Abstract
Photodynamic therapy (PDT) was discovered over 100 years ago when it was observed that certain dyes could kill microorganisms when exposed to light in the presence of oxygen. Since those early days, PDT has mainly been developed as a cancer therapy with regulatory approvals and clinical trials steadily accumulating for different types of cancer and different photosensitizer structures. A very important milestone for PDT was the introduction of 5-aminolevulinic acid (ALA), which functions as a prodrug to induce endogenous porphyrin biosynthesis that acts as an endogenous photosensitizer produced by our cells. PDT with ALA and its derivatives have become mainstays of the clinical dermatologist’s practice covering everything from skin cancer, premalignant lesions, acne, and skin rejuvenation. Another milestone in PDT development was the realization that PDT may also be used as an effective antimicrobial modality and a potential treatment for localized infections. To some extent, this means that PDT has gone full circle and returned to its roots from when it was first discovered in 1900. In this chapter we discuss, in a contextualized fashion, what are the expected characteristics of an ideal photosensitizer and which are the main molecular frameworks used for development of synthetic, natural, and nanostructured photosensitizers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Skin Photosensitivity
- Skin Rejuvenation
- Photosensitizer Structure
- Deep Tissue Penetration
- Photodynamic Reaction
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction
Photodynamic therapy (PDT) was discovered over 100 years ago when it was observed that certain dyes could kill microorganisms when exposed to light in the presence of oxygen (see Chap. 1). Since those early days, PDT has mainly been developed as a cancer therapy with regulatory approvals and clinical trials steadily accumulating for different types of cancer and different photosensitizer structures, starting with Photofrin® [1]. A very important milestone for PDT was the introduction of 5-aminolevulinic acid (ALA), which functions as a prodrug to induce endogenous porphyrin biosynthesis [2]. See Fig. 3.1 for a depiction of how ALA can induce accumulation of protoporphyrin IX (PpIX) in tissue to which it has been topically applied.
Heme biosynthesis cycle and role of ALA-induced PPIX. ALA-S ALA synthase, ALA-D ALA dehydratase, PBG-D porphobilinogen deaminase, UCS uroporphyrinogen synthase, UGD uroporphyrinogen decarboxylase, CPO coproporphyrinogen oxidase, PPO protoporphyrinogen oxidase, FCH ferrochelatase [49]
ALA-PDT and PDT using ALA esters have become mainstays of the clinical dermatologist’s practice covering everything from skin cancer, premalignant lesions, acne, and skin rejuvenation. A second major milestone in the development of PDT was its introduction as a clinical treatment for choroidal neovascularization secondary to age-related macular degeneration. The PS called Visudyne (benzoporphyrin derivative) was injected IV, and soon afterward red light was shone into the back of the eye as a way to destroy proliferating blood vessels in the retina [3]. The third milestone in PDT development was the realization that PDT may also be used as an effective antimicrobial modality and a potential treatment for localized infections [4]. To some extent, this means that PDT has gone full circle and returned to its roots from when it was first discovered in 1900. The antimicrobial applications have been enthusiastically embraced by dentists, who treat periodontitis, peri-implantitis, and endodontics [5]. The fourth milestone in PDT development (which unfortunately has not been widely accepted as yet) is the realization that PDT can stimulate an adaptive and/or innate immune response against both tumors [6] and also against pathogens [7].
3.2 Important Features of Photosensitizers
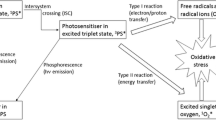
The characteristics of the ideal PS can be summarized as follows. PS should have low levels of dark toxicity to both humans and animals and a low likelihood of adverse pharmacological effects upon administration such as hypotension (decreased blood pressure) or allergic reactions. PS should absorb light in the red or far-red wavelength regions in order for the tissue-damaging effect to reach as deep as possible. It is known that both absorbance and scattering of light are minimized at longer wavelengths that penetrate the tissue deeper. Absorption bands at shorter wavelengths have less tissue penetration and are more likely to lead to skin photosensitivity (the power in sunlight drops off at λ > 600 nm). Absorption bands at too high wavelengths (>800 nm) mean that the photons will not have sufficient energy to promote PS molecules to excited triplet state to allow energy transfer to the ground state oxygen molecule to excite it to the singlet state. They should have relatively high absorption bands (>20,000 M−1cm−1) to minimize the dose of PS and light needed to achieve the desired effect. Synthesis of the PS should be relatively easy and the starting materials readily available to make large-scale production feasible. The PS should be a pure compound with a constant composition and a stable shelf life and be ideally water soluble or soluble in a biocompatible drug delivery vehicle. It should not aggregate unduly in biological environments as this reduces its photochemical efficiency. The pharmacokinetic elimination from the patient should be rapid, i.e., less than 1 day to avoid the necessity for posttreatment protection from light exposure due to prolonged skin photosensitivity. A short interval between injection and illumination is desirable to facilitate outpatient treatment that is both patient-friendly and cost-effective. Pain on treatment is undesirable, as PDT procedures do not usually require anesthesia or heavy sedation.
Although high PDT activity is generally thought to be a good thing, it is possible to have excessively powerful PS that is sometimes considered to be “unforgiving.” With limitations in the effectiveness of both PS and light dosimetry, highly active PS may easily permit treatment overdosage when the surrounding normal tissue is damaged as well as the target tumor forming extensive necrotic areas. It is at present uncertain whether it is better to have a PS “tailored” to a specific indication, and to have families or portfolios of PS designed for various specific diseases or patient types, or alternatively to seek a single PS that works against most diseases. For cancer treatment it has been thought that an ideal PS should selectively accumulate in tumors after intravenous injection. Although the exact mechanisms for this “tumor-localizing effect” are not completely understood, some PS can achieve a 5:1 or higher accumulation in tumors compared to the surrounding normal tissue. Lastly, a desirable feature might be to have an inbuilt method of monitoring PS dosimetry, localization, and following response to treatment by measuring fluorescence and its loss by photobleaching in real time.
Many of the early attempts to kill microorganisms with PDT employed the same photosensitizers that were used for PDT of cancer. However, it was later realized that these structures were not optimal. Because phenothiazinium dyes (such as methylene blue) that have an intrinsic cationic charge were able to photoinactivate many classes of microorganism, it was concluded that the presence of cationic charges was crucial for broad-spectrum antimicrobial effects [8]. Although neutral and anionic PS are able to kill Gram-positive bacteria, in order to kill Gram-negative bacteria, one needs positive charges on the PS to bind and/or penetrate through the outer membrane barrier composed with large amounts of negatively charged lipopolysaccharides.
3.3 Main PS Structures
3.3.1 Tetrapyrroles
The class of tetrapyrrole PS contains (by a considerable margin) the largest number of individual compounds that have been explored for PDT both in the laboratory and also approved and tested clinically. The four most commonly explored backbone structures are porphyrins, chlorins, bacteriochlorins, and phthalocyanines. Reduction of one double bond (green arrow) in a porphyrin leads to a red shift and increase in absorption intensity of the Q-bands (e.g., 600 nm → 660 nm) in the chlorin (name derived from green chlorophyll), while reduction of two double bonds (purple arrows) leads to a further red shift (660 nm → 780 nm) and a intensity increase in the peak of the bacteriochlorin (name derived from the pigment in purple photosynthetic bacteria). The more conjugated nature of the phthalocyanine macrocycle (four additional aromatic rings) leads to a very intense absorption band at 700 nm.
Hematoporphyrin derivative (which later became known as Photofrin® and Photogem®) was approved in 1990 [9] and remains today as the most widely used PS in clinical PDT. As mentioned above, the PpIX production is induced by exogenously administered ALA, a therapeutic strategy widely used by dermatologists [10]. A later pharmacologic strategy uses methylated ALA molecules (M-ALA) to facilitate deeper prodrug diffusion through the skin using topical administration. Other porphyrin derivatives such as hematoporphyrin monomethyl ether (Hemoporfin), 2,4-di(1-methoxyethyl)-deuteroporphyrin-IX (Dimegin), and sinoporphyrin sodium [11] have been tested for PDT of cancer and a wide range of cationic porphyrins for antimicrobial PDT.
Chlorins represent a class of PS containing several examples, which have advanced as far as clinical trials. In addition to mTHPC and BPD mentioned in Table 3.1, 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH), mono-N-aspartyl chlorin (e6) (Npe6) or talaporfin sodium (LS11), photoditazine, and tin(IV) etiopurpurin (SnEt2) are all classified as chlorins. Bacteriochlorins are not as stable as porphyrins and chlorins, and their use has not been as widely studied despite their more advantageous absorption peaks in the infrared region. Besides soluble TOOKAD® (Table 3.1), another compound, LUZ11 (a non-metallated fluorinated bacteriochlorin sulfonamide), has been tested in clinical trials for cancer.
3.3.2 Synthetic Dyes
The dye industry developed a very large number of conjugated heterocyclic compounds with high absorption bands in the visible region of the spectrum (Table 3.2). The vast majority of these dyes were found (or later designed) to be photostable so that they could be used for dyeing fabrics and clothing. However, a few of these dyes that were not photostable but were found to have an appreciable quantum yield to the triplet state that meant they could be used as PS for PDT. There remain some concerns about these synthetic dye compounds on whether they may be darker toxic compared to the tetrapyrrole compounds, which are derived from natural molecules. Most dyes were designed to be water soluble (so clothing could be dyed) that makes them suitable for antimicrobial PDT applications. Moreover, many dyes are cationic and this again encourages their use against a broad spectrum of bacteria (including Gram-negative species). Interestingly, veterinarians already use triarylmethane dyes in some countries to treat local infections and decontaminate fish tank water due to their intrinsic microbicidal activity, even though most people disregard the great antimicrobial activity enhancement that can be provided by photodynamic reactions promoted by the same dyes. In recognition to this potential, we strongly recommend those users to expose the stained water or lesions to either sunlight or artificial light sources.
3.3.3 Other Structures
To illustrate the diversity of different structures that have been investigated as PS in PDT, we will describe some innovative chemical structures (Table 3.3) that have not (yet) progressed as far as those shown in Tables 3.1 and 3.2.
3.3.4 PS Derived from Natural Products
In common with other areas of medicine, in PDT there is also a movement toward the use of natural products and away from synthetic drugs produced by “Big Pharma.” The idea is that natural product-derived PS will have less overall toxicity and fewer side effects (Table 3.4). However, there may be a flaw in that argument in that since nearly all life-forms have evolved in sunlight, they will be unlikely to contain very powerful PS, as the consequent phototoxicity caused by sun exposure would have been selected against.
3.3.5 Inorganic Structures
Inorganic compounds have occasionally been reported to be able to carry out PDT. Two examples are given in Table 3.5. Titanium dioxide (TiO2) presents great photostability and preferentially undergoes through type 1 photodynamic reactions (see Chap. 2). Because it is colorless but efficiently absorbs UV light, construction companies have been using TiO2 to coat external walls of buildings forming a self-cleaning surface. As a surprising consequence, even the air quality has been reported to be better in the surroundings of TiO2-coated buildings adding an environmental appeal for its large-scale application.
Transition metal complexes, such as [Ru(bpy)3]2+, are probably the most studied structures by photophysicists and photochemists over the past 40 years. Other metals such as cobalt and chromium are also often used for the same purposes. Due to spin-orbital coupling governed by the so-called heavy atom effect, intersystem crossing efficiency is extremely favored and can reach values above 99 %. This characteristic makes transition metal complexes particularly potent as PS since their singlet oxygen yield is in general the greatest possible.
3.3.6 Nanostructures
The use of nanotechnology in PDT can be broadly divided into different approaches, for example, when the nanoparticle (NP) is itself the PS (fullerenes, gold NP, silver NP, quantum dots), when the nanoparticle can absorb light to improve PDT efficiency (quantum dots, silver NP, gold NP, upconversion NP), and when the NP acts as a nano-drug delivery vehicle to improve solubility or targeting (dendrimers, liposomes, mesoporous silica NP) (see Chap. 14). Examples of these classes are shown in Table 3.6.
3.3.7 PS Targeting Using Conjugates with Ligand-Receptor Recognition
Since the mechanism of preferential accumulation of various PS in tumors (or other pathological lesions) is not completely understood [38], it is rather challenging to design a PS with a high inherent ability for tumor targeting. However, there is a valid alternative approach to increase the specificity of PS for tumors. This relies on covalent conjugation of PS to various targeting ligands (either macromolecules or small molecules) that show specific molecular recognition ability with some cognate receptor that is over-expressed on tumor or other cells of interest. The advantage is that the PS can be chosen solely based on its photochemical ROS (reactive oxygen species) generation and ability to be conjugated, and the tumor-targeting component can be provided by the ligand. Examples of this ligand-receptor interaction are given in Table 3.7.
3.3.8 Nonlinear Excitation (Two-Photon, Upconversion, Second Harmonic, and Four-Wave Mixing)
The use of long wavelength light (600–1300 nm) provides deeper tissue penetration. Although some PS that absorb in the 700–800 nm range have been described, photons at wavelengths longer than 800 nm do not have enough energy to promote generation of singlet oxygen. There have been some innovative approaches developed to overcome this limitation. The most well-studied is two-photon absorption in which a very short-pulsed laser (ideally below 100 fs pulse duration) can deliver photons with such high peak power that the chances of two of them being absorbed at the same time by the PS become non-negligible [46]. The next most studied is photon upconversion by rare-earth nanoparticles that change NIR laser (often 800 or 980 nm) to visible or ultraviolet light, providing deep tissue penetration allied to site-specific generation of short wavelength light [47]. Optical upconversion can also be accomplished by second-harmonic generation in collagen, and four-wave mixing approaches, including coherent anti-Stokes Raman scattering [48].
References
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–81.
Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci. 2008;7(3):283–9.
Verteporfin Roundtable P. Guidelines for using verteporfin (Visudyne) in photodynamic therapy for choroidal neovascularization due to age-related macular degeneration and other causes: update. Retina. 2005;25(2):119–34.
Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–50.
Santin GC, Oliveira DS, Galo R, Borsatto MC, Corona SA. Antimicrobial photodynamic therapy and dental plaque: a systematic review of the literature. ScientificWorldJournal. 2014;2014:824538.
Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535–45.
Huang YY, Tanaka M, Vecchio D, Garcia-Diaz M, Chang J, Morimoto Y, et al. Photodynamic therapy induces an immune response against a bacterial pathogen. Expert Rev Clin Immunol. 2012;8(5):479–94.
Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol B. 1996;32(3):153–7.
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905.
Kostovic K, Pastar Z, Ceovic R, Mokos ZB, Buzina DS, Stanimirovic A. Photodynamic therapy in dermatology: current treatments and implications. Coll Antropol. 2012;36(4):1477–81.
Wang H, Wang X, Zhang S, Wang P, Zhang K, Liu Q. Sinoporphyrin sodium, a novel sensitizer, triggers mitochondrial-dependent apoptosis in ECA-109 cells via production of reactive oxygen species. Int J Nanomedicine. 2014;9:3077–90.
Kessel D. Photosensitization with derivatives of haematoporphyrin. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49(6):901–7.
Senge MO, Brandt JC. Temoporfin (Foscan(R), 5,10,15,20-tetra(m-hydroxyphenyl)chlorin) – a second-generation photosensitizer. Photochem Photobiol. 2011;87(6):1240–96.
Chan WM, Lim TH, Pece A, Silva R, Yoshimura N. Verteporfin PDT for non-standard indications – a review of current literature. Graefes Arch Clin Exp Ophthalmol. 2010;248(5):613–26.
Moore CM, Azzouzi AR, Barret E, Villers A, Muir GH, Barber NJ, et al. Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU Int. 2015;116(6):888–96.
Anderson CY, Freye K, Tubesing KA, Li YS, Kenney ME, Mukhtar H, et al. A comparative analysis of silicon phthalocyanine photosensitizers for in vivo photodynamic therapy of RIF-1 tumors in C3H mice. Photochem Photobiol. 1998;67(3):332–6.
Wainwright M, Crossley KB. Methylene blue – a therapeutic dye for all seasons? J Chemother. 2002;14(5):431–43.
Cincotta L, Foley JW, MacEachern T, Lampros E, Cincotta AH. Novel photodynamic effects of a benzophenothiazine on two different murine sarcomas. Cancer Res. 1994;54(5):1249–58.
Ali MF. Topical delivery and photodynamic evaluation of a multivesicular liposomal Rose Bengal. Lasers Med Sci. 2011;26(2):267–75.
Kawamoto KS,N, Shimada K, Ito K, Hirano Y, Murai S. Antibacterial effect of yellow He-Ne laser irradiation with crystal violet solution on porphyromonas gingivalis: an evaluation using experimental rat model involving subcutaneous abscess. Lasers Med Sci. 2000;15(4):5.
Prates RA, Yamada Jr AM, Suzuki LC, Eiko Hashimoto MC, Cai S, Gouw-Soares S, et al. Bactericidal effect of malachite green and red laser on Actinobacillus actinomycetemcomitans. J Photochem Photobiol B Biol. 2007;86(1):70–6.
Shafeekh KM, Soumya MS, Rahim MA, Abraham A, Das S. Synthesis and characterization of near-infrared absorbing water soluble squaraines and study of their photodynamic effects in DLA live cells. Photochem Photobiol. 2014;90(3):585–95.
Rice DR, Gan H, Smith BD. Bacterial imaging and photodynamic inactivation using zinc(ii)-dipicolylamine BODIPY conjugates. Photochem Photobiol Sci. 2015;14(7):1271–81.
Cieplik F, Spath A, Regensburger J, Gollmer A, Tabenski L, Hiller KA, et al. Photodynamic biofilm inactivation by SAPYR – an exclusive singlet oxygen photosensitizer. Free Radic Biol Med. 2013;65:477–87.
Theodossiou TA, Hothersall JS, De Witte PA, Pantos A, Agostinis P. The multifaceted photocytotoxic profile of hypericin. Mol Pharm. 2009;6(6):1775–89.
Maisch T, Eichner A, Spath A, Gollmer A, Konig B, Regensburger J, et al. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PLoS ONE. 2014;9(12):e111792.
Tortik N, Steinbacher P, Maisch T, Spaeth A, Plaetzer K. A comparative study on the antibacterial photodynamic efficiency of a curcumin derivative and a formulation on a porcine skin model. Photochem Photobiol Sci. 2016;15(2):187–95.
Lei W, Zhou Q, Jiang G, Zhang B, Wang X. Photodynamic inactivation of Escherichia coli by Ru(II) complexes. Photochem Photobiol Sci Off J Eur Photochem Assoc Eur Soc Photobiol. 2011;10(6):887–90.
El-Hussein A, Mfouo-Tynga I, Abdel-Harith M, Abrahamse H. Comparative study between the photodynamic ability of gold and silver nanoparticles in mediating cell death in breast and lung cancer cell lines. J Photochem Photobiol B. 2015;153:67–75.
Chu CK, Tu YC, Hsiao JH, Yu JH, Yu CK, Chen SY, et al. Combination of photothermal and photodynamic inactivation of cancer cells through surface plasmon resonance of a gold nanoring. Nanotechnology. 2016;27(11):115102.
Meziani MJ, Dong X, Zhu L, Jones LP, LeCroy GE, Yang F, et al. Visible-light-activated bactericidal functions of carbon “Quantum” dots. ACS Appl Mater Interfaces. 2016;8(17):10761–6.
Li S, Chang K, Sun K, Tang Y, Cui N, Wang Y, et al. Amplified singlet oxygen generation in semiconductor polymer dots for photodynamic cancer therapy. ACS Appl Mater Interfaces. 2016;8(6):3624–34.
Jin S, Zhou L, Gu Z, Tian G, Yan L, Ren W, et al. A new near infrared photosensitizing nanoplatform containing blue-emitting up-conversion nanoparticles and hypocrellin A for photodynamic therapy of cancer cells. Nanoscale. 2013;5(23):11910–8.
Spyropoulos-Antonakakis N, Sarantopoulou E, Trohopoulos PN, Stefi AL, Kollia Z, Gavriil VE, et al. Selective aggregation of PAMAM dendrimer nanocarriers and PAMAM/ZnPc nanodrugs on human atheromatous carotid tissues: a photodynamic therapy for atherosclerosis. Nanoscale Res Lett. 2015;10:210.
Bastien E, Schneider R, Hackbarth S, Dumas D, Jasniewski J, Roder B, et al. PAMAM G4.5-chlorin e6 dendrimeric nanoparticles for enhanced photodynamic effects. Photochem Photobiol Sci. 2015;14(12):2203–12.
Senge MO. mTHPC – a drug on its way from second to third generation photosensitizer? Photodiagnosis Photodyn Ther. 2012;9(2):170–9.
Tu J, Wang T, Shi W, Wu G, Tian X, Wang Y, et al. Multifunctional ZnPc-loaded mesoporous silica nanoparticles for enhancement of photodynamic therapy efficacy by endolysosomal escape. Biomaterials. 2012;33(31):7903–14.
Hamblin MR, Newman EL. On the mechanism of the tumour-localising effect in photodynamic therapy. J Photochem Photobiol B. 1994;23(1):3–8.
Goff BA, Hermanto U, Rumbaugh J, Blake J, Bamberg M, Hasan T. Photoimmunotherapy and biodistribution with an OC125-chlorin immunoconjugate in an in vivo murine ovarian cancer model. Br J Cancer. 1994;70(3):474–80.
Kascakova S, Hofland LJ, De Bruijn HS, Ye Y, Achilefu S, van der Wansem K, et al. Somatostatin analogues for receptor targeted photodynamic therapy. PLoS ONE. 2014;9(8):e104448.
Demidova TN, Hamblin MR. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob Agents Chemother. 2005;49(6):2329–35.
Demidova TN, Hamblin MR. Macrophage-targeted photodynamic therapy. Int J Immunopathol Pharmacol. 2004;17(2):117–26.
You H, Yoon HE, Jeong PH, Ko H, Yoon JH, Kim YC. Pheophorbide-a conjugates with cancer-targeting moieties for targeted photodynamic cancer therapy. Bioorg Med Chem. 2015;23(7):1453–62.
El-Akra N, Noirot A, Faye JC, Souchard JP. Synthesis of estradiol-pheophorbide a conjugates: evidence of nuclear targeting, DNA damage and improved photodynamic activity in human breast cancer and vascular endothelial cells. Photochem Photobiol Sci. 2006;5(11):996–9.
Ballut S, Makky A, Chauvin B, Michel JP, Kasselouri A, Maillard P, et al. Tumor targeting in photodynamic therapy. From glycoconjugated photosensitizers to glycodendrimeric one. Concept, design and properties. Org Biomol Chem. 2012;10(23):4485–95.
Zou Q, Zhao H, Zhao Y, Fang Y, Chen D, Ren J, et al. Effective two-photon excited photodynamic therapy of xenograft tumors sensitized by water-soluble bis(arylidene)cycloalkanone photosensitizers. J Med Chem. 2015;58(20):7949–58.
Zhang X, Ai F, Sun T, Wang F, Zhu G. Multimodal upconversion nanoplatform with a mitochondria-targeted property for improved photodynamic therapy of cancer cells. Inorg Chem. 2016;55(8):3872–80.
Kachynski AV, Pliss A, Kuzmin AN, Ohulchanskyy XTY, Baev A, Qu J, et al. Photodynamic therapy by in situ nonlinear photon conversion. Nat Photonics. 2014;8:455–61.
Gryson O. Servier medical art France: servier. 2016. Available from: http://www.servier.com/Powerpoint-image-bank.
Acknowledgments
MR Hamblin was supported by US NIH grant R01AI050875.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hamblin, M.R., Sabino, C.P. (2016). Photosensitizers. In: Sellera, F., Nascimento, C., Ribeiro, M. (eds) Photodynamic Therapy in Veterinary Medicine: From Basics to Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-319-45007-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-45007-0_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45006-3
Online ISBN: 978-3-319-45007-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)