between surfaces. The observation (Hiller 1968, 1971, 1976) that geckos cannot adhere to polytetrafluoroethylene (PTFE or Teflon®) is consistent with the capillary hypothesis, since PTFE is strongly hydrophobic. Indeed, the apparent correlation between adhesive force and hydrophobicity (as indicated by the water contact angle θ) (Hiller 1968, 1971, 1976) suggested that the polarity of the surface might be an important factor in the strength of adhesion (Autumn and Peattie 2002).
A non-mutually exclusive alternative mechanism is dispersive van der Waals (vdW) forces (Stork 1980; Autumn et al. 2000). These vdW forces are strongly dependent on the distance between surfaces, increase with the polarizability of the two surfaces, and are not related directly to surface polarity (Israelachvili 2011). The observation (Hiller 1968) that geckos cannot adhere to PTFE is consistent with both van der Waals and capillary hypotheses, since PTFE is weakly polarizable and hydrophobic.
11.2.4.3 Contact Angle Estimates of Surface Energy
Hiller’s experiments (Hiller 1968, 1969, 1976) were groundbreaking because they provided the first direct evidence for adhesion sensu stricto. The precise nature of the adhesion remained unknown until 2002 (Autumn and Peattie 2002; Autumn et al. 2002b). The intermolecular attraction between any fluid droplet and a surface is due to a combination of dispersive vdW and polar components (Israelachvili 2011; Pocius 2012). Water contact angle by itself cannot be used to determine the relative contributions of vdW and polar interactions. Complete liquid droplet contact angle analyses require a series of fluids ranging from primarily dispersive (e.g., methylene iodide) to primarily polar (e.g., water) in order to partition the relative contributions of the different intermolecular forces (Baier et al. 1968; Israelachvili 2011; Pocius 2012). However, it is possible to test the hypothesis that vdW forces are sufficient for gecko adhesion by reanalyzing Hiller’s data to linearize the relationship between water contact angle and adhesion energy (Autumn and Peattie 2002). Hiller’s data (1968, 1969, 1976) when linearized yield a strong correlation between force and adhesion energy for θ > 60°, consistent with the van der Waals hypothesis (Autumn and Peattie 2002).
11.2.5 Property (4): Material-Independent Adhesion
11.2.5.1 Testing the van der Waals and Capillary Adhesion Hypotheses
To test directly whether capillary adhesion or the dispersive vdW force is a sufficient mechanism of adhesion in geckos, Autumn and colleagues (2002b) measured the hydrophobicity of the setal surface and measured adhesion and friction on two polarizable semiconductor surfaces that varied greatly in hydrophobicity. If capillary adhesive forces dominate, we expect a lack of adhesion on the strongly hydrophobic surfaces. In contrast, if the dispersive vdW forces are sufficient, large adhesive forces on the hydrophobic, but polarizable, GaAs and Si surfaces of the MEMS devices were predicted. In either case, strong adhesion to the hydrophilic SiO2, control surfaces should be present. The tokay gecko setae are ultrahydrophobic (θ = 160.9°) (Autumn et al. 2002b; Autumn and Hansen 2006), probably as a consequence of the hydrophobic side groups of β-keratin proteins (Landmann 1986). The strongly hydrophobic nature of setae suggests that they interact primarily via dispersive vdW forces whether water is present or not.
Shear stress of live gecko toes on GaAs (θ = 110°) and SiO2 (θ = 0°) semiconductors was not significantly different, and adhesion of a single gecko seta on the hydrophilic SiO2 and hydrophobic (HF-etched) Si cantilevers differed by only 2 %. These results reject the hypothesis that the polarity (as indicated by θ) of a surface predicts attachment forces in gecko setae, as suggested by Hiller (1968, 1969), and are consistent with the Hiller data following their reanalysis using adhesion energies (Autumn and Peattie 2002). Since vdW dispersion forces are the only mechanism that can cause two hydrophobic surfaces to adhere in air (Israelachvili 2011), the GaAs and hydrophobic semiconductor experiments provide direct evidence that these vdW forces are a sufficient mechanism of adhesion in gecko setae and that water-based capillary forces are not required. Setal adhesion is strong on polar and nonpolar surfaces, perhaps because of the strongly hydrophobic material they are made of and due to the very large contact areas made possible by the spatular nanoarray. Gecko setae thus have the property of material independence: they can adhere strongly to a wide range of materials, largely independently of surface chemistry.
11.2.5.2 The Role of Water in Gecko Adhesion
Property (4), material-independent adhesion, does not preclude an effect of water on gecko adhesion under some conditions. Water is likely to alter contact geometry and adhesion energies when present between hydrophobic (e.g., spatula) and hydrophilic (e.g., glass) surfaces, but it is exceedingly difficult to predict what the effect will be in gecko setae because of the complexity of the system. Experimental evidence indicates that adhesion is enhanced in environments with high relative humidity (Sun et al. 2005; Huber et al. 2005b; Niewiarowski et al. 2008; Puthoff et al. 2010).
The humidity-dependent adhesion forces appear to contradict the fact that setae are strongly hydrophobic (Autumn et al. 2002b; Autumn and Hansen 2006). This raises the question of how to explain the apparent effect of humidity. Interpretations based on the presence of water bridges predict that the adhesion forces at fixed humidity should be proportional to the absolute temperature (Kim and Bhushan 2008), but these predictions do not agree with the experimental observations (Huber et al. 2005b; Niewiarowski et al. 2008). Water could reduce adhesion on rough surfaces by preventing spatular penetration into gaps, thus decreasing the contact fraction, but the humidity effects are present on atomically flat surfaces (Huber et al. 2005b; Puthoff et al. 2010). The data of Huber et al. rejected “true” capillary forces involving a water bridge since only a few monolayers of water were present at the spatula–substrate interface, even at high humidity. Instead, they concluded that humidity (a) modifies the contact geometry, increasing adhesion, and (b) decreases the Hamaker coefficient (A) that determines the strength of the vdW forces, reducing adhesion. Puthoff et al. (2010) confirmed that contact forces increase with humidity in isolated gecko setal arrays but not because of capillary effects. Contrary to the predictions of a capillary mechanism, contact forces and humidity effects were similar on hydrophobic and hydrophilic substrates and at shear rates that yielded insufficient time for capillary bridges to form.
The adhesion enhancement in the presence of humidity appears to be the result of changes in the properties of the setal material (Chen and Gao 2010). Support for this hypothesis comes with the confirmation that the viscoelastic properties of the setae change with humidity (Puthoff et al. 2010; Prowse et al. 2011). These results suggested that capillarity is absent or has a limited role in the adhesion of geckos under humid conditions. Rather, humidity softens and plasticizes setal β-keratin, which increases true contact area and adhesion forces. This explanation is consistent with the fact that the change in adhesion force from low to high humidity is the same on hydrophobic and hydrophilic substrates.
Research on the role of standing water on the adhesion of geckos is ongoing. Pesika et al. (2009b) found that adhesion of isolated gecko setae is reduced in water considerably, consistent with the predictions of the Lifshiz model of van der Waals adhesion. Stark and colleagues (2012, 2013, 2015a, b) investigated the behavior of the setal arrays on a toe of a live gecko. When submerged, a plastron of air forms underneath the toe, effectively limiting the exposure of the hydrophobic setae to water. After prolonged exposure to water, the scansors are filled with water, substantially reducing adhesion on hydrophilic substrates. The effect of surface water on the clinging ability of whole animals also appears to depend on the surface chemistry of the substrate, since hydrophilic surfaces promote wetting of the setal tissue. As long at toe pads remain in the unwetted state, adhesion on hydrophilic, intermediately wetting, or hydrophobic surfaces is the same (Stark et al. 2013). However, wet hydrophilic surfaces promote toe-pad wetting, decreasing the animal’s overall clinging force (Stark et al. 2013).
11.2.5.3 Dominance of Geometry in VdW Interactions
The theoretical magnitude of the dispersive vdW force (F vdW) between a planar substrate and a circular planar spatula of radius R (Israelachvili 2011) is F vdW = AR 2/6D 3, and for a planar substrate and a curved spatula of radius R, F vdW = AR/6D 2, where A represents the Hamaker coefficient and D is the gap distance (typically 0.2 nm for solids in contact). A is a function of the volume and polarizability of the molecules involved.
For materials interacting in dry air, A ~ 10–19 J. Altering the chemical composition of one or both surfaces can alter A, which can be as low as one-half to one-third this value for some polymer–polymer interactions (e.g., PTFE or polystyrene) and as high as five times this value for some metal-on-metal interactions. In water, A can be reduced by an order of magnitude. Nevertheless, this variation in A is only about an order of magnitude, while gap distance D and contact area may vary by six or more orders of magnitude without macroscopically visible changes at the interface. Moreover, the effect of the gap distance is exponential to a power of at least two. Thus, adhesive surface effects due to vdW interactions are a function primarily of geometry, not of chemistry. A van der Waals mechanism for adhesion in gecko setae suggests that continuum theory models of the mechanics of surface contact (Johnson 1985) may be applicable. Then again, since the complex structure of setae and spatulae differs dramatically from the ideal curved and planar surfaces used in contact mechanics models, one might question the validity of models based on simple geometries to the function of gecko setae.
11.2.5.4 JKR Model of Spatulae
The mechanics of contact have been modeled using continuum theory and highly simplified geometries. For example, the Johnson/Kendall/Roberts (JKR) model considers the force F JKR required to pull an elastic sphere of radius R from a planar surface (Johnson et al. 1971). The predicted adhesion force is F JKR = (3/2)πRγ, where γ is the adhesion energy determined by the materials the sphere and the surface are comprised of. Using an approximate value of R = 100 nm for the spatular size and taking γ = 50 mJ/m2, the predicted pull off force for a gecko spatula is F JKR = 23.6 nN, approx. twice the value measured by AFM (Huber et al. 2005a) (Table 11.1).
Another test of the validity of the JKR model is to begin with the forces measured in single setae and then calculate the size of the associated JKR sphere (Autumn et al. 2002b). Adhesion is ≈ 40 μN per seta on silicon cantilever surfaces (Table 11.1). The setal tip is approximately 43 μm2 in area, and therefore, the adhesive stress (σ) is σ = force/area ≈ 917 kPa. If the spatulae are packed tightly, σ ≈ F JKR/(approx. JKR contact area) = (3/2)πRγ/πR 2 = (3/2)γ/R. Using a typical value of γ for dispersive vdW interactions between surfaces (50–60 mJ/m2), solving for the predicted radius (R JKR) of individual spatular contacts using empirical force measurements gives R JKR as 82–98 nm (or contacts 164–196 nm across). These values are remarkably close to empirical measurements of the size of real gecko spatulae (200 nm across) (Ruibal and Ernst 1965; Autumn et al. 2000), yet obviously spatulae are not spherical (Fig. 11.1e). Note that the preceding estimate of R JKR differs from that of Autumn and colleagues (2002b) in that they estimated the area of one seta using setal density and arrived at a similar but somewhat lower value for σ. The confirmation that the JKR model predicts similar magnitudes of force as observed in setae suggested the extraordinary conclusion that adhesion can be enhanced simply by splitting a surface into small protrusions to increase the surface density of individual contacts (Autumn et al. 2002b) and that adhesive stress is proportional to l/R (Arzt et al. 2002). This assessment was initially supported by a comparative analysis of setae in lizards and arthropods (Arzt et al. 2003) (see Sect. 11.5.1). However, scaling of spatular density as a function of body mass was rejected once phylogenetic relationships were accounted for (Peattie and Full 2007).
11.2.5.5 Kendall Peel Model of Spatulae
Spatulae may also be modeled as nanoscale strips of adhesive tape (Huber et al. 2005a; Spolenak et al. 2005b; Hansen and Autumn 2005). Using the approach of Kendall (1975), the peel force is F peel = γw/(1 – cos θ), assuming there is negligible elastic energy storage in the spatula as it is pulled off. w is the width of the spatula, γ is the adhesion energy as for the JKR model, and θ is the angle that the adhesive strips make with the substrate. Empirical measurements of vertical (θ = 90°) spatular adhesion (Huber et al. 2005a) suggest that each spatula adheres with approximately 10 nN of force. Using γ = 50 mJ/m2, typical of vdW interactions, the Kendall peel model predicts a spatular width of 200 nm, remarkably close to the actual dimension (Ruibal and Ernst 1965; Autumn et al. 2000).
The peel zone model of adhesion (Tian et al. 2006; Pesika et al. 2007) is an extension of the Kendall peel model that places additional emphasis on the nanometer-scale aspects of adhesion. The curvature of the peeling film over the region where intermolecular forces are most significant introduces a geometrical correction to the peeling equation: F peel ≈ 2γwθ/π(1 − cos θ)sin θ. When θ = 90° = π/2 rad, the result is the same as for the Kendall solution, but as θ becomes small, F increases much more rapidly as more intermolecular forces are brought into play.
Theoretical considerations suggest that generalized continuum models of spatulae as spheres or nanotape are applicable to the range of spatula size and β-keratin stiffness of setae found in reptiles and arthropods (Spolenak et al. 2005b). Interestingly, at the 100-nm size scale, the effect of shape on adhesion force may be limited (Gao and Yao 2004; Spolenak et al. 2005b). However, at sizes above 100 nm, and especially above 1 μm, Spolenak et al. (2005b) concluded that shape should have a very strong effect on adhesion force. A phylogenetic comparative analysis of attachment force in lizards and insects will be an important test of this hypothesis.
11.2.6 Property (5): Rate-Dependent Adhesion
The gecko adhesion system also possesses the property of rate-dependent friction and adhesion. All friction is rate dependent, a fact typically reflected in the convention that there are separate coefficients of friction μ static and μ dynamic for the respective cases of a stationary contact and one with relative motion between the surfaces. Detailed models of friction adopt a truly dynamic coefficient of friction μ = μ(v), where v is the velocity of relative motion. Ordinarily, dry, hard materials slip more easily as they slide more rapidly. However, the gecko adhesive setae, also dry and hard, become stickier as they slide faster (Gravish et al. 2010; Puthoff et al. 2010, 2013). Additionally, gecko setae are extremely wear resistant and capable of sliding as far as 300 m without significant deterioration (Gravish et al. 2010).
The adhesion produced follows the friction according to the principles of frictional adhesion. This is a property that might have significant ramifications for the capabilities of the organism, since very strong forces can be exerted by a gecko moving rapidly (i.e., falling) with respect to a substrate. These stable tribological forces in sliding gecko setae may emerge from the stochastic stick–slip of a large population of individual fibrils with high resonant frequencies, and wear resistance may be a consequence of stick–slip motion that minimizes rubbing and involves semi-regular elastic energy release. Moreover, each seta is small enough to be affected by thermal energy, raising the possibility that rate enhancement is in part due to thermally activated kinetics (Gravish et al. 2010; Puthoff et al. 2013).
11.3 Antiadhesive Properties of Gecko Setae
Paradoxical as it may seem, there is growing evidence that gecko setae are strongly antiadhesive. Gecko setae do not adhere spontaneously to surfaces, but instead require a mechanical program for attachment (Autumn et al. 2000). Unlike adhesive tapes, gecko setae do not self-adhere. Pushing the setal surfaces of a gecko’s feet together does not result in strong adhesion. Also unlike conventional adhesives, gecko setae do not seem to stay dirty.
11.3.1 Properties (6) and (7): Self-Cleaning and Anti-Self-Adhesion
Dirt particles are common in nature (Little 1979), yet casual observation suggests that geckos’ feet are quite clean (Fig. 11.1b). Sand, dust, leaf litter, pollen, and plant waxes would seem likely to contaminate gecko setae. Hairlike elements on plants accumulate micron-scale particles (Little 1979) that could come into contact with gecko feet during climbing. Indeed, insects must cope with particulate contamination that reduces the function of their adhesive pads (Gorb and Gorb 2002) and spend a significant proportion of their time grooming (Stork 1983) in order to restore function. On the other hand, geckos have not been observed to groom their feet (Russell and Rosenberg 1981), yet apparently retain the adhesive ability of their setae during the months between shed cycles. How geckos manage to keep their toes dean while walking about with sticky feet has remained a puzzle until recently (Hansen and Autumn 2005). While self-cleaning by water droplets has been shown to occur in plant (Barthlott and Neinhuis 1997) and animal (Baum et al. 2002) surfaces, no adhesive had been shown to self-clean.
Gecko setae are the first known self-cleaning adhesive (Hansen and Autumn 2005). Tokay geckos with 2.5-μm-radius microspheres applied to their feet recovered their ability to cling to vertical surfaces after only a few steps on clean glass. Hansen and Autumn contaminated toes on one side of the animal with an excess of 2.5-μm-radius silica-alumina microspheres and compared the shear stress produced to that of uncontaminated toes on the other side of the animal. Prior researchers had suggested that geckos’ unique toe peeling motion (digital hyperextension) might aid in cleaning of the toe pads (Russell 1979; Bauer et al. 1996; Hu et al. 2012; Xu et al. 2015), so the geckos’ toes were immobilized and applied by hand to the surface of a glass force plate to determine if self-cleaning could occur without toe peeling. After only four simulated steps on a clean glass surface, the geckos recovered enough of their setal function to support their body weight by a single toe. To test the hypothesis that self-cleaning is an intrinsic property of gecko setae and does not require a gecko, Hansen and Autumn isolated arrays of setae, glued them to plastic strips, and simulated steps using a servomanipulation system called RoboToe. The shear stress in clean setal arrays was compared to that in the same arrays with a monolayer of microspheres applied to their adhesive surfaces. Self-cleaning of microspheres occurred in arrays of setae isolated from the gecko. Again as for live gecko toes, isolated setal arrays rapidly recovered the shear force lost due to contamination by microspheres. Presumably, the microspheres were being preferentially deposited on the glass substrate and did not remain strongly attached to the setae.
Contact mechanical models suggest that it is possible that self-cleaning occurs by an energetic disequilibrium between the adhesive forces attracting a dirt particle to the substrate and those attracting the same particle to one or more spatulae (Fig. 11.3) (Hansen and Autumn 2005). The models suggest that self-cleaning may in fact require γ of spatulae to be relatively low (equal to or less than that of the wall), perhaps constraining the spatula to be made of a hydrophobic material. So, geckos may benefit by having setae made of an antiadhesive material: decreasing γ decreases adhesion energy of each spatula, but promoting self-cleaning should increase adhesion of the array as a whole by maximizing the number of uncontaminated spatulae. If γ were to be increased by supplementing the vdW forces with stronger intermolecular forces such as polar or H-bonding types, it is likely that self-cleaning and anti-self-fouling properties would be lost. Thus the self-cleaning and anti-self-fouling properties may represent a sweet spot in the evolutionary design space for adhesive nanostructures. Additionally, the rate dependence of friction and adhesion (Gravish et al. 2010; Puthoff et al. 2013) influences self-cleaning (Xu et al. 2015). As the sliding and detachment velocity are parameters an animal can ideally control, the capability to actively clean the scansor surfaces via a dynamical process is present.
Model of self-cleaning in gecko setae from Hansen and Autumn (2005). If we model spatula as nanoscale strips of adhesive tape (Kendall 1975) that peel during detachment, the particle-spatula pull off force is given by F = 2R s γ ps, where γ ps is the adhesion energy at the particle-spatula interface and 2R is the width of the spatula, assuming negligible elastic energy storage. The pull off force of the dirt particle from a planar wall, using the JKR model (Johnson et al. 1971), is F pw = (3/2)πR p γ pw, where γ ps is the adhesion energy of the particle to the wall. N represents the number of spatulae attached simultaneously to each dirt particle to achieve energetic equilibrium
11.3.2 Property (8): Nonsticky Default State
The discovery that maximal adhesion in isolated setae requires a small push perpendicular to the surface, followed by a small parallel drag (Autumn et al. 2000), explained the load dependence and directionality of adhesion observed at the whole-animal scale by Haase (1900) and Dellit (1934) and was consistent with the structure of individual setae and spatulae (Ruibal and Ernst 1965; Hiller 1968). In their resting state, setal stalks are recurved proximally. When the toes of the gecko are planted, the setae may become bent out of this resting state, flattening the stalks between the toe and the substrate such that their tips point distally. This small preload and a micron-scale displacement of the toe or scansor proximally may serve to bring the spatulae (previously in a variety of orientations) uniformly flush with the substrate, maximizing their surface area of contact. Adhesion results and the setae are ready to bear the load of the animal’s body weight.
To test the hypothesis that the default state of gecko setal arrays is to be nonsticky, Autumn and Hansen (2006) estimated the fraction of area able to make contact with a surface in setae in their unloaded state. Only less than 7 % of the area at the tip of a seta is available for initial contact with a smooth surface, and 93 % is empty air. This suggests that, initially, during a gecko’s foot placement, the contact fraction of the distal region of the setal array must be very low. Yet, the dynamics of the foot must be sufficient to increase the contact fraction substantially to achieve the extraordinary values of adhesion and friction that have been measured in whole animals (Autumn et al. 2002b; Hansen and Autumn 2005; Irschick et al. 1996) and isolated setae (Autumn et al. 2000, 2002b; Hansen and Autumn 2005). Thus gecko setae may be nonsticky by default because only a very small contact fraction is possible without mechanically deforming the setal array.
How much does the contact fraction increase during attachment? While there are no empirical measurements of the number of spatulae in contact as a function of adhesion (or friction) force, it is possible to estimate from measurements of single setae. Empirical measurements and theoretical estimates of spatular adhesion (Autumn et al. 2000, 2002b; Arzt et al. 2003; Huber et al. 2005a; Spolenak et al. 2005b; Hansen and Autumn 2005) suggest that each spatula generates 10–40 nN with an approximate area of 0.02 μm2; this gives an adhesive stress of 500–2000 kPa. A single seta on a Si MEMs cantilever can generate approximately 920 kPa (Table 11.1). The value of 10 nN of adhesion measured in single spatulae using an AFM (Huber et al. 2005a) implies that 4000 spatulae would need to be attached to equal the peak adhesion force (40 μN) measured in single setae (Autumn et al. 2002b). However, each seta contains not more than 1000 spatulae (Ruibal and Ernst 1965; Schleich and Kästle 1986). Therefore, a spatular force of 40 nN corresponds to a conservative estimate of setal contact fraction during attachment. In the case of a spatular adhesion force of 40 nN, the adhesive stress is 2000 kPa; a contact fraction of 46 % is required to yield this stress. This suggests that, unless the force of adhesion of a spatula has been greatly underestimated, the contact fraction must increase from 6 % to 46 %, or by approximately 7.5-fold, following preload and drag.
11.4 Modeling Adhesive Nanostructures
11.4.1 Effective Modulus of a Setal Array
The gecko adhesive is a microstructure in the form of an array of millions of high-aspect-ratio shafts. The effective elastic modulus of this array (E eff) (Persson 2003; Sitti and Fearing 2003) is much lower than Young’s modulus (E) of β-keratin. Thus, arrays of setae should behave as a softer material than bulk β-keratin. The modulus of β-keratin in tension is approx. 2.5 GPa in bird feathers (Bonser and Purslow 1995) and 1.3–1.8 GPa in bird claws (Bonser 2000). Young’s moduli of lizard β-keratins in general (Fraser and Parry 1996) and gecko β-keratins in particular (Alibardi 2003) remain unknown at present. The behavior of a setal array during compression and subsequent relaxation will depend on the mode(s) of deformation of individual setae. Bending is a likely mode of deformation (Simmermacher 1884) (Fig. 11.1d), and a simple approach is to model arrays of setae as arrays of cantilever beams (Persson 2003; Sitti and Fearing 2003; Glassmaker et al. 2004; Hui et al. 2004; Spolenak et al. 2005a; Autumn et al. 2006c; Pesika et al. 2009a). One might question the applicability of models based on a simple geometry for the complex, branched structure of the seta. However, as with the JKR and Kendall models (Sects. 11.2.5.4 and 11.2.5.5) applied to spatulae, the simple cantilever model is surprisingly well supported by empirical measurements of setal arrays (Geisler et al. 2005; Autumn et al. 2006c).
The effective modulus of an array of vertical fibers depends on parameters such as the material, geometric properties, and density of the fibers (Campolo et al. 2003; Sitti and Fearing 2003). A single setal stack is approximately a cantilever beam subject to a lateral load (F) at its tip. The resulting tip displacement due to bending is Δ = FL 3/3EI, where L is the length, E is the elastic modulus of the material, and I = πR 4/4 is the area moment of inertia of the (cylindrical) cantilever (Timoshenko and Gere 1984) (Fig. 11.4a). For a cantilever at an angle ϕ to the substrate and under a normal load F n, the resolved force lateral to the cantilever is F = F n cos ϕ (Fig. 11.4b). This results in a lateral tip displacement of Δ = F n L 3 cos (ϕ)/3EI and a normal tip displacement of Δ n = Δ cos ϕ = F n L 3 cos2 (ϕ)/3EI.
For a system with an effective modulus, Hooke’s law is
where σ is the stress applied perpendicularly to the setal array and ε is the resulting perpendicular strain. For an array with setal density ρ, the stress is F n ρ, and the strain is ε = Δ n/(L sin ϕ). Expanding and substituting these equations into Eq. (11.1) gives
A typical tokay setal array has approx. 14,000 setae per mm2 (ρ = 1.44 × 1010 m–2), so the shaft angle ϕ (Fig. 11.4b) required to yield an effective modulus of 100 kPa (the upper limit of Dahlquist’s criterion; see Sect 11.6) is ϕ = 50° for E = 1.0 GPa and ϕ = 36.65° for E = 2.0 GPa.
We measured the forces resulting from deformation of isolated arrays of tokay gecko setae to determine E eff and tested the validity of the cantilever model. We found that E eff of tokay gecko setae falls near 100 kPa, close to the upper limit of Dahlquist’s criterion for tack (Fig. 11.5) (Geisler et al. 2005; Autumn et al. 2006c). Additionally, we observed values of ϕ for tokay gecko setae near 43°, further supporting the validity of the cantilever model (Fig. 11.4).
Young’s modulus (E) of various materials, including the approximate values of bulk β-keratin and the effective modulus (E eff) of natural setal arrays. A value of E ~ 100 kPa (measured at 1 Hz) is the upper limit of the Dahlquist criterion for tack, which is based on empirical observations of PSAs (Dahlquist 1969; Pocius 2012). A cantilever beam model (Eq. 11.2) predicts a value of E eff near 100 kPa, as observed for natural setae and PSAs. It is notable that geckos have evolved E eff close to the limit of tack. This value of E eff may be tuned to allow strong and rapid adhesion, yet prevent spontaneous or inappropriate attachment
11.4.2 Rough Surface and Antimatting Conditions
Adhesion force has been shown to depend on the roughness of the substrate at the seta level (Huber et al. 2007) and the toe level (Pugno and Lepore 2008; Gillies et al. 2013; Stark et al. 2015b). The cantilever model predicts that a high density of setae should be selected for in increasing adhesive force of setal arrays. First, it follows from the JKR model (Autumn et al. 2002b; Arzt et al. 2003) that packing in more spatulae should increase adhesion in an array of setae. Second, the cantilever model suggests that thinner setal shafts should decrease E eff and promote a greater contact fraction on rough surfaces (Stork 1983; Scherge and Gorb 2001; Jagota and Bennison 2002; Campolo et al. 2003; Persson 2003; Persson and Gorb 2003; Sitti and Fearing 2003; Spolenak et al. 2005a). The cantilever model also suggests that longer and softer setal shafts, and a lower shaft angle ϕ, will result in better adhesion on rough surfaces because these parameters will reduce E eff. On a randomly rough surface, some setal shafts should be bent in compression (concave), while others will be bent in tension (convex). The total force required to pull off a setal array from a rough surface should therefore be determined by the cumulative adhesive force of all the attached spatulae, minus the sum of the forces due to elastic deformation of compressed setal shafts.
If setae mat together (Stork 1983), it is likely that adhesive function will be compromised. Interestingly, the same parameters that promote strong adhesion on rough surfaces should also cause matting of adjacent setae (Persson 2003; Sitti and Fearing 2003; Glassmaker et al. 2004; Hui et al. 2004; Spolenak et al. 2005a). The distance between setae and the stiffness of the shafts will determine the amount of force required to bring the tips together for matting to occur. It follows from the cantilever model that stiffer, shorter, and thicker stalks will allow a greater packing density without matting. As is the case for self-cleaning (Hansen and Autumn 2005), setae should be made of materials with lower surface energy to prevent self-adhesion and matting. Satisfying both antimatting and rough surface conditions may require a compromise of design parameters. Spolenak et al. (2005a) devised “design maps” for setal adhesive structures, an elegant approach to visualizing the parametric tradeoffs needed to satisfy the rough surface and antimatting conditions while at the same time maintaining structural integrity of the material.
11.5 Scaling
Small and large organisms are dominated by different forces (McMahon and Bonner 1983). Inertial forces usually dwarf adhesive forces in organisms gecko-size and above. Geckos are unusual among macroscale organisms in having adhesive forces dominate their world. The astonishing adaptive radiation in geckos and their unique ecologies can be seen as an emergent property of integration across seven orders of magnitude in size (Pianka and Sweet 2005)—from the nanoscale spatula and the microscale seta to the mesoscale scansors and the macroscale body (Fig. 11.1).
While it is tempting to focus on the smallest level in the gecko adhesive system, integration of multiple levels in the compliance hierarchy is needed to achieve reliable and controllable adhesion and friction. Self-cleaning adhesive nanostructures cannot adhere if they never get near the surface. Compliant scansors and the compliant adipose or vascular tissue underlying the scansors may be important in spreading the load during foot placement (Russell 1986, 2002). The complex morphology and musculature of the toes, feet, and limbs play a critical role in bringing the compliant scansors to bear upon the substrate in the appropriate manner and in detaching them without large forces (Russell 1975). Simulation studies of animal-like climbers suggest that tuning limb compliance correctly is much more important for climbing than for running. In particular, the ratios of linear and torsional compliances at the foot and ankle have an enormous effect on climbing stability and efficacy (Autumn et al. 2005).
11.5.1 Scaling of Pad Area and Spatular Size
Shear force of the two front feet of pad-bearing lizards (geckos, anoles, and skinks) is highly correlated with pad area, even when the effects of body size and phylogeny are accounted for (Irschick et al. 1996). However, there is significant variation in shear force among taxa of similar size and pad area, suggesting that other factors are important in determining the strength of the setal adhesive. The JKR model (Autumn et al. 2002b; Arzt et al. 2003) predicts that larger spatulae should result in lower forces; this is supported by an inverse correlation between body mass and the size of the spatula or setal tip in lizards and arthropods (Arzt et al. 2003), but Peattie and Full (Peattie and Full 2007) rejected this correlation using a phylogenetically independent contrasts across broad sample of lizards and arthropods.
11.5.2 Scaling of Stress
Amontons’ first law states that the relationship of shear force (friction) to load is a constant value, μ (the coefficient of friction). Amontons’ second law predicts that μ is independent of the area of contact (Bhushan 2013; Ringlein and Robbins 2004). When pulled in shear (Autumn et al. 2000, 2002b), gecko setae seem to violate Amontons’ laws, as do tacky polymers where the forces of adhesion can be much greater than the external load. Shear stress in setae increases greatly with a decrease in contact area, suggesting that at larger scales, fewer spatulae are attached and/or the contact fraction within spatulae is reduced (Fig. 11.6; Table 11.1). The scaling of shear stress (τ) is exponential and scales as log τ = 1.14 – 0.24 log (area). It is unknown whether stress is uniformly spread across the toe or foot (Russell 2002), or if there are high stress concentrations on the setal arrays of a few scansors. The force of only 2 % of setae, and only 25 % of setal arrays, is required to yield the maximum shear stresses measured at the whole-animal level. However, at the setal level, it appears that most spatulae must be strongly attached to account for theoretical and empirical values of adhesion, suggesting that the seta is highly effective at making contact with a smooth surface. If each spatula can generate 10–40 nN, it would take 1000–4000 spatulae to yield the 40 μN of adhesion measured in single setae. However, each seta bears only 100–1000 spatulae. Clearly further work is needed to resolve this discrepancy. The relationship between adhesion and friction also demands further investigation. Existing data suggest that friction at the seta level is about two to four times the adhesion.
Stress vs. area in the gecko adhesive hierarchy (see Table 11.1 for numerical values and literature sources). JKR and Kendall model predictions for spatular adhesive stress (triangles) bound the measured value of Huber et al. (2005a). Text below the area axis shows the associated level in the gecko adhesive hierarchy (Fig. 11.1)
11.6 Comparison of Conventional and Gecko Adhesives
Conventional adhesives are materials that are used to join two surfaces. Typically, adhesives are liquids that are chemically compatible with both surfaces and have sufficiently low viscosity that wetting of the surfaces occurs either spontaneously or with a small amount of pressure (Baier et al. 1968; Kinloch 1987; Pocius 2012). Surface treatments are often needed to raise the interfacial energies between one or both surfaces and the adhesive. Liquid hard-set adhesives (e.g., epoxy or cyanoacrylate glues) flow easily during application, but cure to make a strong, permanent bond. Because they are stiff when cured, hard-set adhesives can resist plastic creep caused by sustained loading. However, hard-set adhesives are single use: their bonds must be broken or dissolved for removal, and once broken, hard-set adhesives do not rebond.
Conventional PSAs are fabricated from soft, tacky, viscoelastic materials (Gay and Leibler 1999; Gay 2002; Pocius 2012). Tacky materials are those that exhibit spontaneous plastic deformation that increases true area of contact with the surface at the molecular scale. Theoretical considerations (Creton and Liebler 1996) agree with Dahlquist’s (1969; Pocius 2012) empirical observation that Young’s modulus below 100 kPa (at 1 Hz) is needed to achieve a high contact fraction with the substrate. Additives known as tackifiers are commonly used to promote plastic deformation in PSAs during contact (Pocius 2012). PSAs such as masking tape or sticky notes are capable of repeated attachment and detachment cycles without residue because the dominant mechanism of adhesion is weak intermolecular forces. PSAs adhering with weak intermolecular forces can require much more energy to pull off of surfaces than do rigid adhesives relying on strong chemical bonds. As soft polymeric adhesives are pulled apart from a surface, polymer chains or bundles of polymer chains can be elongated into pillars in a process known as crazing. The total fracture energy can greatly exceed the sum of all the bond energies at the interface since work must be done on the craze as well as to break adhesive bonds at the interface. Thus the strong adhesion in polymeric adhesives results from long bonds rather than from strong bonds (Persson 2003). However, because they are soft polymeric materials, PSAs are prone to creep, degradation, self-adhesion, and fouling.
In contrast to the soft polymers of PSAs, the adhesive on the toes of geckos is made of hard protein (β-keratin) with values of E four to five orders of magnitude greater than the upper limit of Dahlquist’s criterion. Therefore, one would not expect a β-keratin structure to function as a PSA by readily deforming to make intimate molecular contact with a variety of surface profiles. However, since the gecko adhesive is a microstructure in the form of an array of millions of high-aspect-ratio shafts (setae), the effective elastic modulus, E eff (Jagota and Bennison 2002; Persson 2003; Sitti and Fearing 2003; Glassmaker et al. 2004; Hui et al. 2004; Spolenak et al. 2005a), is much lower than the value of E of bulk β-keratin. The effective modulus of gecko setal arrays is close to 100 kPa (Geisler et al. 2005; Autumn et al. 2006c). Gecko setal arrays possess some of the properties of PSAs although the bulk material properties of β-keratin place it in the class of stiff, nonviscous materials (Fig. 11.5) (Dahlquist 1969; Creton and Liebler 1996; Gay and Leibler 1999; Gay 2002; Jagota and Bennison 2002; Persson 2003; Persson and Gorb 2003; Sitti and Fearing 2003; Pocius 2012).
There is emerging evidence that an array of gecko setae can act like a tacky, deformable material, while individual setae and spatulae retain the structural integrity of stiff protein fibers. This may enable the gecko adhesive to tolerate heavy, repeated use without creep or degradation. Indeed, theoretical considerations suggest that the fibrillar structure of the gecko adhesive can be thought of as a permanent craze (Jagota and Bennison 2002; Persson 2003) that has a higher fracture energy than a solid layer of adhesive material. As with polymer crazes, setal structures under stress could store energy elastically in each seta of the array, and then as setae are pulled off, elastic energy could be dissipated internally without contributing to propagation of the crack between the adhesive and substrate (Hui et al. 2004; Jagota and Bennison 2002; Persson 2003). Unlike polymer crazes, setal structures may dissipate energy primarily elastically rather than plastically. However, measurements of isolated gecko setae during detachment suggest that the high fracture toughness of the setal interface is not due to elastic losses, but rather to frictional losses (Gravish et al. 2008). As setal arrays are pulled away from a surface, micron-scale sliding increases the net detachment energy.
Gecko setae do not bond spontaneously on contact, as do PSAs. Gecko setae have a nonsticky default state (Autumn and Hansen 2006) and require mechanical deformation to initiate adhesion and friction (Autumn et al. 2000; Autumn and Peattie 2002). Again in contrast to PSAs, gecko setae are anisotropic and possess a built-in release mechanism. Setae are sticky when forces are directed with the curvature of the shaft and released when forces are directed away from the curvature of the shaft (Autumn et al. 2000; Autumn and Peattie 2002; Gao et al. 2005).
11.7 Gecko-Inspired Synthetic Adhesive Nanostructures
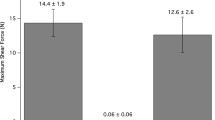
Using a nanostructure to create an adhesive is a novel and bizarre concept. It is possible that if it had not evolved, humans would never have invented it. With the inspiration of biology, materials incorporating adhesive nanostructures are being developed (Fig. 11.1f). The growing list of benchmark properties—eight of which are presented in this chapter—can be used to evaluate the degree of geckolike function of synthetic prototypes. Early synthetic setae (Autumn et al. 2002b; Geim et al. 2003; Sitti and Fearing 2003; Peressadko and Gorb 2004; Northen and Turner 2005), while functional in some sense, possessed few truly geckolike properties. For example, consider the adhesion coefficient μ′ = F adhesion/F preload as a metric for geckolike adhesive function. By this criterion, the material of Geim et al. (Geim et al. 2003) is not geckolike since it required a very large preload of 50 N to yield 3 N and 0.3 atm of adhesion, yielding μ′ = 0.06. The synthetic setae of Northen and Turner (Northen and Turner 2005) perform significantly better with μ′ = 0.125, but still well below the benchmark of real gecko setae with μ′ = 8–16.
Since the development of these early prototype materials, implementation of numerous gecko-inspired synthetic adhesives has been documented in the literature (Kwak et al. 2011). Frequently, these materials exceed the capabilities of the natural system for a single property, such as self-cleaning capabilities (Lee and Fearing 2008; Lee and Bhushan 2012; Abusomwan and Sitti 2014) or surface roughness tolerance (Lee et al. 2009), but fall short in replicating the combination of properties to any degree. Developing materials that exhibit all eight benchmark functional properties of natural gecko adhesives will require considerable theoretical, experimental, and materials-design work.
Applications abound for a dry self-cleaning adhesive that does not rely on soft polymers or chemical bonds. Biomedical applications such as endoscopy and tissue adhesives (Pain 2000; Menciassi and Dario 2003) are one example. However, any materials chosen for synthetic setae in biomedical applications would need to be nontoxic and nonirritating (Baier et al. 1968). Other applications include MEMS switching (Decuzzi and Srolovitz 2004), wafer alignment (Slocum and Weber 2003), micromanipulation (Pain 2000; Jeong et al. 2014), and robotics (Autumn et al. 2005; Daltorio et al. 2007; Kim et al. 2008). Since a nanostructure could be applied directly to a surface, it is conceivable that geckolike structures could replace screws, glues, and interlocking tabs in many assembly applications such as automobile dashboards or mobile phones.
Sports applications such as fumble-free football gloves or rock climbing aids (Irving 1955; Hawkes et al. 2014) could be revolutionary. Using gecko technology to climb is not a new idea. In a seventeenth-century Indian legend, Shivaji and his Hindu warriors used adhesive lizards from the Deccan region as grappling devices to scale a shear rock cliff and mount a surprise attack on a Maharashtrian clifftop stronghold (Ghandi 2002).
11.8 Future Directions in the Study of the Gecko Adhesive System
Adhesion in geckos remains a sticky problem that is generating at least as many new questions as answers. Much of the fertility of this area stems from an integration of biology, physics, and engineering (Autumn et al. 2014). For example, the relationship between friction and adhesion is one of the most fundamental issues in surface science (Ringlein and Robbins 2004; Luan and Robbins 2005). One of the most striking properties (Table 11.2) of the gecko adhesive system is the coupling between adhesion and friction. Without a shear load, setae detach easily. Indeed, without shear loading of opposing toes or legs, a gecko could not hang from the ceiling. Integration of the macroscale system with the as yet undefined relationship between friction and adhesion at the nanoscale could yield important design principles for natural and synthetic setal structures.
Natural surfaces are rarely smooth, and an important next step will be to measure empirically the effect of surface roughness (Vanhooydonck et al. 2005) on friction and adhesion in gecko setae to test the predictions of the new generation of theoretical models for rough surface contacts with micro- and nanostructures (Persson and Gorb 2003). Under real-world conditions where surfaces are fractal (Greenwood 1992; Persson and Gorb 2003), compliance is required at each level of the gecko adhesive hierarchy: spatula, seta, lamella, toe, and leg. Models including a spatular array at the tip of a seta have not yet been developed. Similarly, models of lamellar structure will be needed to explain function on roughness above the micron scale.
Biological diversity of setal and spatular structure is high and poorly documented, though some advances in the arena have been made (Hagey et al. 2014). Basic morphological description will be required. Theory predicts that tip shape affects pull off force less at smaller sizes (Gao and Yao 2004), so it is possible that part of spatular variation is due to phylogenetic effects, but material constraints such as tensile strength of β-keratin must be considered as well (Autumn et al. 2002b; Spolenak et al. 2005a; Puthoff et al. 2010; Prowse et al. 2011). The collective behavior of the setal array will be a productive research topic (Gao and Yao 2004). Diversity of the array parameters, density, dimension, and shape is great but not well documented. In particular, the shape of setal arrays on lamellae demands further investigation. Phylogenetic analysis (Harvey and Pagel 1991; Peattie and Full 2007) of the variation in setal structure and function will be required to tease apart the combined effects of evolutionary history, material constraints, and adaptation (Autumn et al. 2002a).
The molecular structure of setae is not yet known. Setae are made primarily of β-keratin, but a histidine-rich protein or proteins may be present as well (Alibardi 2003). One possible role of non-keratin proteins is as a glue that holds the keratin fibrils together in the seta (Fig. 11.1d) (Alibardi 2003; Rizzo et al. 2006). This suggests a possible role of genes coding for histidine-rich protein(s) in tuning the material properties of the setal shaft. The outer molecular groups responsible for adhesion at the spatular surface will also be an important topic for future research.
Clearly there is a great desire to engineer a material that functions like a gecko adhesive, yet progress has been limited. A biomimetic approach of attempting to copy gecko setae blindly is unlikely to succeed due to the complexity of the system (Fig. 11.1) and the fact that evolution generally produces satisfactory rather than optimal structures. Instead, development of biologically inspired adhesive nanostructures will require careful identification and choice of design principles (Table 11.2) to yield selected geckolike functional properties. As technology and the science of gecko adhesion advance, it may become possible to tune design parameters to modify functional properties in ways that have not evolved in nature.
It is remarkable that the study of a lizard is contributing to understanding the fundamental processes underlying adhesion and friction (Fakley 2001; Urbakh et al. 2004; Autumn et al. 2014) and providing biological inspiration for the design of novel adhesives and climbing robots. Indeed, the broad relevance and applications of the study of gecko adhesion underscore the importance of basic, curiosity-based research.
References
Abusomwan UA, Sitti M (2014) Mechanics of load-drag-unload contact cleaning of gecko-inspired fibrillar adhesives. Langmuir 30(40):11913–11918
Alibardi L (2003) Ultrastructural autoradiographic and immunocytochemical analysis of setae formation and keratinization in the digital pads of the gecko Hemidactylus turcicus (Gekkonidae, Reptilia). Tissue Cell 35(4):288–296
Altevogt R (1954) Probleme eines Fuβes. Kosmos. Gesellschaft der Naturfreunde (Stuttgart) 50:428–430
Aristotle (1910) Historia animalium. Translation by D’Arcy Thompson. The Clarendon Press, Oxford
Arzt E, Enders S, Gorb S (2002) Towards a micromechanical understanding of biological surface devices. Z Met 93(5):345–351
Arzt E, Gorb S, Spolenak R (2003) From micro to nano contacts in biological attachment devices. Proc Natl Acad Sci 100(19):10603–10606
Autumn K, Hansen W (2006) Ultrahydrophobicity indicates a non-adhesive default state in gecko setae. J Comp Physiol A 192(11):1205–1212
Autumn K, Peattie AM (2002) Mechanisms of adhesion in geckos. Integr Comp Biol 42(6):1081–1090
Autumn K, Liang YA, Tonia Hsieh S, Zesch W, Chan WP, Kenny TW, Fearing R, Full RJ (2000) Adhesive force of a single gecko foot-hair. Nature 405(6787):681–685
Autumn K, Ryan MJ, Wake DB (2002a) Integrating historical and mechanistic biology enhances the study of adaptation. Q Rev Biol 77(4):383–408
Autumn K, Sitti M, Liang YA, Peattie AM, Hansen WR, Sponberg S, Kenny TW, Fearing R, Isrealachvili JN, Full RJ (2002b) Evidence for van der Waals adhesion in gecko setae. Proc Natl Acad Sci 99(19):12252–12256
Autumn K, Buehler M, Cutkosky M, Fearing R, Full RJ, Goldman D, Groff R, Provancher W, Rizzi AA, Saranli U, Saunders A, Koditschek DE (2005) Robotics in Scansorial Environments. In: Gerhart GR, Shoemaker CM, Gage DW (eds) Proceedings of the SPIE vol. 5804: unmanned ground vehicle technology VII. SPIE, Bellingham, WA, pp 291–302
Autumn K, Dittmore A, Santos D, Spenko M, Cutkosky M (2006a) Frictional adhesion: a new angle on gecko attachment. J Exp Biol 209(18):3569–3579
Autumn K, Hsieh ST, Dudek DM, Chen J, Chitaphan C, Full RJ (2006b) Dynamics of geckos running vertically. J Exp Biol 209(2):260–272
Autumn K, Majidi C, Groff RE, Dittmore A, Fearing R (2006c) Effective elastic modulus of isolated gecko setal arrays. J Exp Biol 209(18):3558–3568
Autumn K, Niewiarowski PH, Puthoff JB (2014) Gecko adhesion as a model system for integrative biology, interdisciplinary science, and bioinspired engineering. Annu Rev Ecol Evol Syst 45:445–470
Baier RE, Shafrin EG, Zisman WA (1968) Adhesion: mechanisms that assist or impede it. Science 162(3860):1360–1368
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202(1):1–8
Bauer AM (1998) Morphology of the adhesive tail tips of carphodactyline geckos (Reptilia: Diplodactylidae). J Morphol 235(1):41–58
Bauer AM, Russell AP, Powell GL (1996) The evolution of locomotor morphology in Rhoptropus (Squamata: Gekkonidae): Functional and phylogenetic considerations. Afr J Herpetol 45(1):8–30
Baum C, Meyer W, Stelzer R, Fleischer L-G, Siebers D (2002) Average nanorough skin surface of the pilot whale (Globicephala melas, Delphinidae): considerations on the self-cleaning abilities based on nanoroughness. Mar Biol 140(3):653–657
Baumberger T, Berthoud P, Caroli C (1999) Physical analysis of the state- and rate-dependent friction law. II Dynamic friction. Phys Rev B 60(6):3928–3939
Bellairs A (1970) The life of reptiles. Universe Books, New York
Bhushan B (2013) Introduction to tribology. Wiley, New York
Biewener AA, Full RJ (1992) Force platform and kinematic analysis. In: Biewener AA (ed) Biomechanics: structures and systems: a practical approach. IRL Press at Oxford University Press, Oxford, pp 45–73
Blackwall J (1845) On the means by which walk various animals on the vertical surface of polished bodies. Ann Mag Nat Hist Ser 1 15(96):115–119
Bonser RHC (2000) The Young’s modulus of ostrich claw keratin. J Mater Sci Lett 19(12):1039–1040
Bonser RHC, Purslow PP (1995) The Young’s modulus of feather keratin. J Exp Biol 198(4):1029–1033
Brainerd EL (1994) Adhesion force of ants on smooth surfaces. Am Zool 34(5):128A (Abstract from 1994 American Society of Zoologists Annual Meeting.)
Braun M (1878) Zur Bedeutung der Cuticularborsten auf den Haftlappen der Geckotiden. Arbeiten aus dem Zoologisch-Zootomischen Institut in Würzburg 4:231–237
Brörmann K, Barel I, Urbakh M, Bennewitz R (2013) Friction on a microstructured elastomer surface. Tribol Lett 50(1):3–15
Campolo D, Jones S, Fearing RS (2003) Fabrication of gecko foot-hair like nano structures and adhesion to random rough surfaces. In: Proceedings of the third IEEE conference on nanotechnology, vol. 2. IEEE, Los Alamitos, pp 856–859
Cartier O (1872) Studien über den feineren Bau der Epidermis bei den Geckotiden. Verhandlungen der Physikalisch-medincinischen Gesellschaft zu Würzburg 3:7–22
Cartier O (1874a) Studien über den feineren Bau der Haut bei Reptilien. I Die Epidermis der Geckotiden Arbeiten aus dem. Zoologisch-Zootomischen Institut in Würzburg 1:83–96
Cartier O (1874b) Studien über den feineren Bau der Haut bei Reptilien. II Ueber die Wachsthumserscheinimgen der Oberhaut von Schlangen und Eidechsen bei der Häutung Arbeiten aus dem. Zoologisch-Zootomischen Institut in Würzburg 1:239–258
Chen B, Gao H (2010) An alternative explanation of the effect of humidity in gecko adhesion: stiffness reduction enhances adhesion on a rough surface. Int J Appl Mech 2(1):1–9
Chen JJ, Peattie AM, Autumn K, Full RJ (2006) Differential leg function in a sprawled-posture quadrupedal trotter. J Exp Biol 209(2):249–259
Chen B, Wu P, Gao H (2009) Pre-tension generates strongly reversible adhesion of a spatula pad on substrate. J R Soc Interface 6(35):529–537
Chui BW, Kenny TW, Mamin HJ, Terris BD, Rugar D (1998) Independent detection of vertical and lateral forces with a sidewall-implanted dual-axis piezoresistive cantilever. Appl Phys Lett 72(11):1388–1391
Creton C, Liebler L (1996) How does tack depend on contact time and contact pressure? J Polym Sci B Polym Phys 34(3):545–554
Dahlquist CA (1969) Pressure-sensitive adhesives. In: Patrick RL (ed) Treatise on adhesion and adhesives, vol 2. Materials. Dekker, New York, pp 219–260
Daltorio KA, Gorb S, Peressadko A, Horchler AD, Wei TE, Ritzmann RE, Quinn RD (2007) Microstructured polymer adhesive feet for climbing robots. MRS Bull 32(6):504–508
Decuzzi P, Srolovitz DJ (2004) Scaling laws for opening partially adhered contacts in MEMS. J Microelectromech Syst 13(2):377–385
Delaugerre M, Alain G, Leoncini A (2015) One island, two geckos and some powder. Why and how a colonization process can fail? In: X Congresso Nazionale della Societas Herpetologica Italica. Societas Herpetologica Italica, Pavia, Italy, pp 117–121
Dellit W-D (1934) Zur anatomie und physiologie der Geckozehe. Jenaische Zeitschrift für Naturwissenschaft 68:613–656
Dewitz H (1882) Wie ist es den Stubenfliegen und vielen anderen Insecten möglich, an senkrechten Glaswänden emporzulaufen? Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin 17(1):5–7
Edwards JS, Tarkanian M (1970) The adhesive pads of Heteroptera: a re-examination. Proc R Entomol Soc Lond A Gen Entomol 45(1-3):1–5
Emerson SB, Diehl D (1980) Toe pad morphology and mechanisms of sticking in frogs. Biol J Linn Soc 13(3):199–216
Fakley M (2001) Smart adhesives. Chem Ind Mag:691–695
Fraser RDB, Parry DAD (1996) The molecular structure of reptilian keratin. Int J Biol Macromol 19(3):207–211
Gadow H (1901) Amphibia and reptiles. Cambridge natural history, vol 8. Macmillan & Co., Ltd., London
Gao H, Yao H (2004) Shape insensitive optimal adhesion of nanoscale fibrillar structures. Proc Natl Acad Sci 101(21):7851–7856
Gao H, Wang X, Yao H, Gorb S, Arzt E (2005) Mechanics of hierarchical adhesion structures of geckos. Mech Mater 37(2-3):275–285
Gay C (2002) Stickiness—some fundamentals of adhesion. Integr Comp Biol 42(6):1123–1126
Gay C, Leibler L (1999) Theory of tackiness. Phys Rev Lett 82(5):936–940
Geim AK, Dubonos SV, Grigorieva IV, Novoselov KS, Zhukov AA, Shapoval SY (2003) Microfabricated adhesive mimicking gecko foot-hair. Nat Mater 2(7):461–463
Geisler B, Dittmore A, Gallery B, Stratton T, Fearing R, Autumn K (2005) Deformation of isolated gecko setal arrays: bending or buckling? 2. Kinetics. Society for Integrative and Comparative Biology, San Diego (Abstract from 2005 Society for Integrative and Comparative Biology Annual Meeting)
Gennaro JG Jr (1969) The gecko grip. Nat Hist (The Journal of The American Museum of Natural History) 78(7):36–43
Ghandi M (2002) The ughly buglies. Swagat Mag Media Transasia, Bangalore
Gillett JD, Wigglesworth VB (1932) The climbing organ of an insect, Rhodnius prolixus (Hemiptera; Reduviidae). Proc R Soc B 111(772):364–376
Gillies AG, Henry A, Lin H, Ren A, Shiuan K, Fearing RS, Full RJ (2013) Gecko toe and lamellar shear adhesion on macroscopic, engineered rough surfaces. J Exp Biol 217(2):283–289
Glassmaker JN, Jagota A, Hui C-Y, Kim J (2004) Design of biomimetic fibrillar interfaces: 1. Making contact. J R Soc Interface 1(1):23–33
Gorb EV, Gorb SN (2002) Attachment ability of the beetle Chrysolina fastuosa on various plant surfaces. Entomol Exp Appl 105(1):13–28
Gravish N, Wilkinson M, Autumn K (2008) Frictional and elastic energy in gecko adhesive detachment. J R Soc Interface 5(20):339–348
Gravish N, Wilkinson M, Sponberg S, Parness A, Esparza N, Soto D, Yamaguchi T, Broide M, Cutkosky M, Creton C, Autumn K (2010) Rate-dependent frictional adhesion in natural and synthetic gecko setae. J R Soc Interface 7(43):259–269
Green DM (1981) Adhesion and the toe-pads of treefrogs. Copeia 1981(4):790–796
Greenwood JA (1992) Problems with surface roughness. In: Singer IL, Pollock HM (eds) Fundamentals of friction: macroscopic and microscopic processes, Volume 220 of the NATO ASI Series. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 57–76
Haase A (1900) Untersuchungen über den Bau und die Entwicklung der Haftlappen bei den Geckotiden. Archiv für Naturgeschichte 1–2(2):321–346
Hagey TJ, Puthoff JB, Holbrook M, Harmon LJ, Autumn K (2014) Variation in setal micromechanics and performance of two gecko species. Zoomorphology 133(2):111–126
Han D, Zhou K, Bauer AM (2004) Phylogenetic relationships among gekkotan lizards inferred from C-mos nuclear DNA sequences and a new classification of the Gekkota. Biol J Linn Soc 83(3):353–368
Hanna G, Jon W, Jon Barnes WP (1991) Adhesion and detachment of the toe pads of tree frogs. J Exp Biol 155(1):103–125
Hansen WR, Autumn K (2005) Evidence for self-cleaning in gecko setae. Proc Natl Acad Sci 102(2):385–389
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford series in ecology and evolution. Oxford University Press, Oxford, UK
Hawkes EW, Eason EV, Christensen DL, Cutkosky MR (2014) Human climbing with efficiently scaled gecko-inspired dry adhesives. J R Soc Interface 12(102):20140675
Hecht MK (1952) Natural selection in the lizard genus Aristelliger. Evolution 6(1):112–124
Hepworth J (1854) On the structure of the foot of the fly. J Cell Sci s1–2(7):158–163
Hiller U (1968) Untersuchungen zum Feinbau und zur Funktion der Haftborsten von Reptilien. Zeitschrift für Morphologie der Tiere 62(4):307–362
Hiller U (1969) Correlation between corona-discharge of polyethylene films and the adhering power of Tarentola M. mauritanica (Rept.). Forma et Functio 1:350–352
Hiller U (1971) Form und Funktion der Hautsinnesorgane bei Gekkoniden. Forma et Functio 4:240–253
Hiller U (1976) Comparative studies on the functional morphology of two gekkonid lizards. J Bombay Nat Hist Soc 73(2):278–282
Hora SL (1924) The adhesive apparatus on the toes of certain geckos and tree frogs. J Proc Asiat Soc Bengal 9:137–145
Hu C, Alex Greaney P (2014) Role of seta angle and flexibility in the gecko adhesion mechanism. J Appl Phys 116(7):074302
Hu S, Lopez S, Niewiarowski PH, Xia Z (2012) Dynamic self-cleaning in gecko setae via digital hyperextension. J R Soc Interface 9(76):2781–2790
Huber G, Gorb SN, Spolenak R, Arzt E (2005a) Resolving the nanoscale adhesion of individual gecko spatulae by atomic force microscopy. Biol Lett 1(1):2–4
Huber G, Mantz H, Spolenak R, Mecke K, Jacobs K, Gorb SN, Artz E (2005b) Evidence for capillary contributions to gecko adhesion from single spatula nanomechanical measurements. Proc Natl Acad Sci 102(45):16293–16296
Huber G, Gorb SN, Hosoda N, Spolenak R, Arzt E (2007) Influence of surface roughness on gecko adhesion. Acta Biomater 3(4):607–610
Hui C-Y, Glassmaker NJ, Tang T, Jagota A (2004) Design of biomimetic fibrillar interfaces: 2. Mechanics of enhanced adhesion. J R Soc Interface 1(1):35–48
Irschick DJ, Austin CC, Petren K, Fisher RN, Losos JB, Ellers O (1996) A comparative analysis of clinging ability among pad-bearing lizards. Biol J Linn Soc 59(1):21–35
Irving RLG (1955) A history of British mountaineering. B.T. Batsford, London
Israelachvili J (2011) Intermolecular and surface forces. Academic, San Diego, CA, USA
Jagota A, Bennison SJ (2002) Mechanics of adhesion through a fibrillar microstructure. Integr Comp Biol 42(6):1140–1145
Jeong J, Kim J, Song K, Autumn K, Lee J (2014) Geckoprinting: assembly of microelectronic devices on unconventional surfaces by transfer printing with isolated gecko setal arrays. J R Soc Interface 11(99):20140627
Johnson KL (1985) Contact mechanics. Cambridge University Press, Cambridge
Johnson KL, Kendall K, Roberts AD (1971) Surface energy and the contact of elastic solids. Proc R Soc A 324(1558):301–313
Kendall K (1975) Thin-film peeling—the elastic term. J Phys D Appl Phys 8(13):1449–1452
Kim TW, Bhushan B (2008) The adhesion model considering capillarity for gecko attachment system. J R Soc Interface 5(20):319–327
Kim S, Spenko M, Trujillo S, Heyneman B, Santos D, Cutkosky MR (2008) Smooth vertical surface climbing with directional adhesion. IEEE Trans Robot 24(1):65–74
Kinloch AJ (1987) Adhesion and adhesives: science and technology, 1st edn. Chapman & Hall, New York
Kwak MK, Pang C, Jeong H-E, Kim H-N, Yoon H, Jung H-S, Suh K-Y (2011) Towards the next level of bioinspired dry adhesives: new designs and applications. Adv Funct Mater 21(19):3606–3616
Landmann L (1986) The skin of reptiles: epidermis and dermis. In: Bereiter-Hahn J, Matoltsy AG, Richards KS (eds) Biology of the integument, vol 2: Vertebrates. Springer Science + Business Media, Berlin, Germany
Lee H, Bhushan B (2012) Fabrication and characterization of hierarchical nanostructured smart adhesion surfaces. J Colloid Interface Sci 372(1):231–238
Lee J, Fearing RS (2008) Contact self-cleaning of synthetic gecko adhesive from polymer microfibers. Langmuir 24(19):10587–10591
Lee YI, Kogan M, Larsen JR (1986) Attachment of the potato leafhopper to soybean plant surfaces as affected by morphology of the pretarsus. Entomol Exp Appl 42(2):101–107
Lee J, Bush B, Maboudian R, Fearing RS (2009) Gecko-inspired combined lamellar and nanofibrillar array for adhesion on nonplanar surface. Langmuir 25(21):12449–12453
Lees AD, Hardie J (1988) The organs of adhesion in the Aphid Megoura Viciae. J Exp Biol 136(1):209–228
Little P (1979) Particle capture by natural surfaces. Agric Aviat 20:129–144
Luan B, Robbins MO (2005) The breakdown of continuum models for mechanical contacts. Nature 435(7044):929–932
Maderson PFA (1964) Keratinized epidermal derivatives as an aid to climbing in gekkonid lizards. Nature 203(4946):780–781
Mahendra BC (1941) Contributions to the bionomics, anatomy, reproduction and development of the Indian house-gecko, Hemidactylus flaviviridis Rüppel. Part II. The problem of locomotion. Proc Ind Acad Sci B 13(5):288–306
McMahon TA, Bonner JT (1983) On size and life. Scientific American Books - W. H. Freeman & Co., New York
Menciassi A, Dario P (2003) Bio-inspired solutions for locomotion in the gastrointestinal tract: background and perspectives. Philos Trans R Soc A 361(1811):2287–2298
Niewiarowski PH, Lopez S, Ge L, Hagen E, Dhinojwala A (2008) Sticky gecko feet: the role of temperature and humidity. PLoS One 3(5), e2192
Northen MT, Turner KL (2005) A batch fabricated biomimetic dry adhesive. Nanotechnology 16(8):1159–1156
Pain S (2000) Sticking power. New Sci 168(2270/2271):62–67
Peattie AM, Full RJ (2007) Phylogenetic analysis of the scaling of wet and dry biological fibrillar adhesives. Proc Natl Acad Sci 104(47):18595–18600
Peattie AM, Fearing RS, Full RJ (2004) Using a simple beam model to predict morphological variation in adhesive gecko hairs. Society for Integrative and Comparative Biology, New Orleans (Abstract from 2004 Society for Integrative and Comparative Biology Annual Meeting)
Peressadko A, Gorb SN (2004) When less is more: experimental evidence for tenacity enhancement by division of contact area. J Adhes 80(4):247–261
Persson BNJ (1995) Theory of friction: stress domains, relaxation, and creep. Phys Rev B 51(19):13568–13585
Persson BNJ (2003) On the mechanism of adhesion in biological systems. J Chem Phys 118(16):7614–7621
Persson BNJ, Gorb S (2003) The effect of surface roughness on the adhesion of elastic plates with application to biological systems. J Chem Phys 119(21):11437–11444
Pesika NS, Yu T, Zhao B, Rosenberg K, Zeng H, McGuiggan P, Autumn K, Israelachvili JN (2007) Peel-zone model of tape peeling based on the gecko adhesive system. J Adhes 83(4):383–401
Pesika NS, Gravish N, Wilkinson M, Zhao B, Zeng H, Yu T, Israelachvili J, Autumn K (2009a) The crowding model as a tool to understand and fabricate gecko-inspired dry adhesives. J Adhes 85(8):512–525
Pesika NS, Zeng H, Kristiansen K, Zhao B, Yu T, Autumn K, Israelachvili J (2009b) Gecko adhesion pad: a smart surface? J Phys Condens Matter 21(46):464132
Peterson JA, Williams EE (1981) A case history in retrograde evolution: the onca lineage in Anoline lizards II Subdigital fine structure. Bull Museum Comp Zool 149(4):215–268
Pianka ER, Sweet SL (2005) Integrative biology of sticky feet in geckos. BioEssays 27(6):647–652
Pocius AV (2012) Adhesion and adhesive technology. Carl Hanser Verlag, Munich, Germany
Prowse M, Puthoff JB, Wilkinson M, Autumn K (2011) Effects of humidity on the mechanical properties of gecko setae. Acta Biomater 7(2):733–738
Pugno NM, Lepore E (2008) Observation of optimal gecko’s adhesion on nanorough surfaces. BioSystems 94(3):218–222
Puthoff JB, Prowse MS, Wilkinson M, Autumn K (2010) Changes in materials properties explain the effects of humidity on gecko adhesion. J Exp Biol 213(21):3699–3704
Puthoff JB, Holbrook M, Wilkinson MJ, Jin K, Pesika NS, Autumn K (2013) Dynamic friction in natural and synthetic gecko setal arrays. Soft Matter 9(19):4855–4863
Ringlein J, Robbins MO (2004) Understanding and illustrating the atomic origins of friction. Am J Phys 72(7):884–891
Rizzo NW, Gardner KH, Walls DJ, Keiper-Hrynko NM, Ganzke TS, Hallahan DL (2006) Characterization of the structure and composition of gecko adhesive setae. J R Soc Interface 3(8):441–451
Röll B (1995) Epidermal fine structure of the toe tips of Sphaerodactylus cinereus (Reptilia, Gekkonidae). J Zool 235(2):289–300
Rosenberg HI, Rose R (1999) Volar adhesive pads of the feathertail glider, Acrobates pygmaeus (Marsupialia; Acrobatidae). Can J Zool 77(2):233–248
Ruibal R, Ernst V (1965) The structure of the digital setae of lizards. J Morphol 117(3):271–293
Russell AP (1975) A contribution to the functional analysis of the foot of the Tokay, Gekko gecko (Reptilia: Gekkonidae). J Zool 176(4):437–476
Russell AP (1976) Some comments concerning the interrelationships amongst gekkonine geckos. In: Bellairs AA, Cox CB (eds) Morphology and biology of reptiles, number 3 in Linnean Society Symposium Series. Academic, London, pp 217–244
Russell AP (1979) Parallelism and integrated design in the foot structure of Gekkonine and Diplodactyline Geckos. Copeia 1979(1):1–21
Russell AP (1981) Descriptive and functional anatomy of the digital vascular system of the tokay, Gekko gecko. J Morphol 169(3):293–323
Russell AP (1986) The morphological basis of weight-bearing in the scansors of the tokay gecko (Reptilia: Sauria). Can J Zool 64(4):948–955
Russell AP (2002) Integrative functional morphology of the Gekkotan adhesive system (Reptilia: Gekkota). Integr Comp Biol 42(6):1154–1163
Russell AP, Bauer AM (1988) Paraphalangeal elements of gekkonid lizards: a comparative survey. J Morphol 197(2):221–240
Russell AP, Bauer AM (1990a) Digit I in pad-bearing gekkonine geckos: alternate designs and the potential constraints of phalangeal number. Mem Qld Mus 29(2):453–472
Russell AP, Bauer AM (1990b) Oedura and Afroedura (Reptilia: Gekkonidae) revisited: similarities of digital design, and constraints on the development of multiscansorial subdigital pads? Mem Qld Mus 29(2):473–486
Russell AP, Rosenberg HI (1981) Self-grooming in Diplodactylus spinigerus (Reptilia: Gekkonidae), with a brief review of such behaviour in reptiles. Can J Zool 59(3):564–566
Sauer RA (2009) Multiscale modelling and simulation of the deformation and adhesion of a single gecko seta. Comput Methods Biomech Biomed Engin 12(6):627–640
Scherge M, Gorb S (2001) Biological micro- and nanotribology: natures solutions nanoscience and technology series. Springer, Berlin, Germany
Schleich HH, Kästle W (1986) Ultrastrukturen an Gecko-Zehen (Reptilia: Sauria: Gekkonidae). Amphibia-Reptilia 7(2):141–166
Schmidt H (1904) Zur Anatomie und Physiologie der Geckopfote. Jenaische Zeitschrift für Naturwissenschaft 39:551–580
Simmermacher G (1884) Haftapparate bei Wirbeltieren. Der Zoologische Garten 25(10):289–301
Sitti M, Fearing RS (2003) Synthetic gecko foot-hair micro/nano-structures as dry adhesives. J Adhes Sci Technol 17(8):1055–1073
Slocum AH, Weber AC (2003) Precision passive mechanical alignment of wafers. J Microelectromech Syst 12(6):826–834
Spolenak R, Gorb S, Arzt E (2005a) Adhesion design maps for bio-inspired attachment systems. Acta Biomater 1(1):5–13
Spolenak R, Gorb S, Gao H, Arzt E (2005b) Effects of contact shape on the scaling of biological attachments. Proc R Soc A 461(2054):305–319
Stark AY, Sullivan TW, Niewiarowski PH (2012) The effect of surface water and wetting on gecko adhesion. J Exp Biol 215(17):3080–3086
Stark AY, Badge I, Wucinich NA, Sullivan TW, Niewiarowski PH, Dhinojwala A (2013) Surface wettability plays a significant role in gecko adhesion underwater. Proc Natl Acad Sci 110(16):6340–6345
Stark AY, Ohlemacher J, Knight A, Niewiarowski PH (2015a) Run don’t walk: locomotor performance of geckos on wet substrates. J Exp Biol 218(15):2435–2441
Stark AY, Palecek AM, Argenbright CW, Bernard C, Brennan AB, Niewiarowski PH, Dhinojwala A (2015b) Gecko adhesion on wet and dry patterned substrates. PLoS One 10(12), e0145756
Stewart WJ, Higham TE (2014) Passively stuck: death does not affect gecko adhesion strength. Biol Lett 10(12):20140701
Stork NE (1980) Experimental analysis of adhesion of Chrysolina Polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J Exp Biol 88(1):91–108
Stork NE (1983) A comparison of the adhesive setae on the feet of lizards and arthropods. J Nat Hist 17(6):829–835
Sun W, Neuzil P, Kustandi TS, Sharon O, Samper VD (2005) The nature of the gecko lizard adhesive force. Biophys J 89(2):14–17
Tian Y, Pesika N, Zeng H, Rosenberg K, Zhao B, McGuiggan P, Autumn K, Israelachvili J (2006) Adhesion and friction in gecko toe attachment and detachment. Proc Natl Acad Sci 103(51):19320–19325
Timoshenko SP, Gere SM (1984) Mechanics of materials. Thomson Brooks/Cole, Independence, KY, USA
Urbakh M, Klafter J, Gourdon D, Israelachvili J (2004) The nonlinear nature of friction. Nature 430(6999):525–528
Vanhooydonck B, Andronescu A, Herrel A, Irschick DJ (2005) Effects of substrate structure on speed and acceleration capacity in climbing geckos. Biol J Linn Soc 85(3):385–393
Vinson J, Vinson J-M (1969) The saurian fauna of the Mascarene Islands. In: Mauritius institute bulletin, vol 6. The Mauritius Institute, Port Louis, Mauritius, pp 203–320
Vitt LJ, Zani PA (1997) Ecology of the nocturnal lizard Thecadactylus rapicauda (Sauria: Gekkonidae) in the Amazon region. Herpetologica 53(2):165–179
von Wittich (1854) Der Mechanismus der Haftzehen von Hyla arborea. Archiv für Anatomie, Physiologie und Wissenschaftliche Medicin 8:170–183
Wagler J (1830) Natürliches System der Amphibien: mit vorangehender Classification der Säugethiere und Vögel: ein Beitrag zur vergleichenden Zoologie, 1st edn. J.G. Cotta’schen Buchhandlung, München, Germany
Wainwright SA, Biggs WD, Currey JD, Gosline JM (1982) Mechanical design in organisms. Princeton University Press, Princeton, NJ, USA
Weitlaner F (1902) Eine Untersuchung fiber den Haftfuβ des Gecko. Verhandlungen der Zoologisch-Botanischen Gesellschaft in Wien 52:328–332
Williams EE, Peterson JA (1982) Convergent and alternative designs in the digital adhesive pads of scincid lizards. Science 215(4539):1509–1511
Xu Q, Wan Y, Hu TS, Liu TX, Tao D, Niewiarowski PH, Yu T, Liu Y, Dai L, Yang Y, Xia Z (2015) Robust self-cleaning and micromanipulation capabilities of gecko spatulae and their bio-mimics. Nat Commun 6:8949
Yao H, Gao H (2006) Mechanics of robust and releasable adhesion in biology: Bottom-up designed hierarchical structures of gecko. J Mech Phys Solids 54(6):1120–1146
Acknowledgments
I am grateful to many colleagues for their help with this chapter, including Eduard Arzt, Sanford Autumn, Violeta Autumn, Emerson De Soma, Andrew Dittmore, Ron Fearing, Valeurie Friedman, Bob Full, Bill Geisler, Stas Gorb, Gerrit Huber, Jacob Israelachvili, Carmel Majidi, Anne Peattie, Holger Pfaff, Tony Russell, Andy Smith, and Simon Sponberg. Thanks to Stas Gorb and MPI Stuttgart for the Cryo-SEM image of a single seta. Thanks also to Carmel Majidi and Ron Fearing for Eqs. (11.1) and (11.2). Supported by DARPA N66001-03-C-8045, NSF-NIRT 0304730, DCI/NGIA HM1582-05-2022, and Johnson & Johnson Dupuy-Mitek Corp.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Autumn, K., Puthoff, J. (2016). Properties, Principles, and Parameters of the Gecko Adhesive System. In: Smith, A. (eds) Biological Adhesives. Springer, Cham. https://doi.org/10.1007/978-3-319-46082-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-46082-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46081-9
Online ISBN: 978-3-319-46082-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)