Abstract
Over the past decade, several approaches have been employed to develop cell and gene therapy strategies that generate artificial insulin-producing cells (IPCs) for potential therapeutic applications in the treatment of type 1 diabetes mellitus (T1D) . The genetic engineering of functional IPCs necessitates a broad understanding of the pancreatic developmental process and the β cell transcription factors that govern mature β cell differentiation and function. To successfully obtain functional IPCs, the type of vectors utilised for gene transfer and the selection of a suitable target cell for subsequent differentiation into IPCs is of fundamental importance. Techniques for manufacturing IPCs include the dedifferentiation and directed transdifferentiation of autologous or allogeneic cells ex vivo followed by transplantation and the in vivo transdifferentiation of target tissue via viral gene transfer. Ultimately, the goal is to construct IPCs that have the capacity to process, store and secrete insulin in response to fluctuating blood glucose levels, whilst avoiding the administration of immunosuppressants and recurrent autoimmune destruction, thereby indefinitely restoring normoglycaemia.
Dario Gerace and Rosetta Martiniello-Wilks have contributed equally to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Several approaches have been employed to develop cell and gene therapy strategies that generate artificial insulin-producing cells (IPCs) for potential therapeutic applications in the treatment of type 1 diabetes mellitus (T1D). The genetic engineering of functional IPCs necessitates a broad understanding of the pancreatic developmental process and the β-cell transcription factors that govern mature β-cell differentiation and function. To successfully obtain functional IPCs, the type of vectors utilized for gene transfer and the selection of a suitable target cell for subsequent differentiation into IPCs is of fundamental importance. Techniques for manufacturing IPCs include the de-differentiation and directed differentiation of autologous or allogeneic cells ex vivo followed by transplantation and the in vivo differentiation of target tissue via viral gene transfer. Ultimately, the goal is to construct IPCs that have the capacity to process, store and secrete insulin in response to and relative to the circulating blood glucose levels, whilst avoiding the administration of immunosuppressants and recurrent autoimmune destruction, thereby restoring normoglycaemia.
Selecting an Ideal Viral Vector for T1D Gene Therapy
The use of viruses as tools for the correction of genetic disorders due to their ability to infect and deliver genetic material to cells has paved the way for a number of promising cell and gene therapies. By engineering viral vectors that are replication deficient and efficiently transduce genes into target cells, key challenges encountered in the generation of successful therapies can be circumvented. Selecting a suitable viral vector is contingent on the nature of gene expression required (sustained or short-term expression), whether the vector is integrative or non-integrative, and the type of cells targeted for gene transfer (dividing or non-dividing) (Table 10.1). Ideally, the generation of artificial IPCs would utilise integrating viral vectors that offer long-term gene expression over the life of the patient, resulting in a persisting therapeutic benefit.
Retroviral Vectors
Retroviral vectors are derived from disabled murine viruses and are the most commonly used gene delivery vector due to their ability to integrate chromosomally and sustain expression of the transgene (Morgan and Anderson 1993). However, the risk of site-specific insertional mutagenesis near oncogenic locations could increase predisposition to tumour development, limiting their utility (Bushman 2007; Laufs et al. 2004). This feature of retroviral vectors was demonstrated in 1999 following the treatment of nine severe combined immunodeficiency (Scid) patients which led to the development of leukaemia in four of the patients (Cavazzana-Calvo et al. 2000). The primary disadvantage with retroviral gene transfer is the requirement for target cells to be in an active state of division for transduction; therefore, target tissues composed predominantly of non-dividing cells represent a challenge. A successful use of retroviral vectors to generate IPCs was performed by Xu et al. (2007), who transduced bone marrow-derived mesenchymal stem cells (BMSCs) with an insulin gene under the control of the cytomegalovirus (CMV) promoter. Following transplantation into streptozotocin (STZ)-diabetic mice, they discovered that the transduced BMSCs expressed insulin and were able to maintain normoglycaemia for at least 42 days whilst evading autoimmune destruction.
Adenoviral and Adeno-Associated Vectors
Due to their ability to transduce dividing and non-dividing cells with high efficiency, adenoviral vectors have been studied intensively, particularly for the targeted therapy of cystic fibrosis (Zabner et al. 1993; Knowles et al. 1995). However, challenges associated with the administration of adenoviral vectors such as the development of immune responses against the viral capsid proteins, and in some cases the transgene itself, have limited their use in the clinic (Wold et al. 1999; McCaffrey et al. 2008; Nayak and Herzog 2010). In addition, adenoviral vectors transfer their genetic cargo episomally and subsequently offer only transient gene expression (Morgan and Anderson 1993; Volpers and Kochanek 2004). To address the immunogenicity of the viral capsid proteins, a “gutless” adenovirus was constructed where the genes encoding the common viral capsid proteins were deleted (Alba et al. 2005). Although the new generation vectors reduce immunogenicity, the administration of immunosuppressants is still required following treatment (Zhou et al. 2004).
Adeno-associated (AAV) viral vectors are becoming an attractive alternative for gene therapy as they are able to transduce both dividing and non-dividing cells, whilst also preferentially integrating their genetic cargo at a specific site on chromosome 19 (Muzyczka 1992). Despite possessing a limited gene cargo capacity of less than 5 kb, AAV vectors have been utilised to deliver the preproinsulin gene to livers of STZ-diabetic mice (Sugiyama et al. 1997), resulting in the transient stabilisation of blood glucose levels.
Lentiviral Vectors
Lentiviral vectors (LV) are derived from the human immunodeficiency virus (HIV) and are an attractive candidate for gene therapy as they are able to transduce both dividing and non-dividing cells (Yoon and Jun 2002). Due to their derivation from HIV, biosafety was a concern for their application as therapeutics; however, engineered deletions in the long terminal repeat (LTR) promoter reduce the possibility of producing replication competent virus and thereby improve safety (Zufferey et al. 1998). LV is at present the vector of choice for gene therapy within our laboratory, and we have successfully used a lentiviral vector (HMD) to deliver furin-cleavable insulin (INS-FUR) to the livers of STZ-diabetic rats (Ren et al. 2007), non-obese diabetic (NOD) mice (Ren et al. 2013) and pancreatectomised Westran pigs (Gerace et al. 2013). In the rodent animal models, we observed liver to pancreas transdifferentiation characterised by spontaneous expression of β cell transcription factors, formation of insulin storage granules, normal glucose tolerance and permanent reversal of diabetes.
Selecting a Suitable Target Cell for T1D Gene Therapy
Somatic cell gene therapy for T1D was first performed in monkey kidney cells and fibroblasts (Laub and Rutter 1983; Iwata et al. 1993). However, due to their intrinsic lack of β cell characteristics, these cells were not able to produce biologically active insulin despite successfully transcribing and translating the insulin gene. Likewise, the targeting of muscle cells has been studied sparingly due to their lack of β cell characteristics. Nevertheless, a study utilising vascular smooth muscle cells transduced with INS-FUR was able to reduce blood glucose levels in spontaneously diabetic congenic BioBreeding (BB) rats for a period of 6 weeks (Barry et al. 2001). Of more success was the reversal of diabetes following the dual expression of insulin and glucokinase (GK) in the muscle of STZ-diabetic mice for >4 months (Mas et al. 2006). However, a suitable target cell for the production of functional artificial IPCs would possess characteristics similar to those of normal β cells (Table 2) such as a glucose-sensing system, proinsulin-processing enzymes and an exocytosis system (Zaret and Grompe 2008).
Endocrine Cells
Pituitary cells contain proinsulin-processing enzymes and secretory granules. In a study where a murine pituitary cell line (AtT20) was transfected with a recombinant plasmid containing a human preproinsulin cDNA (AtT20Ins-1.4), expression of biologically active insulin was demonstrated; however, glucose responsiveness was absent (Stewart et al. 1994). Upon the insertion of GLUT2 and glucokinase, the AtT20Ins-1.4 cells became glucose responsive; however, the secretion of adenocorticotropic hormone stimulated glucocorticoid synthesis which inhibited insulin function, and therefore limited their effectiveness (Hughes et al. 1993).
K cells, a type of endocrine cell found in the gut, possess many β cell characteristics and have drawn a significant attention as a potential target for gene therapy (Yoon and Jun 2002). An in vivo study targeting K cells with a construct of human insulin under the control of the glucose-dependent insulinotropic polypeptide (GIP) regulatory promoter resulted in the production of biologically active insulin and the restoration of normoglycaemia (Cheung et al. 2000). Despite this encouraging result, an efficient method of delivery to these cells is yet to be established.
Liver Cells
Considering liver cells are derived from the same endodermal origin as the pancreas (Zaret 2008), increasing effort has been focused on engineering hepatocytes to function as artificial IPCs . The possession of GLUT2 and glucokinase in hepatocytes permits a response to fluctuating glucose concentrations similar to that in normal β cells and hence their preferred use in gene therapy protocols for the treatment of T1D . Although hepatocytes do not contain proinsulin-processing enzymes and lack secretory granules, these functions can be induced in certain circumstances (e.g. via the expression of β cell transcription factors or INS-FUR) (Ren et al. 2007, 2013; Gerace et al. 2013; Tuch et al. 2003).
Mesenchymal Stem Cells (MSCs)
Within the last decade, the targeting of MSCs for genetic manipulation has become increasingly popular owing to their wide-ranging immunomodulatory properties and reported ability to elude immune detection (Gebler et al. 2012; Vija et al. 2009; Abdi et al. 2008; da Silva Meirelles et al. 2009). Due to the immunomodulatory capacities of MSC, their predominant use in the correction of diabetes has been in the form of transplantation of native MSCs intended to control immune responses (Lee et al. 2006; Ezquer et al. 2008). However, at the present time, MSCs are more commonly becoming utilised as target cells for the generation of artificial IPCs via chemically induced differentiation protocols or direct transfer of genetic material (Tang et al. 2004). With regard to the chemical induction of MSCs to generate IPCs, it has been shown that BMSCs cultured in high-glucose medium (Oh et al. 2004) or nicotinamide-enriched medium are inclined to differentiate into IPCs (Wu et al. 2007). Similarly, BMSCs subjected to a three-step chemically induced differentiation protocol produced glucose-responsive IPCs with high expression levels of Pdx-1, insulin and glucagon (Sun et al. 2007). On the other hand, BMSCs targeted for gene therapy with a retroviral vector containing the β cell transcription factor Pdx-1 generated IPCs that reduced blood glucose concentrations after transplantation in STZ-diabetic/Scid mice and exhibited a normal glucose tolerance until 6–8 weeks post-transplantation (Karnieli et al. 2007).
Viral-Mediated Transfer of β Cell Transcription Factors
Currently, pancreas and islet transplantation are the only “cures” for diabetes mellitus. However, the limitations associated with the current therapeutic options necessitate the requirement for an alternative IPC that is also capable of evading recurrent immune destruction. During normal pancreatic development, islet cell differentiation is regulated by the expression of β cell transcription factors (Fig. 10.1), and during adult life, the transcription factors regulate the expression of pancreatic hormones. As a result, the utilisation of β cell transcription factors as mediators of IPC generation became of considerable interest as an alternative therapy for the treatment of diabetes mellitus. The production of IPCs for diabetes reversal via viral-mediated transfer of β cell transcription factors has been comprehensively examined in liver tissue as it is derived from the same endodermal origin as the pancreas (Zaret and Grompe 2008), making it more amenable to the transdifferentiation process.
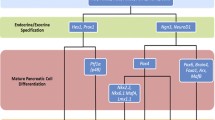
Hierarchical representation of the transcription factors involved in pancreatic islet cell differentiation. Signals from the mesenchyme and notochord induce the early gut endoderm to form the pancreatic buds, where expression of Pdx-1 then drives differentiation of the pancreatic precursor cells. Exocrine and endocrine differentiation are then specified by the expression of Neurog3 and Hes1, respectively, with subsequent expression of NeuroD1 maintaining the endocrine cell lineage. α-Cell and β cell differentiation is mediated by Pax6 and Pax4, respectively. Final differentiation of β cells is governed by the expression of Nkx2.2, Nkx6.1 and MafA
Pdx-1
The direct in vivo delivery of the transcription factor Pdx-1 to the livers of diabetic mice was studied by Ferber et al. (2000) as a potential method of correcting hyperglycaemia via the induced expression of insulin. The study reported the restoration of normoglycaemia in the mice for a period of 8 days as a consequence of the expression of Pdx-1 which induced insulin expression and secretion. However, uncontrolled transdifferentiation led to the undesirable development of exocrine tissue which resulted in hepatitis in the liver (Ferber et al. 2000; Kojima et al. 2003).
Exocrine differentiation following delivery of Pdx-1 to the livers of STZ-diabetic mice was also reported by Kojima et al. (2003) and was presumably a consequence of the continuous expression of high levels of Pdx-1. Thus far, Pdx-1-mediated transdifferentiation from hepatocytes to pancreatic tissue has been accomplished on numerous occasions with varying degrees of success (Fodor et al. 2007; Ber et al. 2003; Wang et al. 2007; Sapir et al. 2005; Nagaya et al. 2009). Other target cells for direct delivery of Pdx-1 include mouse pancreas via the bile duct (Taniguchi et al. 2003), rat intestinal epithelium-derived cells (IEC-6) (Yoshida et al. 2002) and primary duct cells (Noguchi et al. 2006). A combinatorial approach utilising the transcription factors Pdx-1, Neurog3 and MafA successfully converted pancreatic exocrine cells into IPCs in vivo, providing support for the delivery of combinations of pancreatic transcription factors (Zhou et al. 2008). However, glucose tolerance in the animals differed significantly from normal.
As a result of the success of utilising Pdx-1 as a mediator of pancreatic transdifferentiation , stem cells emerged as potential targets for gene transfer due to their regenerative capabilities and plasticity. As mentioned in “Mesenchymal Stem Cells (MSCs)” section, MSCs became of particular interest due to their unique immune-evading capabilities. MSCs from a variety of sources have been targeted for Pdx-1 delivery, including BMSC (Karnieli et al. 2007; Sun et al. 2006; Limbert et al. 2011; Moriscot et al. 2005; Li et al. 2007, 2008), umbilical cord MSC (UC-MSC) (He et al. 2011) and adipose-derived MSC (AMSC) (Baer 2011; Lin et al. 2009; Kajiyama et al. 2010). A study by Miyazaki et al. (2004) targeted a murine embryonic stem cell (ESC) line (EB3) for Pdx-1 gene transfer and found that it could be induced to differentiate into IPCs ; however, the cells lacked expression of pancreatic genes in vivo; therefore, the cells would ultimately be of no therapeutic potential. Consequently, an effort was made to improve the generation of IPCs in a number of other ESC lines that could be of more benefit for the treatment of diabetes mellitus (Lavon et al. 2006; Vincent et al. 2006; Raikwar and Zavazava 2012).
Neurog3
The implications of Neurog3 in specifying endocrine cell lineage suggest that it could potentially overcome the undesirable development of exocrine differentiation associated with the use of Pdx-1 as a mediator of pancreatic transdifferentiation . Unfortunately, most studies employing the transfer of Neurog3 have reported low levels of insulin production and a lack of glucose responsiveness (Noguchi et al. 2006; Kaneto et al. 2005; Wang et al. 2007; Song et al. 2007; Heremans et al. 2002). Adenoviral transfer of Neurog3 and betacellulin to oval cells did result in the production of insulin and pancreatic transdifferentiation (Yechoor et al. 2009); however, the most effective use of Neurog3-induced transdifferentiation was in combination with other transcription factors (Zhou et al. 2008).
NeuroD1
Kojima et al. (2003) expressed NeuroD1 and betacellulin in the livers of STZ-treated diabetic mice and showed a return to normoglycaemia for greater than 120 days, in addition to the expression of a number of pancreatic transcription factors . Most importantly, there was no evidence of any exocrine differentiation or hepatotoxicity. Further studies have also shown the ability of NeuroD1 to strongly induce insulin expression, supporting its utility as a means for the production of IPCs (Noguchi et al. 2006; Yatoh et al. 2007).
Success utilising NeuroD1 has also been reported within our laboratory, where the endocrine-specific transcription factor has been delivered to a genetically modified rat liver cell line (H4IIE) which does not endogenously express β cell transcription factors . Following the delivery of both insulin and NeuroD1 to the H4IIE cell line, the cells were able to transcribe and translate insulin, process the translated protein into its mature form and package the mature insulin with storage granules (Swan MA 2009). After transplantation in NOD/Scid mice, the cells secreted insulin in response to increasing concentrations of glucose and restored normoglycaemia. Most importantly, they also displayed the hallmark characteristics of pancreatic transdifferentiation with expression of Pdx-1, NeuroD1, Pax6, Nkx2.2 and Nkx6.1, in addition to rat insulin 1 and 2, glucagon, somatostatin, proconvertase 1 and 2 (PC1/2) and pancreatic polypeptide. Consequently, this study supports the already available evidence for the prospective use of NeuroD1 to produce IPCs that are safe for use as a therapeutic for diabetes mellitus.
Pax4
Pax4 is a lower-hierarchy transcription factor that is essential for directing β cell differentiation and therefore is a suitable candidate for the generation of IPCs . Liew et al. (2008) demonstrated that human ESCs engineered to overexpress Pax4 have an enhanced propensity to form putative β cells. A study supporting this finding showed that IPCs created from mouse ESCs via the overexpression of Pax4 and selected for nestin expression were proficient in restoring normoglycaemia for 14 days (Blyszczuk et al. 2003). However, the propensity for ESCs to form teratomas limits their potential for clinical application (Stachelscheid et al. 2013; Hentze et al. 2009).
Nkx6.1
The disruption in the differentiation of β cells in mice has been demonstrated by knockouts of the Nkx6.1 transcription factor, implicating it as an essential part of the β cell developmental pathway. As a result, it has the potential to be a successful mediator of IPC generation. However, ectopic expression of Nkx6.1 alone has been shown to poorly induce the expression of vital upper-hierarchy β cell transcription factors . Only upon co-expression with Pdx-1 are the upper-hierarchy transcription factors expressed, which subsequently leads to insulin expression and glucose-responsive insulin release (Gefen-Halevi et al. 2010). As a result of the poor capacity of Nkx6.1 to induce the expression of the full repertoire of β cell transcription factors, it is not a promising choice for the generation of IPCs .
Viral-Mediated Transfer of Insulin
As mentioned in section “Lentiviral Vectors”, our laboratory has successfully employed the use of lentiviral vectors to transfer insulin to hepatocytes as an alternative therapeutic strategy for the treatment of T1D . The development of exocrine differentiation associated with liver-directed gene therapy using Pdx-1 (Ferber et al. 2000; Kojima et al. 2003) has never been observed in our studies, presumably due to the diverse choice of genes utilised for gene delivery. In the human liver cell line (Huh7), which endogenously expresses β cell transcription factors, we were able to induce the development of insulin storage granules and glucose-responsive insulin secretion following the delivery of the insulin gene (Huh7ins). Diabetes in NOD/Scid mice was corrected following transplantation of the Huh7ins cells (Tuch et al. 2003).
Insulin Transfer in Rodent Models
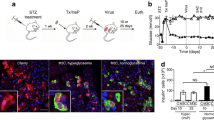
Permanent reversal of diabetes in STZ-diabetic rats (Ren et al. 2007) and spontaneously diabetic NOD mice (Ren et al. 2013) following liver-directed lentiviral delivery of INS-FUR has been demonstrated within our laboratory. In these rodent models, we showed the spontaneous expression of the essential upper-hierarchy β cell transcription factors (Pdx-1, Neurog3 and NeuroD1), some lower-hierarchy β cell transcription factors (Pax4 and Nkx2.2) and the development of glucose-responsive insulin secretion (Nathwani et al. 2011; Lisowski et al. 2014; Apelqvist et al. 1999; Sommer et al. 1996). Permanent reversal of diabetes was characterised by normal intravenous glucose tolerance tests in the STZ-diabetic rat and NOD mouse study (Fig. 10.2). The insulin-secreting liver cells and NOD mouse livers engineered to express insulin generated within our laboratory are also resistant to the detrimental effects of β cell cytotoxins and proinflammatory cytokines that play a principle role in the pathogenesis of T1D (Ren et al. 2013; Tabiin et al. 2001, 2004; Tuch et al. 1997).
Plasma glucose levels following an intravenous glucose tolerance test (IVGTT) in diabetic non-obese diabetic (NOD) mice treated with furin-cleavable human insulin (INS-FUR) within a lentiviral vector (HMD). An IVGTT was performed on non-diabetic NOD (12–16 weeks), non-obese-resistant (NOR) mice and HMD/INS-FUR-treated NOD mice, 5 months after reversal of diabetes. (n = 5, data were examined by one-way analysis of variance after log transformation of data and expressed as the mean ± SEM). [Reproduced from Ren et al. (2013)]
Due to the success shown by the viral delivery of INS-FUR within our laboratory, a number of successive studies utilising viral delivery of INS-FUR showing amelioration of hyperglycaemia in rodent models were reported (Han et al. 2011; Tatake et al. 2007; Tudurí et al. 2012; Hsu et al. 2008). In contrast to our research, Elsner et al. (2012) applied lentiviral delivery of INS-FUR to the liver of diabetic rats and observed reversal of hyperglycaemia; however, pancreatic transdifferentiation and development of secretory granules were absent. Their gene therapy approach also lacked closely regulated control of blood glucose observed within normally functioning β cells, therefore requiring islet supplementation for clinical application.
Insulin Transfer in a Porcine Model
In order to more appropriately translate the rodent studies performed within our laboratory to the clinical setting, we also reversed diabetes in a large animal model that more closely resembles human physiology. Reversal of diabetes following liver-directed lentiviral delivery of INS-FUR was characterised by the expression of β cell transcription factors and normal glucose tolerance (Gerace et al. 2013). Unexpectedly, the complexity of applying the surgical procedure (which isolates the liver from the circulation) in a large animal model led to difficulties in reproducing the successful reversal of diabetes. This suggested that translation of the surgical procedure in the clinical setting may also raise a number of challenges; therefore, it would likely be of more benefit to transplant cells modified ex vivo so as to overcome the surgical obstacles.
Conclusion
Viral-mediated gene transfer of β cell transcription factors and insulin represents an alternative approach to generating artificial IPCs for the treatment of T1D . Currently, one of the challenges facing the translation of these potential alternative cell therapies to the clinic is the generation of sufficient quantities of IPCs on a large scale. The success of liver-directed gene therapy in the past decade is nowadays becoming overshadowed by the greater understanding of the regenerative and therapeutic potential of stem cells. It is understandable that the emerging cell and gene therapy approaches are targeting stem cells for ex vivo modification as they overcome the surgical difficulties associated with in vivo gene therapy. The source of stem cells is also to be considered, as autologous cell therapies would require considerable effort to generate a single therapy. In addition, the increased likelihood of developing a full repertoire of β cell autoantigens from autologous cell modification would increase susceptibility of the grafts to recurrent autoimmunity. As discussed, the success of generating IPCs via gene therapy that are functionally equivalent to normal β cells is closely related to the choice of β cell transcription factor, viral vector and gene promoter, as unwanted exocrine differentiation can lead to tissue destruction. Ideally, targeting of an allogeneic source of cells that are capable of circumventing the autoimmune response for ex vivo gene therapy and subsequent differentiation into IPCs would overcome these limitations.
References
Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH (2008) Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 57(7):1759–1767
Alba R, Bosch A, Chillon M (2005) Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Ther 12:18–27
Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T et al (1999) Notch signalling controls pancreatic cell differentiation. Nature 400(6747):877–881
Baer PC (2011) Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev 20(10):1805–1816
Barry SC, Ramesh N, Lejnieks D, Simonson WT, Kemper L, Lernmark A et al (2001) Glucose-regulated insulin expression in diabetic rats. Hum Gene Therapy 12(2):131–139
Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I et al (2003) Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem 278(34):31950–31957
Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L et al (2003) Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA 100(3):998–1003
Bushman FD (2007) Retroviral integration and human gene therapy. J Clin Investig 117(8):2083–2086
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P et al (2000) Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288(5466):669–672
Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M et al (2000) Glucose-dependent insulin release from genetically engineered K cells. Science 290(5498):1959–1962
da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI (2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20(5):419–427
Elsner M, Terbish T, Jorns A, Naujok O, Wedekind D, Hedrich H-J et al (2012) Reversal of diabetes through gene therapy of diabetic rats by hepatic insulin expression via lentiviral transduction. Mol Ther 20(5):918–926
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA (2008) Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transpl 14(6):631–640
Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I et al (2000) Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 6(5):568–572
Fodor A, Harel C, Fodor L, Armoni M, Salmon P, Trono D et al (2007) Adult rat liver cells transdifferentiated with lentiviral IPF1 vectors reverse diabetes in mice: an ex vivo gene therapy approach. Diabetologia 50(1):121–130
Gebler A, Zabel O, Seliger B (2012) The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med 18(2):128–134
Gefen-Halevi S, Rachmut IH, Molakandov K, Berneman D, Mor E, Meivar-Levy I et al (2010) NKX6.1 promotes PDX-1-induced liver to pancreatic beta-cells reprogramming. Cell Reprogram 12(6):655–664
Gerace D, Ren B, Hawthorne WJ, Byrne MR, Phillips PM, O’Brien BA et al (2013) Pancreatic transdifferentiation in porcine liver following lentiviral delivery of human furin–cleavable insulin. Transpl Proc 45(5):1869–1874
Han J, McLane B, Kim E-H, Yoon J-W, Jun H-S (2011) Remission of diabetes by insulin gene therapy using a hepatocyte-specific and glucose-responsive synthetic promoter. Mol Ther 19(3):470–478
He D, Wang J, Gao Y, Zhang Y (2011) Differentiation of PDX1 gene-modified human umbilical cord mesenchymal stem cells into insulin-producing cells in vitro. Int J Mol Med 28(6):1019–1024
Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR (2009) Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res 2(3):198–210
Heremans Y, Van De Casteele M, Gradwohl G, Serup P, Madsen O et al (2002) Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol 159(2):303–312
Hsu P-J, Kotin R, Yang Y-W (2008) Glucose- and metabolically regulated hepatic insulin gene therapy for diabetes. Pharm Res 25(6):1460–1468
Hughes SD, Quaade C, Johnson JH, Ferber S, Newgard CB (1993) Transfection of AtT-20ins cells with GLUT-2 but not GLUT-1 confers glucose-stimulated insulin secretion. Relationship to glucose metabolism. J Biol Chem 268(20):15205–15212
Iwata H, Ogawa N, Takagi T, Mizoguchi J (1993) Preparation of insulin-releasing Chinese hamster ovary cell by transfection of human insulin gene. In: Polymers of biological and biomedical significance, vol 540. American Chemical Society, pp 306–313
Kajiyama H, Hamazaki TS, Tokuhara M, Masui S, Okabayashi K, Ohnuma K et al (2010) Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. Int J Dev Biol 54(4):699–705
Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M et al (2005) PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes 54(4):1009–1022
Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S (2007) Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells 25(11):2837–2844
Knowles MR, Hohneker KW, Zhou Z, Olsen JC, Noah TL, Hu P-C et al (1995) A controlled study of adenoviral-vector–mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med 333(13):823–831
Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M et al (2003) NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med 9(5):596–603
Laub O, Rutter WJ (1983) Expression of the human insulin gene and cDNA in a heterologous mammalian system. J Biol Chem 258(10):6043–6050
Laufs S, Nagy KZ, Giordano FA, Hotz-Wagenblatt A, Zeller WJ, Fruehauf S (2004) Insertion of retroviral vectors in NOD/SCID repopulating human peripheral blood progenitor cells occurs preferentially in the vicinity of transcription start regions and in introns. Mol Ther 10(5):874–881
Lavon N, Yanuka O, Benvenisty N (2006) The effect of overexpression of Pdx1 and Foxa2 on the differentiation of human embryonic stem cells into pancreatic cells. Stem Cells 24(8):1923–1930
Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD et al (2006) Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA 103(46):17438–17443
Li L, Li F, Qi H, Feng G, Yuan K, Deng H et al (2008) Coexpression of Pdx1 and betacellulin in mesenchymal stem cells could promote the differentiation of nestin-positive epithelium-like progenitors and pancreatic islet-like spheroids. Stem Cells Dev 17(4):815–823
Li Y, Zhang R, Qiao H, Zhang H, Wang Y, Yuan H et al (2007) Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol 211(1):36–44
Liew CG, Shah NN, Briston SJ, Shepherd RM, Khoo CP, Dunne MJ et al (2008) PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS One 3(3):e1783
Limbert C, Path G, Ebert R, Rothhammer V, Kassem M, Jakob F et al (2011) PDX1- and NGN3-mediated in vitro reprogramming of human bone marrow-derived mesenchymal stromal cells into pancreatic endocrine lineages. Cytotherapy 13(7):802–813
Lin G, Wang G, Liu G, Yang LJ, Chang LJ, Lue TF et al (2009) Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem Cells Dev 18(10):1399–1406
Lisowski L, Dane AP, Chu K, Zhang Y, Cunningham SC, Wilson EM et al (2014) Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506(7488):382–386
Mas A, Montané J, Anguela XM, Muñoz S, Douar AM, Riu E et al (2006) Reversal of type 1 diabetes by engineering a glucose sensor in skeletal muscle. Diabetes 55(6):1546–1553
McCaffrey AP, Fawcett P, Nakai H, McCaffrey RL, Ehrhardt A, Pham TT et al (2008) The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol Ther 16(5):931–941
Miyazaki S, Yamato E, Miyazaki JI (2004) Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes 53(4):1030–1037
Morgan RA, Anderson WF (1993) Human gene therapy. Annu Rev Biochem 62:191–217
Moriscot C, de Fraipont F, Richard M-J, Marchand M, Savatier P, Bosco D et al (2005) Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells 23(4):594–603
Muzyczka N (1992) Use of adeno-associated virus as a general transduction vector for mammalian cells. In: Muzyczka N (ed) Viral expression vectors, vol 158. Springer, Berlin, pp 97–129
Nagaya M, Katsuta H, Kaneto H, Bonner-Weir S, Weir GC (2009) Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrinol 201(1):37–47
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC et al (2011) Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365(25):2357–2365
Nayak S, Herzog RW (2010) Progress and prospects: immune responses to viral vectors. Gene Ther 17(3):295–304
Noguchi H, Xu G, Matsumoto S, Kaneto H, Kobayashi N, Bonner-Weir S et al (2006) Induction of pancreatic stem/progenitor cells into insulin-producing cells by adenoviral-mediated gene transfer technology. Cell Transpl 15(10):929–938
Oh S-H, Muzzonigro TM, Bae S-H, LaPlante JM, Hatch HM, Petersen BE (2004) Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest 84(5):607–617
Raikwar SP, Zavazava N (2012) PDX1-engineered embryonic stem cell-derived insulin producing cells regulate hyperglycemia in diabetic mice. Transpl Res 1(1):1440–2047
Ren B, O’Brien BA, Byrne MR, Ch’ng E, Gatt PN, Swan MA et al (2013) Long-term reversal of diabetes in non-obese diabetic mice by liver-directed gene therapy. J Gene Med 15(1):28–41
Ren B, O’Brien BA, Swan MA, Koina ME, Nassif N, Wei MQ et al (2007) Long-term correction of diabetes in rats after lentiviral hepatic insulin gene therapy. Diabetologia 50(9):1910–1920
Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E et al (2005) Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci USA 102(22):7964–7969
Simpson AM, Tao C, Swan MA, B R, O’Brien BA (2009) An engineered rat liver cell line H4IIEins/ND reverses diabetes in mice. In: International diabetes federation world diabetes congress, Montreal, Abstract no. MT-0996
Sommer L, Ma Q, Anderson DJ (1996) neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol Cell Neurosci 8(4):221–241
Song YD, Lee EJ, Yashar P, Pfaff LE, Kim SY, Jameson JL (2007) Islet cell differentiation in liver by combinatorial expression of transcription factors neurogenin-3, BETA2, and RIPE3b1. Biochem Biophys Res Commun 354(2):334–339
Stachelscheid H, Wulf-Goldenberg A, Eckert K, Jensen J, Edsbagge J, Bjorquist P et al (2013) Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors. J Tissue Eng Regen Med 7(9):729–741
Stewart C, Taylor NA, Green IC, Docherty K, Bailey CJ (1994) Insulin-releasing pituitary cells as a model for somatic cell gene therapy in diabetes mellitus. J Endocrinol 142(2):339–343
Sugiyama A, Hattori S, Tanaka S, Isoda F, Kleopoulos S, Rosenfeld M et al (1997) Defective adenoassociated viral-mediated transfection of insulin gene by direct injection into liver parenchyma decreases blood glucose of diabetic mice. Horm Metab Res 29(12):599–603
Sun Y, Chen L, Hou XG, Hou WK, Dong JJ, Sun L et al (2007) Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J 120(9):771–776
Sun J, Yang Y, Wang X, Song J, Jia Y (2006) Expression of Pdx-1 in bone marrow mesenchymal stem cells promotes differentiation of islet-like cells in vitro. Sci China C Life Sci 49(5):480–489
Tabiin MT, Tuch BE, Bai L, Han XG, Simpson AM (2001) Susceptibility of insulin-secreting hepatocytes to the toxicity of pro-inflammatory cytokines. J Autoimmun 17(3):229–242
Tabiin MT, White CP, Morahan G, Tuch BE (2004) Insulin expressing hepatocytes not destroyed in transgenic NOD mice. J Autoimmune Dis 1(1):3
Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA et al (2004) In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes 53(7):1721–1732
Taniguchi H, Yamato E, Tashiro F, Ikegami H, Ogihara T, Miyazaki J (2003) beta-cell neogenesis induced by adenovirus-mediated gene delivery of transcription factor pdx-1 into mouse pancreas. Gene Ther 10(1):15–23
Tatake RJ, O’Neill MM, Kennedy CA, Reale VD, Runyan JD, Monaco K-AD et al (2007) Glucose-regulated insulin production from genetically engineered human non-beta cells. Life Sci 81(17–18):1346–1354
Tuch BE, Beynon S, Tabiin MT, Sassoon R, Goodman RJ, Simpson AM (1997) Effect of beta-cell toxins on genetically engineered insulin-secreting cells. J Autoimmun 10(3):239–244
Tuch BE, Szymanska B, Yao M, Tabiin MT, Gross DJ, Holman S et al (2003) Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Ther 10(6):490–503
Tudurí E, Bruin JE, Kieffer TJ (2012) Restoring insulin production for type 1 diabetes. J Diabetes 4(4):319–331
Vija L, Farge D, Gautier JF, Vexiau P, Dumitrache C, Bourgarit A et al (2009) Mesenchymal stem cells: stem cell therapy perspectives for type 1 diabetes. Diabetes Metab 35(2):85–93
Vincent R, Treff N, Budde M, Kastenberg Z, Odorico J (2006) Generation and characterization of novel tetracycline-inducible pancreatic transcription factor-expressing murine embryonic stem cell lines. Stem Cells Dev 15(6):953–962
Volpers C, Kochanek S (2004) Adenoviral vectors for gene transfer and therapy. J Gene Med 6(S1):S164–S171
Wang AY, Ehrhardt A, Xu H, Kay MA (2007a) Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther J Am Soc Gene Ther 15(2):255–263
Wang AY, Ehrhardt A, Xu H, Kay MA (2007b) Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther 15(2):255–263
Wold WS, Doronin K, Toth K, Kuppuswamy M, Lichtenstein DL, Tollefson AE (1999) Immune responses to adenoviruses: viral evasion mechanisms and their implications for the clinic. Curr Opin Immunol 11(4):380–386
Wu XH, Liu CP, Xu KF, Mao XD, Zhu J, Jiang JJ et al (2007) Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World J Gastroenterol 13(24):3342–3349
Xu J, Lu Y, Ding F, Zhan X, Zhu M, Wang Z (2007) Reversal of diabetes in mice by intrahepatic injection of bone-derived GFP-murine mesenchymal stem cells infected with the recombinant retrovirus-carrying human insulin gene. World J Surg 31(9):1872–1882
Yatoh S, Akashi T, Chan PP, Kaneto H, Sharma A, Bonner-Weir S et al (2007) NeuroD and reaggregation induce β-cell specific gene expression in cultured hepatocytes. Diabetes/Metab Res Rev 23(3):239–249
Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H et al (2009) Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell 16(3):358–373
Yoon JW, Jun HS (2002) Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med 8(2):62–68
Yoshida S, Kajimoto Y, Yasuda T, Watada H, Fujitani Y, Kosaka H et al (2002) PDX-1 induces differentiation of intestinal epithelioid IEC-6 into insulin-producing cells. Diabetes 51(8):2505–2513
Zabner J, Couture LA, Gregory RJ, Graham SM, Smith AE, Welsh MJ (1993) Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 75(2):207–216
Zaret KS (2008) Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat Rev Genet 9(5):329–340
Zaret KS, Grompe M (2008) Generation and regeneration of cells of the liver and pancreas. Science 322(5907):1490–1494
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA (2008) In vivo reprogramming of adult pancreatic exocrine cells to b-cells. Nature 455(7213):627–632
Zhou HS, Liu DP, Liang CC (2004) Challenges and strategies: the immune responses in gene therapy. Med Res Rev 24(6):748–761
Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L et al (1998) Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 72(12):9873–9880
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gerace, D., Martiniello-Wilks, R., Simpson, A.M. (2016). Viral-Mediated Gene Therapy for the Generation of Artificial Insulin-Producing Cells as a Therapeutic Treatment for Type 1 Diabetes Mellitus. In: A. Hardikar, A. (eds) Pancreatic Islet Biology. Stem Cell Biology and Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-45307-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-45307-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45305-7
Online ISBN: 978-3-319-45307-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)