Abstract
In eukaryotic cells, AMP-activated protein kinase (AMPK) generally promotes catabolic pathways that produce ATP and at the same time inhibits anabolic pathways involved in different processes that consume ATP. As an energy sensor, AMPK is involved in the main cellular functions implicated in cell fate, such as cell growth and autophagy.
Recently, AMPK has been connected with apoptosis regulation, although the molecular mechanism by which AMPK induces and/or inhibits cell death is not clear.
This chapter reviews the essential role of AMPK in signaling pathways that respond to cellular stress and damage, highlighting the complex and reciprocal regulation between AMPK and their targets and effectors. The therapeutic implications of the role of AMPK in different pathologies such as diabetes, cancer, or mitochondrial dysfunctions are still controversial, and it is necessary to further investigate the molecular mechanisms underlying AMPK activation.
The original version of this chapter was revised. An erratum to this chapter can be found at DOI 10.1007/978-3-319-43589-3_21.
An erratum to this chapter can be found at http://dx.doi.org/10.1007/978-3-319-43589-3_21
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Every cell uses ATP as the main source of energy; therefore, the ATP levels must be matched by the energy demand needed to carry on different functions. In order to maintain the equilibrium between nutrient supply and energy demand, the eukaryotic cell uses an ATP sensor coupled to different pathways that are initiated when a decrease in ATP levels is detected. AMPK exerts this function in the eukaryotic cells; therefore, it is considered a master regulator of cell metabolism (Viollet and Andreelli 2011).
Activation of AMPK occurs in response to stress circumstances such as low glucose, hypoxia, ischemia, heat shock, increased reactive oxygen species (ROS), and exercise (Luo et al. 2005), although AMPK can be activated in response to several pharmacological agents as well. Under these situations, the intracellular AMP + ADP/ATP ratio is increased, and then AMPK is phosphorylated in its kinase domain to become a catalytically competent protein. By sensing changes in adenine nucleotide ratios, AMPK is activated by different stresses that diminish cellular energy levels and/or increase cellular ATP consumption. Once activated, AMPK is able to stimulate catabolic processes, ATP-generating pathways, and inhibits anabolic processes such as synthesis of lipids, carbohydrates, and proteins in order to assure cell survival.
The involvement of AMPK in many regulatory pathways makes it considered as a master metabolic switch. In fact, in mammals, AMPK has an essential role in coordinating survival, growth, and metabolism under conditions of low energy. Therefore, AMPK regulates in a coordinate way catabolism, cell growth, autophagy, and apoptosis, since all these cell functions cannot be balanced independently.
The AMPK signaling network covers numerous genes involved in survival and cell growth. Among them, mammalian Target Of Rapamycin (mTOR) regulation is the axis of numerous cell processes since it plays an essential role in metabolism regulation, autophagy, cell growth, and apoptosis (Xu et al. 2012). This relation is complex but essential to understand the dialogue and the interplay between autophagy and apoptosis, which has been gaining importance in the last years and is closely related with cell growth and survival.
2 AMPK Regulation of Cell Growth
AMPK is situated in the center of an energy-sensing cascade that is activated by ATP depletion. AMPK promotes catabolic pathways that produce ATP, and at the same time AMPK inhibits anabolic pathways involved in cell growth and other processes that consume ATP (Grahame Hardie 2014).
Moreover, AMPK is part of a tumor suppressor network that regulates cell growth and proliferation in stress situations; in fact, it is assumed that AMPK is responsible for the tumor suppressor effects of LKB1 (Liver Kinase B1) (Hardie 2011). Consistent with these functions, AMPK acts as a cell growth inhibitor in different stress situations, such as glucose deprivation, energy depletion, tumor proliferation, or DNA damage.
How does activation of AMPK result in cell growth suppression? Cell growth requires balanced nutrient availability and energy production. Therefore, protein, rRNA, and lipid synthesis are needed, in addition to replication of DNA (Vander Heiden et al. 2009). In this sense, AMPK prevents cell growth by inhibiting protein, rRNA, and lipid synthesis, and it also induces cell cycle arrest in G1, preventing DNA replication (Table 3.1).
2.1 AMPK Inhibits Protein and rRNA Synthesis
AMPK is considered an antigrowth molecule, among other reasons, due to its relationship with the tumor suppressor gene Tuberous Sclerosis Complex 2 (TSC2), also known as Tuberin. AMPK directly phosphorylates TSC2 on T1227 and S1345 to enhance its Rheb-GAP activity, which causes an inhibition of mammalian Target Of Rapamycin Complex 1 (mTORC1) kinase activity (Inoki et al. 2003). Moreover, AMPK is able to directly inhibit mTORC1 by phosphorylating raptor, the mTORC1-binding partner (Gwinn et al. 2008). The phosphorylation of two conserved serines (Ser722 and Ser792) of raptor by AMPK blocks the ability of the mTORC1 kinase complex to phosphorylate its substrates.
By inhibiting mTORC1, AMPK prevents two major biosynthetic pathways required for cell growth, such as protein and rRNA synthesis. The objective of the relationship between AMPK and mTORC1 is adjusting energy required by anabolic process to energy availability (Alexander and Walker 2011).
mTORC1 regulates two key proteins which are enhancers of protein synthesis: p70S6K (p70S6 Kinase) and 4EBP1 (eIF-4E-binding protein 1) (Zoncu et al. 2011). p70S6K is a positive regulator of translation initiation and elongation. When it is phosphorylated by mTORC1, p70S6K phosphorylates multiple substrates including eukaryotic translation initiation factor 4B (eIF4B) (Holz et al. 2005). In contrast, 4EBP1 inhibits mRNA translation. When phosphorylated by mTORC1, it dissociates from eIF4E, allowing the latest to recruit eukaryotic translation initiation factor 4 gamma (eIF4G) to the 5′ end of most mRNAs (Ma and Blenis 2009).
AMPK inhibits protein synthesis in an mTOR-independent manner by promoting phosphorylation of eukaryotic elongation factor 2 (eEF2). eEF2 phosphorylation regulates the peptide chain elongation (Browne and Proud 2002). Phosphorylation of eEF2, which inhibits its activity, is catalyzed by a specific calcium/calmodulin-dependent protein kinase termed eEF2 kinase. AMPK inactivates eEF2 by directly phosphorylating eEF2 kinase (Browne et al. 2004).
By this way, AMPK and mTOR provide the link between cellular energy status and protein synthesis, a major consumer of metabolic energy.
Related to rRNA synthesis, mTORC1 regulates RNA Pol I-mediated transcription by enhancing the activity and localization of TIF-IA, a transcription factor for RNA polymerase-1 that senses nutrient availability (Mayer et al. 2004).
AMPK also inhibits rRNA synthesis in an mTOR-independent manner: it directly phosphorylates TIF-1A at Ser635, and by this way the assembly of functional transcription initiation complexes becomes impaired (Hoppe et al. 2009).
By the mechanisms described above, AMPK allows the adaptation of protein and rRNA synthesis (and finally cell growth) to changes in cellular energy supply.
2.2 AMPK Induces Cell Cycle Arrest
Besides protein and rRNA synthesis, cell growth needs DNA replication. AMPK uses different mechanisms to induce cell cycle arrest. First, AMPK is able to phosphorylate MDMX, which regulates p53 proteasomal turnover since it is a component of the E3 ubiquitin ligase complex (He et al. 2014). AMPK-mediated phosphorylation of MDMX on Ser342 promotes the association between MDMX and 14-3-3. This inhibits p53 ubiquitylation, preventing its turnover by the proteasome. Moreover, inhibition of p53 ubiquitylation induces cell cycle arrest by promoting stabilization and activation of p53.
Second, AMPK directly phosphorylates p53 on Ser15 to stabilize it and initiate AMPK-dependent cell cycle arrest (Jones et al. 2005). In stress conditions, such as glucose deprivation, cell cycle arrest promoted by p53 allows cell to survive. Upon glucose restoration, cells reenter the cell cycle. Interestingly, p53 activation also regulates AMPK. It has been demonstrated that the products of Sestrin1 and Sestrin2, which are two p53 target genes, activate AMPK (Budanov and Karin 2008). Furthermore, p53 inhibits mTORC1 by increasing expression of genes that negatively regulate its function, such as insulin-like growth factor-binding protein 3 (igf-bp3), PTEN (phosphatase and tensin homolog), and TSC2 (Buckbinder et al. 1995; Feng et al. 2007). Thus, the stabilization of p53 by AMPK not only induces cell cycle arrest but also indirectly promotes protein and rRNA synthesis inhibition.
At this point, we can consider that the connection between AMPK, mTORC1, and p53 balances the growth-inhibiting response to cellular stress.
2.3 AMPK Inhibits Lipid Synthesis
During cell division, cells must double their lipid content. Among different lipid classes, fatty acids are particularly important for cell growth, since cells need them for membrane generation, protein modification, and bioenergetic requirements. Therefore, fatty acid synthesis plays an essential role in cell growth.
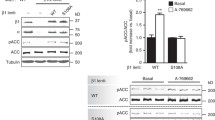
It has been described that AMPK inhibits fatty acid synthesis by different mechanisms. First, AMPK inhibits lipogenesis by phosphorylating and inactivating the acetyl-CoA carboxylase ACC1 on Ser79 (Davies et al. 1992; Hardie and Pan 2002). ACC1 catalyzes the synthesis of malonyl-CoA, the substrate for fatty acid synthesis. Moreover, AMPK also downregulates the expression of fatty acid synthase (FAS), which is also important in fatty acid synthesis (Foretz et al. 1998).
Second, AMPK downregulates the expression of enzymes involved in fatty acid synthesis at the transcriptional level by phosphorylating the transcription factor Sterol regulatory element-binding protein-1c (SREBP-1c). SBREP activity controls fatty acid and sterol synthesis; therefore, activation of SREBP and expression of SREBP target genes are required for efficient cell growth in mammalian cells (Porstmann et al. 2008). AMPK phosphorylates SREBP-1c on Ser372 to prevent its translocation to the nucleus, leading to inhibition of lipogenesis and lipid accumulation (Li et al. 2011).
Besides, AMPK phosphorylates and inactivates other enzymes involved in lipid synthesis, such as HMG-CoA reductase, essential for cholesterol synthesis (Henin et al. 1995), or glycerol-3-phosphate acyltransferase (GPAT), which participates in glycerolipid and glycerophospholipid metabolism (Muoio et al. 1999).
3 AMPK Regulation of Autophagy
As we have exposed above, AMPK mainly stimulates catabolic pathways and prevents anabolic pathways, and it exerts its function via direct phosphorylation of different downstream enzymes or indirect regulation of transcription factors activities (Hardie 2007). Autophagy is one of the most important processes in cell catabolism, since cytosol and organelles are sequestered within double-membrane vesicles (pre-autophagosomes) that deliver the contents to the lysosome/vacuole (autolysosome) for hydrolytic degradation and recycling of the resulting macromolecules. Moreover, autophagy is considered a survival mechanism of the cell in stress situations (Goldman et al. 2010); hence, an appropriate regulation of autophagy is essential for cellular homeostasis, since excessive self-digestion can be harmful (Mizushima 2007).
Autophagy can be a nonselective or selective process because autophagy proteins and receptors of the autophagosome can interact specifically with the cytoplasmic component that needs to be eliminated (Johansen and Lamark 2011). Whereas autophagy usually refers to the nonselective elimination of any component of the cytoplasm, different terms have been coined to define the selective degradation of different organelles such as mitochondria (mitophagy) (Lemasters 2005), peroxisomes (pexophagy) (Till et al. 2012), or even lipids (lipophagy) (Liu and Czaja 2013).
Autophagy is generally activated by starvation and nutrient deprivation to generate metabolic intermediates to maintain ATP production. In fact, the autophagy checkpoint is a major mechanism for the maintenance of intracellular homeostasis that can be upregulated by nutrient deprivation and/or organelle damage. Therefore, autophagy is controlled by several metabolites, including the ATP/ADP ratio and the availability of acetyl-CoA, which affect the activity of various acetyltransferases (Green et al. 2014).
Since AMPK was primarily characterized as a kinase allosterically activated by AMP (Yeh et al. 1980), it was logical to hypothesize that AMPK would have a critical role in autophagy regulation.
Energy levels manifested in the form of ADP/ATP ratio trigger autophagy by activating AMPK. AMP enhances kinase activity of AMPK by binding to its γ subunits. On the contrary, ATP inhibits the allosteric activation of AMPK by AMP (Hardie 2007). Moreover, oxidative stress, which manifests in the form of high levels of ROS or oxidizing agents, activates AMPK by different pathways, some of them in an AMP-independent manner (Hwang et al. 2004; Emerling et al. 2009; Mungai et al. 2011).
In addition to energy levels, it has been hypothesized that other stimuli trigger autophagy by AMPK activation, under normal conditions indeed. Intracellular calcium (Ca2+) levels seem to be crucial in AMPK activity, since Calmodulin-Dependent Kinase Kinase β (CaMKKβ) is suggested as an upstream kinase that activates AMPK (Hawley et al. 2005; Woods et al. 2005). It has been observed that different Ca2+ mobilizing agents, such as vitamin D compounds, induce autophagy by CaMKKβ and AMPK phosphorylation and activation. Moreover, inhibition of CaMKKβ by siRNA causes the inactivation of AMPK and an attenuation of autophagy (Høyer-Hansen et al. 2007).
Therefore, although AMPK activation is necessary to trigger autophagy, it is not known if it is sufficient or there are other molecular pathways which induce the change between basal autophagy and a huge autophagy in response to a cellular stress.
3.1 AMPK Regulation of Nonselective Autophagy
Independently of the stimuli, AMPK activation can induce the autophagic process through two different mechanisms: inhibition of mTOR and direct phosphorylation of ULK1 (Unc-51 Like Kinase 1, a mammalian orthologue of Atg1).
mTOR complex is a main regulator of diverse intracellular pathways that controls growth, proliferation, and survival. It is an effector in the PI3K/AKT pathway, and it carries out its action by two different complexes called mTORC1 and mTORC2. mTORC1 promotes protein synthesis, lipid biogenesis, cell growth, and anabolism and inhibits cellular catabolism by preventing autophagy. Instead, mTORC2 regulates cell survival, cell proliferation, and metabolism.
Although mTOR name comes from its sensitivity to rapamycin, rapamycin inhibits autophagy by affecting solely mTORC1, which is formed by the serine–threonine kinase mTOR, mLST8, PRAS40, and raptor, a regulatory protein which recruits downstream substrates such as 4EBP1 and ribosomal S6 kinase. mTORC1 inhibits autophagy by blocking the autophagy initiator complex activity, which is formed by ULK1, Atg13, Atg101, and FIP200 (Hosokawa et al. 2009; Jung et al. 2009). ULK1 is the essential protein for autophagy initiation in mammalian cells (Kuroyanagi et al. 1998; Chan et al. 2007; Wong et al. 2013).
The autophagy initiator complex is assembled independently of mTOR activity or nutrient conditions. However, under fed conditions, mTORC1 phosphorylates ULK1 in Ser638 and Ser758 and inhibits its kinase activity, preventing autophagy initiation (Shang and Wang 2011). Atg13 is also a substrate of mTOR kinase, and its phosphorylation has negative effects on ULK1 activity (Hosokawa et al. 2009). Moreover, ULK1 phosphorylation by mTOR disrupts its interaction with AMPK, preventing autophagy. Under starvation conditions, mTORC1-dependent phosphorylation of ULK1 is removed and ULK1 autophosphorylates and phosphorylates Atg13 and FIP200, initiating autophagy.
Since activation of mTORC1 is the principal mechanism to inhibit autophagy in mammalian cells, one of the mechanisms of AMPK-dependent induction of autophagy is inhibition of mTORC1 activity. AMPK is able to inhibit mTORC1 in a direct way by phosphorylating raptor (Gwinn et al. 2008) and in an indirect way by phosphorylating Tuberous Sclerosis Complex 2 (TSC2 or Tuberin) (Inoki et al. 2003).
When energy levels are low or there is a starvation situation, AMPK is phosphorylated and activated AMPK is able to bind and phosphorylate raptor, the mTORC1-binding partner (Gwinn et al. 2008). Gwinn et al. firstly revealed by bioinformatics techniques that raptor could be a direct substrate of AMPK since it presents two serine residues (Ser722 and Ser792) which match with the AMPK consensus motif. Secondly, the bioinformatics data were corroborated by biological evidences in vivo. Under energy stress conditions (low ATP levels), activated AMPK phosphorylates raptor in the predicted serine residues and this phosphorylation causes mTORC1 inhibition, so the autophagy initiator ULK1 can start the autophagic cascade. The mechanism by which raptor phosphorylation inhibits mTORC1 kinase function is a common mechanism for phosphorylation-based inactivation of target proteins. Phosphorylation of Ser722 and Ser792 induces 14-3-3 binding to raptor. 14-3-3 binding do not cause a disruption of mTORC1 complex but an inhibition of mTORC1 kinase activity.
Moreover, activated AMPK phosphorylates TSC2, an upstream inhibitor of mTORC1, since it inactivates GTPase Rheb, an activator of mTORC1 (Inoki et al. 2002). TSC2 forms a functional complex with TSC1 to inhibit two key regulators of translation: S6K and 4EBP1, which are phosphorylated by mTOR. It has been observed that under energy stress or starvation conditions, AMPK directly phosphorylates TSC2 on T1227 and S1345 to enhance its Rheb-GAP activity, which causes an inhibition of mTOR kinase activity (Inoki et al. 2003).
Therefore, energy stress results in AMPK phosphorylation and subsequently AMPK activation, which phosphorylates the mTORC1 inhibitor TSC2 and the mTORC1 regulatory subunit Raptor to inhibit mTORC1 and regulate catabolism, principally by initiating autophagy (Hardie 2008; Shaw 2009).
More recently, it has been observed that AMPK can also activate autophagy by direct phosphorylation of ULK1 (Guan et al. 2011; Egan et al. 2011b). However, there are some important discrepancies between the two investigations. The principal one is that the key phosphorylation sites identified by the different groups are not the same. Moreover, recently it was published that ULK1 forms a stable complex with AMPK (Behrends et al. 2010). Guan et al. described that this complex is disrupted when mTOR is activated and phosphorylates ULK1, but Egan et al. did not observe this mechanism. Therefore, more investigation about regulation of autophagy by AMPK is needed to resolve these differences.
Furthermore, in recent times it has been suggested that phosphorylation of ULK1 by AMPK stabilizes a negative regulation of autophagy initiation (Löffler et al. 2011). ULK1 is able to phosphorylate the three subunits of AMPK, and this effect reduces the phosphorylation of AMPKα Thr172, preventing AMPK kinase activity. By this way, ULK1 participates in the elimination of the initial autophagy signal, in addition to initiate the autophagic cascade.
Thus, we can conclude that AMPK uses a dual mechanism to activate autophagy by inactivating mTOR and also directly activating ULK1 by direct phosphorylation. Interestingly, the patterns of activation of AMPK and mTOR are opposite, regulating by this way the activation of autophagy depending on the nutrient conditions (Egan et al. 2011a; Alers et al. 2012) (Fig. 3.1).
3.2 AMPK Activation of Selective Autophagy of Mitochondria
Besides activating macroautophagy, AMPK promotes a selective form of autophagy called mitophagy, by which dysfunctional mitochondria are engulfed in autophagosomes and transported to lysosomes where they are degraded to recycle their components. In mammals, loss of AMPK resulted in aberrant accumulation of the autophagy adaptor p62 and defective mitophagy (Egan et al. 2011b). p62 binds to specific cargo targeted for autophagy-mediated degradation. Especially, p62 is bound to dysfunctional mitochondria targeted for mitophagy and is involved in mitochondrial aggregation and clearance (Geisler et al. 2010). Moreover, number of mitochondria per cell is significantly increased in AMPK-deficient cells, suggesting that AMPK plays an important role in mitochondria turnover.
Moreover, although the best-known function of the autophagy-initiating factor ULK1 is the autophagy induction (Mizushima 2010; Wirth et al. 2013), recent studies suggest that ULK1 has a more selective function in mitophagy (Kundu et al. 2008; Itakura et al. 2012). Recently, it has been described that ULK1 translocates to dysfunctional mitochondria in response to hypoxia or FCCP. Translocated ULK1 phosphorylates FUNDC1 (a mitophagy receptor located in external mitochondrial membrane) allowing its interaction with LC3 and inducing mitochondria degradation by autophagy (Wu et al. 2014).
In relation with this novel role of ULK1, it has been demonstrated that specific phosphorylation of ULK1 at Ser555 by activated AMPK is crucial for ULK translocation to the mitochondria (Tian et al. 2015). Therefore, AMPK-mediated phosphorylation of ULK1 is essential for mitophagy to initiate under stress conditions such as hypoxia.
For these reasons, AMPK is considered essential for the regulation of selective autophagy, besides its role as a macroautophagy inductor.
4 AMPK Regulation of Apoptosis
Although the best-known functions of AMPK are maintaining ATP homeostasis and regulating metabolism, AMPK has recently been proposed as a regulator of cell apoptosis or survival under stress conditions.
However, it is not clear whether AMPK is a proapoptotic or pro-survival molecule. In fact, AMPK participates in cell death or survival depending on the kind of stress, the cell type, and the duration of the activation of the signaling cascade. All these features make the role of AMPK in apoptosis controversial and complex.
4.1 AMPK as a Proapoptotic Molecule
Several metabolic checkpoints convert metabolic changes (signals), which are detected by specific systems (sensors), into stimuli which regulate the function of components of the cell death-regulatory machinery (effectors).
AMPK activation has been shown to mediate its proapoptosis effects primarily through modulation of several downstream signaling events by regulating JNK (c-Jun N-terminal protein kinase) and p53, inhibiting mTORC1 or directly enhancing the activation of proapoptotic proteins, among other different pathways (Fig. 3.2).
4.1.1 AMPK Induces Apoptosis by Activating JNK Pathway
The first demonstration of a proapoptotic effect of AMPK was observed in liver rat cells. It was observed that AMPK activation by high concentrations of AICAR triggers apoptosis in liver cells by activating JNK and Caspase 3 (Meisse et al. 2002). JNK pathway regulates proteins involved in apoptosis such as p53, c-myc, and members of the Bcl-2 family.
AICAR activation of apoptosis mediated by JNK and caspase 3 has been observed in different cell types. Kefas et al. observed that prolonged stimulation of AMPK induced apoptosis of insulin-producing cells (MIN6) of mouse. It is interesting that apoptosis induction was proportional to concentration of AICAR and time of exposure. Prolonged exposure to low glucose concentration induced apoptosis by AMPK activation too (Kefas et al. 2003a). The same response was observed in pancreatic beta cells (Kefas et al. 2003b).
4.1.2 AMPK Induces Apoptosis by Regulating and Stabilizing p53
Apart from its involvement in JNK activation, it has been described how AICAR induced p53 activation by AMPK stimulation. One of the first evidences that AMPK could be involved in regulating p53 function was observed in human hepatocellular carcinoma cell lines (HepG2) (Imamura et al. 2001). Later, it has been described how AMPK relates glucose availability to the p53 pathway, a master regulator of survival and proliferation (Jones et al. 2005). Prolonged cell cycle arrest induced by p53 may be followed by apoptosis activation. Glucose deprivation also induces phosphorylation of AMPK and subsequent activation of p53 and bax, a proapoptotic protein, promoting thymocytes and osteosarcoma cells to undergo apoptosis (Okoshi et al. 2008). Moreover, AICAR also induces apoptosis in other cancer cells such as glioblastomas due to AMPK-dependent inhibition of mTORC1 (Guo et al. 2009). Therefore, the same compound can activate AMPK and induce apoptosis in different types of cells by various mechanisms.
AMPK also contributes to UV- and H2O2-induced apoptosis in human skin keratinocytes (Cao et al. 2008). Chiefly, on UV- or H2O2-treated cells, AMPK induces apoptosis by inhibiting mTORC1 and positively regulating p53 and p38 MAPK, which mediates apoptosis elicited by both energy imbalance and pro-oxidant conditions.
4.1.3 AMPK Induces Apoptosis by Inhibiting mTORC1
One of the most important metabolic checkpoints that control cell death is the AMPK–mTORC1 pathway, which is based on the short half-life of antiapoptotic proteins such as FLIPL and MCL-1. In this sense, mTORC1 inhibition by AMPK activation causes the inhibition of protein translation; therefore, the abundance of short-lived antiapoptotic proteins such as MCL1 decreases and the cell is sensitized to mitochondrial apoptosis (Meynet et al. 2013).
Moreover, since mTOR inhibits apoptosis by the mediation of p53 and p27 (Faivre et al. 2006), AMPK-induced inhibition of mTORC1 could sensitize cells to undergo apoptosis too.
4.1.4 AMPK Directly Upregulates Proapoptotic Proteins Such as BH3-Only Proteins
For instance, it has been shown that prolonged AMPK activation can result in the direct induction of apoptotic excitotoxicity injury in neocortical neurons. This effect is dependent on Bim (proapoptotic BH3-only protein essential for the initiation of cell death). AMPK couples energy depletion to Bim mRNA induction and the subsequent activation of the Bcl-2-regulated apoptotic pathway in neurons (Concannon et al. 2010).
Moreover, AMPK activates the proapoptotic BH3-only proteins either through an indirect mechanism, by the AKT–FOXO3A pathway, because it has been suggested that FOXO3A is able to induce the translocation of Bim to mitochondria (Weisová et al. 2011).
4.1.5 AMPK Can Induce Apoptosis by Inhibiting the Unfolded Protein Response Pathway
Apart from inhibition of mTORC1, p53 stabilization, and JNK pathway activation, recently it has been proposed a new mechanism to explain AMPK-induced apoptosis. Metformin-induced activation of AMPK triggers apoptosis in acute lymphoblastic leukemia (ALL) cells by inhibiting the Unfolded Protein Response (UPR) pathway (Leclerc et al. 2013), which is triggered in response to the accumulation of unfolded/misfolded proteins in the ER lumen. First, metformin induces ER stress by accumulation of unfolded/misfolded proteins in the ER as a consequence of ATP depletion. At the same time, metformin activates AMPK, which acts as a negative regulator of the UPR, preventing cells from effectively engaging the UPR to overcome ER stress, leading to apoptotic death by inositol-requiring enzyme 1α (IRE1α), C/EBP homologous protein (CHOP), caspases, and JNK activation.
4.1.6 AMPK Involvement in Apoptosis Is Controversial
Recently, AICAR-induced apoptosis has been controversial since it has been shown that AICAR can induce an apoptosis in an AMPK- and p53-independent mechanism (Santidrián et al. 2010; Gonzalez-Girones et al. 2013). It has been shown that AICAR induced apoptosis in chronic lymphoid leukemia (CLL) through the mitochondrial apoptotic pathway, by inducing the modulation of RNA levels of Bcl-2, Bim, and Noxa, independently of AMPK and p53.
In the same way, AMPK sustained activation causes apoptosis induction in beta cells due to enhanced production of mitochondria-derived oxygen radicals which induce the activation of the intrinsic mitochondrial apoptosis pathway (Cai et al. 2007). Besides this mechanism, it is suggested an induction of the intrinsic apoptosis pathway by sustained AMPK activation, since dysregulation of BH3 members of the Bcl-2 family can be observed.
Other AMPK activators such as metformin have been demonstrated to induce apoptosis by different mechanisms that could be dependent or independent on AMPK. Metformin induces apoptosis in different cancer cell types (Leclerc et al. 2013; Queiroz et al. 2014), but it is important to notice that metformin has other targets apart from AMPK, for example, it functions as an inhibitor of complex I of the electron transport chain (Batandier et al. 2006). Moreover, the AMPK inhibitor, compound C, has been shown to compromise cell survival by AMPK-dependent and AMPK-independent mechanisms, since inhibition of AMPK is not sufficient to induce apoptosis in some cell types (Vucicevic et al. 2009).
Therefore, role of AMPK in AICAR/metformin-mediated apoptosis remains unclear and more investigations are needed to understand the real function and importance of AMPK in apoptosis induction (Bonini and Gantner 2013). What is clear is that AMPK is necessary (although in some cases is not sufficient) to promote apoptosis in different cell types.
4.1.7 Involvement of AMPK in Anticancer Drug Effects
Besides metformin, other anticancer drugs have been demonstrated to activate AMPK-dependent cell apoptosis pathway, although the molecular mechanisms involved in apoptosis initiation are different between the diverse compounds.
Vincristine, a drug that binds to tubulin and causes microtubule depolymerization, induces apoptosis in cells undergoing mitosis by AMPK activation. In B16 melanoma cells, high levels of ROS and LKB1 and AMPK activation have been observed, which induce both p53 activation and stabilization and mTORC1 inhibition, which are necessary mechanisms to mediate melanoma cell apoptosis (Chen et al. 2011).
Taxol (paclitaxel) is another drug that also affects microtubules but by a different mechanism. Taxol stabilizes the microtubule polymer and protects it from disassembly. Due to its action, chromosomes are unable to achieve a metaphase spindle configuration. It has been observed that taxol activates AMPK and downstream ACC in ovarian cancer cells and AMPK activation is implicated in the apoptosis induction mechanism (Sun et al. 2011). Apoptosis induction could be caused by ROS augmentation, mTORC1 inhibition, and activation of JNK pathway.
Temozolomide (TMZ) is another anticancer drug that exerts its action by a different mechanism, due to its ability to alkylate/methylate DNA. This methylation damages the DNA and has been demonstrated to induce apoptosis in primary cultured human glioblastoma cell lines. TMZ-mediated apoptosis is induced by AMPK activation, among other mechanisms. AMPK phosphorylation and activation in turn stabilize and activate p53 and induce the upregulation of p21, Noxa, and Bax. Activation of AMPK by TMZ also inhibits mTORC1 signaling and promotes antiapoptosis protein Bcl-2 downregulation (Zhang et al. 2010).
However, other drugs that induce DNA damage responses, such as doxorubicin (intercalating agent), induce AMPK activation in a ROS-dependent manner, which in turn contributes to apoptotic cell death by mTORC1 inhibition (Ji et al. 2010). It is interesting that inhibition of ROS production and AMPK activation by antioxidants such as N-acetylcysteine (NAC) or manganese (III) tetrakis (4-benzoic acid) porphyrins (MnTBAP) inhibits doxorubicin-induced AMPK activation and cell death. Therefore, the high level of ROS is the activating signal for AMPK in this situation.
Therefore, there are so many signals that are able to activate AMPK under stress situations for cells to undergo apoptosis.
4.2 AMPK as a Pro-survival Molecule
AMPK-mediated apoptosis has been reported in numerous cell types as we have exposed above. Conversely, it is recognized that activation of this kinase leads to metabolic alterations that can prevent ATP depletion in certain cell types, resulting in improved cell survival under different stress situations and protection from apoptosis. In fact, in conditions like starvation, energy deprivation, and oxidative stress, AMPK activation is required for cell survival. The molecular targets and effectors of the AMPK-mediated cell survival are diverse and different depending on the stress which induces the ATP depletion situation (Fig. 3.3).
4.2.1 AMPK Induces Cell Survival in Oxidative Stress Conditions
In oxidative stress conditions, for example, cell exposure to H2O2, AMPK is required for cell survival and inhibits apoptosis in osteoblasts (She et al. 2014). AMPK activation inhibits H2O2-induced oxidative stress through inhibiting NADPH depletion. Moreover, H2O2 is demonstrated to induce autophagy in MG63 cells. AMPK-dependent ULK1 activation and mTORC1 inactivation involve autophagy activation, which exerts a pro-survival effect instead of apoptosis induction. Therefore, activation of AMPK by H2O2 is pro-survival in osteoblasts through two mechanisms: activation of autophagy and inhibition of NADPH depletion.
It is important to observe that in the presence of the same condition (e.g., oxidative stress), AMPK can act as a pro-survival or proapoptotic molecule. It depends on the type of cell, the time of exposure, and the duration of the AMPK activation, among other features. Therefore, AMPK regulation of cell survival and death is very complex, and it is important to study every case separately.
4.2.2 AMPK Induces Cell Survival in Starvation Conditions
TSC2 and its phosphorylation by AMPK protect cells from glucose deprivation-induced apoptosis. As we have mentioned above, one of the major cellular functions of TSC1/TSC2 is to prevent protein translation by inhibiting the phosphorylation of S6K and 4EBP1. AMPK directly phosphorylates TSC2 on T1227 and S1345. Although TSC2 phosphorylation by AMPK plays an essential role in inhibiting cell growth, it is important for regulating cell survival under glucose starvation conditions too. Energy starvation activates TSC2, which then prevents cell growth and promotes cell survival. Activation of TSC2 by AMPK-dependent phosphorylation prepares cells for an unfavorable growth environment and results in protection from cell death. In fact, a mutant version of TSC2, TSC2-3A, which cannot be phosphorylated by AMPK, has demonstrated to be incapable to protect cells from glucose deprivation-induced apoptosis (Inoki et al. 2003).
4.2.3 AMPK Protects from Energy Stress-Induced Apoptosis
AMPK activation under an energy stress situation extends cell survival by redox regulation. In glucose limitation conditions, NADPH generation by the pentose phosphate pathway (PPP) is reduced, and this situation generates oxidative stress, since NADPH is needed for the regeneration of reduced glutathione (GSH), which is used by glutathione peroxidase (GPX) to eliminate H2O2. However, AMPK is able to induce alternative routes to maintain NADPH and inhibit cell death. By this way, AMPK plays an essential role in NADPH maintenance, which is critical for cancer cell survival under energy stress conditions. AMPK maintains NADPH levels by inhibiting the acetyl-CoA carboxylases ACC1 and ACC2. These effects induce a decrease in NADPH consumption (inhibition of fatty acid synthesis) and an increase in NADPH generation (induction of fatty acid oxidation) (Jeon et al. 2012).
Recently, it has been shown that AMPK promotes cell survival and suppresses apoptosis by directly increasing the expression of antiapoptotic proteins (Blc2 and Survivin) via NF-κB activation in endothelial cells exposed to hypoxia and glucose deprivation (Liu et al. 2010).
NF-κB has been proposed to induce the expression of different genes whose products can enhance cell survival and protect cells from apoptosis (Mitsiades 2002; Angileri et al. 2008). Nevertheless, the underlying mechanisms by which AMPK activation induces NF-κB-mediated cell survival remain poorly defined.
5 AMPK Regulation of Bioenergetics
Bioenergetics is the study of the transformation of energy in living organisms and the different cellular processes that can lead to production and usage of energy in different forms such as ATP.
AMPK has an essential role in bioenergetics regulation because it is a cellular energy sensor activated by conditions of metabolic stress characterized by an increase in the AMP/ATP ratio. AMPK activation restores cellular bioenergetics by inhibiting anabolic pathways that consume ATP and by activating specific catabolic reactions that generate ATP (Hardie 2011; Hindupur et al. 2015). Under stress conditions, the objective of AMPK activation is the conservation of cellular energy in order to avoid bioenergetic catastrophe (Faubert et al. 2015).
AMPK activation changes the way used by the cells to obtain energy in the form of ATP molecules. Predominantly, AMPK upregulates the production of ATP by glycolysis and fatty acid oxidation, for this reason, AMPK is crucial for the metabolic adaptation of cells which present some dysfunctions of the OXPHOS (Oxidative Phosphorylation) system (Wu and Wei 2012; Garrido-Maraver et al. 2015).
Activated AMPK switches off anabolic pathways such as fatty acid, glycogen, RNA, and protein synthesis to avoid ATP waste. However, in this section, we are focusing on the different catabolic pathways that are upregulated upon AMPK activation, in order to maintain energetic homeostasis (Fig. 3.4) (Hardie 2014), such as glucose uptake, fatty acid uptake, glycolysis, and fatty acid oxidation.
5.1 Activation of Glucose Uptake by AMPK
AMPK activation induces the upregulation of two glucose transporter: GLUT1 and GLUT4. GLUT1 is responsible for the low level of basal glucose uptake required to maintain respiration in cells, so AMPK is involved in basal glucose uptake maintenance (GLUT1 upregulation). Moreover, AMPK is responsible for insulin-dependent glucose uptake in adipose tissues and striated muscle, due to its role in GLUT4 upregulation.
5.1.1 GLUT1 Upregulation by AMPK
First studies in glucose uptake regulation by AMPK demonstrated that AMPK activation increased the Vmax of GLUT1-dependent glucose uptake (Barnes et al. 2002). More recently, a possible explanation for these results has been found. It has been suggested that AMPK activation increases the levels of GLUT1 mRNA due to the AMPK-dependent phosphorylation of Thioredoxin-interacting protein (TXNIP) (Wu et al. 2013).
TXNIP is induced in response to glucose elevation and suppresses glucose uptake directly, by binding to GLUT1 and inducing GLUT1 internalization, as well as indirectly, by reducing the level of GLUT1 mRNA. AMPK phosphorylation of TXNIP induces its rapid degradation by proteasome system. Therefore, TXNIP is released from GLUT1, and there is an increase in GLUT1 mRNA levels and function.
5.1.2 GLUT4 Upregulation by AMPK
First studies in glucose uptake regulation showed that AMPK activation induced the GLUT4 translocation from an intracellular location to the plasma membranes (Kurth-Kraczek et al. 1999). Recently, it has been demonstrated that AMPK-mediated translocation of GLUT4 is due in part to phosphorylation of the Rab GAP protein TBC1D1 by AMPK (Pehmøller et al. 2009). TBC1D1 is a component of the insulin-signaling cascade downstream of Akt that regulates GLUT4 translocation. Phosphorylation of TBC1D1 by AMPK promotes its 14-3-3 binding, which could increase basal and insulin-stimulated GLUT4 translocation.
Moreover, AMPK activation promotes upregulation of GLUT4 by a second mechanism because AMPK induces the transcription of the GLUT4 gene, due to phosphorylation of histone deacetylase-5 (HDAC5), a transcriptional repressor of GLUT4 (McGee et al. 2008). AMPK-mediated phosphorylation of HDAC5 reduces HDAC5 association with the GLUT4 promoter, increasing the GLUT4 mRNA levels.
5.2 AMPK Activation of Fatty Acids Uptake
Long chain fatty acid (LCFA) uptake is mostly dependent on the translocase CD36 (Koonen et al. 2005; Habets et al. 2007). AMPK activation stimulates LCFA uptake inducing the translocation of CD36 to the sarcolemma, which is essential for AMPK-induced fatty acid (FA) metabolism (Luiken et al. 2003; Bonen et al. 2007; Habets et al. 2009). Recently, it has been proposed that CD36 also interacts with AMPK to coordinate FA uptake. When exogenous FA levels are low, CD36 maintains AMPK inactive by indirectly promoting LKB1 transport to the nucleus. When exogenous FA levels increase, CD36-induced LKB1 transport to the nucleus is disrupted, and the cytosolic enrichment in LKB1 promotes AMPK activation and FA uptake (Samovski et al. 2015).
5.3 AMPK Activation of Glycolysis
Under cellular stress conditions (glucose deprivation, hypoxia, or oxidative stress), AMPK signaling drives glycolytic flux to maintain energy homeostasis and cellular ATP levels through glucose catabolism. AMPK promotes glycolysis via phosphorylation and activation of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB). PFKFB is a bifunctional enzyme with different tissue-dependent isoforms. The phosphofructo-2-kinase (PFK-2) domain functions to promote glycolysis by increasing levels of fructose-2,6-bisphosphate through phosphorylation of fructose-6-phosphate, and the fructose-2,6-biphosphatase (FB) domain catalyzes the reverse reaction producing fructose-6-phosphate.
In fact, AMPK is responsible for the increased glycolytic flux present after an ischemia in the heart, since it phosphorylates PFK-2 at Ser466 (Marsin et al. 2000). AMPK also induces glycolysis under hypoxia conditions (Marsin et al. 2002). It is important to remark that oxidative stress also promotes AMPK to increase glycolytic flux (Wu and Wei 2012).
5.4 AMPK Activation of Fatty Acid Oxidation
AMPK plays an important role in the regulation of FA oxidation because AMPK activation induces a change in different steps involved in FA oxidation pathway. First, as we commented above, AMPK controls fatty acid transport across the cell membrane by inducing CD-36 translocation.
Second, activated AMPK phosphorylates and inactivates the ACC2 isoform of acetyl-CoA carboxylase, which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the limiting step in fatty acid synthesis. Malonyl-CoA is also an inhibitor of fatty acid absorption into mitochondria (Merrill et al. 1997). Therefore, AMPK activation increases the rate of fatty acid oxidation.
Third, very recently, it has been shown that AMPK phosphorylation promotes lipid droplets (LDs) mobility, dispersion, and consumption by affecting the microtubules network, increasing the rate of FA oxidation (Herms et al. 2015). By this way, AMPK promotes FAs from LDs to be channeled preferentially to different metabolic fates depending on the cellular energetic status: in the absence of glucose, FAs are directed to beta oxidation in mitochondria.
5.5 Induction of Mitochondrial Biogenesis by AMPK
Besides promoting different catabolic pathways, AMPK activates mitochondrial biogenesis to maintain the overall ATP-generating capacity of cells and avoid cell death. The most important regulator of mitochondrial biogenesis is PGC1α (Hardie et al. 2012). It has been observed that PGC1α is directly phosphorylated and transactivated by AMPK (Jäger et al. 2007). PGC1α can also be activated by Sirt-1-mediated deacetylation, which is induced by AMPK activation too (Cantó et al. 2009). PGC1α upregulates the activity of transcription factors involved in mitochondrial biogenesis, such as NRF-1, which in turn modulates the expression of other factors such as mtTFA, important for the regulation of mtDNA replication and transcription.
5.6 Inhibition of Lipolysis by AMPK
In response to glucose deprivation, AMPK inhibits lipolysis in adipocytes. One of the most important enzymes involved in lipolysis, hormone-sensitive lipase (HSL) is phosphorylated by AMPK on a key serine residue that antagonizes phosphorylation of HSL by cyclic AMP-dependent protein kinase (PKA) (Garton and Yeaman 1990), causing suppression of lipolysis. Although it is a catabolic pathway, its inhibition by AMPK is considered as a way to maintain cell energy homeostasis because an excessive accumulation of fatty acids promotes the synthesis of triglycerides, an ATP-consuming process (Garton 1989; Daval 2005).
6 Conclusion
AMPK is a critical regulator of energy homeostasis at both the cellular and whole-body levels. However, as a result of the wide range of pathways and signaling in which AMPK is involved, diverse contradictions arise about the role of AMPK in cell death and survival. For example, AMPK has different roles in apoptosis regulation depending on the duration of stimulation, dosage of induction, the type of cell, and the type of stress that induce its activation. Thus, AMPK phosphorylation induces apoptosis in mouse astrocytoma but may protect normal brain cells from apoptosis under similar energy stress condition (Mukherjee et al. 2008). Therefore, for now, it is necessary to study every case separately because we do not have all the knowledge about AMPK pathways, signaling, and targets.
Understanding the complex role of AMPK in regulation of ATP balance is important because disturbances in energy homoeostasis underlie a number of diseases in humans, e.g., cancer, mitochondrial dysfunctions, diabetes, etc. In this sense, discovering new targets of AMPK is a research field which is gaining importance in the last years. For example, the involvement of AMPK in the insulin/IGF1 and MAPK–ERK pathways to control cell growth is being extensively studied.
As more targets of AMPK are known, we can discover which effectors are responsible for the beneficial effects of AMPK activation seen in diverse diseases. It is accepted that AMPK is emerged as a potential therapeutic target in different human diseases, but it is necessary to investigate about the molecular mechanisms underlying its therapeutic function.
Moreover, it is logical to think that AMPK regulation of cell growth, bioenergetics, and cell fate (death or survival) is closely related with the aging process regulation. Some investigations suggest that aging is linked to a loss of sensibility of AMPK—it loses its capacity to sense changes in AMP/ATP ratio—which induce a generalized energy imbalance situation. Then, the complete understanding of AMPK-mediated regulation of cell metabolism might lead to improve the quality of life in the elderly and to fight different diseases which have not curative treatments yet.
References
Alers S, Loffler AS, Wesselborg S, Stork B (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32:2–11. doi:10.1128/MCB.06159-11
Alexander A, Walker CL (2011) The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett 585:952–957. doi:10.1016/j.febslet.2011.03.010
Angileri FF, Aguennouz M, Conti A et al (2008) Nuclear factor-κB activation and differential expression of survivin and Bcl-2 in human grade 2-4 astrocytomas. Cancer 112:2258–2266. doi:10.1002/cncr.23407
Barnes K, Ingram JC, Porras OH et al (2002) Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115:2433–2442
Batandier C, Guigas B, Detaille D et al (2006) The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr 38:33–42. doi:10.1007/s10863-006-9003-8
Behrends C, Sowa ME, Gygi SP, Harper JW (2010) Network organization of the human autophagy system. Nature 466:68–76. doi:10.1038/nature09204
Bonen A, Han X-X, Habets DDJ et al (2007) A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am J Physiol Endocrinol Metab 292:E1740–E1749. doi:10.1152/ajpendo.00579.2006
Bonini MG, Gantner BN (2013) The multifaceted activities of AMPK in tumor progression – why the “one size fits all” definition does not fit at all? IUBMB Life 65:889–896. doi:10.1002/iub.1213
Browne GJ, Proud CG (2002) Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem 269:5360–5368. doi:10.1046/j.1432-1033.2002.03290.x
Browne GJ, Finn SG, Proud CG (2004) Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem 279:12220–12231. doi:10.1074/jbc.M309773200
Buckbinder L, Talbott R, Velasco-Miguel S et al (1995) Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 377:646–649. doi:10.1038/377646a0
Budanov AV, Karin M (2008) p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134:451–460. doi:10.1016/j.cell.2008.06.028
Cai Y, Martens GA, Hinke SA et al (2007) Increased oxygen radical formation and mitochondrial dysfunction mediate beta cell apoptosis under conditions of AMP-activated protein kinase stimulation. Free Radic Biol Med 42:64–78. doi:10.1016/j.freeradbiomed.2006.09.018
Cantó C, Gerhart-Hines Z, Feige JN et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060. doi:10.1038/nature07813
Cao C, Lu S, Kivlin R et al (2008) AMP-activated protein kinase contributes to UV- and H2O2-induced apoptosis in human skin keratinocytes. J Biol Chem 283:28897–28908. doi:10.1074/jbc.M804144200
Chan EYW, Kir S, Tooze SA (2007) siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem 282:25464–25474. doi:10.1074/jbc.M703663200
Chen M-B, Shen W-X, Yang Y et al (2011) Activation of AMP-activated protein kinase is involved in vincristine-induced cell apoptosis in B16 melanoma cell. J Cell Physiol 226:1915–1925. doi:10.1002/jcp.22522
Concannon CG, Tuffy LP, Weisová P et al (2010) AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress induced apoptosis. J Cell Biol 189:83–94. doi:10.1083/jcb.200909166
Daval M (2005) Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 280:25250–25257. doi:10.1074/jbc.M414222200
Davies SP, Carling D, Munday MR, Hardie DG (1992) Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem 203:615–623
Egan D, Kim J, Shaw RJ, Guan KL (2011a) The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7:643–644
Egan DF, Shackelford DB, Mihaylova MM et al (2011b) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461. doi:10.1126/science.1196371
Emerling BM, Weinberg F, Snyder C et al (2009) Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med 46:1386–1391. doi:10.1016/j.freeradbiomed.2009.02.019
Faivre S, Kroemer G, Raymond E (2006) Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 5:671–688. doi:10.1038/nrd2062
Faubert B, Vincent EE, Poffenberger MC, Jones RG (2015) The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett 356:165–170. doi:10.1016/j.canlet.2014.01.018
Feng Z, Hu W, de Stanchina E et al (2007) The regulation of AMPK 1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res 67:3043–3053. doi:10.1158/0008-5472.CAN-06-4149
Foretz M, Carling D, Guichard C et al (1998) AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem 273:14767–14771
Garrido-Maraver J, Paz MV, Cordero MD et al (2015) Critical role of AMP-activated protein kinase in the balance between mitophagy and mitochondrial biogenesis in MELAS disease. Biochim Biophys Acta 1852:2535–2553. doi:10.1016/j.bbadis.2015.08.027
Garton A (1989) Phosphorylation of bovine hormone‐sensitive lipase by the AMP‐activated protein kinase. Eur J Biochem 254:249–254
Garton AJ, Yeaman SJ (1990) Identification and role of the basal phosphorylation site on hormone-sensitive lipase. Eur J Biochem 191:245–250
Geisler S, Holmström KM, Skujat D et al (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12:119–131. doi:10.1038/ncb2012
Goldman SJ, Taylor R, Zhang Y, Jin S (2010) Autophagy and the degradation of mitochondria. Mitochondrion 10:309–315. doi:10.1016/j.mito.2010.01.005
Gonzalez-Girones DM, Moncunill-Massaguer C, Iglesias-Serret D et al (2013) AICAR induces Bax/Bak-dependent apoptosis through upregulation of the BH3-only proteins Bim and Noxa in mouse embryonic fibroblasts. Apoptosis 18:1008–1016. doi:10.1007/s10495-013-0850-610.1007/s10495-013-0850-6
Grahame Hardie D (2014) AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med 276:543–559. doi:10.1111/joim.12268
Green DR, Galluzzi L, Kroemer G (2014) Cell biology. Metabolic control of cell death. Science 345(80):1250256. doi:10.1126/science.1250256
Guan JK, Mondira K, Benoit V, Kun L (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141. doi:10.1038/ncb2152
Guo D, Hildebrandt IJ, Prins RM et al (2009) The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci 106:12932–12937. doi:10.1073/pnas.0906606106
Gwinn DM, Shackelford DB, Egan DF et al (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226. doi:10.1016/j.molcel.2008.03.003
Habets DDJ, Coumans WA, Voshol PJ et al (2007) AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem Biophys Res Commun 355:204–210. doi:10.1016/j.bbrc.2007.01.141
Habets DDJ, Coumans WA, El Hasnaoui M et al (2009) Crucial role for LKB1 to AMPKalpha2 axis in the regulation of CD36-mediated long-chain fatty acid uptake into cardiomyocytes. Biochim Biophys Acta 1791:212–219. doi:10.1016/j.bbalip.2008.12.009
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8:774–785. doi:10.1038/nrm2249
Hardie DG (2008) AMPK and raptor: matching cell growth to energy supply. Mol Cell 30:263–265. doi:10.1016/j.molcel.2008.04.012
Hardie DG (2011) AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 25:1895–1908. doi:10.1101/gad.17420111
Hardie DG (2014) AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr 34:31–55. doi:10.1146/annurev-nutr-071812-161148
Hardie DG, Pan DA (2002) Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 30:1064–1070. doi:10.1042/
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13:251–262. doi:10.1038/nrm3311
Hawley SA, Pan DA, Mustard KJ et al (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2:9–19. doi:10.1016/j.cmet.2005.05.009
He G, Zhang Y-W, Lee J-H et al (2014) AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Mol Cell Biol 34:148–157. doi:10.1128/MCB.00670-13
Henin N, Vincent MF, Gruber HE, Van den Berghe G (1995) Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J 9:541–546
Herms A, Bosch M, Reddy BJN et al (2015) AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat Commun 6:7176. doi:10.1038/ncomms8176
Hindupur SK, González A, Hall MN (2015) The opposing actions of target of rapamycin and AMP-activated protein kinase in cell growth control. Cold Spring Harb Perspect Biol. doi:10.1101/cshperspect.a019141
Holz MK, Ballif BA, Gygi SP, Blenis J (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123:569–580. doi:10.1016/j.cell.2005.10.024
Hoppe S, Bierhoff H, Cado I et al (2009) AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc Natl Acad Sci USA 106:17781–17786. doi:10.1073/pnas.0909873106
Hosokawa N, Hara T, Kaizuka T et al (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20:1981–1991. doi:10.1091/mbc.E08-12-1248
Høyer-Hansen M, Bastholm L, Szyniarowski P et al (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and Bcl-2. Mol Cell 25:193–205. doi:10.1016/j.molcel.2006.12.009
Hwang J-T, Lee M, Jung S-N et al (2004) AMP-activated protein kinase activity is required for vanadate-induced hypoxia-inducible factor 1alpha expression in DU145 cells. Carcinogenesis 25:2497–2507. doi:10.1093/carcin/bgh253
Imamura K, Ogura T, Kishimoto A et al (2001) Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun 287:562–567. doi:10.1006/bbrc.2001.5627
Inoki K, Li Y, Zhu T et al (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4:648–657. doi:10.1038/ncb839
Inoki K, Zhu T, Guan K-L (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Itakura E, Kishi-Itakura C, Koyama-Honda I, Mizushima N (2012) Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J Cell Sci 125:1488–1499. doi:10.1242/jcs.094110
Jäger S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104:12017–12022. doi:10.1073/pnas.0705070104
Jeon S-M, Chandel NS, Hay N (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485:661–665. doi:10.1038/nature11066
Ji C, Yang B, Yang Y-L et al (2010) Exogenous cell-permeable C6 ceramide sensitizes multiple cancer cell lines to Doxorubicin-induced apoptosis by promoting AMPK activation and mTORC1 inhibition. Oncogene 29:6557–6568. doi:10.1038/onc.2010.379
Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7:279–296
Jones RG, Plas DR, Kubek S et al (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18:283–293. doi:10.1016/j.molcel.2005.03.027
Jung CH, Jun CB, Ro S-H et al (2009) ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20:1992–2003. doi:10.1091/mbc.E08-12-1249
Kefas BA, Cai Y, Ling Z et al (2003a) AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol 30:151–161. doi:10.1677/jme.0.0300151
Kefas BA, Heimberg H, Vaulont S et al (2003b) AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia 46:250–254. doi:10.1007/s00125-002-1030-3
Koonen DPY, Glatz JFC, Bonen A, Luiken JJFP (2005) Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta 1736:163–180. doi:10.1016/j.bbalip.2005.08.018
Kundu M, Lindsten T, Yang C-Y et al (2008) Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112:1493–1502. doi:10.1182/blood-2008-02-137398
Kuroyanagi H, Yan J, Seki N et al (1998) Human ULK1, a novel serine/threonine kinase related to UNC-51 kinase of Caenorhabditis elegans: cDNA cloning, expression, and chromosomal assignment. Genomics 51:76–85
Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW (1999) 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48:1667–1671
Leclerc GM, Leclerc GJ, Kuznetsov JN et al (2013) Metformin induces apoptosis through AMPK-dependent inhibition of UPR signaling in ALL lymphoblasts. PLoS One 8:e74420. doi:10.1371/journal.pone.0074420
Lemasters JJ (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8:3–5. doi:10.1089/rej.2005.8.3
Li Y, Xu S, Mihaylova MM et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13:376–388. doi:10.1016/j.cmet.2011.03.009
Liu K, Czaja MJ (2013) Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ 20:3–11. doi:10.1038/cdd.2012.63
Liu C, Liang B, Wang Q et al (2010) Activation of AMP-activated protein kinase α1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins Bcl-2 and survivin. J Biol Chem 285:15346–15355. doi:10.1074/jbc.M110.102491
Löffler AS, Alers S, Dieterle AM et al (2011) Ulk1-mediated phosphorylation of AMPK constitutes a negative regulatory feedback loop. Autophagy 7:696–706. doi:10.4161/auto.7.7.15451
Luiken JJFP, Coort SLM, Willems J et al (2003) Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 52:1627–1634. doi:10.2337/diabetes.52.7.1627
Luo Z, Saha AK, Xiang X, Ruderman NB (2005) AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 26:69–76. doi:10.1016/j.tips.2004.12.011
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10:307–318. doi:10.1038/nrm2672
Marsin AS, Bertrand L, Rider MH et al (2000) Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10:1247–1255
Marsin A-S, Bouzin C, Bertrand L, Hue L (2002) The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem 277:30778–30783. doi:10.1074/jbc.M205213200
Mayer C, Zhao J, Yuan X, Grummt I (2004) mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev 18:423–434. doi:10.1101/gad.285504
McGee SL, van Denderen BJW, Howlett KF et al (2008) AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 57:860–867. doi:10.2337/db07-0843
Meisse D, Van de Casteele M, Beauloye C et al (2002) Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett 526:38–42. doi:10.1016/S0014-5793(02)03110-1
Merrill GF, Kurth EJ, Hardie DG, Winder WW (1997) AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 273:E1107–E1112
Meynet O, Zunino B, Happo L et al (2013) Caloric restriction modulates Mcl-1 expression and sensitizes lymphomas to BH3 mimetic in mice. Blood 122:2402–2411. doi:10.1182/blood-2013-01-478651
Mitsiades N (2002) Biologic sequelae of nuclear factor-kappa B blockade in multiple myeloma: therapeutic applications. Blood 99:4079–4086. doi:10.1182/blood.V99.11.4079
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873. doi:10.1101/gad.1599207
Mizushima N (2010) The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22:132–139. doi:10.1016/j.ceb.2009.12.004
Mukherjee P, Mulrooney TJ, Marsh J et al (2008) Differential effects of energy stress on AMPK phosphorylation and apoptosis in experimental brain tumor and normal brain. Mol Cancer 7:37. doi:10.1186/1476-4598-7-37
Mungai PT, Waypa GB, Jairaman A et al (2011) Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 31:3531–3545. doi:10.1128/MCB.05124-11
Muoio DM, Seefeld K, Witters LA, Coleman RA (1999) AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J 338(Pt 3):783–791
Okoshi R, Ozaki T, Yamamoto H et al (2008) Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J Biol Chem 283:3979–3987. doi:10.1074/jbc.M705232200
Pehmøller C, Treebak JT, Birk JB et al (2009) Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab 297:E665–E675. doi:10.1152/ajpendo.00115.2009
Porstmann T, Santos CR, Griffiths B et al (2008) SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8:224–236. doi:10.1016/j.cmet.2008.07.007
Queiroz EAIF, Puukila S, Eichler R et al (2014) Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One 9:e98207. doi:10.1371/journal.pone.0098207
Samovski D, Sun J, Pietka T et al (2015) Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation. Diabetes 64:353–359. doi:10.2337/db14-0582
Santidrián AF, González-gironès DM, Iglesias-serret D et al (2010) AICAR induces apoptosis independently ofAMPK and p53 through up-regulation of the BH3-only proteins BIM and NOXAin chronic lymphocytic leukemia cells. Blood 116:3023–3032. doi:10.1182/blood-2010-05-283960
Shang L, Wang X (2011) AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy 7:924–926
Shaw RJ (2009) LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol 196:65–80. doi:10.1111/j.1748-1716.2009.01972.x
She C, Zhu L, Zhen Y et al (2014) Activation of AMPK protects against hydrogen peroxide-induced osteoblast apoptosis through autophagy induction and NADPH maintenance: new implications for osteonecrosis treatment? Cell Signal 26:1–8. doi:10.1016/j.cellsig.2013.08.046
Sun H, Yu T, Li J (2011) Co-administration of perifosine with paclitaxel synergistically induces apoptosis in ovarian cancer cells: more than just AKT inhibition. Cancer Lett 310:118–128. doi:10.1016/j.canlet.2011.06.010
Tian W, Li W, Chen Y et al (2015) Phosphorylation of ULK1 by AMPK regulates translocation of ULK1 to mitochondria and mitophagy. FEBS Lett 589:1847–1854. doi:10.1016/j.febslet.2015.05.020
Till A, Lakhani R, Burnett SF, Subramani S (2012) Pexophagy: the selective degradation of peroxisomes. Int J Cell Biol 2012:512721. doi:10.1155/2012/512721
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. doi:10.1126/science.1160809
Viollet B, Andreelli F (2011) AMP-activated protein kinase and metabolic control. Handb Exp Pharmacol 303–330. doi:10.1007/978-3-642-17214-4_13
Vucicevic L, Misirkic M, Janjetovic K et al (2009) AMP-activated protein kinase-dependent and -independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol 77:1684–1693. doi:10.1016/j.bcp.2009.03.005
Weisová P, Dávila D, Tuffy LP et al (2011) Role of 5′-adenosine monophosphate-activated protein kinase in cell survival and death responses in neurons. Antioxid Redox Signal 14:1863–1876. doi:10.1089/ars.2010.3544
Wirth M, Joachim J, Tooze SA (2013) Autophagosome formation – the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin Cancer Biol 23:301–309. doi:10.1016/j.semcancer.2013.05.007
Wong PM, Puente C, Ganley IG, Jiang X (2013) The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy 9:124–137
Woods A, Dickerson K, Heath R et al (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2:21–33. doi:10.1016/j.cmet.2005.06.005
Wu S-B, Wei Y-H (2012) AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: Implication of the cell survival in mitochondrial diseases. Biochim Biophys Acta 1822:233–247. doi:10.1016/j.bbadis.2011.09.014
Wu N, Zheng B, Shaywitz A et al (2013) AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 49:1167–1175. doi:10.1016/j.molcel.2013.01.035
Wu W, Tian W, Hu Z et al (2014) ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep 15:566–575. doi:10.1002/embr.201438501
Xu J, Ji J, Yan X-H (2012) Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr 52:373–381. doi:10.1080/10408398.2010.500245
Yeh L, Lee K, Kim K (1980) Regulation of rat liver acetyl-coA carboxylase. J Biol Chem 255:2308–2315
Zhang W-B, Wang Z, Shu F et al (2010) Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem 285:40461–40471. doi:10.1074/jbc.M110.164046
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35. doi:10.1038/nrm3025
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Villanueva-Paz, M. et al. (2016). AMPK Regulation of Cell Growth, Apoptosis, Autophagy, and Bioenergetics. In: Cordero, M., Viollet, B. (eds) AMP-activated Protein Kinase. Experientia Supplementum, vol 107. Springer, Cham. https://doi.org/10.1007/978-3-319-43589-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-43589-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43587-9
Online ISBN: 978-3-319-43589-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)