Abstract
The pulmonary system develops from a series of complex events that involve coordinated growth and differentiation of distinct cellular compartments. After lung specification of the anterior foregut endoderm, branching morphogenesis occurs generating an intricate arrangement of airways. This process depends on epithelial-mesenchymal interactions tightly controlled by a network of conserved signaling pathways. These signaling events regulate cellular processes and control the temporal-spatial expression of multiple molecular players that are essential for lung formation. Additionally, remodeling of the extracellular matrix establishes the appropriate environment for the delivery of diffusible regulatory factors that modulate these cellular processes. In this chapter, the molecular mechanisms underlying avian lung development are thoroughly revised. Fibroblast growth factor, WNT, sonic hedgehog, transforming growth factor-β, bone morphogenetic protein, vascular endothelial growth factor, and regulatory mechanisms such as microRNAs control cell proliferation, differentiation, and patterning of the embryonic chick lung. With this section, we aim to provide a snapshot of the current knowledge of the molecular aspects of avian lung development.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 General Considerations
The avian respiratory tract is a remarkably efficient system that accurately responds to the high metabolic rate of birds. It is constituted by the parabronchial lung that is involved in gas exchange and air sacs which control air movements. During development, the larynx, trachea, and lungs originate from the gut and are constituted by a thin layer of endoderm surrounded by dense mesoderm. To obtain a fully functional organ, a series of highly regulated molecular interactions must occur between these two cellular compartments to give rise to the parabronchial tree (endoderm-derived) and the muscles, connective tissue, and blood and lymphatic vessels (mesoderm-derived) (Bellairs and Osmond 2014).
In the chicken embryo, Gallus gallus, the embryonic lung springs up from the laryngotracheal groove around day 3 of incubation as a small, ridgelike outgrowth. After fusing along the ventral midline, it divides into right and left primordial bud looking like a pair of sacs lying on either side of the esophagus (Maina 2003). In the developing lung bud, the primary bronchus (mesobronchus) grows distally, and secondary bronchi sprout laterally from its dorsal surface into the surrounding mesenchyme, in a craniocaudal sequence. This lateral (or monopodial) branching is similar to the domain branching observed during mammalian lung development (Metzger et al. 2008). The initial stages of chick lung development are named according to the number of secondary buds formed: b1 stage corresponds to lungs with only one secondary bronchus, b2 stage presents two secondary bronchi, b3 stage presents three secondary bronchi per mesobronchus, and so on (Moura et al. 2011). Later, secondary bronchi branch and invade the mesenchyme to originate parabronchi (tertiary bronchi) that will then anastomose and finally connect the secondary bronchi. In the chick lung, the air sacs, a cyst-like structure, are formed ventrally during development as dilations of the mesobronchi and are named according to their position along the craniocaudal axis of the primary bronchus (cervical, intra-clavicular, thoracic, or abdominal) (Bellairs and Osmond 2014). Altogether, these processes depend on epithelial-mesenchymal interactions that rely on the activity of conserved signaling pathways that ultimately regulate cell proliferation, migration, and differentiation and that contribute to proper lung development.
5.2 Lung Specification
The epithelial compartment of the avian respiratory tract has its origin in endoderm progenitor cells of the foregut. This primitive structure possesses regional anterior-posterior (AP) information due to the differential expression of unique transcription factors (in visceral mesoderm and endoderm) that defines discrete domains that will then give rise to different organ primordia. The establishment of respiratory cell fate, in the case of the mammalian lung, is associated with the endodermal expression of ttf-1 (thyroid transcription factor-1, or nkx2.1) that provides AP and dorsoventral (DV) positional information to the gut endoderm (Lazzaro et al. 1991; Minoo et al. 1999). Similarly, nkx2.1 endodermal expression distinguishes the respiratory progenitors as early as the HH14 stage in the chick embryo (Sakiyama et al. 2003). Additionally, tbx4 (a member of the T-box transcription factor family) is specifically expressed in the mesoderm surrounding the prospective lung primordium, indicating that it is involved in the initial specification of lung mesoderm (Sakiyama et al. 2003). After the establishment of the lung primordium, TBX4 can induce endodermal budding by eliciting fgf10 (fibroblast growth factor 10) expression, thus defining its AP mesodermal expression domain, and triggers nkx2.1 expression, hence inducing endoderm differentiation; in fact, tbx4-fgf10 expression overlaps with the posterior border of the nkx2.1 expression domain at early lung development. Conversely, nkx2.1 expression before the appearance of the lung bud primordium does not seem to be regulated by the tbx4-fgf10 system. Furthermore, the tbx4-fgf10 system is essential for the anatomic separation of the respiratory tract and the esophagus, namely, for the formation of the tracheoesophageal septum (Sakiyama et al. 2003).
nkx2.1 is expressed in the respiratory epithelial cells (trachea and main bronchi) of the early lung bud and all through embryonic lung development (Zeng et al. 1998). The hepatocyte nuclear factor 3β (HNF-3β) that plays a major role in foregut endoderm formation is also expressed in endodermal derivatives at the onset of lung bud formation. It was demonstrated that HNF-3β can activate nkx2.1 in vitro. The fact that they coexist spatially in early lung organogenesis may indicate a possible role of these transcription factors in the specification of respiratory epithelium along the foregut axis (Zeng et al. 1998).
5.3 Dorsal-Ventral Patterning of the Chick Lung
The avian lung is organized in two distinct morphological and physiological regions, the bronchial tree and the air sacs (cyst-like) that appear dorsally and ventrally, respectively. The regional cyst-branch difference is defined early in development through the coordinated mesenchymal expression of hoxb genes (Sakiyama et al. 2000). Moreover, the disparity between the dorsal and ventral side of the chick lung might also be explained by changes in the diffusion rate of morphogens (namely, FGF10) as a result of local extracellular matrix modifications (Miura et al. 2009).
5.3.1 Hox Genes
hox genes, which are clustered on four chromosomes, are a family of transcription factors that specify local differences along the AP axis, therefore guiding the axial regionalization of the vertebrate embryo. There is an association between their location in the chromosome and the timing of their expression during embryogenesis (temporal collinearity) and the position of their expression domain along the AP axis (spatial collinearity). This characteristic temporal-spatial distribution is crucial for patterning the vertebrate body plan since it provides the correct identity to each vertebra (Wellik 2007).
In the chick respiratory tract, hoxb-5 to hoxb-9 genes are expressed in restricted domains and regulate the establishment of regional morphological subdivisions. Before the commencement of bronchial branching, the mesenchymal expression domain of hoxb-5, hoxb-6, hoxb-7, and hoxb-8 exhibits a nested pattern with 5′-located genes on the cluster displaying a more ventral-distal restricted domain. Moreover, in the lung primordium, hoxb-6 expression domain defines the identity of ventral vs. dorsal pulmonary mesenchyme that, consequently, presents different inductive ability regarding the epithelium (air saclike vs. branching morphogenesis, respectively). At later stages of lung development, the expression domains of these genes seem to correlate with the morphological subdivisions of the bronchial tree and the air sacs. hoxb genes might be upstream regulators of the cytodifferentiation of both mesenchyme and epithelium, and might have a role in the determination of the appropriate morphological subdivisions along the proximodistal axis of the lung (Sakiyama et al. 2000).
5.3.2 Diffusion Gradient
The pattern difference between dorsal and ventral chick lung can be explained by the different levels of mesenchymal FGF10, which are a consequence of different morphogen diffusion rates on both sides. FGF10 protein, a chemoattractant of lung epithelium (Bellusci et al. 1997; Park et al. 1998), is present in the dorsal (branched) lung mesenchyme forming a proximal-distal gradient, whereas its expression is weak in the ventral (cystic) mesenchyme. Additionally, the ventral tissue is looser than the dorsal one because it has lower cell density, causing FGF10 to diffuse faster in that region (Miura et al. 2009). On the other hand, the presence of elevated levels of heparan sulfate proteoglycan (HSPG) on the dorsal side, when compared to the ventral region, limits FGF10 diffusion (causing its accumulation). The dorsal-ventral difference in tissue architecture and HSPG concentration contributes to the difference of FGF10 diffusion coefficient between the two regions and is responsible for the developmental dichotomy that leads to branching morphogenesis dorsally and cyst formation ventrally. These findings suggest a possible mechanism as how HOXb transcription factors regulate cyst-branch morphology, probably through the regulation of HSPG expression levels and, eventually, cell proliferation in chick lung mesenchyme cells.
5.4 Branching Morphogenesis
After the establishment of the lung primordium, primary bronchi lengthen caudally and, from the epithelium, secondary bronchi bud laterally into the adjacent mesenchyme in a process known as branching morphogenesis. The new branches appear on the dorsal side of the primary bronchus in an anterior-posterior sequence whereas the mesobronchus continues to extend distally. It has been shown by tissue recombination that the mesenchyme governs the branching pattern of the epithelial primordium of the chick lung (Hilfer et al. 1985), suggesting that epithelial-mesenchymal interactions are crucial for chick lung development. In vitro lung explant cultures have been widely used to determine the role of different growth factors in epithelial branching and mesenchyme morphogenesis. One of the first examples is the work of Goldin and Opperman (1980) who reported the induction of supernumerary tracheal buds by epidermal growth factor (EGF). It has been successively demonstrated that tissue cross talk relies on the activation of conserved signaling pathways that control cellular processes such as extracellular matrix remodeling, growth, and differentiation, in a temporal-spatial manner.
5.4.1 Secondary Bud Initiation
The epithelium of the mesobronchus must go through a synchronized bending process to originate a new secondary bud, which requires the generation of forces conveyed among adjacent cells. It has been demonstrated that monopodial branching is initiated by the contraction of the apical/lumen side of epithelial cells (Kim et al. 2013). Apical constriction is driven by a contractile actomyosin network that triggers the narrowing of cellular apices of the dorsal airway epithelium, which causes tissue deformation and culminates with the appearance of a new bud. In fact, the inhibition of actomyosin contractility reduced the initiation of new buds. Moreover, in vitro FGF signaling inhibition by SU5402 (an FGF receptor antagonist) inhibits lung branching (Moura et al. 2011; Kim et al. 2013) and blocks apical constriction (Kim et al. 2013). Taken together these results suggest that, in the avian lung, apical actomyosin contraction might be FGF signaling dependent. Curiously, despite the high proliferation levels observed along the primary bronchus, blocking cell cycle progression in the mesobronchus did not affect the bud initiation process; nevertheless, proliferation might have a morphogenetic role within and adjacent to the new branches. Notwithstanding, apical constriction and differential cell proliferation are not enough to completely shape the developing lung bud (Kim et al. 2013), pointing to a potential physical role for the mesenchyme (Blanc et al. 2012).
5.4.2 Extracellular Matrix
For branching morphogenesis to occur, the extracellular matrix (ECM) undergoes a series of remodeling processes to suit epithelial growth. The particular composition of ECM throughout lung development directly affects the availability and, consequently, the activity of soluble factors since it influences not only diffusion rates but also the accessibility of their cognate receptors.
The basement membrane, which lies at the interface between epithelial and mesenchymal compartment, has two main components: the basal lamina, directly adjacent to the basal surface of the epithelial cells, and the fibrillar meshwork containing type I collagen. During branching morphogenesis, the basal lamina becomes thinner at the tip of newly formed branches due to reduced glycosaminoglycan (GAG) synthesis. In fact, decreased levels of hyaluronic acid (HA), chondroitin sulfate (CS), keratan sulfate (KS), and collagen are observed at the tip (Gallagher 1986; Abbott et al. 1991). On the other hand, in the interbud region, there is an increase in collagen deposition and GAG accumulation due to stabilization by the collagen (Gallagher 1986). Regarding the mesenchymal compartment, GAGs and glycoproteins (GPs) present a distinct distribution pattern throughout development. At early branching stages, mesenchymal ECM displays visible amounts of HA in the growing regions, near the main bronchus and the branch tip, and this pattern is repeated every time a new bud is formed. As the new branch grows, the surrounding mesenchyme becomes enriched in CS. Later in development, differentiated secondary bronchi accumulate mainly GPs (Becchetti et al. 1988).
At points where a secondary branch will emerge, mesenchymal cells adjoining lung epithelium are flattened, and this feature seems to be correlated with bud initiation. It has been suggested that modification of cell shape might be responsible for the loss of collagen observed in the basement membrane, probably due to the presence of collagenase. This structural weakening facilitates the bulging of new buds. After bud initiation, the new branch must elongate into the surrounding mesenchyme which encompasses cell proliferation. The accumulation of tenascin in the mesenchyme at the incipient bud tip, and in the distal mesobronchus tip, unveils a potential role for this molecule in bud extension (Abbott et al. 1991).
Throughout lung development, GAG/GP levels and distribution (in both mesenchymal ground substance and basement membrane) vary in a spatial-temporal manner to enable epithelial morphogenesis and, later on, the differentiation of thin structures (like the air-blood barrier). There are several molecules capable of changing ECM composition and that hence contribute to the regulation of the branching pattern. For instance, it has been demonstrated, in vitro, that polyamines (PAs) promote sulfate GAG accumulation in the mesenchymal compartment and have a positive effect on lung branching. It has been suggested that PAs are involved in the signal transduction cascade of TGFβ1 (transforming growth factor beta 1) (Evangelisti et al. 1997); TGFβ1 stimulates mesenchyme sulfate GAG deposition but has an opposite effect on lung branching (Stabellini et al. 2001).
On the other hand, ECM remodeling also involves degradation processes through the action of lysosomal enzymes, glycosidases that specifically degrade GAGs. In the chick lung, glycosidase activity is tightly associated with epithelial proliferation, bronchial tubule lengthening, and infiltration of the surrounding mesenchyme. There is a relationship between HA/CS/HS mesenchymal levels and the expression of glycosidases that specifically degrade each of these glycosaminoglycans and alter non-sulfated/sulfated GAG ratio (Stabellini et al. 2002; Calvitti et al. 2004). Enzyme activity, and consequently ECM features, is regulated by growth factors such as TGFβ2 and interleukin-1 (IL-1) that are known to promote tissue stabilization or growth, respectively. In fact, the expression pattern of these growth factors colocalizes with specific glycosaminoglycans that support tissue stabilization and growth (Stabellini et al. 2002, 2007; Calvitti et al. 2004).
To summarize, glycosaminoglycan content differs along the craniocaudal axis of the embryonic chick lung contributing to the regulation of cellular events (such as cell proliferation and differentiation). This distinctive spatial-temporal distribution creates the precise extracellular environment needed for the availability and activation of specific growth factors for the lung morphogenesis process.
5.4.3 FGF Signaling
Fibroblast growth factor (FGF) signaling pathway plays critical roles not only during organogenesis but also in the adult organism. FGF canonical ligands are secreted proteins that act in a paracrine fashion by interacting with neighboring FGF receptors (FGFRs). Canonical FGF ligands bind to HSPGs that function as cofactors for their interaction with FGFRs. FGFR1–FGFR4 are receptor tyrosine kinases that activate intracellular signaling cascades that are, in its turn, regulated by specific proteins as, for instance, Sprouty (SPRY) (reviewed by Ornitz and Itoh 2015).
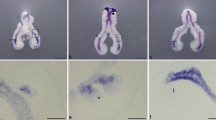
Fibroblast growth factor signaling pathway, specifically FGF10 and its cognate receptor FGFR2, plays a crucial role in lung development (reviewed by El Agha and Bellusci 2014). In the chick lung, the tbx4-fgf10 system is involved in the induction of endodermal budding, and it is essential for the formation of the tracheoesophageal septum (please consult Section 5.2). Additionally, fgf10, fgfr1–fgfr4, and spry2 are expressed at early stages of chick lung branching (Moura et al. 2011). fgf10 and fgfr2 expression pattern is in agreement with the mammalian fetal lung suggesting a similar role for mesenchymal FGF10 as a proliferative factor that stimulates distal epithelial growth through the activation of epithelial FGFR2 (Fig. 5.1a, b). Furthermore, in vitro FGF signaling inhibition by SU5402 (an FGF receptor antagonist) elicited the formation of secondary bronchi with a cystic shape without an increase in their number, and a disruption of the mesenchymal tissue (Moura et al. 2011). Moreover, fgfr1 is ubiquitously expressed and may be responsible for capturing proliferative factors in both compartments; fgfr3 has a more proximal expression, whereas fgfr4 is nearly absent at early stages; spry2 is expressed in the distal tip supporting its association with FGF10 signaling center (Fig. 5.1c). This study demonstrates the importance of FGFRs in the epithelial-mesenchymal interactions that determine epithelial branching and mesenchyme growth in early chick lung development. It is plausible to believe that the disruption of the mesenchymal scaffold contributes to the cystic phenotype if one considers that a lower cell density substantially contributes to ventral cyst formation since it increases FGF diffusion rate (Miura et al. 2009). Another member of the FGF family, FGF2, has been described to be diffusely expressed in epithelial and mesenchymal lung cells from as early as day 3.5 (Maina et al. 2003). This expression pattern is in agreement to what is described for fetal rat lung (Han et al. 1992), unveiling a potential, yet unknown role for this ligand in the avian lung.
Expression of FGF signaling members in the embryonic chick lung. Whole-mount in situ hybridization of stage b2 lungs probed with fgf10 (a), fgfr2 (b), and spry2 (c). fgf10 is present in the mesenchyme adjacent to the emerging new buds and in the distal mesenchyme surrounding the main bronchus; fgfr2 is expressed in the distal epithelium of the main bronchus and secondary bronchi; spry2 is present in the peri-epithelial mesenchyme bordering the main bronchus and secondary bronchi. Scale bar: 500 μm. Adapted from Moura et al. (2011)
5.4.4 SHH Signaling
The hedgehog (HH) signaling pathway is crucial for embryonic development and influences the organogenesis of several organs, specifically the lung. Cell surface transmembrane receptor Patched (PTCH) constitutively represses HH signaling because it blocks the activity of G protein-coupled transmembrane protein Smoothened (SMO). Hedgehog ligands, such as sonic hedgehog (SHH), are secreted proteins that bind PTCH, thus releasing SMO inhibition and allowing the expression of glioblastoma (GLI) zinc finger transcription factors that can either activate or inhibit transcription of target genes. Ligand availability regulates SHH signaling pathway since membrane proteins such as PTCH and HHIP (Hedgehog-interacting protein) bind to SHH and limit its diffusion (reviewed by Briscoe and Thérond 2013).
In the chick embryonic lung, shh is present in the trachea at embryonic day (E)6 (Davey et al. 2014) and in the proximal and distal epithelium at E10 (Loscertales et al. 2008). Moreover, in earlier stages (E4.5–E5.5) all the canonical elements of SHH signaling pathway are expressed in the same cellular compartments as their mammalian counterparts, although their proximodistal distribution is slightly changed. shh is expressed in the epithelium of the main bronchus and secondary bronchi, but it is absent from the tip of the growing buds (Fig. 5.2a). smo, ptch1 (Fig. 5.2b), hhip, and gli1 (Fig. 5.2c) expression mirror shh pattern, as it occurs in the mouse fetal lung (Moura et al. 2016). It is expected that SHH signaling is involved in the epithelial-mesenchymal interactions that regulate chick lung branching. In fact, in lung explants, exogenous SHH protein supplementation induces pulmonary hyperplasia mainly due to the expansion of mesenchyme. In vitro inhibition by cyclopamine that inhibits HH signaling through direct interaction with SMO causes a loss of lung epithelial branching which leads to pulmonary hypoplasia (Loscertales et al. 2008). In talpid 3 chicken mutants, which have a defective SHH signaling, lung morphogenesis is seriously disturbed. talpid 3 lungs do not express shh and display a hypoplastic phenotype with severe abnormalities in both epithelial and mesenchymal compartments (Davey et al. 2014). Altogether, these phenotypes disclose a role for SHH signaling in chick lung branching as it occurs in the mammalian lung, although it might not be required for the formation of the tracheoesophageal septum (Litingtung et al. 1998; Pepicelli et al. 1998). In the mammalian lung, FGF and SHH signaling are engaged in a twisted negative feedback interaction that is crucial to secondary branching (Pepicelli et al. 1998; Chuang et al. 2003). In the chick lung, shh is not a downstream target of FGF signaling as it occurs in the mammalian lung (Moura et al. 2016). Notwithstanding, the absence of shh epithelial expression coincides with fgf10 expression regions, whereas the presence/activity of shh corresponds to pulmonary areas without fgf10 mesenchymal expression. FGF-Shh signaling interplay in the mammalian lung promotes the same local fgf10 expression differences that will contribute to proper branching (Chuang et al. 2003). Despite the fact that shh is not FG10-dependent, the regional differences are maintained in the chick lung. The chick lung seems to have some features that are species-specific regarding SHH signaling and that may probably be because avian secondary bronchi undergo epithelial outgrowth without tip splitting.
Expression of SHH signaling members in the embryonic chick lung. Whole-mount in situ hybridization of stage b2 lungs probed with shh (a), ptch1 (b), and gli1 (c). shh is expressed in the tracheal region and in the epithelium except the distal tip of the main bronchus and secondary bronchi; ptch1 and gli1 are detected in the peri-epithelial mesenchyme of the main bronchial tree, and they are absent from the distal mesenchyme. Scale bar: 500 μm. Adapted from Moura et al. (2016)
5.4.5 WNT Signaling
The canonical WNT/β-catenin pathway regulates numerous developmental processes, as for instance cell fate and morphogenesis. WNT-secreted glycoproteins bind to Frizzled (FZD) transmembrane receptors and their co-receptors LRP5/LRP6, low-density lipoprotein receptor-related protein (LRP). The WNT-FZD-LRP complex triggers a series of intracellular reactions that finally leads to the stabilization of cytoplasmic β-catenin and its translocation to the nucleus. Once in the nucleus, it binds to TCF transcriptional complexes and regulates gene expression. This signaling pathway is modulated by secreted Frizzled-related proteins (SFRPs) and Dickkopf (DKK) proteins (reviewed by Baarsma et al. 2013). The noncanonical Wnt signaling is β-catenin independent and activates planar cell polarity (PCP) and WNT/Ca2+ pathways, either via FZD receptors or other receptors, including the orphan tyrosine kinase ROR2 (Semenov et al. 2007).
Several WNT ligands (wnt-1, wnt-2b, wnt-3a, wnt-5a, wnt-7b, wnt-9a) are expressed in early stages of chick lung development (Moura et al. 2014). Their expression is, overall, in agreement with the mammalian counterparts and uncovers a similar function for these ligands in the avian pulmonary system. For instance, wnt-7b is expressed in the developing airway epithelium (Fig. 5.3a) as it occurs in the mouse model (Shu et al. 2002). Likewise, lrp5, lrp6, sfrp1, dkk1, β-catenin (Fig. 5.3b), and axin2 (Fig. 5.3c) are also expressed at early stages of chick lung development. Moreover, phospho/non-phospho forms of LRP6 and β-catenin are present in this embryonic tissue, which proves that WNT signaling is active. In vitro inhibition of canonical WNT signaling leads to an impairment of lung branching as it happens with the mammalian lung. Collectively, these results indicate that canonical WNT signaling is implicated in the molecular mechanisms required for chick lung branching morphogenesis (Moura et al. 2014). Branching morphogenesis is an intricate process that relies on a network of conserved signaling pathways that regulate each other’s components to fine-tune the overall process. In this sense, a WNT-FGF cross talk is not unforeseen. In fact, WNT signaling inhibition induces a decrease of spry2 (the downstream target of FGF signaling) in the chick lung, and it acts upstream FGF signaling (Moura et al. 2014).
Expression of WNT signaling members in the embryonic chick lung. Whole-mount in situ hybridization of stage b2 lungs probed with wnt-7b (a), lrp6 (b), and β-catenin (c). wnt-7b is expressed all over the pulmonary epithelium of the main bronchus, markedly in the distal tip and the secondary bronchi; lrp6 is present in lung mesothelium; β-catenin is expressed throughout all lung mesenchyme and in the epithelial tip of secondary buds and main bronchus. Scale bar: 500 μm. Adapted from Moura et al. (2014)
Noncanonical WNT signaling seems to be involved in mid-developmental stages of chick lung development. In the developing avian lung, WNT-5a acts noncanonically, through the ROR2 receptor, to regulate pulmonary distal airway and vasculature development (Loscertales et al. 2008). Misexpression of wnt-5a results in changes of the vascular pattern, lung hypoplasia, and altered expression patterns of shh, fgf10, bmp4 (bone morphogenetic protein 4), fibronectin, and vegf (vascular endothelial growth factor). wnt-5a most likely guides pulmonary vascular patterning through the regulation of VEGF pathway via its effect on fibronectin. Branching dysfunction is probably due to an increase in fibronectin levels and a decrease in cell adhesion. The hypoplastic phenotype is rescued by SHH supplementation or inhibition of fibronectin function. These results indicated that wnt5a acts upstream of shh (in addition to fgf10 and bmp4) and that fibronectin levels are regulated directly by the WNT ligand and indirectly through its regulation of SHH. wnt-5a might also have a role in tracheal morphogenesis and, eventually, in the inhibition of branching in this particular region since it is expressed in the tracheal mesenchyme around E5 (Sakiyama et al. 2000; Moura et al. 2014). In fact, wnt-5a -/- mouse lungs display a reduced number of cartilage rings that cause the reduction of the trachea size (Li et al. 2002).
5.4.6 TGFβ-BMP Signaling
The transforming growth factor-β (TGFβ) superfamily of secreted cytokines plays critical roles in a diverse set of cellular processes during embryogenesis as well as in mature tissues. Bone morphogenetic proteins (BMPs) constitute the largest subgroup of the TGFβ superfamily. TGFβ and BMP ligands bind to specific membrane receptors that transmit the signal to intracellular SMAD proteins that then trigger the appropriate cellular response by regulating the transcription of target genes. Typically, these cytokines act as inhibitory morphogens (Massagué 2012).
In the embryonic chick lung, TGFβ1 and BMP4 influence branching and growth of the airways in a different manner (Gleghorn et al. 2012). In vitro supplementation with recombinant TGFβ1 or a TGFβ receptor inhibitor causes a decrease/increase in branching, respectively, and in both cases a reduction in lung size. On the other hand, BMP4 supplementation has no effect on branching, but it diminishes organ growth; BMP signaling inhibition significantly reduces branching and causes an increase in overall lung growth. In conclusion, TGFβ1 preferentially prevents lung branching, while BMP4 affects lung growth, and together contribute to defining the dynamics of lung morphogenesis. Despite the alterations in the gross morphology of the lungs, the relative positioning of secondary buds along the primary bronchi remains unaltered which means that branch sites scale according to the size of the organ (rather than being established at fixed positions) (Gleghorn et al. 2012). tgfβ1 has also been described as a potential target of regulating nuclear factor-kB (NF-kB) (Muraoka et al. 2000). NF-kB increased mesenchymal activity leads to a decrease in epithelial branching, a decrease in cellular proliferation, and an increase in mesenchymal tgfβ1 expression which most likely exerts an inhibitory action on the adjacent epithelium. This mechanism is in agreement with TGFβ1 inhibitory role described by Gleghorn et al. (2012). Conversely, hyperactivation of mesenchymal NF-kB repressed mesenchymal fgf10 and bmp4 expression which contributes to epithelial branching inhibition (Muraoka et al. 2000). This mechanism is a clear example of the epithelial-mesenchymal interactions that take place during branching morphogenesis, in which the transcriptional regulation of growth factors produced in the proximal mesenchyme affects epithelial branching of the neighboring region.
5.4.7 miRNAs
MicroRNAs (miRNAs) have emerged as important regulators of development. These small noncoding RNAs function posttranscriptionally by interacting with the 3′ untranslated region of specific mRNAs in a sequence-specific manner and, therefore, cause mRNA degradation or translational inhibition. Mature miRNAs are formed by a multistep process that involves different enzymes and proteins: DROSHA/DGCR8 nuclear complex, exportin-5 nuclear membrane protein, and cytoplasmic DICER enzyme (reviewed by Ivey and Srivastava 2015). There is also an alternative noncanonical miRNA biosynthesis pathway that can be DROSHA/DGCR8 independent or DICER independent (Abdelfattah et al. 2014).
It has been recently shown that miRNA processing machinery is expressed in the developing chick lung, supporting the previously recognized regulatory role of this mechanism in epithelial and mesenchymal morphogenesis (Moura et al. 2015). The dorsal and distal mesenchymal expression of the biogenesis machinery may well indicate a potential regulatory role in mesenchymal morphogenesis, through the regulation of specific genes. exportin-5 and dicer1 are also expressed on the apical side of the distal lung epithelium and emerging secondary bronchi, which points toward the existence of a noncanonical miRNA pathway in the chick embryonic lung (Moura et al. 2015).
Sanford et al. (2016) have revealed that miR-449a is expressed in the distal lung epithelium, and not in the mesenchyme, at embryonic day 12. Moreover, they showed that cdc20b (a proxy for chick miR-449a) expression was highest at E20 and that transcription factor N-Myc (a predicted target for this miRNA and related with the proliferation of undifferentiated progenitors) was negatively correlated with CDC20B/miR-449a. miR-449a overexpression, by in ovo retroviral infection, disrupted lung growth leading to lung hypoplasia. At E13, chick lungs displayed a decreased number of airways. At E15, less severe affected lungs presented reduced proliferation levels and compromised airway epithelial differentiation. This study unveiled a role for miR-449a in the regulation of chick lung differentiation and proliferation most likely through N-Myc (Sanford et al. 2016).
miR-15a seems to be associated with the mechanisms that are responsible for hypoxia-induced changes in lung development, probably through the translational inhibition of the antiapoptotic protein BCL-2 (Hao et al. 2014). miR-15a expression has its peak around E19-20 that coincides with the formation of the crosscurrent gas exchange system (CCGS); at the same time, BCL-2 protein levels are diminished. In the embryonic chick lung, the expression of miR-15a is induced by hypoxia stress, whereas BCL-2 protein levels decline. Hao et al. (2014) demonstrated that chicken miR-15a posttranscriptionally silences chick bcl-2. In this scenario, antiapoptotic mechanisms are inhibited causing an increase in mesenchymal apoptosis. The reduction in the mesenchymal compartment is necessary for the development of a thin blood-gas barrier that determines, together with CCGS, the avian lung air-diffusing capacity. The hypoxia-induced miR-15a-bcl-2 system mediates CCGS/BGB establishment and is essential for understanding the adaptational development of the chick lung (Hao et al. 2014).
5.5 Surfactant Synthesis
Pulmonary surfactant is constituted by a combination of lipids and proteins that reduce surface tension, therefore, preventing mammalian lung alveoli to collapse at the end of expiration. The avian lung does not exhibit alveoli. Nonetheless, it contains the surfactant that functions to sustain airflow in the air capillaries (Bernhard et al. 2001).
The major class of phospholipid in avian surfactant is phosphatidylcholine (PC), and, quantitatively, the most important subcomponent is disaturated phosphatidylcholine (DSPC) (Fujiwara et al. 1970), namely, dipalmitoylphosphatidylcholine (PC16:0/16:0). Regarding protein content, it is enriched in surfactant protein B (SP-B), whereas SP-C is absent (Bernhard et al. 2001). Recently, SP-A has been identified as an extracellular component of the lung lining fluid of the avian tertiary bronchi (Zhang et al. 2016). The differences in surfactant protein content most likely reflect the particulars of the avian tubular lung.
At the molecular level, pneumocytes can be distinguished by specific protein markers as, for instance, SP-B for cuboidal type II epithelial cells and aquaporin 5 for type I pneumocytes from E17 onward (Bjørnstad et al. 2014), similarly to the mammalian lung. The transcription of the surfactant genes is controlled by nuclear proteins including TTF-1 and HNF-3β, which can bind to regulatory regions of these target genes (Clevidence et al. 1993; Zhang et al. 1997). In late stages of chick lung development (E15), SP-A and SP-B expression pattern overlaps with TTF-1 and HNF-3β suggesting that the transcription regulatory mechanism might be conserved in the avian lung (Zeng et al. 1998).
At the cellular level, flattened type I pneumocytes that facilitate gas exchange line the avian respiratory membrane. Regarding cuboid (type II) pneumocytes, they are visible around E17 and located in the atrial walls, air sacs, and parabronchi but not in the air capillaries (Bernhard et al. 2001; Maina 2003). It has been demonstrated that avian surfactant, produced by type II cells, is capable of efficiently adsorbing and extending into an air/liquid interface like the air capillaries where it exerts its role (Bernhard et al. 2001). Surfactant-producing cells also present storage organelles for surfactant (known as lamellar bodies) that are visible around E16, increase at E18, and decrease post-hatching (Hylka 1989). Glycogen granules are also present in this cell type, and they are depleted between E14 and post-hatching which support the concept that they may contribute with the precursor material to form pulmonary DSPC; nevertheless, the synthesis of DSPC near the end of incubation may rely upon other substrates as lipids of yolk (Hylka 1989).
5.5.1 Hormonal Regulation
Around E18, before pipping, both PC and DSPC contents increase which indicates the beginning of breathing. This increase coincides with a peak of corticoids, thyroid hormone, and prolactin. Hylka and Doneen (1983) have demonstrated that corticosterone inhibits cellular proliferation (leading to a reduction in lung size) and stimulates surfactant phospholipid synthesis (increasing total pulmonary phospholipid and PC content), comparable to mammals. Moreover, the removal of pituitary by hypophysectomy triggers a decrease in lipid content in the embryonic lung, probably due to delayed appearance of lamellar bodies (Hylka and Doneen 1983). Besides, pituitary hormones seem to be responsible for acquisition of maximum content of glycogen by the lung before day 16 (Hylka 1989).
Thyroid hormones are known to control organ growth and development. Nonetheless, thyroid hormone (TH) has no effect on surfactant production, despite the described increase of thrβ (TH receptor-β) mRNA at E19 (Forrest et al. 1990). This feature might indicate that TH acts by enhancing the sensitivity to glucocorticoids or that its effects were already maximal at the ages tested meaning that further stimulation with exogenous THs does not result in an additional effect (De Groef et al. 2013). Nonetheless, in a chemically induced model of hypothyroidism, lung maturation was hindered, specifically pneumocyte and vascular differentiation (Bjørnstad et al. 2016). TRβ1 expression levels are elevated in lungs of methimazole (MMI)-treated embryos; on the other hand, Kruppel-like factor 2 (klf2) expression levels remain unaltered. KLF2 is a TH-dependent transcription factor involved in type I pneumocyte differentiation program in the mammalian lung (Pei et al. 2011), but that seems to be TH-independent in the avian lung. Notwithstanding, in MMI-induced hypothyroidism, type I (and type II) pneumocyte-specific cell markers are reduced which points to an impairment in pneumocyte differentiation. Moreover, specific miRNAs showed upregulated upon MMI treatment highlighting their role as regulators of developmental lung processes (Bjørnstad et al. 2016).
On the other hand, the embryonic chick lung might function as an extrapituitary production site of pituitary hormones. For instance, luteinizing hormone (LH) has been detected in the trachea and lung of E3–E7 embryos (Shirasawa et al. 1996) and β-TSH (thyrotropin) in the bronchus of E7 developing lung (Murphy and Harvey 2001). Growth hormone (GH) and its receptor (GHR) are also present in the embryonic chick lung as early as E7 (Beyea et al. 2005). Overall these results suggest a potential, so far undetermined, autocrine/paracrine role for these hormones in lung development, before the appearance of the pituitary gland.
5.5.2 Regulation by Oxygen Levels
Surfactant levels are regulated by fetal oxygen tension, and exposure to abnormal levels of oxygen has diverse effects depending on the developmental window in which the insult occurs. In normoxic conditions (21% O2), disaturated phospholipid (DSPL) content increases between E16 and E19 which reflects the physiologic increase in surfactant production in this developmental window. Mild hypoxic conditions (17% O2), from E10 forward, speed up surfactant maturation when compared to normoxic conditions. Moreover, corticosterone levels increase, while T3 levels remain unaltered in response to hypoxia, so it is possible that the maturation process is mediated by glucocorticoids (Blacker et al. 2004).
Furthermore, it has been shown that both chronic prenatal hypoxia (15% O2) and hyperoxia (60% O2) occurring during late stages of development (from E15 onwards) trigger surfactant synthesis (at E19) which points to an acceleration of lung maturation. In these hypoxic conditions (at E16), expression of vegf isoforms 122, 146, and 190 increases transitorily before the stimulation of surfactant production disclosing a potential, yet still concealed, role for VEGF in this process. Conversely, in hyperoxic conditions vegf expression levels remained unaltered. On the other hand, chronic long-term hypoxia (from E6 ahead) does not significantly affect surfactant synthesis and VEGF expression. The maturation of the surfactant system displays a high level of plasticity since it can adjust according to fetal oxygenation (Been et al. 2010).
5.6 Hypoxic Adaptation
Embryonic development is highly dependent on oxygen levels. Hypoxia can lead to profound developmental abnormalities, but the severity of these anomalies depends on the duration and onset of the insult (Zhang and Burggren 2012), so hypoxic adaptation is crucial for the survival of avian embryos in such adverse conditions. Chicken populations that usually live at high altitudes have developed mechanisms that help them to cope with lower oxygen concentrations. For instance, the expression in the embryonic lung of endothelial nitric oxide synthase (eNOS), which is responsible for the synthesis of nitric oxide (NO, a potent vasodilator), is higher in a highland breed when compared to a lowland breed (Peng et al. 2012). The authors suggested that, from the genetic point of view, the highland chicken lung may have more efficient respiration capability compared with the lowland chicken lung against high-altitude hypoxia due to NO-induced vasodilation although embryonic lungs do not perform ventilation function until pipping at E19 (Peng et al. 2012).
At the cellular level, hypoxia increases oxidative stress and accumulation of reactive oxygen species (ROS) (Duranteau et al. 1998). The catalytic products of heme oxygenase 1 (HO-1) have a cytoprotective role under hypoxia since they can scavenge ROS. The ho-1 pulmonary expression has its highest level at E19 probably due to the degree of tissue hypoxia reached in the chicken embryo at late stages of embryonic development (Gou et al. 2014). Moreover, when subjected to hypoxic conditions (13% O2), ho-1 expression shows upregulated in lung tissues especially at E19 of highland chickens. HO-1 has an indirect role in erythropoiesis since it supplies iron, from heme recycling, needed for the formation of new red blood cells. Taking this into consideration, the augmented levels of HO-1 as a response to hypoxia contribute to relieving hypoxic damage since they contribute to enhancing blood oxygen transport capacity (Gou et al. 2014).
Several studies have reported that embryonic lung mass is virtually unaffected by hypoxic incubation which reflects the absolute need of a proper respiratory surface area to survive (Chan and Burggren 2005; Lewallen and Burggren 2015). Moreover, lung morphology is not significantly altered under hypoxic conditions (15% O2) since pulmonary morphometric parameters as well as blood vessel density are very similar to normoxic conditions. Nonetheless, at the molecular level, the expression of hypoxia-inducible factor (HIF-1) is increased and, consequently, vegf transcription is upregulated. In hyperoxic conditions (30% O2), the vegf expression is also upregulated at E18 (Lewallen and Burggren 2015). VEGF is a key factor in promoting pulmonary angiogenesis and vasculogenesis and contributes to enhancing perfusion and tissue oxygenation (reviewed by Woik and Kroll 2015). However, in the case of the avian lung, its presence does not correlate with an increase in blood vessel formation, and in this context, its role is poorly understood.
5.7 Concluding Remarks
The molecular mechanisms underlying avian lung development are being increasingly more explored. Nonetheless, they are still less studied than mammalian ones. Table 5.1 compiles the expression pattern of chick lung’s signaling molecules, transcription factors, and other molecular factors mentioned in this chapter as well as their putative or known role. The chick model is a suitable and appealing animal model for research since it circumvents major ethical issues; is affordable, with shorter gestation times; and is more easily maintained/manipulated than mammalian models. Despite the major structural morphological differences, when compared to mouse/rat adult lung, early avian pulmonary development presents several molecular similarities. On the other hand, molecular differences may account for the specifics of the avian tubular lung. Nevertheless, further studies are still needed to dissect and to unveil mechanisms responsible for the divergence between mammals and birds.
References
Abbott LA, Lester SM, Erickson CA. Changes in mesenchymal cell-shape, matrix collagen and tenascin accompany bud formation in the early chick lung. Anat Embryol. 1991;183(3):299–311. doi:10.1007/BF00192217.
Abdelfattah AM, Park C, Choi MY. Update on non-canonical microRNAs. Biomol Concepts. 2014;5(4):275–87. doi:10.1515/bmc-2014-0012.
Baarsma HA, Königshoff M, Gosens R. The WNT signaling pathway from ligand secretion to gene transcription: molecular mechanisms and pharmacological targets. Pharmacol Ther. 2013;138(1):66–83. doi:10.1016/j.pharmthera.2013.01.002.
Becchetti E, Evangelisti R, Stabellini G, Pagliarini A, del Borrello E, Calastrini C, Carinci P. Developmental heterogeneity of mesenchymal glycosaminoglycans (GAG) distribution in chick embryo lung anlagen. Am J Anat. 1988;181(1):33–42. doi:10.1002/aja.1001810105.
Been JV, Zoer B, Kloosterboer N, Kessels CG, Zimmermann LJ, van Iwaarden JF, Villamor E. Pulmonary vascular endothelial growth factor expression and disaturated phospholipid content in a chicken model of hypoxia-induced fetal growth restriction. Neonatology. 2010;97(3):183–9. doi:10.1159/000252970.
Bellairs R, Osmond M. Atlas of Chick Development. 3rd ed. Oxford: Academic Press, Elsevier; 2014.
Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124(23):4867–78.
Bernhard W, Gebert A, Vieten G, Rau GA, Hohlfeld JM, Postle AD, Freihorst J. Pulmonary surfactant in birds: coping with surface tension in a tubular lung. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R327–37.
Beyea JA, Olson DM, Vandergriend RA, Harvey S. Expression of growth hormone and its receptor in the lungs of embryonic chicks. Cell Tissue Res. 2005;322(3):379–92. doi:10.1007/s00441-005-0040-0.
Bjørnstad S, Paulsen RE, Erichsen A, Glover JC, Roald B. Type I and II pneumocyte differentiation in the developing fetal chicken lung: conservation of pivotal proteins from birds to human in the struggle for life at birth. Neonatology. 2014;105(2):112–20. doi:10.1159/000355346.
Bjørnstad S, Samara A, Erichsen A, Paulsen RE, Glover JC, Roald B. Hampered lung maturation in methimazole-induced hypothyroidism in fetal chicken: morphological and molecular correlates to human fetal development. Neonatology. 2016;110(2):83–92. doi:10.1159/000444656.
Blacker HA, Orgeig S, Daniels CB. Hypoxic control of the development of the surfactant system in the chicken: evidence for physiological heterokairy. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R403–10. doi:10.1152/ajpregu.00399.2003.
Blanc P, Coste K, Pouchin P, Azaïs JM, Blanchon L, Gallot D, Sapin V. A role for mesenchyme dynamics in mouse lung branching morphogenesis. PLoS One. 2012;7(7):e41643. doi:10.1371/journal.pone.0041643.
Briscoe J, Thérond PP. The mechanisms of hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14(7):416–29. doi:10.1038/nrm3598.
Calvitti M, Baroni T, Calastrini C, Lilli C, Caramelli E, Becchetti E, Carinci P, Vizzotto L, Stabellini G. Bronchial branching correlates with specific glycosidase activity, extracellular glycosaminoglycan accumulation, TGF beta(2), and IL-1 localization during chick embryo lung development. J Histochem Cytochem. 2004;52(3):325–34. doi:10.1177/002215540405200303.
Chan T, Burggren W. Hypoxic incubation creates differential morphological effects during specific developmental critical windows in the embryo of the chicken (Gallus gallus). Respir Physiol Neurobiol. 2005;145(2–3):251–63. doi:10.1016/j.resp.2004.09.005.
Chuang PT, Kawcak TN, McMahon AP. Feedback control of mammalian hedgehog signaling by the hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17:342–7. doi:10.1101/gad.1026303.
Clevidence DE, Overdier DG, Tao W, Qian X, Pani L, Lai E, Costa RH. Identification of nine tissue-specific transcription factors of the hepatocyte nuclear factor 3/forkhead DNA-binding-domain family. Proc Natl Acad Sci U S A. 1993;90(9):3948–52. doi:10.1073/pnas.90.9.3948.
Davey MG, McTeir L, Barrie AM, Freem LJ, Stephen LA. Loss of cilia causes embryonic lung hypoplasia, liver fibrosis, and cholestasis in the talpid3 ciliopathy mutant. Organogenesis. 2014;10(2):177–85. doi:10.4161/org.28819.
De Groef B, Grommen SV, Darras VM. Hatching the cleidoic egg: the role of thyroid hormones. Front Endocrinol (Lausanne). 2013;4:63. doi:10.3389/fendo.2013.00063.
Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273(19):11619–24. doi:10.1074/jbc.273.19.11619.
El Agha E, Bellusci S. Walking along the fibroblast growth factor 10 route: a key pathway to understand the control and regulation of epithelial and mesenchymal cell-lineage formation during lung development and repair after injury. Scientifica. 2014;2014:538379. doi:10.1155/2014/538379.
Evangelisti R, Valeno V, Bodo M, Bosi G, Stabellini G, Carinci P. Involvement of polyamines in the action of transforming growth factor beta and interleukin-1 on cultured chick embryo fibroblasts. Cell Biochem Funct. 1997;15(1):47–51. doi:10.1002/(SICI)1099-0844(199703)15:1<47::AID-CBF719>3.0.CO;2-F.
Forrest D, Sjöberg M, Vennström B. Contrasting developmental and tissue-specific expression of alpha and beta thyroid hormone receptor genes. EMBO J. 1990;9(5):1519–28.
Fujiwara T, Adams FH, Nozaki M, Dermer GB. Pulmonary surfactant phospholipids from Turkey lung: comparison with rabbit lung. Am J Phys. 1970;218(1):218–25.
Gallagher BC. Basal laminar thinning in branching morphogenesis of the chick lung as demonstrated by lectin probes. J Embryol Exp Morphol. 1986;94:173–88.
Gleghorn JP, Kwak J, Pavlovich AL, Nelson CM. Inhibitory morphogens and monopodial branching of the embryonic chicken lung. Dev Dyn. 2012;241(5):852–62. doi:10.1002/dvdy.23771.
Goldin GV, Opperman LA. Induction of supernumerary tracheal buds and the stimulation of DNA synthesis in the embryonic chick lung and trachea by epidermal growth factor. J Embryol Exp Morphol. 1980;60:235–43.
Gou W, Peng J, Wu Q, Zhang Q, Zhang H, Wu C. Expression pattern of heme oxygenase 1 gene and hypoxic adaptation in chicken embryos. Comp Biochem Physiol B Biochem Mol Biol. 2014;174:23–8. doi:10.1016/j.cbpb.2014.05.005.
Han RN, Liu J, Tanswell AK, Post M. Expression of basic fibroblast growth factor and receptor: immunolocalization studies in developing rat fetal lung. Pediatr Res. 1992;31(5):435–40. doi:10.1203/00006450-199205000-00004.
Hao R, Hu X, Wu C, Li N. Hypoxia-induced miR-15a promotes mesenchymal ablation and adaptation to hypoxia during lung development in chicken. PLoS One. 2014;9(6):e98868. doi:10.1371/journal.pone.0098868.
Hilfer SR, Rayner RM, Brown JW. Mesenchymal control of branching pattern in the fetal mouse lung. Tissue Cell. 1985;17(4):523–38. doi:10.1016/0040-8166(85)90029-1.
Hylka VW. Ultrastructural and biochemical evidence of glycogen in the developing lung of the chick embryo: possible contribution to surfactant. Comp Biochem Physiol A Comp Physiol. 1989;93(4):677–83. doi:10.1016/0300-9629(89)90483-0.
Hylka VW, Doneen BA. Ontogeny of embryonic chicken lung: effects of pituitary gland, corticosterone, and other hormones upon pulmonary growth and synthesis of surfactant phospholipids. Gen Comp Endocrinol. 1983;52(1):108–20. doi:10.1016/0016-6480(83)90163-6.
Ivey KN, Srivastava D. microRNAs as developmental regulators. Cold Spring Harb Perspect Biol. 2015;7(7):a008144. doi:10.1101/cshperspect.a008144.
Kim HY, Varner VD, Nelson CM. Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Development. 2013;140(15):3146–55. doi:10.1242/dev.093682.
Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–104.
Lewallen MA, Burggren WW. Chronic hypoxia and hyperoxia modifies morphology and VEGF concentration of the lungs of the developing chicken (Gallus gallus variant domesticus). Respir Physiol Neurobiol. 2015;219:85–94. doi:10.1016/j.resp.2015.08.004.
Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248(1):68–81. doi:10.1006/dbio.2002.0729.
Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20(1):58–61. doi:10.1038/1717.
Loscertales M, Mikels AJ, Hu JK, Donahoe PK, Roberts DJ. Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development. 2008;135(7):1365–76. doi:10.1242/dev.010504.
Maina JN. A systematic study of the development of the airway (bronchial) system of the avian lung from days 3 to 26 of embryogenesis: a transmission electron microscopic study on the domestic fowl, Gallus gallus variant domesticus. Tissue Cell. 2003;35(5):375–91. doi:10.1016/S0040-8166(03)00058-2.
Maina JN, Madan AK, Alison B. Expression of fibroblast growth factor-2 (FGF-2) in early stages (days 3–11) of the development of the avian lung, Gallus gallus variant domesticus: an immunocytochemical study. J Anat. 2003;203(5):505–12. doi:10.1046/j.1469-7580.2003.00236.x.
Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30. doi:10.1038/nrm3434.
Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453(7196):745–50. doi:10.1038/nature07005.
Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209(1):60–71. doi:10.1006/dbio.1999.9234.
Miura T, Hartmann D, Kinboshi M, Komada M, Ishibashi M, Shiota K. The cyst-branch difference in developing chick lung results from a different morphogen diffusion coefficient. Mech Dev. 2009;126(3–4):160–72. doi:10.1016/j.mod.2008.11.006.
Moura RS, Carvalho-Correia E, da Mota P, Correia-Pinto J. Canonical Wnt signaling activity in early stages of chick lung development. PLoS One. 2014;9(12):e112388. doi:10.1371/journal.pone.0112388.
Moura RS, Coutinho-Borges JP, Pacheco AP, Damota PO, Correia-Pinto J. FGF signaling pathway in the developing chick lung: expression and inhibition studies. PLoS One. 2011;6(3):e17660. doi:10.1371/journal.pone.0017660.
Moura RS, Silva-Gonçalves C, Vaz-Cunha P, Correia-Pinto J. Expression analysis of Shh signaling members in early stages of chick lung development. Histochem Cell Biol. 2016;146(4):457–66. doi:10.1007/s00418-016-1448-1.
Moura RS, Vaz-Cunha P, Silva-Gonçalves C, Correia-Pinto J. Characterization of miRNA processing machinery in the embryonic chick lung. Cell Tissue Res. 2015;362(3):569–75. doi:10.1007/s00441-015-2240-6.
Muraoka RS, Bushdid PB, Brantley DM, Yull FE, Kerr LD. Mesenchymal expression of nuclear factor-kappaB inhibits epithelial growth and branching in the embryonic chick lung. Dev Biol. 2000;225(2):322–38. doi:10.1006/dbio.2000.9824.
Murphy AE, Harvey S. Extrapituitary beta TSH and GH in early chick embryos. Mol Cell Endocrinol. 2001;185(1–2):161–71. doi:10.1016/S0303-7207(01)00615-3.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–66. doi:10.1002/wdev.176.
Park WY, Miranda B, Lebeche D, Hashimoto G, Cardoso WV. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev Biol. 1998;201(2):125–34. doi:10.1006/dbio.1998.8994.
Pei L, Leblanc M, Barish G, Atkins A, Nofsinger R, Whyte J, Gold D, He M, Kawamura K, Li HR, Downes M, Yu RT, Powell HC, Lingrel JB, Evans RM. Thyroid hormone receptor repression is linked to type I pneumocyte-associated respiratory distress syndrome. Nat Med. 2011;17(11):1466–72. doi:10.1038/nm.2450.
Peng JF, Ling Y, Gou WY, Zhang H, Wu CX. Identification of chicken eNOS gene and differential expression in highland versus lowland chicken breeds. Poult Sci. 2012;91(9):2275–81. doi:10.3382/ps.2012-02197.
Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8(19):1083–6. doi:10.1016/S0960-9822(98)70446-4.
Sakiyama J, Yamagishi A, Kuroiwa A. Tbx4-Fgf10 system controls lung bud formation during chicken embryonic development. Development. 2003;130(7):1225–34. doi:10.1242/dev.00345.
Sakiyama J, Yokouchi Y, Kuroiwa A. Coordinated expression of hoxb genes and signaling molecules during development of the chick respiratory tract. Dev Biol. 2000;227(1):12–27. doi:10.1006/dbio.2000.9880.
Sanford EL, Choy KW, Donahoe PK, Tracy AA, Hila R, Loscertales M, Longoni M. MiR-449a affects epithelial proliferation during the pseudoglandular and canalicular phases of avian and mammal lung development. PLoS One. 2016;11(2):e0149425. doi:10.1371/journal.pone.0149425.
Semenov MV, Habas R, Macdonald BT, He X. SnapShot: noncanonical Wnt signaling pathways. Cell. 2007;131(7):1378. doi:10.1016/j.cell.2007.12.011.
Shirasawa N, Shiino M, Shimizu Y, Nogami H, Ishii S. Immunoreactive luteinizing hormone (ir-LH) cells in the lung and stomach of chick embryos. Cell Tissue Res. 1996;283(1):19–27. doi:10.1007/s004410050508.
Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–42.
Stabellini G, Calvitti M, Baroni T, Marinucci L, Calastrini C, Carinci P, Becchetti E. Glycosidases during chick embryo lung development and their colocalization with proteoglycans and growth factors. Eur J Histochem. 2002;46(1):41–52. doi:10.4081/1653.
Stabellini G, Calvitti M, Becchetti E, Carinci P, Calastrini C, Lilli C, Solmi R, Vizzotto L, Baroni T. Lung regions differently modulate bronchial branching development and extracellular matrix plays a role in regulating the development of chick embryo whole lung. Eur J Histochem. 2007;51(1):33–41. doi:10.4081/1009.
Stabellini G, Locci P, Calvitti M, Evangelisti R, Marinucci L, Bodo M, Caruso A, Canaider S, Carinci P. Epithelial-mesenchymal interactions and lung branching morphogenesis. Role of polyamines and transforming growth factor beta1. Eur J Histochem. 2001;45(2):151–62. doi:10.4081/1625.
Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev Dyn. 2007;236(9):2454–63. doi:10.1002/dvdy.21286.
Woik N, Kroll J. Regulation of lung development and regeneration by the vascular system. Cell Mol Life Sci. 2015;72(14):2709–18. doi:10.1007/s00018-015-1907-1.
Zeng X, Yutzey KE, Whitsett JA. Thyroid transcription factor-1, hepatocyte nuclear factor-3β and surfactant protein A and B in the developing chick lung. J Anat. 1998;193(3):399–408. doi:10.1046/j.1469-7580.1998.19330399.x.
Zhang H, Burggren WW. Hypoxic level and duration differentially affect embryonic organ system development of the chicken (Gallus gallus). Poult Sci. 2012;91(12):3191–201. doi:10.3382/ps.2012-02449.
Zhang W, Cuperus T, van Dijk A, Skjødt K, Hansen S, Haagsman HP, Veldhuizen EJ. Developmental regulation of chicken surfactant protein A and its localization in lung. Dev Comp Immunol. 2016;61:80–7. doi:10.1016/j.dci.2016.03.010.
Zhang L, Whitsett JA, Stripp BR. Regulation of Clara cell secretory protein gene transcription by thyroid transcription factor-1. Biochim Biophys Acta. 1997;1350(3):359–67. doi:10.1016/S0167-4781(96)00180-7.
Acknowledgments
Funding sources: FEDER funds, through the Competitiveness Factors Operational Program (COMPETE), and by National funds, through the Foundation for Science and Technology (FCT), under the scope of the project POCI-01-0145-FEDER-007038; project NORTE-01-0145-FEDER-000013, supported by the Northern Portugal Regional Operational Program (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Moura, R.S., Correia-Pinto, J. (2017). Molecular Aspects of Avian Lung Development. In: Maina, J. (eds) The Biology of the Avian Respiratory System. Springer, Cham. https://doi.org/10.1007/978-3-319-44153-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-44153-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-44152-8
Online ISBN: 978-3-319-44153-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)