Abstract
Congenital pulmonary lesions encompass congenital pulmonary airway malformations (CPAMs), bronchopulmonary sequestration (BPS), and congenital lobar emphysema (CLE). These lesions are typically diagnosed prenatally, and prognosis is related to the size of the lesion and the associated physiologic sequelae. Postnatally, cross-sectional imaging consisting of computed tomography or magnetic resonance imaging (MRI) is advantageous to look at the extent of the lesion as well as for multifocal disease. CLE management is solely based upon the presence of symptoms and does not require surgical resection in the asymptomatic patient. Current recommendations are for resection of CPAM and BPS even in the asymptomatic patient. Lobectomy has become the cornerstone of surgical treatment due to the risk of recurrent pneumonia, respiratory disease, and malignancy. Over the last decade and a half, thoracoscopic resection has gained favor and has been proven to be a technically feasible and safe alternative to thoracotomy. More recently, segmental resection has been recognized as a potential treatment for congenital pulmonary lesions. In this chapter we briefly describe the pathophysiology and preoperative workup for patients with congenital pulmonary lesions. We further describe the operative approach for thoracoscopic resection of congenital pulmonary lesions.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Thoracoscopy
- Congenital pulmonary lesion
- Neonate
- Congenital pulmonary airway malformation
- CPAM
- Congenital cystic adenomatoid lesion
- CCAM
- Bronchopulmonary sequestration
- Congenital lobar emphysema
- Lobectomy
- Segmental resection
Introduction
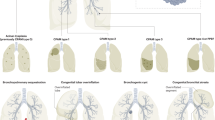
Cystic pulmonary lesions of the newborn are a rare congenital anomaly and in one study were found to have an incidence of 1 per 25,000–35,000 births [1]. These lesions consist of congenital pulmonary airway malformations (CPAMs), bronchopulmonary sequestrations (BPS), and congenital lobar emphysema (CLE). CPAM, previously known as congenital cystic adenomatoid malformation (CCAM), is characterized by a lack of normal alveolarization with an increased number of terminal bronchioles that are cystic in nature. The cysts within these lesions range from less than 1 mm in size to greater than 10 cm. Based upon size of the lesions, CPAMs can be described as macrocystic (lesions greater than 5.0 cm) or microcystic. While the abnormal bronchioles do not participate in normal gas exchange, they maintain their connection with the normal tracheobronchial tree [2, 3]. This communication can lead to overinflation during aggressive attempts at resuscitation in the neonate. Inadequate clearing of normal respiratory bacterial pathogens may lead to recurrent pneumonias [2]. Unlike BPS , CPAMs only receive blood supply from the pulmonary artery. CPAMs may show malignant degeneration if left unresected (pulmonary blastoma and rhabdomyosarcoma in infants and young children, bronchoalveolar carcinoma in older children) [4].
Bronchopulmonary sequestration consists of lung parenchyma that does not communicate with the tracheobronchial tree. These lesions can be extralobar or intralobar. As mentioned previously, the lesion derives its arterial supply from an aberrant systemic vessel [5]. Care must be taken during operative resection to identify and control this vessel as it may originate from the abdominal cavity [2]. Similar to CPAM, symptoms of BPS may include respiratory distress and recurrent pneumonia. As in the case of CPAM, elective resection is recommended.
Congenital lobar emphysema is characterized by overdistention of the affected lobe due to emphysematous changes resulting from a variety of causes. This is fundamentally due to normal passage of air into the lung with decreased expulsion of air on expiration. The underlying pathology may be due to abnormal bronchial cartilage, partial obstruction due to inspissated mucous or mucosal proliferation, or external compression of the cartilage. The majority of the cases of CLE affect the left upper lobe [2]. Resection of the emphysematous lobe is dependent upon symptomatology. Unlike CPAM or BPS, children with mild or no symptoms do not require resection.
Prenatal Diagnosis
Pulmonary lesions are routinely diagnosed on prenatal ultrasound. The differential diagnosis includes congenital diaphragmatic hernia, congenital lobar emphysema, bronchopulmonary sequestration, foregut duplication cyst, and mediastinal cystic teratoma. Postnatally they can be confused with congenital diaphragmatic hernia or pneumatocele. Fetal MRI may be used to differentiate between these lesions ultimately allowing for improved prenatal and postnatal care as well as prenatal counseling of the family [6] (Fig. 14.1). The size of the pulmonary lesion is significant prognostically. Compression of the esophagus may lead to polyhydramnios due to abnormal fetal swallowing of amniotic fluid. Compression of the mediastinum by a large lesion may result in compression of the heart and great vessels ultimately leading to hydrops fetalis. CPAM volume ratio (CVR) can be determined using prenatal ultrasound by determining the CPAM volume and dividing by the head circumference to standardize for fetal size. A CVR greater than 1.6 is predictive of increased risk for hydrops [7]. CVR can then be useful for determining which CPAM’s require increased level of surveillance prenatally. CPAMs reach maximal growth before 28 weeks of gestation. Following this time period most CPAMs either plateau in size or regress [2].
Preoperative Evaluation and Treatment
Management of a fetus with a cystic pulmonary lesion is dependent upon the symptoms present. Prenatal management of CPAM may consist of steroid treatment in fetuses with a CVR greater than 1.4. Betamethasone has been shown to arrest growth of CPAM with subsequent improvement in hydrops symptoms [8]. A fetus that has been diagnosed with a macrocystic CPAM complicated by hydrops may be treated with thoracoamniotic shunting. Microcystic or solid CPAMs that present with hydrops have been approached with fetal surgery [9]. A late gestation fetus with hydrops may benefit from an ex utero intrapartum therapy (EXIT) approach [4, 10]. The fetus with a CPAM without hydrops should be managed with planned delivery and neonatal evaluation and eventual surgery.
All newborns prenatally diagnosed with a congenital pulmonary lesion should have a baseline radiograph at the time of birth. Surgical management should be based upon whether the newborn is symptomatic from the lesion. Infants with hemodynamic or significant respiratory compromise may need immediate resection. Extracorporeal membrane oxygenation (ECMO) has been used in some of these cases [2]. Persistent tachypnea, oxygen requirement, poor weight gain, and inability to feed orally are indications for early resection prior to discharge from the hospital. Asymptomatic children may be discharged and followed up as an outpatient. At our institutions, we typically perform preoperative imaging using computed tomographic scans at 3 months of age. Lesions are typically isolated to a single lobe; however, multilobar CPAM has been documented and will affect surgical decision-making (Fig. 14.2). It may be difficult to differentiate an extralobar sequestration from an intralobar sequestration radiographically. Attention should be paid to look for a systemic blood supply to the lesion to assist in differentiation of CPAM from BPS. Additionally, identification of a subdiaphragmatic feeding vessel will assist with operative planning (Figs. 14.3 and 14.4).
Technique
Resection of pulmonary lesions has been classically performed through a posterolateral thoracotomy . Over the last decade and a half, surgical resection through a minimally invasive approach has become more commonplace. We recommend surgical resection, using a minimally invasive approach, when the infant is 3–6 months old. Children who present with infected lesions are recommended to undergo adequate antibiotic therapy for the infection prior to resection of the lesion. Minimally invasive approach is still a viable option in children who have had pneumonia; however, there has been a documented increase in conversion to open thoracotomy in these children [11].
Minimally Invasive Approach
The child should be placed in the lateral decubitus position with the affected side up. Adequate padding of all bony prominences as well as proper positioning of an axillary roll should be ensured. Small gel rolls, placed anteriorly and posteriorly, are adequate to prevent patient movement in younger children. In older children, we use a beanbag underneath the patient as our preferred method of stabilization. Central venous lines, arterial lines, and bladder catheters are not required intraoperatively. Management of the airway requires an anesthesiologist experienced in pediatric airways to ensure adequate single-lung ventilation. A techniques available for isolating the contralateral lung includes double-lumen endotracheal tube in older children and adolescents. In younger children, a Fogarty balloon catheter (Edwards Lifesciences, Irvine, CA) may be used as an endobronchial blocker with placement of a single-lumen endotracheal tube. This technique is more difficult in children less than ~5 kg because the bronchial blocker itself fills much of the lumen of the endotracheal tube making ventilation more difficult. We have passed the blocker extraluminally in order to alleviate this problem. Infants may require main stem intubation of the contralateral bronchus due to the narrow airway [11]. The use of a mild tension pneumothorax may also be useful in helping to collapse the ipsilateral lung and improve visualization. We use a pressure of no more than 4–5 mmHg. Flexible bronchoscopy is necessary to ensure proper placement of the endotracheal tube and bronchial blocker during initial placement and after repositioning of the patient. We do not place epidural catheters for pain management except in rare instances when conversion to an open procedure is needed.
The surgeon and assistant both stand on the same side of the patient depending on the lobe to be resected. We prefer to stand at the front of the patient with the monitor at the patient’s back for lower lobes and the reverse for upper lobes. Local anesthetic is infiltrated at each trocar site. A veress needle is used to enter the chest cavity through a Step radially expanding sheath (Medtronic, Minneapolis, MN). Alternatively, a direct cutdown and placement of the initial trocar and reusable trocars may be used. The hemithorax is then insufflated with low-flow, low-pressure carbon dioxide to aid in collapse of the affected lung. This initial entry site is typically through the fifth or sixth intercostal space beneath the tip of the scapula in the anterior axillary line for lower lobes and the posterior axillary line for upper lobes. Entry at this site will allow for visualization of the major fissure and the underlying pulmonary parenchyma [12] (Fig. 14.5). Subsequent ports are then placed such that the camera port overlies the fissure. We typically use three 5-mm ports (two working ports and one camera port). In smaller babies we often use two 3-mm ports and one 5 mm to allow for use of the Ligasure (Covidien Energy Devices, Boulder, CO). For upper lobes all ports are placed in line in the posterior axillary line, while lower lobes have the ports placed in line in the anterior axillary line (Figs. 14.6 and 14.7). A fourth stab incision for insertion of an instrument to assist with parenchymal retraction or suction is placed in the lower chest in the 8–9th interspace. We use the anterior and posterior axillary lines as landmarks because it allows the operating surgeon to place the venous anatomy of the lung lobe between themselves and the monitor while the arterial anatomy is always fixed within the fissure. Alternatively, surgeons may choose to adopt a triangulation method with placement of ports in the anterior, posterior, and midaxillary line using the location of the lesion as the focal point of the triangle.
Resection of pulmonary lesions is typically through formal lobectomy. In the case of multiple lesions affecting multiple lobes, segmental resection may be appropriate to preserve pulmonary parenchyma. The steps in dissection vary depending upon the affected lobe and follow the same principles as open thoracotomy [13]. Completion of the major and minor fissure allows for visualization of the pulmonary vasculature and segmental artery branches. Division of the arterial branches is followed by division of the pulmonary veins (Fig. 14.8). Initial division of the pulmonary artery prevents parenchymal congestion and preserves the intrathoracic work space. The Ligasure has proven to be an effective method of dividing pulmonary parenchyma to complete division of the fissure. This device has also been shown to be an effective method of division of pulmonary vessels <7 mm in size. We currently use either the LS 1500 5-mm laparoscopic sealer/divider or the LF 1737 laparoscopic sealer. The LS 1500 has a blunt dolphin-tip while the LF 1737 has a Maryland tip. The Maryland tip we find beneficial for dissection especially around vessels. Multiple vessel sealing devices are available currently (Harmonic, Gyrus, JustRight sealer); however, we have routinely utilized the Ligasure system. For larger vessels, control with an endoscopic hemoclip (Auto Suture ENDO CLIP, Covidien) or with intracorporeal suture ligation followed by division using an energy-based sealing device has been described [11, 14].
Following division of the pulmonary vasculature, attention is turned to the segmental bronchus. In larger children, an endoscopic stapler can be utilized; however, this may require placement of a 12-mm port. In some instances, the newer 5-mm staplers may be used. In infants we prefer to use a locking hemoclip such as the Hem-o-lok system (Teleflex Medical, Research Triangle Park, NC) [15]. This ensures closure of the bronchus (Fig. 14.9). Alternatively, the bronchus can also be divided and sutured with a monofilament, absorbable suture or closed with an endoloop (Fig. 14.10). After division of the bronchus, the specimen can then be removed by enlarging the most inferior trocar site. We do not routinely utilize an endoscopic pouch for specimen retrieval. An evaluation is then made for any significant bronchial leaks by partially filling the chest with saline and ventilating to a pressure of 20-cm H2O. If one is discovered, this is suture ligated. An appropriately sized chest tube is placed in the most inferior incision. All wounds are then closed with absorbable sutures. The patient is extubated in the operating room and monitored overnight. We routinely remove the chest tube on the first postoperative day if there is no air leak, with discharge on the same day.
Management of bronchopulmonary sequestration is accomplished in the same manner. Identification of the systemic blood supply is paramount. We typically place a suture ligature or titanium clip (Fig. 14.11) around the artery to ensure adequate control and utilize the Ligasure to divide the vessel. For intralobar sequestration , lobectomy then proceeds in the same manner as described previously (Figs. 14.12 and 14.13). For extralobar sequestration , the lesion is invested in the pleura. Resection of the lesion can be accomplished using the Ligasure to divide the pleura without performing a formal pulmonary resection. Chest tubes may not be necessary in these cases and often just evacuate the hemithorax with a rubber catheter prior to final closure.
Recent literature has focused on segmental resection for the treatment of congenital pulmonary lesions. Segmental resection has been an accepted standard in the case of multilobar disease that allows for preservation of pulmonary parenchyma. However, recent literature has suggested that even in the case of focal disease that is peripherally located, segmental resection is a safe alternative. Advocates against nonanatomic resection point to the microscopic disease that may be left behind as it can be difficult to visualize the diseased lung when the lung is deflated [16, 17]. To perform a segmental resection thoracoscopically, port placement proceeds as described previously; however, segmental blood vessels can be divided using a Ligasure. Pulmonary parenchyma can be divided safely with the Ligasure in small patients, obviating the need for an endoscopic stapler (Figs. 14.14, 14.15, and 14.16). We have adopted the use of a fibrin sealant over the cut edge of parenchyma to prevent an air leak. Postoperative management to include chest tube placement remains the same as previously described.
Summary
-
Thoracoscopic resection of pulmonary lesions is a safe alternative to open resection.
-
Despite longer operative times, the minimally invasive approach has been shown in multiple studies to lead to decreased length of hospital stay, a shorter required time for chest tube, and an overall lower postoperative complication rate [18–20].
-
Lobectomy remains the current standard of care for resection of pulmonary lesions; however, an increasing body of literature suggests that consideration for segmental resection for peripheral lesions may be a safe alternative with decreased morbidity [16, 17].
-
Current limitations for a thoracoscopic approach include patient size and inflammation from recurrent pneumonias making dissection more difficult. However, an attempt at a minimally invasive approach is still recommended in these cases and can be accomplished safely.
-
Newborn infants who present with symptomatic lesions may best treated with an open approach depending upon the associated physiologic sequelae.
-
If a child has hemodynamic instability or compromise, an open approach may be the best alternative.
-
For those children with respiratory difficulty or failure to thrive from feeding intolerance, a minimally invasive approach is our preferred operative approach.
-
-
Thoracoscopy in infants and children can be technically demanding but remains a viable option in the management of CCAM in the pediatric population.
The views expressed in this publication are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, nor the US Government.
I am a military service member. This work was prepared as part of my official duties. Title 17 USC 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 USC 101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
References
Laberge JM, Flageole H, Pugash D, et al. Outcome of the prenatally diagnosed congenital cystic adenomatoid lung malformation: a Canadian experience. Fetal Diagn Ther. 2001;16:178–86.
Adzick NS, Farmer DL. Cysts of the lungs and mediastinum. In: Coran AG, Caldamone A, Adzick NS, et al. editors. Pediatric surgery. 7th ed. Philadelphia: Mosby; 2012. p. 825–35.
Stocker JT, Manewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung: classification and morphologic spectrum. Hum Pathol. 1977;8:155–61.
Chiu B, Flake AW. Congenital lung lesions. In: Mattei P, editor. Fundamentals of pediatric surgery. New York: Springer; 2011.
Puligandla PS, Laberge JM. Congenital lung lesions. Clin Perinatol. 2012;39:331–47.
Pacharn P, Kline-Fath B, Calvo-Garcia M, et al. Congenital lung lesions: prenatal MRI and postnatal findings. Pediatr Radiol. 2013;43(9):1136–43.
Crombleholme TM, Coleman BG, Howell LJ, et al. Elevated cystic adenomatoid malformation volume ratio (CVR) predicts outcomes in prenatal diagnosis of cystic adenomatoid malformation of the lung. J Pediatr Surg. 2002;37:331–8.
Peranteau WH, Wilson W, Liechty KW, et al. The effect of maternal betamethasone on prenatal congenital cystic adenomatoid malformation growth and fetal survival. Fetal Diagn Ther. 2005;20:74–8.
Adzick NS, Harrison MR, Crombleholme TM, et al. Fetal lung lesions: management and outcome. Am J Obstet Gynecol. 1998;179:884–9.
Hedrick HL, Flake AW, Crombleholme TM, et al. The EXIT procedure for high risk fetal lung lesions. J Pediatr Surg. 2005;40:1038–43.
Seong YW, Kang CH, Kim JT, et al. Video-assisted thoracoscopic lobectomy in children: safety, efficacy, and risk factors for conversion to thoracotomy. Ann Thorac Surg. 2013;95:1236–42.
Rothenberg SS. First decade’s experience with thoracoscopic lobectomy in infants and children. J Pediatr Surg. 2008;43:40–5.
Waldhausen JA, Pierce WS, Campbell DB. Surgery of the chest. St Louis: Mosby Year-Book; 1995.
Bignon H, Buela E, Martinez-Ferro M. Which is the best vessel-sealing method for pediatric thoracoscopic lobectomy? J Laparoendosc Adv Surg Tech. 2010;20(4):395–8.
Kunisaki SM, Powelson IA, Haydar B, et al. Thoracoscopic vs. open lobectomy in infants and young children with congenital lung malformations. J Am Coll Surg. 2014;218(2):261–70.
Bagrodia N, Cassel S, Liao J, et al. Segmental resection for the treatment of congenital pulmonary malformations. J Pediatr Surg. 2014;49:905–9.
Rothenberg SS, Shipman K, Kay S, et al. Thoracoscopic segmentectomy for congenital and acquired pulmonary disease: a case for lung-sparing surgery. J Laparoendosc Adv Surg Tech. 2014;24(1):50–4.
Vu LT, Farmer DL, Nobuhara KK, et al. Thoracoscopic versus open resection for congenital cystic adenomatoid malformations of the lung. J Pediatr Surg. 2008;43:35–9.
Albanese CT, Syorak RM, Tsao KJ, et al. Thoracoscopic lobectomy for prenatally diagnosed lung lesions. J Pediatr Surg. 2003;38:553–5.
Koontz CS, Oliva V, Gow KW, et al. Video-assisted thoracoscopic surgical excision of cystic lung disease in children. J Pediatr Surg. 2005;40:835–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ricca, R.L., Waldhausen, J.H.T. (2017). Thoracoscopic Approaches to Congenital Lung Lesions. In: Walsh, D., Ponsky, T., Bruns, N. (eds) The SAGES Manual of Pediatric Minimally Invasive Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-43642-5_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-43642-5_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43640-1
Online ISBN: 978-3-319-43642-5
eBook Packages: MedicineMedicine (R0)