Abstract
The focus of this chapter is cutaneous infections due to fungi, in particular deep fungal infections, and the available evidence supporting specific treatments, both medical and surgical, for these conditions in children and adolescents. For some infections, adjunctive therapies are recommended in addition to first-line, second-line, and third-line treatments.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Subcutaneous Mycoses: Sporotrichosis, Chromoblastomycosis, Mycetoma, Rhinosporidiosis
Clinical Features
Sporotrichosis is chronic infection caused by the dimorphic fungus Sporothrix schenkii, which is transmitted by direct inoculation of soil through skin. The most common form of sporotrichosis is lymphocutaneous, characterized by the development of an erythematous papule or nodule at the site of inoculation. Subsequently, additional lesions, with or without ulceration, occur proximally along lymphatic channels. The most common site of involvement is the upper extremity (Fig. 17.1). Fixed cutaneous lesions, in the form of verrucous or ulcerative plaques, may occur on the face or extremities. In immunocompromised states, visceral spread, such as pulmonary disease, may occur [1].
Chromomycosis is caused by inoculation of a dematiaceous fungus from the soil into skin. Typical lesions of chromomycosis in children or adolescents are erythematous nodules or verrucous plaques, most often located on the upper extremity. This is of notable contrast to disease presentation in adults, which usually occurs on the lower extremity. In one study of chromomycosis in South American children, Cladophialophora carrionii was the most common cause, while most studies in adults have show Fonsecaea pedrosoi to be the most common cause [2, 3].
Eumycetoma is a chronic mycotic infection of the skin and soft tissue. Infection follows inoculation injury from a contaminated thorn or splinter. At least 30 species of molds are implicated; Pseudallescheria boydii is the most common causative species in the United States, while Madurella mycetomatis is the most common cause worldwide. Infection most often involves the feet or lower extremities, and is characterized by large verrucous nodules with abscesses, sinus tracts, and macroscopic grains (Fig. 17.2). Eumycetomas are usually confined to subcutaneous tissues, but can involve fascia, bone, and regional lymph nodes via contiguous dissemination. Fibrosis, deformity, and lymphedema eventually result if untreated [4].
Rhinosporidiosis is a non-contagious chronic granulomatous infection caused by Rhinosporidium seeberi, and is characterized by polyps which are sessile or pedunculated, and mainly affect the nasal mucosa and, less commonly, the conjunctival or ocular mucosa. Cutaneous lesions may occur due to spread from adjacent mucosa, direct inoculation, or hematogenous spread. Infection in adolescents and young adults is common [5, 6].
Specific Investigations
For diagnosis |
Fungal culture |
KOH |
Histopathology |
For treatment |
CBC, LFTs, lipid panel, metabolic panel (potassium, blood glucose), kidney function tests (BUN and creatinine), EKG |
Thyroid function tests (TFTs) |
Fungal culture is the most sensitive method for the diagnosis of sporotrichosis. Aspirate from a nodule or tissue from a punch biopsy should be inoculated onto Sabourad dextrose agar; growth occurs within 5 days at room temperature. Histopathology may be supportive, but is rarely diagnostic, given the difficulty of finding the sparse organisms, which are 3 μm or smaller in diameter. Nonspecific findings, including suppurative granulomas and asteroid bodies, are often present [7]. An enzyme immunoassay for serologic testing of S. schenkii has demonstrated up to 90 % sensitivity and 80 % specificity, but is not available widely [8].
In chromomycosis, KOH is highly sensitive in identifying the characteristic sclerotic or medlar bodies. Fungal culture on Sabourad or Mycosel agar is sensitive and specific. Histopathology often demonstrates granulomatous inflammation, pseudocarcinomatous hyperplasia, and pigmented yeast forms with single septations [9].
Eumycetoma can be diagnosed by the clinical findings in combination with black macroscopic grains, which are only found with fungal infections; yellow or white grains indicate fungal or bacterial infection. KOH demonstrates broad, septate, and branching hyphae. Culture should be performed but requires 6–8 weeks for growth. Histopathology is also helpful and demonstrates hyaline or pigmented hyphae in microscopic grains. Radiography, including X-ray, computed tomography, and magnetic resonance imaging should be considered in extensive cases, to exclude bony and soft tissue involvement [10].
In rhinosporidiosis, KOH or histopathology are diagnostic, demonstrating large sporangia 200 μm in diameter and filled with smaller endospores. Culture is not helpful, as R. seeberi is intractable to isolation or growth in microbiologic culture media [11].
Given the lengthy duration of systemic azole therapy required for theses diseases, baseline and periodic evaluation of CBC and LFTs is recommended. In the case of itraconazole, periodic evaluation of serum lipids is recommended, given the risk of hypertriglyceridemia. SSKI therapy requires monitoring of thyroid function tests, including TSH and FT4. Kidney function tests as well as potassium levels should be monitored during amphotericin B treatment; EKG is also recommended, given the risk of arrhythmia.
Clinical cure was obtained in almost 95 % of patients with cutaneous sporotrichosis, including children, treated with oral itraconazole. In another study, clinical response rate to itraconazole was over 80 %.
In a randomized non-blinded study, clinical cure rates were high and similar (above 89 %) for pediatric patients treated with either daily dosing or four times daily dosing of SSKI.
Initial treatment with intravenous amphotericin B followed by long-term therapy with itraconazole is reserved for disseminated sporotrichosis and based on case reports.
Complete clinical and mycologic remission was achieved in 85 % of the patients treated with itraconazole or 5-FU cream. Similar results were obtained for electrodesiccation or fulguration, but given the more invasive nature and potential for scarring, this is considered an alternative to itraconazole or 5-FU. Ajoene gel (isolated from alcoholic extracts of garlic) also demonstrated a high rate of efficacy, but may be difficult to obtain.
Low cure rates (31 %) were observed in a study of 51 cases, but cryosurgery and itraconazole produced the best results overall, sometimes in combination.
In one study, itraconazole was moderately efficacious, associated with improvement in 42 % of cases, although none showed mycologic or clinical remission. Voriconazole is the treatment of choice for eumycetoma caused by P. boydii. Posaconazole can be used as an equally effective alternative to itraconazole and voriconazole.
Radical surgical procedures should be avoided, but combined medical therapy and conservative excision have produced good results. Relapse rates after surgery alone are high (over 50 %), so antifungals should be administered for at least 6 months prior to surgery, and then in the post-surgical period to reduce recurrence. In a small study of oral ketoconazole, 5 of 13 patients were completely cured, and 4 improved following at least 6 months of treatment. Despite its historical role as the preferred agent for this disease, toxicity limits its use, and it is now only considered an alternative agent.
Local surgical excision is the treatment of choice, but has been associated with 10 % recurrence rate. Concurrent medical treatment with dapsone has been used to decrease this risk.
Case reports have described long courses of dapsone being used as monotherapy or in combination with surgery. It has also been used in combination with surgery or other antimicrobials such as cycloserine and ketoconazole for disseminated disease.
Systemic Mycoses: Blastomycosis, Coccidioidomycosis, Paracoccidioidomycosis, Histoplasmosis
Clinical Features
Blastomycosis is caused by inhalation of the conidia of the dimorphic fungus Blastomyces dermatitidis. The lungs are the most common site of disease, and infection may be asymptomatic or severe. Cutaneous disease results from hematogenous spread from the lungs, and occurs in up to one-fifth of patients. Verrucous lesions with irregular borders and microabscesses, ulcerative plaques with elevated borders, subcutaneous nodules, and cold abscesses may be seen [28].
Coccidioidomycosis is caused by the dimorphic fungi, Coccidioides immitis, or Coccidioides posadasii, which are endemic to arid regions. Patients of African or Filipino ancestry or those with a history of immunosuppression are at increased risk of infection. Cutaneous lesions are either due to disseminated disease via hematogenous spread from a pulmonary nidus or, less commonly, primary infection. Organism-specific manifestations include nodules, pustular lesions, verrucous plaques, abscesses, and fistulae. Reactive cutaneous manifestations include erythema nodosum, erythema multiforme, an acute exanthem, Sweet’s syndrome, and interstitial granulomatous dermatitis [29, 30].
Paracoccidioidomycosis is a systemic mycotic disease caused by the dimorphic fungus Paracoccidioides brasiliensis. It is endemic in Central and South America, where it is widely present as a soil saprophyte. Exposure is often occupational, and the main portal of entry is inhalation. Acute or subacute disease is most often seen in children and adolescents: features include lymphadenopathy, hepatosplenomegaly, fever, and bone marrow dysfunction, but skin and pulmonary involvement are uncommon. In contrast, the chronic form of the disease involves the lungs, mucosa, skin, lymph nodes, and adrenal glands. Mucosal and skin findings simulate those of leishmaniasis. Painful ulcers with ragged borders and petechiae are seen most often in the mouth or larynx. Ulcerative or verrucous nodules or plaques are seen in the skin [31, 32].
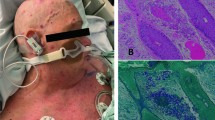
Histoplasma capsulatum is a dimorphic intracellular fungus found worldwide. Cutaneous lesions are present in up to 15 % of patients with disseminated histoplasmosis (Figs. 17.3 and 17.4). A variety of manifestations are seen, including nodules, plaques, ulcers, pustules, abscesses, erythroderma, cellulitis and panniculitis, and purpura [33].
Specific Investigations
For diagnosis |
KOH |
Fungal culture |
Histopathology |
Antigen detection (EIA) |
Serology (EIA, immunodiffusion, complement fixation) |
Skin testing (Coccidioidomycosis) |
Imaging (Computed tomography or x-ray, for paracoccidioidomycosis) |
For treatment |
Repeat serology to monitor treatment response (paracoccidioidomycosis) |
CBC, LFTs, lipid panel, metabolic panel (potassium, blood glucose), kidney function tests (BUN and creatinine), EKG |
For blastomycosis, KOH preparation has a diagnostic yield of less than 50 %, despite multiple specimens. Histopathology demonstrates suppurative granulomas, but yeast forms may be difficult to visualize. When identified, they are 8–15 μm in diameter, with refractile walls and single broad-based buds. Definitive diagnosis requires fungal culture, and B. dermatitidis grows within 1–4 weeks [34]. Antigen detection assays for blastomycosis demonstrate overall sensitivity of 90 %, but specificity is less than 80 % due to cross-reactive antigens in histoplasmosis, paracoccidioidomycosis, and penicilliosis. Sensitivity is higher in urine than in serum. Given the lower specificity, culture is still the gold standard [35].
Cutaneous coccidioidomycosis should be diagnosed either by direct visualization of the organism or by culture. Coccidioides spp will grow on routine media, but may take more than 1 week to isolate. Spherules of Coccidioides are large, up to 70 μm in diameter, and can be detected with KOH prep or in histologic sections. Despite their size, organisms are often sparse, so multiple level sections should be examined by the dermatopathologist [36]. Overall, serologic tests for the detection of IgG and IgM antibodies against Coccidioides are highly specific, but sensitivity is variable in early infection, as antibody production may not occur for weeks to months after illness onset. Immunodiffusion testing is the most specific serology available, while enzyme-linked immunoassay (EIA) has a sensitivity of 100 %. Thus, EIA should be used a screening test and immunodiffusion should be used for confirmation [37–39]. Complement fixation and tube precipitin-type assays are less accurate than these two newer methods. EIA for antigenuria is over 70 % sensitive, but also detects Histoplasma antigen. Skin testing for coccidioidomycosis is not recommended to diagnose current illness. Skin tests are positive for life, even in healthy patients with adequate prior treatment. In contrast, skin tests can be negative in infected patients with anergy. Thus, it is more useful as a prognostic test [40].
Paracoccidioidomycosis is diagnosed via direct microscopic visualization and/or by culturing P. brasiliensis from clinical specimens. KOH prep is positive in over 90 % of cases. Skin biopsy may be obtained in chronic disease, and demonstrates suppurative granulomas in most cases; P. brasiliensis is seen as a round or oval yeast 4–40 μm, with two or more narrow-necked budding cells (resembling a “pilot’s wheel” or “Mickey mouse head”). Quantitative immunodiffusion is the most useful serologic test for diagnosis and for monitoring response to therapy, given its high sensitivity and specificity—up to 97 and 100 %, respectively. Cultures are positive in up to 80 % of cases, but can take up to 30 days to grow. In addition to imaging of affected areas (computed tomography or X-ray) to evaluate lymphadenopathy and pulmonary lesions, all patients with suspected paracoccidioidomycosis should have specimens submitted for direct microscopy, culture, and serology [41].
In a study of HIV-infected patients with disseminated histoplasmosis, skin biopsy with special stains for fungi (gomori methenamine silver or PAS) allowed direct visualization of Histoplasma in over 86 % of cases. The most common histologic pattern is a diffuse infiltrate of macrophages parasitized by yeast 2–3 μm in size. However, organisms may also be extracellular [42, 43]. EIA antigen testing in disseminated histoplasmosis demonstrates a sensitivity ranging from 75 % to 100 %, with increased sensitivity in serum compared to urine, and in immunocompromised patients. However, false-positive tests can occur in patients with blastomycosis or coccidioidomycosis [44]. PCR is positive in over 70 % of culture-positive tissue samples [45]. Immunodiffusion and complement fixation methods detect anti-Histoplasma antibodies in 70 % of immunocompromised and 90 % of immunocompetent patients with disseminated infection, but are often negative in patients on tumor necrosis-alpha inhibitor therapy [46]. Blood cultures are positive in 65 % of patients with disseminated histoplasmosis; tissue cultures from skin biopsy specimens can also be submitted.
Blastomycosis
For patients with severe disseminated infection, amphotericin B should be used. Pooled retrospective data show that amphotericin B is up to 91 % effective for blastomycosis. Although liposomal amphotericin B does not have as much supportive data, it should be used when available, and is particularly preferred in cases with CNS involvement. Itraconazole may be used in patients with mild to moderate disease not involving the CNS. In an open study, 90 % of patients with blastomycosis demonstrated clinical response to treatment with 6 months of itraconazole, making this a first-line therapy for blastomycosis. Although ketoconazole has strong supportive trial data for its curative success in blastomycosis, its use can be associated with severe hepatotoxicity as well as infection relapse. Therefore it is not recommended for the treatment of any endemic mycosis, including blastomycosis.
Fluconazole was 65 % effective at doses under or equal to 400 mg/day, but was successful in 87 % of patients treated with doses above 400 mg/day for 6 months in open studies. Voriconazole has been successful in small series for the treatment of refractory blastomycosis with CNS involvement. Posaconazole has also been used.
Coccidioidomycosis
A randomized, controlled trial demonstrated 72 % response rate for itraconazole and 57 % response rate for fluconazole after 12 months of treatment. While the majority of patients included were adults, there were a few cases in children as young as 6 years old.
Case series support the use of posaconazole, which has shown up to 73 % efficacy in refractory infections. Voriconazole demonstrated similar results for the treatment of resistant disease, albeit following a shorter treatment course.
Amphotericin B treatment is reserved for patients with rapidly worsening or CNS disease. Otherwise, treatment with oral azoles is preferred. Of note, in children with primary cutaneous disease and solitary lesions in whom disseminated disease has been excluded, observation or conservation excision, if feasible, can be considered.
Paracoccidioidomycosis
Oral antifungal therapy can be used in most (mild to moderate) cases of paracoccidioidomycosis. Among children and adults with paracoccidioidomycosis treated with itraconazole for an average of 6 months, 91 % of patients showed either marked improvement or resolution. In a small randomized trial, itraconazole, ketoconazole, and sulfadiazine were roughly equivalent in efficacy following treatment for at least 24 months. For patients with severe infection, including hypotension, respiratory failure, or severe malnutrition, therapy should be started with amphotericin B and then transitioned to oral therapy once improved.
Measured in terms of complete or partial treatment response, itraconazole was over 94 % effective compared to voriconazole, which was over 88 % effective. Voriconazole also has excellent in vitro activity against P. brasiliensis, but better data is available to support the use of itraconazole as a first-line therapy. In a separate open study, TMP-SMX was as effective as itraconazole, but treatment duration was four times as long with TMP-SMX.
Therapy with amphotericin B is reserved for severe or refractory disease, and retrospective reviews have supported the use of this agent in this context.
Histoplasmosis
In adult patients with AIDS and moderate-severe disseminated histoplasmosis, liposomal amphotericin B demonstrated better clinical success (88 %) than conventional amphotericin deoxycholate (64 %), in addition to improved survival and reduced nephrotoxicity; however, in children, amphotericin B deoxycholate is usually well tolerated, and the lipid preparations are not preferred. If liposomal formulations are not available, then amphotericin B deoxycholate should be used for induction.
Itraconazole demonstrated 85 % clinical response in adult patients with AIDS and histoplasmosis. However, patients with moderate to severe disease responded poorly. Additionally, clearance of fungemia is slower with itraconazole than with amphotericin B. Therefore, itraconazole is reserved as induction therapy for patients with mild disease without fungemia, and for maintenance therapy after successful induction. Maintenance therapy should continue for 1 year, to reduce the risk of relapse.
Itraconazole is superior to fluconazole in terms of clearance of fungemia as well as clinical response. Additionally, fluconazole is not as active as itraconazole against H. capsulatum in vitro. Although 74 % of patients responded to induction therapy with fluconazole in a large open study, almost half of patients demonstrated a relapse of their disease at 1 year while on maintenance therapy. Thus, fluconazole is reserved as a second-line therapy when amphotericin B and itraconazole cannot be tolerated. In a small case series, posaconazole has been effective for severe refractory infection as salvage therapy. Posaconazole demonstrates high in vitro activity against H. capsulatum. Voriconazole has also been used successfully as salvage therapy in disseminated histoplasmosis, but has inferior in vitro activity compared to itraconazole, and like posaconazole, has not been evaluated in a high-quality study.
Opportunistic Mycoses: Aspergillosis, Cryptococcosis, Fusariosis, Mucormycosis
Clinical Features
Aspergillus species are ubiquitous, and inhalation occurs often without sequelae in healthy hosts. In the setting of immunosuppression, most often during treatment for hematologic malignancies, or stem cell or solid organ transplantation, A. fumigatus, A. flavus, and A. terreus invade pulmonary or cutaneous tissue and may disseminate widely in the presence of angioinvasion. Neutropenia, high-dose corticosteroids, burns, and the neonatal period are also risk factors. Cutaneous aspergillosis may be primary, resulting from inoculation from trauma, or secondary, resulting from contiguous or hematogenous spread. Primary cutaneous aspergillosis may present as acute paronychia, necrotic plaques or nodules at the site of catheter insertion, or an erythematous edematous plaque. Secondary cutaneous aspergillosis may present with inflammatory or necrotic nodules, periorbital cellulitis, or ulcers [76, 77].
Cryptococcus neoformans and Cryptococcus gattii are encapsulated yeasts found worldwide in soil and bird guano that cause infections predominantly in patients with immunosuppression: HIV/AIDS, corticosteroids, organ transplantation, sarcoidosis, and malignancy. Following inhalation, meningoencephalitis, pulmonary infection, or disseminated disease may occur. Cutaneous lesions are seen in up to 15 % of patients with disseminated cryptococcosis. Plaques, purpura, ulcers, abscesses, cellulitis, and molluscum contagiosum-like lesions in patients with HIV may be seen [78]. Primary cutaneous disease is also possible following inoculation by minor trauma and, unlike secondary cutaneous lesions, may occur in immunocompetent hosts and is associated with favorable prognosis [79, 80].
Fusarium species are hyaline fungi present worldwide in soil, plant parts, and water. Superficial infections such as keratitis, onychomycosis, and intertrigo occur in immunocompetent hosts, while invasive infections occur only in patients with immunosuppression including neutropenia, hematologic malignancy, stem cell transplantation, and corticosteroid therapy. Sinusitis, pneumonia, fungemia, and dissemination can occur. Invasive infections occur via inhalation, direct inoculation, or spread from a superficial infection [81]. In this context, cutaneous lesions may be localized, as in cellulitis, or disseminated, with multiple necrotic painful lesions resembling those of ecthyma gangrenosum. Lesions at different stages of evolution, lymphangitic spread, target lesions, and blisters may be seen. Primary cutaneous disease in otherwise healthy hosts occurs at sites of burns or trauma, and presents with cellulitis, ulcers, verrucous nodules, and abscesses [82].
Rhizopus, Mucor, and Rhizomucor are genera of ubiquitous fungi that belong to the order Mucorales and cause most mucormycosis infections. Almost all infections occur in the context of immunosuppression, including poorly-controlled diabetes with ketoacidosis. Other risk factors include corticosteroid treatment, stem cell transplantation, hematologic malignancy, iron overload or deferoxamine treatment, HIV/AIDS, and burns. Inhalation of spores in susceptible individuals can lead to rhino-orbital-cerebral and pulmonary infections, the most common forms of the disease [83]. In contrast, cutaneous disease is always due to direct inoculation, and may occur following minor iatrogenic trauma such as intravenous line placement. Rarely, primary cutaneous disease may occur in immunocompetent individuals. Cutaneous disease usually presents with single cellulitis-like or ecthyma-like lesion. As with other forms of mucormycosis, rapidly progressive tissue necrosis often ensues due to infarction resulting from angioinvasion. Dissemination from cutaneous lesions can also occur [84].
Specific Investigations
For diagnosis |
Culture |
Histopathology with GMS, PAS, mucicarmine, alcian blue, or India ink |
EIA for galactomannan or beta-D-glucan polysaccharides |
Cryptococcal antigen testing (EIA, latex agglutination, lateral flow assays) |
PCR |
Imaging (computed tomography) |
For treatment |
CBC, LFTs, lipid panel, metabolic panel (potassium, blood glucose), kidney function tests (BUN and creatinine), EKG |
Flucytosine levels (for induction treatment in cryptococcosis) |
Definitive diagnosis of aspergillosis requires culture in combination with the histopathologic demonstration of tissue invasion by hyphae. Organisms are observed in biopsy specimens as narrow (3–6 μm wide), septate, and hyaline hyphae, with branching at an acute angle (45°). GMS or PAS may be useful to recognize hyphae, which can be seen invading blood vessels of the dermis or subcutis. It is important to note that histopathology alone is very nonspecific, since other hyaline molds such as Scedosporium and Fusarium have the same appearance, although Mucorales can be distinguished morphologically. The polysaccharide galactomannan can be detected in serum by EIA, which has demonstrated up to 71 % sensitivity and 93 % specificity in cases of aspergillosis [85]. False-positive results may occur in patients with infections due to Fusarium, Penicillium, or Histoplasma species, or in patients who have received intravenous piperacillin-tazobactam. The beta-D-glucan assay is less specific (positive in candidiasis), but more sensitive than EIA for galactomannan; both tests are useful detecting invasive aspergillosis prior to the onset of clinical findings in susceptible patients [86]. PCR demonstrates sensitivity up to 84 %; when two PCR tests are positive, the specificity is 95 % [87]. Given that the lungs are the most common site in invasive aspergillosis, CT imaging is an important component of evaluation.
Cutaneous cryptococcosis is best diagnosed by visualization of encapsulated yeast forms (5–7 μm in diameter) and isolation in culture. Mucicarmine and alcian blue highlight the capsule, while Fontana-Masson highlights the cell wall. In contrast, india ink demonstrates the yeast as halos against a black background [87]. Depending on the host response, histopathology may demonstrate suppurative granulomas with fewer organisms, or abundant organisms with minimal inflammation (gelatinous). Various methods are available for the evaluation of disseminated or systemic infection: serum cryptococcal antigen, culture, imaging, and PCR. The sensitivity of serum cryptococcal antigen testing is over 94 % for CNS disease and 90 % for lung disease; the sensitivity of CSF testing is over 87–100 % with a specificity of 100 %. Of note, cryptococcal antigen testing cannot distinguish between C. neoformans and C. gattii and is not useful for monitoring response to treatment. Standard assays utilize EIA or latex agglutination, while lateral flow assays offer rapid screening and are also highly sensitive [88]. Blood, sputum, CSF, and tissue from skin biopsies can be cultured on standard media. Cerebral and lung CT should be performed if disease in those locations is suspected. PCR is sensitive and can distinguish between C. neoformans and C. gattii, but is reserved for use in cases where direct visualization and culture are negative [89].
Histopathology and culture are the best methods for diagnosis of cutaneous fusariosis. In tissue, Fusarium species appear similar to Aspergillus and Scedosporium: septate hyaline hyphae branching at acute angles (45°). Thus, the finding of an angioinvasive hyalohyphomycosis by histopathology is nonspecific, and definitive diagnosis requires culture. Cutaneous lesions are present in over 80 % of patients with disseminated disease, are the only source of diagnostic material over half of cases, and often precede fungemia. Thus skin biopsies should always be performed, and submitted for histology and culture, when this diagnosis is entertained. Fusarium grows rapidly on media that lack cycloheximide; blood cultures are positive in 40 % of patients with invasive disease [90]. Beta-D-glucan is released by Fusarium but also by Candida. Galactomannan antigen assay has a sensitivity of 83 % but a specificity of 67 %, since it is also positive in aspergillosis [91].
Given that attempted cultures of Mucorales often yield no growth, the need for rapid diagnosis, and the importance of empiric therapy, histopathologic identification may provide the only direct evidence of mucormycosis. Presumptive diagnosis is made based on the presence of broad (up to 15 μm in diamater) aseptate hyphae with irregular branching patterns. While speciation should not be attempted by the dermatopathologist, distinction from Aspergillus is helpful, if possible, as Mucorales are not sensitive to voriconazole, the treatment of choice for aspergillosis [92]. PCR may also be helpful when cultures are negative, and can be applied with high sensitivity to histologic specimens [93].
Aspergillosis
In a large open trial of patients with invasive aspergillosis, voriconazole treatment resulted in survival rate over 70 %, while amphotericin B deoxycholate achieved a survival rate of less than 60 %. Voriconazole is also the preferred treatment given the lower risk of severe adverse effects.
In patients who are intolerant of, or refractory to, therapy with voriconazole or amphotericin B, posaconazole is an alternative treatment; in one open trial, posaconazole was successful in 42 % of patients treated. Isavuconazole was non-inferior to voriconazole in a randomized, controlled trial of patients with invasive aspergillosis; however, this study also evaluated patients with infections due to filamentous molds other than Aspergillus.
Itraconazole has demonstrated efficacy comparable to that of amphotericin B, but has inferior activity in vitro against Aspergillus. Caspofungin is approved for the treatment of aspergillosis, and has equivalent activity to that of the other echinocandins micafungin and anidulafungin. In patients intolerant of or refractory to standard treatment, overall clinical response to caspofungin was 45 %.
Echinocandins should not be used as monotherapy for aspergillosis, but can be used in combination with other treatments, including voriconazole. Several trials have produced data supportive of therapy combining voriconazole with echinocandins over monotherapy with voriconazole or amphotericin B alone. Similarly, the combination of liposomal amphotericin B and echinocandins has also demonstrated superiority to polyene therapy alone. However, retrospective data and in vitro studies do not support the use of amphotericin B in combination with azoles. In fact, azole therapy may be antagonistic toward the mechanism of action of amphotericin B.
Cryptococcosis
Management Strategies
Therapy regarding treatment of pediatric cryptococcosis is based on data from studies in adults. Given that most children with cutaneous cryptococcosis have underlying disseminated disease, the following treatment recommendations are best-suited for children with disseminated disease, but without a history of HIV or organ transplantation [107–109].
Several high-quality studies of HIV-infected patients with cryptococcosis have demonstrated that higher dose amphotericin B in conjunction with flucytosine provides improved clinical response, sterilization of cerebrospinal fluid, and survival benefit compared to induction monotherapy with lower dose amphotericin B and without flucytosine. Given the risk of myelosuppression with flucytosine induction, flucytosine peak levels should be maintained between 30 and 80 mcg/mL, and CBC should monitored regularly.
Consolidation and maintenance therapy should follow induction in order to reduce the risk of relapse. Comparative studies have shown that risk of relapse of cryptococcosis is 15–20 times greater without consolidation therapy. Fluconazole is preferred over itraconazole, due to it superior ability to sterilize the cerebrospinal fluid. Itraconazole is used for consolidation and maintenance when fluconazole cannot be tolerated.
For patients with persistent or relapsed infection that is not susceptible to fluconazole, voriconazole or posaconazole may be used for salvage consolidation therapy. Several open trials have supported the use of these alternative agents in this context.
Fusariosis
This section focuses on invasive infection associated with cutaneous lesions, rather than primary superficial infections such as onychomycosis.
Retrospective data supports the use of liposomal amphotericin B for fusariosis, and this is the first-line preferred therapy for invasive or disseminated infection, demonstrating improvement or cure in 46 %. Conventional amphotericin B deoxycholate should not be used for fusariosis, as it is associated with a higher case-fatality rate.
Voriconazole treatment was associated with up to 52 % clinical response rate in retrospective studies. Voriconazole may be used as monotherapy, as step-down therapy following induction treatment, or as combination treatment with amphotericin B. Strictly speaking, retrospective data do not demonstrate a clear benefit for combination therapy, but survival of patients with invasive fusariosis has improved in recent years with an increased use of voriconazole and combination therapy.
In open studies, posaconazole salvage treatment was associated with a successful outcome in 48 % of patients with fusariosis refractory to standard treatment. In small series and reports, isavuconazole has produced partial or complete response in patients with invasive fusariosis.
Mucormycosis
Primary cutaneous disease is associated with a favorable prognosis, and rarely disseminates. Prognosis is very poor in pulmonary or disseminated disease [92].
The initial treatment of choice for mucormycosis is liposomal amphotericin B, based on retrospective data, historical experience, and in vitro data. The liposomal formulation of the drug is preferred if available. It is important to note that treatment should be initiated when this diagnosis is suspected, and not delayed until identification by culture or microscopy is available, given a twofold increase in mortality with delayed treatment. Additionally, when microscopy reveals an angioinvasive hyphal infection, amphotericin B should be selected for treatment until culture results are available, given that Aspergillus is sensitive to voriconazole while Mucorales are not. Surgical debridement should also be undertaken at the time of presumptive diagnosis.
Posaconazole and isavuconazole both have in vitro activity against Mucorales, and data supportive for their use as step-down therapy after induction or for salvage therapy in patients with disease refractory to treatment with amphotericin B. In a retrospective study of patients requiring salvage therapy, clinical response occurred in 60 % of patients treated with posaconazole. Given issues with bioavailability of the oral solution formulation of posaconazole, only the IV formulation or extended-release oral tablets should be used. Isavuconazole demonstrated efficacy in a single-arm open study.
In a small retrospective study, patients with mucormycosis who received combination therapy with caspofungin and amphotericin B had better outcomes than those who received monotherapy alone. However, echinocandins do not have in vitro activity against Mucorales, and this data suggests utility as an adjunct treatment only.
Given that the iron chelator deferoxamine is a risk factor for mucormycosis, deferasirox has been used as adjunctive therapy to amphotericin B, but with mixed results. In a small open study, survival rate was high, but in a randomized, controlled trial, survival was poorer than with placebo.
References
da Rosa AC, Scroferneker ML, Vettorato R, Gervini RL, Vettorato G, Weber A. Epidemiology of sporotrichosis: a study of 304 cases in Brazil. J Am Acad Dermatol. 2005;52(3 Pt 1):451–9.
Pérez-Blanco M, Hernández Valles R, García-Humbría L. Yegres Chromoblastomycosis in children and adolescents in the endemic area of the Falcón State. Venezuela Med Mycol. 2006;44(5):467–71.
Silva JP, de Souza W, Rozental S. Chromoblastomycosis: a retrospective study of 325 cases on Amazonic Region (Brazil). Mycopathologia. 1998;143(3):171–5.
Zijlstra EE, van de Sande WW, Welsh O, el Mahgoub S, Goodfellow M, Fahal AH. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16(1):100–12.
Sudarshan V, Goel NK, Gahine R, Krishnani C. Rhinosporidiosis in Raipur, Chhattisgarh: a report of 462 cases. Indian J Pathol Microbiol. 2007;50(4):718–21.
Pal DK, Mallick AA, Majhi TK, Biswas BK, Chowdhury MK. Rhinosporidiosis in southwest Bengal. Trop Doct. 2012;42(3):150–3.
Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29(2):231–6. quiz 7.
Bernardes-Engemann AR, Costa RC, Miguens BR, Penha CV, Neves E, Pereira BA, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43(6):487–93.
Bonifaz A, Carrasco-Gerard E, Saúl A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44(1–2):1–7.
Ibrahim AI, El Hassan AM, Fahal A, van de Sande WW. A histopathological exploration of the Madurella mycetomatis grain. PLoS One. 2013;8(3):e57774.
Das S, Kashyap B, Barua M, Gupta N, Saha R, Vaid L, et al. Nasal rhinosporidiosis in humans: new interpretations and a review of the literature of this enigmatic disease. Med Mycol. 2011;49(3):311–5.
de Lima Barros MB, Schubach AO, de Vasconcellos Carvalhaes de Oliveira R, Martins EB, Teixeira JL, Wanke B. Treatment of cutaneous sporotrichosis with itraconazole--study of 645 patients. Clin Infect Dis. 2011;52(12):e200.
Sharkey-Mathis PK, Kauffman CA, Graybill JR, Stevens DA, Hostetler JS, Cloud G, et al. Treatment of sporotrichosis with itraconazole. NIAID Mycoses Study Group. Am J Med. 1993;95(3):279.
Kauffman CA, Bustamante B, Chapman SW, Pappas PG, Infectious Diseases Society of America. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(10):1255.
Cabezas C, Bustamante B, Holgado W, Begue RE. Treatment of cutaneous sporotrichosis with one daily dose of potassium iodide. Pediatr Infect Dis J. 1996;15(4):352.
Gottlieb GS, Lesser CF, Holmes KK, Wald A. Disseminated sporotrichosis associated with treatment with immunosuppressants and tumor necrosis factor-alpha antagonists. Clin Infect Dis. 2003;37(6):838.
al-Tawfiq JA, Wools KK. Disseminated sporotrichosis and Sporothrix schenckii fungemia as the initial presentation of human immunodeficiency virus infection. Clin Infect Dis. 1998;26(6):1403.
Hay RJ, Mahgoub ES, Leon G, al-Sogair S, Welsh O. Mycetoma. J Med Vet Mycol. 1992;30(Suppl):1–41.
Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother. 2009;53(1):24.
McGinnis MR. Mycetoma. Dermatol Clin. 1996;14(1):97.
Welsh O. Mycetoma. Current concepts in treatment. Int J Dermatol. 1991;30(6):387.
Mahgoub ES, Gumaa SA. Ketoconazole in the treatment of eumycetoma due to Madurella mycetomii. Trans R Soc Trop Med Hyg. 1984;78(3):376.
Welsh O, Salinas MC, Rodríguez MA. Treatment of eumycetoma and actinomycetoma. Curr Top Med Mycol. 1995;6:47.
US Food and Drug Administration. FDA Drug Safety Communication: FDA limits usage of Nizoral (ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal gland problems. [Online] Available at: http://www.fda.gov/drugs/drugsafety/ucm362415.htm. Accessed 21 Dec 2015.
França Jr GV, Gomes CC, Sakano E, Altemani AM, Shimizu LT. Nasal rhinosporidiosis in children. J Pediatr (Rio J). 1994;70(5):299–301.
George L, Dincy P, Chopra M, Agarwala M, Maheswaran S, Deodhar D, et al. Novel multidrug therapy for disseminated rhinosporidiosis, refractory to dapsone – case report. Trop Doct. 2013;43(3):110–2.
Job A, Venkateswaran S, Mathan M, Krishnaswami H, Raman R. Medical therapy of rhinosporidiosis with dapsone. J Laryngol Otol. 1993;107(9):809–12.
Chapman SW, Lin AC, Hendricks KA, Nolan RL, Currier MM, Morris KR, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12(3):219–28.
Garcia Garcia SC, Salas Alanis JC, Flores MG, Gonzalez Gonzalez SE, Vera Cabrera L, Ocampo Candiani J. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015;90(5):610–9.
McCarty JM, Demetral LC, Dabrowski L, Kahal AK, Bowser AM, Hahn JE. Pediatric coccidioidomycosis in central California: a retrospective case series. Clin Infect Dis. 2013;56(11):1579–85.
Bicalho RN, Santo MF, de Aguiar MC, Santos VR. Oral paracoccidioidomycosis: a retrospective study of 62 Brazilian patients. Oral Dis. 2001;7(1):56–60.
Ramos ESM, Saraiva Ldo E. Paracoccidioidomycosis. Dermatol Clin. 2008;26(2):257–69. vii.
Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore). 2007;86(3):162–9.
Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23(2):367–81.
Wheat LJ. Antigen detection, serology, and molecular diagnosis of invasive mycoses in the immunocompromised host. Transpl Infect Dis. 2006;8(3):128–39.
Monheit JE, Cowan DF, Moore DG. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch Pathol Lab Med. 1984;108(8):616–8.
Pappagianis D, Zimmer BL. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990;3(3):247–68.
Zartarian M, Peterson EM, de la Maza LM. Detection of antibodies to Coccidioides immitis by enzyme immunoassay. Am J Clin Pathol. 1997;107(2):148–53.
Durkin M, Connolly P, Kuberski T, Myers R, Kubak BM, Bruckner D, et al. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin Infect Dis. 2008;47(8):e69–73.
Johnson R, Kernerman SM, Sawtelle BG, Rastogi SC, Nielsen HS, Ampel NM. A reformulated spherule-derived coccidioidin (Spherusol) to detect delayed-type hypersensitivity in coccidioidomycosis. Mycopathologia. 2012;174(5–6):353–8.
Restrepo A, Tobon AM, Agudelo CA. Paracoccidioidomycosis. In: Hospenthal DR, Rinaldi MG, editors. Diagnosis and treatment of human mycoses. 1st ed. Totowa: Humana Press; 2008. p. 331.
Cohen PR, Bank DE, Silvers DN, Grossman ME. Cutaneous lesions of disseminated histoplasmosis in human immunodeficiency virus-infected patients. J Am Acad Dermatol. 1990;23(3 Pt 1):422–8.
Eidbo J, Sanchez RL, Tschen JA, Ellner KM. Cutaneous manifestations of histoplasmosis in the acquired immune deficiency syndrome. Am J Surg Pathol. 1993;17(2):110–6.
Hage CA, Ribes JA, Wengenack NL, Baddour LM, Assi M, McKinsey DS, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53(5):448–54.
Babady NE, Buckwalter SP, Hall L, Le Febre KM, Binnicker MJ, Wengenack NL. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J Clin Microbiol. 2011;49(9):3204–8.
Hage CA, Bowyer S, Tarvin SE, Helper D, Kleiman MB, Wheat LJ. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50(1):85–92.
Chapman SW, Dismukes WE, Proia LA, Bradsher RW, Pappas PG, Threlkeld MG, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(12):1801–12.
Parker JD, Doto IL, Tosh FE. A decade of experience with blastomycosis and its treatment with amphotericin B. A National Communicable Disease Center Cooperative Mycoses Study. Am Rev Respir Dis. 1969;99(6):895–902.
Perfect JR. Treatment of non-Aspergillus moulds in immunocompromised patients, with amphotericin B lipid complex. Clin Infect Dis. 2005;40 Suppl 6:S401–8.
Dismukes WE, Bradsher Jr RW, Cloud GC, Kauffman CA, Chapman SW, George RB, et al. Itraconazole therapy for blastomycosis and histoplasmosis. NIAID Mycoses Study Group. Am J Med. 1992;93(5):489–97.
National Institute of Allergy and Infectious Diseases Mycoses Study Group. Treatment of blastomycosis and histoplasmosis with ketoconazole. Results of a prospective randomized clinical trial. Ann Intern Med. 1985;103(6 (Pt 1):861–72.
Bradsher RW, Rice DC, Abernathy RS. Ketoconazole therapy for endemic blastomycosis. Ann Intern Med. 1985;103(6 (Pt 1):872–9.
Pappas PG, Bradsher RW, Kauffman CA, Cloud GA, Thomas CJ, Campbell Jr GD, et al. Treatment of blastomycosis with higher doses of fluconazole. The National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin Infect Dis. 1997;25(2):200–5.
Pappas PG, Bradsher RW, Chapman SW, Kauffman CA, Dine A, Cloud GA, et al. Treatment of blastomycosis with fluconazole: a pilot study. The National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin Infect Dis. 1995;20(2):267–71.
Borgia SM, Fuller JD, Sarabia A, El-Helou P. Cerebral blastomycosis: a case series incorporating voriconazole in the treatment regimen. Med Mycol. 2006;44(7):659–64.
Proia LA, Harnisch DO. Successful use of posaconazole for treatment of blastomycosis. Antimicrob Agents Chemother. 2012;56(7):4029.
Galgiani JN, Catanzaro A, Cloud GA, Johnson RH, Williams PL, Mirels LF, et al. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Mycoses Study Group. Ann Intern Med. 2000;133(9):676–86.
Stevens DA, Rendon A, Gaona-Flores V, Catanzaro A, Anstead GM, Pedicone L, et al. Posaconazole therapy for chronic refractory coccidioidomycosis. Chest. 2007;132(3):952–8.
Kim MM, Vikram HR, Kusne S, Seville MT, Blair JE. Treatment of refractory coccidioidomycosis with voriconazole or posaconazole. Clin Infect Dis. 2011;53(11):1060–6.
Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41(9):1217–23.
Shikanai-Yasuda MA, Telles Filho Fde Q, Mendes RP, Colombo AL, Moretti ML. Guidelines in paracoccidioidomycosis. Rev Soc Bras Med Trop. 2006;39(3):297–310.
Naranjo MS, Trujillo M, Munera MI, Restrepo P, Gomez I, Restrepo A. Treatment of paracoccidioidomycosis with itraconazole. J Med Vet Mycol. 1990;28(1):67–76.
Shikanai-Yasuda MA, Benard G, Higaki Y, Del Negro GM, Hoo S, Vaccari EH, et al. Randomized trial with itraconazole, ketoconazole and sulfadiazine in paracoccidioidomycosis. Med Mycol. 2002;40(4):411–7.
Queiroz-Telles F, Goldani LZ, Schlamm HT, Goodrich JM, Espinel-Ingroff A, Shikanai-Yasuda MA. An open-label comparative pilot study of oral voriconazole and itraconazole for long-term treatment of paracoccidioidomycosis. Clin Infect Dis. 2007;45(11):1462–9.
Cavalcante Rde S, Sylvestre TF, Levorato AD, de Carvalho LR, Mendes RP. Comparison between itraconazole and cotrimoxazole in the treatment of paracoccidioidomycosis. PLoS Negl Trop Dis. 2014;8(4):e2793.
de Campos EP, Sartori JC, Hetch ML, de Franco MF. Clinical and serologic features of 47 patients with paracoccidioidomycosis treated by amphotericin B. Rev Inst Med Trop Sao Paulo. 1984;26(4):212–7.
Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 2002;137(2):105–9.
Wheat J, Hafner R, Korzun AH, Limjoco MT, Spencer P, Larsen RA, et al. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trial Group. Am J Med. 1995;98(4):336–42.
Wheat LJ, Cloud G, Johnson PC, Connolly P, Goldman M, Le Monte A, et al. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrob Agents Chemother. 2001;45(8):2354–7.
Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807–25.
Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrob Agents Chemother. 2002;46(1):248–50.
Wheat J, MaWhinney S, Hafner R, McKinsey D, Chen D, Korzun A, et al. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Acquired Immunodeficiency Syndrome Clinical Trials Group and Mycoses Study Group. Am J Med. 1997;103(3):223–32.
Restrepo A, Tobon A, Clark B, Graham DR, Corcoran G, Bradsher RW, et al. Salvage treatment of histoplasmosis with posaconazole. J Infect. 2007;54(4):319–27.
Freifeld A, Proia L, Andes D, Baddour LM, Blair J, Spellberg B, et al. Voriconazole use for endemic fungal infections. Antimicrob Agents Chemother. 2009;53(4):1648–51.
Wheat LJ, Connolly P, Smedema M, Durkin M, Brizendine E, Mann P, et al. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. J Antimicrob Chemother. 2006;57(6):1235–9.
van Burik JA, Colven R, Spach DH. Cutaneous aspergillosis. J Clin Microbiol. 1998;36(11):3115–21.
Bernardeschi C, Foulet F, Ingen-Housz-Oro S, Ortonne N, Sitbon K, Quereux G, et al. Cutaneous invasive aspergillosis: retrospective multicenter study of the French Invasive-Aspergillosis Registry and literature review. Medicine (Baltimore). 2015;94(26):e1018.
Thomas I, Schwartz RA. Cutaneous manifestations of systemic cryptococcosis in immunosupressed patients. J Med. 2001;32(5–6):259–66.
Du L, Yang Y, Gu J, Chen J, Liao W, Zhu Y. Systemic review of published reports on primary cutaneous cryptococcosis in immunocompetent patients. Mycopathologia. 2015;180(1–2):19–25.
Lenz D, Held J, Goerke S, Wagner D, Tintelnot K, Henneke P, et al. Primary cutaneous cryptococcosis in an eight-year-old immunocompetent child: how to treat? Klin Padiatr. 2015;227(1):41–4.
Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20(4):695–704.
Hay RJ. Fusarium infections of the skin. Curr Opin Infect Dis. 2007;20(2):115–7.
Adam RD, Hunter G, DiTomasso J, Comerci Jr G. Mucormycosis: emerging prominence of cutaneous infections. Clin Infect Dis. 1994;19(1):67–76.
Arnaiz-Garcia ME, Alonso-Pena D, Gonzalez-Vela Mdel C, Garcia-Palomo JD, Sanz-Gimenez-Rico JR, Arnaiz-Garcia AM. Cutaneous mucormycosis: report of five cases and review of the literature. J Plast Reconstr Aesthet Surg. 2009;62(11):e434–41.
Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42(10):1417–27.
Sulahian A, Porcher R, Bergeron A, Touratier S, Raffoux E, Menotti J, et al. Use and limits of (1–3)-beta-d-glucan assay (Fungitell), compared to galactomannan determination (Platelia Aspergillus), for diagnosis of invasive aspergillosis. J Clin Microbiol. 2014;52(7):2328–33.
Cruciani M, Mengoli C, Loeffler J, Donnelly P, Barnes R, Jones BL, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev. 2015;10:CD009551. Lazcano O, Speights Jr VO, Strickler JG, Bilbao JE, Becker J, Diaz J. Combined histochemical stains in the differential diagnosis of Cryptococcus neoformans. Mod Pathol. 1993;6(1):80–4.
Lindsley MD, Mekha N, Baggett HC, Surinthong Y, Autthateinchai R, Sawatwong P, et al. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis. 2011;53(4):321–5.
Sidrim JJ, Costa AK, Cordeiro RA, Brilhante RS, Moura FE, Castelo-Branco DS, et al. Molecular methods for the diagnosis and characterization of Cryptococcus: a review. Can J Microbiol. 2010;56(6):445–58.
Liu K, Howell DN, Perfect JR, Schell WA. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am J Clin Pathol. 1998;109(1):45–54.
Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, et al. Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41(5):654–9.
Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53.
Hammond SP, Bialek R, Milner DA, Petschnigg EM, Baden LR, Marty FM. Molecular methods to improve diagnosis and identification of mucormycosis. J Clin Microbiol. 2011;49(6):2151–3.
Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the infectious diseases society of America. Clin Infect Dis. 2008;46(3):327–60.
Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531–40.
Patterson TF, Boucher HW, Herbrecht R, Denning DW, Lortholary O, Ribaud P, et al. Strategy of following voriconazole versus amphotericin B therapy with other licensed antifungal therapy for primary treatment of invasive aspergillosis: impact of other therapies on outcome. Clin Infect Dis. 2005;41(10):1448–52.
Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–15.
Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44(1):2–12.
Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–9.
Denning DW, Lee JY, Hostetler JS, Pappas P, Kauffman CA, Dewsnup DH, et al. NIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97(2):135–44.
Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004;39(11):1563–71.
Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81–9.
Singh N, Limaye AP, Forrest G, Safdar N, Munoz P, Pursell K, et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation. 2006;81(3):320–6.
Caillot D, Thiebaut A, Herbrecht R, de Botton S, Pigneux A, Bernard F, et al. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: a randomized pilot study (Combistrat trial). Cancer. 2007;110(12):2740–6.
Kontoyiannis DP, Boktour M, Hanna H, Torres HA, Hachem R, Raad II. Itraconazole added to a lipid formulation of amphotericin B does not improve outcome of primary treatment of invasive aspergillosis. Cancer. 2005;103(11):2334–7.
Meletiadis J, Petraitis V, Petraitiene R, Lin P, Stergiopoulou T, Kelaher AM, et al. Triazole-polyene antagonism in experimental invasive pulmonary aspergillosis: in vitro and in vivo correlation. J Infect Dis. 2006;194(7):1008–18.
Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322.
Gonzalez CE, Shetty D, Lewis LL, Mueller BU, Pizzo PA, Walsh TJ. Cryptococcosis in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1996;15(9):796–800.
Manfredi R, Coronado OV, Mastroianni A, Chiodo F. Liposomal amphotericin B and recombinant human granulocyte-macrophage colony-stimulating factor (rHuGM-CSF) in the treatment of paediatric AIDS-related cryptococcosis. Int J STD AIDS. 1997;8(6):406–8.
Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363(9423):1764–7.
van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997;337(1):15–21.
Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368(14):1291–302.
Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O, French Cryptococcosis Study G. Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS One. 2008;3(8):e2870.
Cryptococcus neoformans infections (cryptococcosis). In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red book: 2015 report of the Committee on Infectious Diseases, 30th ed. Elk Grove Village: American Academy of Pediatrics; 2015. p. 309.
Soltani M, Tobin CM, Bowker KE, Sunderland J, MacGowan AP, Lovering AM. Evidence of excessive concentrations of 5-flucytosine in children aged below 12 years: a 12-year review of serum concentrations from a UK clinical assay reference laboratory. Int J Antimicrob Agents. 2006;28(6):574–7.
Singh N, Lortholary O, Alexander BD, Gupta KL, John GT, Pursell KJ, et al. Antifungal management practices and evolution of infection in organ transplant recipients with cryptococcus neoformans infection. Transplantation. 2005;80(8):1033–9.
Dismukes WE, Cloud G, Gallis HA, Kerkering TM, Medoff G, Craven PC, et al. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N Engl J Med. 1987;317(6):334–41.
Pitisuttithum P, Negroni R, Graybill JR, Bustamante B, Pappas P, Chapman S, et al. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother. 2005;56(4):745–55.
Perfect JR, Marr KA, Walsh TJ, Greenberg RN, DuPont B, de la Torre-Cisneros J, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36(9):1122–31.
Nucci M, Anaissie EJ, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, et al. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer. 2003;98(2):315–9.
Nucci M, Marr KA, Vehreschild MJ, de Souza CA, Velasco E, Cappellano P, et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin Microbiol Infect. 2014;20(6):580–5.
Stanzani M, Tumietto F, Vianelli N, Baccarani M. Update on the treatment of disseminated fusariosis: focus on voriconazole. Ther Clin Risk Manag. 2007;3(6):1165–73.
Lortholary O, Obenga G, Biswas P, Caillot D, Chachaty E, Bienvenu AL, et al. International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob Agents Chemother. 2010;54(10):4446–50.
Raad II, Hachem RY, Herbrecht R, Graybill JR, Hare R, Corcoran G, et al. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis. 2006;42(10):1398–403.
Cornely OA, Ostrosky-Zeichner L, Rahav G, et al. Outcomes in patients with invasive mold disease caused by Fusarium or Scedosporium spp treated with isavuconazole: experience from the VITAL and SECURE trials. (Abstract M-1760) In: Program and abstracts of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC; 2014.
McCarthy M, Rosengart A, Schuetz AN, Kontoyiannis DP, Walsh TJ. Mold infections of the central nervous system. N Engl J Med. 2014;371(2):150–60.
Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503–9.
Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother. 2002;46(5):1581–2.
van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61–5.
Pettit NN, Carver PL. Isavuconazole: a new option for the management of invasive fungal infections. Ann Pharmacother. 2015;49(7):825–42.
Reed C, Bryant R, Ibrahim AS, Edwards Jr J, Filler SG, Goldberg R, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364–71.
Pfaller MA, Marco F, Messer SA, Jones RN. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis. 1998;30(4):251–5.
Spellberg B, Andes D, Perez M, Anglim A, Bonilla H, Mathisen GE, et al. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother. 2009;53(7):3122–5.
Spellberg B, Ibrahim AS, Chin-Hong PV, Kontoyiannis DP, Morris MI, Perfect JR, et al. The Deferasirox-AmBisome therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715–22.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Motaparthi, K. (2017). Infectious Diseases: Deep Fungal Infections. In: Teng, J., Marqueling, A., Benjamin, L. (eds) Therapy in Pediatric Dermatology. Springer, Cham. https://doi.org/10.1007/978-3-319-43630-2_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-43630-2_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43628-9
Online ISBN: 978-3-319-43630-2
eBook Packages: MedicineMedicine (R0)