Abstract
During host–pathogen interactions, a complex web of events is crucial for the outcome of infection. Pathogen recognition triggers powerful cellular signaling events that is translated into the induction and maintenance of innate and adaptive host immunity against infection. In opposition, pathogens employ active mechanisms to manipulate host cell regulatory pathways toward their proliferation and survival. Among these, subversion of host cell energy metabolism by pathogens is currently recognized to play an important role in microbial growth and persistence. Extensive studies have documented the role of AMP-activated protein kinase (AMPK) signaling, a central cellular hub involved in the regulation of energy homeostasis, in host–pathogen interactions. Here, we highlight the most recent advances detailing how pathogens hijack cellular metabolism by suppressing or increasing the activity of the host energy sensor AMPK. We also address the role of lower eukaryote AMPK orthologues in the adaptive process to the host microenvironment and their contribution for pathogen survival, differentiation, and growth. Finally, we review the effects of pharmacological or genetic AMPK modulation on pathogen growth and persistence.
Inês Mesquita and Diana Moreira are contributed equally with all other contributors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Adenosine 5′ monophosphate‐activated protein kinase (AMPK) is a heterotrimeric serine/threonine kinase consisting of a catalytic subunit (α) and two regulatory subunits (β and γ) (Xiao et al. 2011). AMPK is considered a pivotal regulator of cellular metabolism known as a metabolic “master regulator,” switching on catabolic pathways that generate ATP, while switching off anabolic pathways that consume ATP. Therefore, upon activation, AMPK downregulates several anabolic enzymes through phosphorylation leading to the inhibition of both translation initiation by restraining mammalian target of rapamycin complex 1 (mTORC1) activity, as well as translation elongation through the inactivation of Eukaryotic Elongation Factor 2 (eEF2), which will ultimately decrease cellular ATP consumption. In opposition, AMPK activates a catabolic state by inducing oxidative pathways generating energy through the activation of glucose uptake (via activation of both glucose transporter 1 (GLUT1) and GLUT4) (Barnes et al. 2002; Holmes et al. 1999), glycolysis (via phosphorylation and activation of two of four isoforms of 6-phosphofructo-2-kinase) (Marsin et al. 2002), fatty acid uptake (via translocation of the fatty acid transporter FAT/CD36) (Bonen et al. 2007), and fatty acid oxidation (via phosphorylation of the isoform 2 of acetyl-CoA carboxylase (ACC2)) (Winder and Hardie 1996). Classically, the canonical upstream activator that phosphorylates AMPK on Thr 172 (Stein et al. 2000) is the constitutively active tumor suppressor liver kinase B1 (LKB1) accompanied by two accessory subunits, sterile 20 protein‐related adaptor (STRAD) and mouse protein 25 (MO25) (Hawley et al. 2003; Woods et al. 2003), but additional activators such as the Ca2+-calmodulin-dependent kinase kinase (CaMKK) has been also identified (Woods et al. 2005; Hawley et al. 2005).
AMPK is capable of sensing changes in the energy status of the cell, and therefore it is not surprising that it plays a pivotal role during infections (Moreira et al. 2015b). Nutritional immunity has been described as a defence mechanism employed by host cells to prevent the acquisition of essential nutrients by intracellular pathogens, thus preventing pathogen scavenging and further utilization for replication and survival (Hood and Skaar 2012). Furthermore, recent advances have underlined the role of AMPK in macrophage, T cell, and DC functions in distinct settings, providing a molecular link between bioenergetics homeostasis, viability, and effector functions of adaptive and innate immune cells (Blagih et al. 2015; Kelly and O’Neill 2015; Yang and Chi 2015). These concepts are the core of host–pathogen dynamics. Herein, we review the contribution of AMPK sensor in regulating viral, bacterial, parasitic, and fungal infections. Furthermore, given the high degree of conservation among eukaryotes, we intend to discuss the crucial role of AMPK in parasites and fungi. The most recent findings concerning the AMPK role in the parasite and fungal biology during host–pathogen interaction will be also addressed.
2 AMPK: A Regulator in Viral Infection
2.1 Modulation of AMPK Activity by Viruses
Simian virus 40 (SV40) is a polyoma virus that encodes for small and large T antigens implicated in tumor formation. SV40 small T antigen has been shown to protect human cells in glucose deprivation. This survival advantage for cancer cells was demonstrated to be correlated with an increased phosphorylation of AMPK and consequent downregulation of mTORC1, which ultimately triggered autophagy (Kumar and Rangarajan 2009). In vitro infection with avian reovirus (ARV) resulted in an increase in AMPK phosphorylation on Thr172 (Ji et al. 2009) and a concomitant increase in mitogen-activated protein kinase (MAPK) p38 phosphorylation. AMPK phosphorylation has also been described to be increased early after infection with Rift Valley fever virus (RVFV), an important reemerging arthropod-borne human pathogen (Moser et al. 2012).
On the contrary, infections with Hepatitis C virus (HCV), Human cytomegalovirus (HCMV), Herpes simplex virus (HSV), Epstein–Barr virus (EBV), and human immunodeficiency virus-1 (HIV-1) have been reported to inhibit AMPK activity. In this context, cells infected with HCV or harboring an HCV subgenomic replicon inhibited the phosphorylation of AMPK at Thr172. This was consistent with the concomitant reduction on AMPK activity and increased hepatic lipid accumulation required for virus replication (Mankouri et al. 2010). Inhibition of Thr172 phosphorylation was associated with an increased phosphorylation of AMPK at an alternative site, Ser485, which is phosphorylated by protein kinase B (PKB/Akt) (Horman et al. 2006). Interestingly, both HCV NS4B and NS5A proteins have been shown to activate the protein kinase AKT (Park et al. 2009; Street et al. 2004). In addition to HCV, HCMV, a beta herpes virus that establishes chronic infections, activates mTOR signaling by inhibiting AMPK Thr172 phosphorylation at early time points postinfection (Kudchodkar et al. 2007). Interestingly, at later time points of infection, a kinome RNAi screen performed in HCMV-infected MRC5 fibroblasts identified 106 cellular kinases that influenced the growth of the virus, including multiple elements of the AMPK pathway (Terry et al. 2012). The effector proteins responsible for the AMPK inhibition still remain elusive, although the inhibition of CaMKK was reported to block HCMV-mediated AMPK activation (McArdle et al. 2011, 2012). Furthermore, it has been shown that HCMV blocked the function of the mitochondrial trifunctional protein (TFP), a key enzyme during fatty acid-β-oxidation (Seo and Cresswell 2013), through the redistribution of viperin to the mitochondria. The resulting decrease in cellular ATP levels and consequent increase in AMP-activated AMPK. The latent membrane protein 1 (LMP1) encoded by EBV, which is associated with the development of nasopharyngeal carcinoma, inhibited the phosphorylation of LKB1 at serine 428 leading to the inhibition of AMPK phosphorylation (Plummer et al. 2013).
Sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide-dependent class III protein deacetylase, and AMPK are activated during the cellular response to nutrient deprivation and exhibit a reciprocal regulation (Fulco et al. 2008). In the context of HCV core protein expression in HepG2 cells, it has been shown that the activity of SIRT1 along with AMPK is decreased (Yu et al. 2013). In the context of HIV infection, it has been proposed that the transactivator Tat protein mediates its effect on AMPK via the inhibition of SIRT1 (Zhang and Wu 2009). However, a certain complexity is associated with the fact that SIRT1 deacetylates Tat (Pagans et al. 2005) and Tat inhibits SIRT1 (Kwon et al. 2008). More recently, it has been proposed that curcumin reversed Tat-mediated reduction in AMPK activation and downstream ACC activation (Zhang et al. 2011). Therefore, the relationship between HIV infection and AMPK modulation merits to be further explored in the context of viral infection.
2.2 Role of AMPK in Virus Entry and Replication
Since some viruses manipulate host AMPK toward immune evasion, a valuable therapeutic antiviral strategy may pass through the pharmacological manipulation of AMPK activity. In this context, Vaccinia virus infects a wide range of host cells, and all three subunits of AMPK facilitated virus entry (Moser et al. 2010). AMPK is involved in poxvirus entry in a manner that is independent of its role as a metabolic regulator. The authors point to a novel role of AMPK in promoting macropinocytosis and cellular motility by regulating actin dynamics, independently of LKB1 or CaMKK (Moser et al. 2010). AMPK deficiency attenuated vaccinia infection by interfering with viral entry. This supports the development of selective AMPK inhibitors or other inhibitors of macropinocytosis against poxviruses, as well as for other viruses that hijack this endocytic route for their entry mechanism. In vitro treatment of hepatoma cells with the AMPK activator 5-aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR) was shown to suppress HCV replication (Nakashima et al. 2011; Mankouri et al. 2010). The importance of AMPK in HCMV replication is supported by the observation that the inhibition of AMPK kinase with compound C attenuates early and late HCMV replication (Hutterer et al. 2013; McArdle et al. 2012; Terry et al. 2012). Moreover, the addition of 2-octynoic acid (2-OA) to HCV-infected cells inhibited viral replication in a process involving the activation of AMPK and the inhibition of ACC, the first rate-limiting enzyme in fatty acid synthesis (Yang et al. 2013). Importantly, it has been shown that AMPK and its upstream activator LKB1 are essential for the control of RVFV infection, which is associated with the phosphorylation and inhibition of ACC. Treatment with the fatty acid palmitate bypasses this restriction, demonstrating that AMPK restricts RVFV infection through its inhibition of fatty acid biosynthesis (Moser et al. 2012). Furthermore, the authors showed that AMPK restricts the growth of multiple arboviruses from disparate families, including the Flavivirus Kunjin virus (KUNV), the Togavirus Sindbis virus (SINV), and the Rhabdovirus Vesicular stomatitis virus (VSV) (Moser et al. 2012). Therefore, this process represents a novel mechanism of interest regarding viruses’ control mechanisms. Because many viruses require complex and unique interactions with cellular lipid metabolism through both synthesis and degradation pathways, it cannot be excluded that viruses inhibit AMPK activation in order to stimulate lipid synthesis within the infected cells. Thus, AMPK activation is broadly antiviral and may provide a novel antiviral therapeutic target.
Finally, it has been proposed that AMPK is involved in the reactivation of latent viruses, such as HIV-1, through bryostatin-induced activation, a protein kinase C activator (Mehla et al. 2010). This was further confirmed by the use of compound C that partially reduced HIV-1 reactivation. In the same study, the authors showed that metformin, an oral antidiabetic drug that activates AMPK indirectly through mitochondrial complex I inhibition (Zhou et al. 2001), inhibits HIV-1 replication. These results underline differential effects either on the reactivation of HIV-1 or the productive viral replication (Mehla et al. 2010). Furthermore, metformin also inhibits in vitro HCV replication (Huang et al. 2013). The addition of metformin to current HCV treatment regimens had promising, albeit modest, effects on reducing patient viral loads (Romero-Gomez et al. 2009). Moreover, several AMPK activating drugs have been shown to reduce morbidity and mortality during lethal influenza infection in mice (Moseley et al. 2010).
2.3 AMPK and Autophagy During Viral Infections
Autophagy becomes particularly important during cellular starvation as a means to recycle amino acids and cellular components for use as catabolic fuels. mTOR forms two functionally distinct complexes in mammals, mTORC1 and mTORC2. In particular, mTORC1 senses nutrient deprival. This is an essential information to take into account when addressing host–pathogen interactions, given that AMPK is an established negative regulator of the mTOR signaling cascade (Inoki et al. 2003; Shaw et al. 2004). AMPK can directly phosphorylate Raptor, a positive regulatory subunit of the mTORC1 complex, and, in addition, it has been shown that AMPK phosphorylates the protein kinase UNC-51-like kinase 1 (ULK1), which is a key signaling complex required for autophagosome formation (Egan et al. 2011; Kim et al. 2011). During the last two decades, autophagy has been analyzed more in detail and described to regulate host–pathogen interactions. Growing evidences indicate that autophagy acts both in the antiviral and proviral pathway (Rey-Jurado et al. 2015; Santarelli et al. 2015) Viral infections may result in the inhibition of host cell metabolism, thus leading to a concomitant reduction in energy demands. This could happen either by blocking host cell protein synthesis, while in the opposite way, by placing increased energetic demands upon infection, which might result in an activation of AMPK. Further studies are necessary to assess the contributing role of the AMPK pathway in regulating autophagy in the context of viral infections. Therefore, it cannot be excluded that AMPK is a critical regulator of cell autophagy in the context of host–pathogen interactions that need to be further addressed.
2.4 AMPK and Immune Effector Functions Cells During Viral Infection
Immune response against pathogens and in particular viruses leads to a rapid modification of cellular metabolism. It has been initially identified that signal transduction pathways, which modulate T-cell metabolism, involve LKB1 and AMPK as key regulators (Blagih et al. 2015; Blagih et al. 2012). Thus, LKB1-deficient T cells exhibited defects in cell proliferation and viability, accompanied by altered glycolytic and lipid metabolism. Interestingly, loss of LKB1 promoted an increase in T cell activation and inflammatory cytokine production by both CD4+ and CD8+ T cells (MacIver et al. 2011). However, although AMPKα1 activity is dispensable for proliferation and differentiation of cytotoxic T lymphocytes (CTLs), AMPK knockout (KO) mice show a striking defect in their ability to generate memory CD8+ T-cell responses in vivo, along with a lower survival of CTLs following withdrawal of immune stimulation (Rolf et al. 2013). The role of metformin has also been addressed in arthritis. It was recently shown that the attenuation of the disease in AMPK KO mice could result from decreased levels of pro-inflammatory cytokines. Interestingly, the impact of AMPK on the differentiation of T cells into T helper 17 (Th17) cells upon stimulation was also demonstrated (Kang et al. 2013). Recently, it has been demonstrated that AMPKα1 is essential for Th1 and Th17 cell development and primary T cell responses to viral and bacterial infections. A reduction of both Th1 and Th17 responses (Estaquier et al. 1996; Estaquier et al. 1995; Raffatellu et al. 2008; Brenchley et al. 2008; Cecchinato et al. 2008; Campillo-Gimenez et al. 2010) were associated with a higher propensity of the cells to die by apoptosis observed during the course of HIV-1 infection (Estaquier et al. 1994; Monceaux et al. 2003; Hurtrel et al. 2005; Cumont et al. 2007). This originates the question as whether or not metformin could restore functional immune effector T cells through AMPK activation and if this approach could have potential interest in the development of therapeutic strategy against acquired immunodeficiency syndrome (AIDS). Thus, given the critical role played by CD4+ and CD8+ T cells in controlling viruses, regulation of metabolic homeostasis represents a quite remarkable field to explore in the context T cell immunity.
In addition to adaptive immunity, a role for AMPK in regulating innate immunity has been proposed. AMPK activation promotes macrophage polarization toward an anti-inflammatory M2 phenotype (Sag et al. 2008) and modulates inflammatory gene expression through activation of SIRT1 (Yang et al. 2010b). However, one important effect of AMPK is the suppression of signal transducers and activators of transcription 1 (STAT1) signaling induced by interferon-gamma (IFN-γ), which inhibits inflammation and chemokines in primary astrocytes and microglia (Meares et al. 2013). This could be particularly important in the context of neuronal infections because inflammation in the central nervous system contributes to neurologic disorders. Furthermore, type I IFN-derived immune responses, which are essential for controlling virus spread, decreases the phosphorylation of AMPK. Consistently, metformin enhances the antiviral effect of IFN-β in Coxsackievirus B3 virus infection (Burke et al. 2014). It has been also proposed that 2-OA antiviral activity against HCV is associated with AMPK activation, through induction of interferon stimulated genes (ISGs) and the inhibition of miR-122 expression (Yang et al. 2013). In the context of RVFV, however, the authors have excluded a role of type I IFN in regulating virus infection downstream AMPK (Moser et al. 2012). Therefore, AMPK appears to be a central player in regulating innate and adaptive immune responses, which are essential in controlling viral infections. Figure 12.1 illustrates the differential regulation of AMPK activity by viruses.
Viral infection induces a differential regulation of AMPK activity. Host AMPK may be modulated in order to promote and ease virus entry (e.g., by macropinocytosis) and immune evasion. AMPK modulation results in the activation of several downstream targets and consequent host metabolism reprograming. Catabolic processes, as fatty acid oxidation and glucose metabolism, are activated through phosphorylation of ACC and increased expression of GLUT4, respectively. On the other side, anabolic processes, as protein synthesis and fatty acid synthesis, are inhibited. NAMPT-induced activation of SIRT1 originates increased PGC1α-induced transcription of mitochondrial genes. Mitophagy may also be induced by AMPK activation, although its role in viral infections has yet to be addressed. ACC acetyl-coA carboxylase, AMPK AMP-activated protein kinase, CaMKKβ calcium/calmodulin-dependent protein kinase kinase β, GLUT4 glucose transporter 4, NAD nicotinamide adenine dinucleotide, Nampt nicotinamide phosphoribosyltransferase, MKK3/6 MAPK kinase 3/6, mTOR mechanistic target of rapamycin, LKB1 liver kinase B1, p38 MAPK p38 mitogen-activated protein kinase, PGC1α peroxisome proliferator-activated receptor γ co-activator-1α, SIRT1 Sirtuin 1, TSC tuberous sclerosis, ULK1 Unc-51-like kinase 1
3 AMPK in the Control of Bacterial Infection
Pathogenic intracellular bacteria infect their hosts by exploiting its cytoplasmic milieu toward survival, growth, and dissemination. During infection, intracellular bacteria must compete with the host in order to obtain the necessary energy and carbon sources. The specific scavenge of nutritional sources by the bacteria may increase their survival odds and consequently can be detrimental to the survival of the permissive host. In parallel, the host cell is forced to respond vigorously to the internal threat by using specific microbicidal mechanisms at the same time that need to maintain bioenergetic homeostatic levels to prevent failure and death. Yet, AMPK beneficial or prejudicial role during bacterial infections appears to be pathogen-specific (Fig. 12.2).
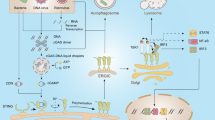
AMPK signaling pathways during host response to bacterial infection. The release of bacterial products such as TLR agonists and PFT or the infectious process itself may lead to AMPK activation. This occurs through alteration of the intracellular calcium concentration and consequent CAMKKβ activation, decrease of ATP/AMP ratios, or ROS production. Additionally, AMPK may also be activated by chemical compounds such as AICAR, metformin, and compound. The phosphorylation of AMPK in the threonine residue 172 (Thr172) during bacterial infections has multiple effects in energy metabolism and autophagy that ultimately contribute to the resolution or progression of the infection. AMPK is a negative regulator of mTOR, so, upon Thr172 phosphorylation, mTOR is inhibited, as well as protein synthesis. Furthermore, mitochondrial metabolism is impacted during infection: mitochondrial respiration and biogenesis may be indirectly increased during bacterial-induced AMPK activation. Furthermore, the transcriptional co-activator PGC-1α is directly modulated by AMPK and originates the increase in transcription of autophagy-related genes, thus leading to increased xenophagy. However, the role of xenophagy in the disclosure of a bacterial infection is still controversial. On one hand, this antibacterial process may cause bacterial death and elimination, through acidification of the intracellular niches and consequent degradation of pathogen. On the other hand, it may increase nutrient availability, thus contributing to pathogen survival. Similarly, the increase in mitochondrial mass may cause an increase in lipid oxidation, which contributes to increased intracellular nutrient availability and, consequently, to proliferation and survival of the pathogen. AICAR 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, AMP adenosine monophosphate, AMPK AMP-activated protein kinase, ATP adenosine triphosphate, CaMKKβ calcium/calmodulin-dependent protein kinase kinase β, mTOR mechanistic target of rapamycin, PFT pore-forming toxins, PGC1α peroxisome proliferator-activated receptor γ co-activator-1α, ROS reactive species of oxygen, TLR Toll-like receptor
3.1 Autophagy: Is Self-Eating Saving or Killing the Bacteria?
Such as in the context of viral infections, it has been shown that selective recognition of intracellular bacteria and their targeting to the autophagic machinery for degradation in lysosomes, also referred as xenophagy, is of crucial importance for bacterial control (Cemma and Brumell 2012). Xenophagy has been put forward as a key component of cell-autonomous innate immunity defence mechanisms against infections (Sorbara and Girardin 2015). If in one hand, xenophagy may contribute to pathogen elimination, in the other, it may also provide the necessary intracellular metabolites that sustain pathogen growth. Amino acid (AA) starvation induced by bacterial pathogens is sensed by the host to trigger protective innate immune and stress responses. The bacteria Shigella and Salmonella are able to trigger an acute AA starvation in HeLa cells, as a consequence of host membrane remodeling and damage. Thus, the AA depletion led to the inhibition of the mTOR pathway emphasizing a possible activation of AMPK (Tattoli et al. 2012). Francisella tularensis is an example of a cytosolic bacterium that activates host autophagic system to increase cytosolic pools of nutrients for its own advantage (Steele et al. 2013). On the other hand, bacterial pathogens have suffered selective pressure leading to the acquisition of skills to manipulate the host enzymatic machinery in their own advantage. In this sense, bacteria can not only prevent the initiation of autophagic mechanisms and maturation of phagosomes, but they are also able to use some components of xenophagy toward its replication and survival (Levine et al. 2011; Deretic et al. 2013). Mycobacterium tuberculosis (Mtb) and Yersinia pseudotuberculosis represent well this last group. Y. pseudotuberculosis can replicate inside autophagosomes, which are blocked in their maturation and thus unable to fuse with lysosomes (Moreau et al. 2010). M. tuberculosis is capable of evading autophagy in macrophages through the inhibition of xenophagic pathways (Kumar et al. 2010). In this case, treatment with Isoniazid, a first-line drug against tuberculosis, during the first 24 h of infection is capable to restore autophagy in M. tuberculosis-infected bone marrow-derived macrophages (BMDMs) in an AMPK-dependent manner leading to pathogen elimination (Kim et al. 2012).
Nonetheless, the replenishment of essential substrates for the pathogen may be assured through modulation of AMPK activity (Brunton et al. 2013). Mycobacterium tuberculosis is capable of utilizing fatty acids as the major energy source (Rhee et al. 2011). While fatty acids and cholesterol are viewed as the predominant carbon source throughout infection (Lee et al. 2013; McKinney et al. 2000; Munoz-Elias and McKinney 2005; Pandey and Sassetti 2008), triglycerides could represent an energetic reservoir during bacteria dormancy (Daniel et al. 2004). M. tuberculosis is capable of inhibiting AMPK and activating mTOR by inducing lipid synthesis. It was recently shown that Mtb accumulated intracellular triacylglycerides (TAG) whose composition is nearly identical to host TAG. Accordingly, it was demonstrated that Mtb TAG were synthesized using free fatty acids released from host macrophages. This lipid scavenging created an energetic reservoir that ultimately allows the bacteria to enter in a latent stage (Daniel et al. 2011). Recently, these kinases arose as a potential trigger of autophagy during infection. Escherichia coli (ETEC) exploits host’s autophagic machinery through the inhibition of mTOR pathway and ERK1/2 and AMPK phosphorylation (Tang et al. 2014). Similarly, the peroxisome proliferator-activated receptor-gamma coactivator 1α (PPARGC1A)-dependent activation of AMPK in M. tuberculosis infection results on increased transcription of autophagy-related genes via CCAAT/enhancer binding protein β (CEBPB). This activation was also associated with increased mitochondrial respiration and biogenesis (Yang et al. 2014). TLR 1/2 has been associated with the initiation of autophagic mechanisms during mycobacterial infections (Yuk et al. 2009). This was shown to be mediated by the mycobacterial lipoprotein LqpH, which activates TLR1/2 inducing a rapid and transient increase in intracellular calcium concentration leading to AMPK activation, possibly through upstream activation of CaMKK. Furthermore, AMPK activation was shown to be essential for autophagy, resulting in the phosphorylation of p38-MAPK (Shin et al. 2010). Similarly to LqpH, pore-forming toxins (PFT) are bacterial virulence factors that have been implicated in the prompting of autophagy, although the underlining mechanisms remain elusive (Gonzalez et al. 2008). Kloft and colleagues observed that several PFT (streptolysin O, Vibrio cholera cytolysin, Staphylococcus aureus α-toxin, and Escherichia coli haemolysin A) cause a drop in intracellular ATP levels leading to AMPK activation (Kloft et al. 2010). It has been hypothesized that the formation of membrane pores leads to the activation of downstream targets, as the AMPK signalling pathway, which may contribute for the maintenance of cellular homeostasis.
Further studies suggest that AMPK signaling may regulate and facilitate the intracellular replication of this pathogen. Although xenophagy may be presented as an antibacterial mechanism, it is important to reckon that other evasion mechanisms driven by a chronic activation of AMPK in the infected cells can be used by invading bacteria, such as Salmonella, to subvert the immune response (Tattoli et al. 2012). Likewise, Legionella pneumophila, the causative agent of Legionnaires’ disease, is capable of inhibiting the phagosome-lysosome fusion, in order to subvert host cell immune response (Horwitz 1983). Macrophage mitochondria and endoplasmic reticulum-derived vesicles are recruited to the vicinity of the phagosome within the first minutes of L. pneumophila infection (Tilney et al. 2001). The consequent encapsulation of Legionella-containing phagosomes increases the survival odds of this bacteria, since it prevents its fusion with lysosomes or acidifying vesicles. This subservient mechanism clearly suggests the utilization of mitochondria and other organelles for support in early stages of infection. Francione et al. (2009) using a Dictyostelium discoideum model for mitochondrial disease observed a faster growth and higher bacterial burden in mitochondrial disease strains. AMPK activation may also be beneficial for Helicobacter pylori and Neisseria meningitidis infections. These gram-negative bacteria colonize the gastric mucosa causing several local distresses as gastritis, gastric ulcers, or gastric cancer. AMPK activation during gastric epithelial cells (GEC) cell infection is induced by transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1), resulting in decreased apoptosis of GEC cells and consequent bacterial survival (Lv et al. 2014). The decreased level of apoptosis originates persistence of infection and GEC proliferation, contributing to the development of carcinogenesis. Accordingly with these results, administration of compound 13, a novel α1-selective activator of AMPK, was shown to decrease H. pylori-induced GEC apoptosis through reactive oxygen species (ROS) scavenging and activation of the AMPK-heme oxygenase (HO-1) pathway (Zhao et al. 2015). Similarly, activation and overexpression of AMPK improve human brain microvascular endothelial cells (HBMECs) permeability after a challenge with lipopolysaccharide (LPS) through suppression of the induction of NAD(P)H oxidase-derived ROS (Zhao et al. 2014). AMPK was activated at 24 h postinfection, while PKB-Akt protein analysis showed dephosphorylation and thus inactivation (Schubert-Unkmeir et al. 2007). In spite of being demonstrated that N. meningitidis regulates gene transcription, translation, and cell metabolism, it is necessary to understand the virulence factors involved.
3.2 Bacterial Host Immune Response: A Double-Edge Sword Controlled by AMPK
Nutrient availability is an important factor when evaluating effector T cell function, since their dysfunction can impair the appropriate responses that culminate with pathogen elimination. Mice with T cell-specific deletion of AMPKα1 infected with an overexpressing OVA Listeria monocytogenes showed a decreased number of total CD4+ and CD8+ T cells at 7 days postinfection. Not only their numbers were reduced as their effector function, since ex vivo stimulation of infected splenocytes in the conditional KO mice displayed lower levels of IFNγ+ (Blagih et al. 2015). This work highlights the importance of AMPK in allowing the proper function of T cells in response to pathogens, which is ultimately connected to the metabolic pathways established in these cells. ROS production is one of the hallmarks of innate host response against intracellular pathogens. Concomitant administration of the AMPK inhibitor compound C with LPS originated an impaired immune response to endotoxemia via the decrease of nuclear-factor kB (NF-kB) activation pathway. This was correlated with a decreased chemotaxis of macrophages and neutrophils to the liver, with decreased ROS production and TNFα levels in the serum (Guo et al. 2014). Furthermore, upon AMPK activation, neutrophils showed an enhancement in E. coli and S. aureus uptake and consequent killing. These observations were supported in vivo using murine models for peritonitis-induced sepsis where metformin-treated mice were found to have fewer viable bacteria (Park et al. 2013). In opposition, in tuberculosis (TB) patients, AMPK-expressing neutrophils were shown to be able to secrete higher quantities of metalloproteinase-8 (MMP-8), in a NF-kB-dependent fashion, leading to matrix destruction and collagen. This study demonstrated that AMPK activation has a role in the secretion of MMP-8 contributing to lung immunopathology during M. tuberculosis infection (Ong et al. 2015). In a very elegant work, Chakrabarti et al. showed that the disruption of Drosophila flies gut homeostasis upon Pseudomonas entomophila infection was caused by the impairment of barrier repair pathways. The mechanism of subversion of gut immune response was mediated by an activation of AMPK-Tuberous sclerosis complex (TSC) stress pathways, which are responsible for TOR inhibition and consequent repression of host translation mechanisms. Ultimately, this contributes to the entrance of infected cells in a quiescent state, more prone for tissue repair but that also allows P. entomophila survival (Chakrabarti et al. 2012).
3.3 Modulation of Host AMPK as a Pharmacological Approach to Target Bacterial Infection
Metformin treatment restricts the intracellular growth of multidrug-resistant Mycobacterium tuberculosis strains in THP-1 and human monocyte-derived macrophages through mitochondrial ROS induction. Moreover, metformin administration ameliorates lung pathology and reduces chronic inflammation while enhancing the efficacy of conventional anti-tuberculosis drugs (isoniazid and ethionamide) in mice models of acute and chronic tuberculosis. In this retrospective study, metformin treatment was associated with improved control of infection and decreased disease severity (Singhal et al. 2014). Moreover, administration of metformin suppresses the inflammatory effects of LPS via induction of activating transcription factor-3 (ATF-3) and AMPK activation. Thus, mice with LPS-induced endotoxemia treated with metformin displayed lower levels of IL-6 and TNF-α, thus decreasing inflammation in vivo (Kim et al. 2014). Furthermore, metformin-induced activation of AMPK inhibited the release of high mobility group box 1 (HMGB1), a protein involved in severe sepsis. This inhibition originated the observed anti-inflammatory effect in LPS-treated RAW264.7 macrophages and in endotoxemic mice, characterized by decreased levels of IL-6, TNF-α, IL-1β, prostaglandin E2 (PGE2), and nitric oxide (NO) (Tsoyi et al. 2011). The activation of AMPK can also be obtained by treatment with AICAR. Similarly, in vivo treatment with this drug resulted in decreased lipoteichoic acid (LTA)-driven lung inflammation (Hoogendijk et al. 2013). Using cecal ligation and puncture (CPL) as a model of sepsis, it was shown that AICAR prevented CLP-induced liver and kidney damage. Additionally, AMPK inhibition in hepatic and renal cells with compound c exacerbated cytokine production and prevented autophagy, thus culminating in increased tissue injury (Escobar et al. 2015). The role of a new bioactive lignan, sauchinone, in AMPK modulation has been studied. The activation of LKB1-AMPK pathway by this anti-inflammatory molecule has been shown to decrease liver toxicity through prevention of iron accumulation (Kim et al. 2009). Recently, Jeong and colleagues explored the role of sauchinone-induced AMPK activation in macrophage phagocytosis (Jeong et al. 2014). The authors demonstrated that sauchinone increased phosphorylation of AMPK and p38 MAPK, which correlated with increased E. coli phagocytic uptake by macrophages in mice lungs.
4 Interaction Between AMPK and Parasites
4.1 Host–Parasite Nutrient Sensing
All the organisms have the ability to sense their surroundings in search for nutrients through a variety of strategies that have evolved to suit the particular needs of each living form. In particular, protozoan parasites need to adapt to more than one host to survive and multiply. Therefore, a dynamic interplay between the host and the parasite is necessary to efficiently coordinate the various parasite developmental stages. The best example would be the maintenance of limited parasite density inside the host or in a distinct host niche that will be responsible for initiating a specific response by ensuring space and nutrients to the entire parasite community (Mony and Matthews 2015; van Zandbergen et al. 2010). This is quite remarkable in African trypanosomes, Trypanosoma brucei spp., where a morphological and molecular alteration into a “slender” form without proliferative capacity is critical to restrain parasitemia and also to have an efficient transmission to the insect host (Gjini et al. 2010; Vassella et al. 1997; Vickerman 1985). Moreover, the confirmed presence of programmed cell death (PCD) in parasites such as Tetrahymena thermophila, Leishmania spp., Trypanosoma cruzi, Plasmodium spp., Trypanosome brucei, Giardia lamblia, Dictyostelium discoideum, Trichomonas vaginalis, Peridinium gatunense, and Blastocystis hominis (Al-Olayan et al. 2002; Ameisen et al. 1995; Pollitt et al. 2011; Rousset and Roze 2007; Zangger et al. 2002) is another mechanism to control parasite density. As a result, the premature death of host or vectors is prevented and the right number of parasites are then able to dampen the host immune system and avoid the limitation of nutrients and resources (Ameisen et al. 1995; Reece et al. 2011). Thus, nutrient availability is a key factor for parasite fitness during infection.
These mechanisms will ultimately ensure a physical space and a nutrient-rich niche that support parasite survival and high growth rate, while maintaining the viability of its host reservoir. During the evolutionary process, unicellular organisms developed strategies to sense extracellular nutrients and intracellular metabolite concentrations since they are often exposed to relevant variations. Some nutrient sensing pathways, as chemoreceptors and PII proteins in bacteria, MEP2 sensor, SPS pathway, and Snf3/Rgt2 sensors in fungi and PII proteins in plants, are unique for these organisms. Such pathways respond to nitrogen, amino acids, ribose, galactose, glucose, and ammonium fluctuations (Chantranupong et al. 2015). Three important pathways, highly conserved from yeast to man, are responsible to sense different nutrient pools. These are general amino acid control non-derepressible 2 (GCN2), TOR kinase and AMPK. Amino-acid levels are sensed by GCN2 and mTOR, while glucose is regulated by mTOR and AMPK and energetic fluctuations are uniquely controlled by AMPK (Chantranupong et al. 2015). Thus, while host and parasites can actually share some nutrient sensors, this section will focus only in AMPK.
4.2 AMPK in the Context of Parasite Infection: Host–Parasite Nutrient Dynamics
The exquisite host–parasite interaction is the result of a coevolution network that has been established continuously since the very first ancient association. The parasite and host coevolution has been developed in parallel throughout time although parasites possess an advantage due to their shorter generation time and to the higher growth rate. To preserve its higher proliferative capacity, parasites have to acquire nutrients from the host, its nutritional requirement being one of the key features in the host–parasite interaction. Within host parasite this dynamic, a nutrient competition is established between the manipulative parasite trying to obtain usable energy and metabolites and the host attempting to sequester the same precursors from the pathogen. The heavy metabolic pressure established dictates the parasite auxotrophy to several nutrients, the host being the only viable source for acquisition. Interestingly, parasites are auxotrophic for several amino acids that are essential for the host. The nutritional pressure on extracellular and intracellular parasites is differently exerted. Extracellular parasites can salvage the nutrients directly from the extracellular fluids, while intracellular parasites need to import nutrients across two or three host membrane systems. Indeed, intracellular parasites need to gain access to nutrients present in the cytosol or inside the intra-vacuolar structures where the threshold levels of amino acids do not match with the high demands of these organisms for carbon, nitrogen, and energy to support its high proliferative rate (Abu Kwaik and Bumann 2013). However, despite their localization, parasites developed several strategies to acquire sufficient nutrients in response to their biological needs. Plasmodium spp. (intracellular), T. brucei (extracellular), and Leishmania spp. (intracellular) acquire nutrients from their hosts by employing different transport proteins, named permeases, located inside their plasma membrane. The nutrients imported from the host include hexoses, purines, iron, polyamines, carboxylates, and amino acids. The different niches occupied by these parasites in their hosts dictate ultimately the mode of nutrient acquisition (Landfear 2011). As an example, during L. major amastigotes persistence in the macrophage phagolysosomes, the parasite exploits the macrophage function on extracellular matrix turnover and remodeling in terms of internalization and degradation of glycosaminoglycans (hyaluronan). This mechanism constitutes another process to exploit additional nutrients such as amino sugars as a carbon source. The dependency of Leishmania on a higher nutrient variety suggests a nutritional diversity inside phagolysosomes that could ultimately represent an advantage to colonize such harsh environment (Naderer et al. 2015). The response of the host against the diversion of nutrients by the parasite is through the restriction of parasite access to nutrient sources. Thus, the process of nutritional immunity limits the availability of iron, zinc, and manganese from the invading pathogens (Crawford and Wilson 2015). An iron-depleted niche is established during L. major infection through the transporter NRAMP1 located at the phagolysosomal compartment. Indeed, NRAMP1 mutation renders the host macrophages more susceptible to Leishmania infections (Appelberg 2006; Forbes and Gros 2001). The strategies developed by the host and parasite in terms of nutrients dynamic will impact the infection outcome.
4.3 AMPK on the Core of Host–Parasite Metabolic Coupling
The nutritional flow established within host–parasite communities will impact the host metabolic background. Several reports have become to identify the pathways involved in the metabolic manipulation of host metabolism required for intracellular pathogen growth. Recent studies with Toxoplasma gondii (MacRae et al. 2012), Trypanosoma brucei (Wang et al. 2008), Leishmania spp. (Moreira et al. 2015a; Rabhi et al. 2012), Schistosoma mansoni (Wang et al. 2008), and Plasmodium berghei (Li et al. 2008) have paved the way to the understanding on the molecular mechanisms used by parasites to take advantage of the nutritive host resources. At the core of these metabolic dynamic interactions, AMPK was identified as having a crucial role to balance the energetic status of the host with a positive or negative impact on parasite survival. Nevertheless, the role of AMPK during the metabolic host–parasite crosstalk still remains largely unexplored.
A microarray analysis performed on mice livers of experimental P. berghei infection revealed significant variations within genes related to carbohydrate and energetic metabolisms. This approach demonstrated an upregulation of gluconeogenesis pathways while glycolysis appears downregulated on liver extracts from mice infected with P. berghei. Interestingly, these modifications were accompanied by an increase in the energetic sensors, Prkaa2 (AMPKα) and Prkag2 (AMPKγ) transcripts (Sales-Dias 2011). Analyzing AMPK translational levels will be important to clarify the actual role during P. berghei liver stage infection. A potential role of AMPK during an experimental model of cerebral malaria (ECM) was recently suggested (Gordon et al. 2015). The mTOR inhibitor rapamycin protects against ECM when administered within the first 4 days of infection. Rapamycin increased survival, blocked the breakdown of the blood–brain barrier and brain hemorrhaging, and decreased the influx of both CD4+ and CD8+ T cells into the brain and the accumulation of parasitized red blood cells in the brain. The impact of mTOR-controlled metabolic pathways and the recent knowledge regarding the activation of metabolic pathways in T cells upon antigen recognition led the authors to suggest a possible impact of some metabolic pathways, such as AMPK, in the control of this disease.
An intense inflammatory reaction accompanies infection with Trypanosoma cruzi, the etiologic agent of Chagas disease. T. cruzi targets the adipose tissue leading to a release of inflammatory cytokines as well as a decrease in adiponectin and PPAR-γ contributing to the establishment of an inflammatory niche. Interestingly, given that adiponectin activates AMPK, it was suggested that a downregulation of the former could be a evasion mechanism to keep AMPK in check and favor parasite growth (Nagajyothi et al. 2008). A recent work confirmed a protective role for AMPK during T. cruzi infection (Caradonna et al. 2013). A genome-wide RNA interference screen identified that a sustained AKT-mTORC1 pathway regulate intracellular T. cruzi growth. The maintenance of cellular ATP/ADP ratios at higher levels provide a distinct advantage for the parasite-limiting AMPK activity. Further, the acute silencing of AMPK catalytic (Prkaa1) or the regulatory subunit (Prkab1) in vitro provides a more favorable growth environment for intracellular T. cruzi. Although it has not been confirmed in vivo, AMPK inhibition is suggested to contribute for T. cruzi survival.
Recently, we demonstrated that AMPK is, in contrast, crucial for the establishment of a microenvironment more prone for L. infantum survival in macrophages (Moreira et al. 2015a). Previous analysis on the transcriptomic signature of L. major-infected macrophages revealed that carbohydrate and lipid metabolism were among the most altered pathways during infection. Increased mRNA levels of glucose transporters as well as key glycolytic enzymes encoding genes, such as hexokinases (Hk), pyruvate kinase M2 (Pkm2), and lactate dehydrogenase a (Ldha), were induced in the presence of live but not heat-killed L. major promastigotes. L. major also induced a downregulation of a number of genes implicated in the tricarboxylic acid (TCA) cycle and oxidative phosphorylation suggesting that infected macrophages mainly rely on increased glycolytic flow for energy production. On the other hand, L. major led to cholesterol and triglycerides accumulation on infected macrophages by enhancing the expression of scavenger receptors involved in the uptake of low-density lipoprotein (LDL), inhibiting cholesterol efflux and increasing the synthesis of triacylglycerides (Rabhi et al. 2012). The accumulation of lipid droplets in close proximity to parasitophorous vacuoles advocates the former structure as a potential high-energy substrate source for the intracellular parasite. We further observed that following L. infantum infection, macrophages switch from an early glycolytic to an oxidative metabolism, in a process requiring SIRT1 and LKB1/AMPK. In the absence of SIRT1 or LKB1, infected macrophages are not able to induce AMPK activation leading to an impairment of the metabolic switch. In that sense, AICAR-induced AMPK activation contributes to parasite survival while inhibition of AMPK using compound C resulted in lower parasite numbers in vitro. This phenotype was further corroborated in vivo as shown by a significantly reduced parasite burden in mice with a myeloid-specific AMPK deficiency (Moreira et al. 2015a).
Gastrointestinal (GI) nematodes also affect profoundly host metabolism. The infection of CD11c-specific AMPKα–/– (DC-AMPK–/–) mice with the gastrointestinal roundworm Nippostrongylus brasiliensis led to a dysregulation of Th2 immune response concomitantly with a failure to regenerate tissue damage mediated by the pathogen. Imbalanced responses generated in DC AMPK–/– mice were associated with increased Type-1 responses, greater numbers of Th17 cells, and defects in the generation of alternatively activated macrophages. Therefore, AMPK activity in myeloid cells was shown to regulate host protection against this GI parasite (Nieves et al. 2014). The manipulation of AMPK activities by distinct parasites are summarized in Fig. 12.3. Although still insufficient, these examples highlight the strategies used by the parasites to explore host resources shedding some light on cellular metabolic subversion mechanisms induced by microbe infections within the host. In this context, the modulation of AMPK activity has been put forward as a possible therapeutic target against parasitic diseases.
Host AMPK dictates parasite infection outcome. (a) In a context of malaria liver stage infection, Plasmodium berghei modulates the transcriptional program of the host hepatocytes. Within the host cells, P. berghei increases gluconeogenesis enzymes and Prkaa2 (AMPKα), Prkag2 (AMPKγ) transcripts, decreasing in parallel the transcription of Pfk glycolytic enzyme. At this stage, an increase of glucose levels is established generating a permissive niche for sporozoites survival, which are highly dependent on glucose. (b) During Leishmania infantum infection, the promastigote form induces a metabolic alteration toward a glycolytic environment with a decrease of energetic (ATP/AMP) and redox status of the host. The increased AMP levels triggers the activation of AMPK that will be phosphorylated by SIRT1-LKB1 pathway which, in this context, is considered an AMPK upstream activator. Afterwards, a metabolic rearrangement occurs through the increase of respiration and PGC-1α activation. At this point, the energetic and redox pools are recovered leading ultimately to the parasite survival. (c) A detrimental effect of AMPK was defined for Nippostrongylus brasiliensis persistence within the host. The specific ablation of AMPK in dendritic cells deregulated the type 2 response establishing a type 1 through the augmentation of Th17 cells. Simultaneously, the ability to alternatively activate macrophages is dampened, concomitantly with the decrease capacity to regenerate tissue damage induced by the pathogen. (d) AMPK in a context of T. cruzi infection has also a deleterious effect for the pathogen survival and growth. Inside the host, trypomastigotes supports AKT-mTORC1 pathway activation maintaining in parallel, a high energetic level that consequently ablates AMPK activity. Ultimately, the established niche becomes permissive for the amastigotes survival and growth inside the host. Pfk phosphofructokinase 1, ECAR extracellular acidification rate, OCR oxygen consumption, SIRT1 silent mating type information regulation 2 homolog 1, LKB1 liver kinase B1, AMPK-p AMP-activated protein kinase α-phosphorylated, PGC-1alpha peroxisome proliferator-activated receptor gamma coactivator 1-alpha and mTORC1, mammalian target of rapamycin complex 1
5 Conservation of the AMPK Machinery in Eukaryote Pathogens
5.1 SNF1/AMPK Pathways in Parasites
The AMPK family of protein kinases is highly conserved among eukaryotes. This protein has been extensively studied in mammals and yeast, where it was demonstrated to have a huge similarity from a structural and functional point of view. The conserved kinase family present in yeast and plants is denominated as sucrose non-fermenting 1 (SNF1) and SNF1-related protein kinase 1 (SnRK1), respectively (Hardie 2007; Polge and Thomas 2007). Genes encoding orthologues of the three domains (α, β, and γ subunits) are described in all eukaryotic parasite species and even in the primitive Giardia lamblia, which lacks mitochondria (Adam 2000; Hardie et al. 2003). The exception is the obligate intracellular parasite Encephalitozoon cuniculi that does not possess an identifiable AMPK orthologue (Miranda-Saavedra et al. 2007).
SNF1 and SnRK1 homologs develop similar functions in what concerns the surveillance of the metabolic status in response to nutrient and environmental stress through the induction of catabolic processes and a general repression of anabolism. Similarly to these organisms, some reports have defined the presence of AMPK/SNF1 protein kinases in eukaryote parasites. The first evidence of the presence of an SNF1 homologue in parasites was described in apicomplexa phylum for Plasmodium falciparum (Bracchi et al. 1996). In this work, an SNF1 homologous gene defined as PfKIN was found to be increased, at transcriptional level, in the gametocyte stage which is involved in the transmission and adaptation of the malaria parasite from the human bloodstream to the mosquito midgut. The deduced protein sequence of PfKIN contains all the characteristic sequence motifs of the eukaryotic protein kinases including the ones involved in ATP-binding, substrate recognition, and catalysis. The PfKIN catalytic domain shows 40 % of homology with the SNF1 sequence from S. cerevisiae. It is important to underline that no significant amino acid sequence similarities to any other proteins were found outside the catalytic domain. This suggests that PfKIN can be modulated by other signals that differ from the ones found in other eukaryotes (Bracchi et al. 1996). A systematic functional analysis of protein kinases in P. berghei identified a SNF1/KIN protein. This orthologue was shown to be relevant for sporozoite development, particularly in the egression to the salivary gland of the mosquito Anopheles stephensi, acting as a regulator of energy metabolism (Tewari et al. 2010). The existence of a SNF1/AMPK orthologue was observed in Cryptosporidium parvum (Artz et al. 2011) that lacks Krebs cycle and oxidative phosphorylation. In Eimeria acervulina, a putative protein belonging to the SNF1 family was identified and correlated with invasion and evasion mechanisms in chicken duodenal epithelial cells (Zhang et al. 2015). Finally, still in the apicomplexa phylum, a potential AMPK homolog (ToxPK1) was observed in Toxoplasma gondii genome with 58 % identity to human AMPK alpha. ToxPK1 gene was shown to be transiently expressed to upregulate glycogen biosynthesis during the development of tachyzoites into bradyzoites (Ghosh et al. 2012; Ng et al. 1995, 1997).
A comparative analysis of the kinomes of representative members of pathogenic trypanosomatids, namely Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major, have highlighted that AMPK homologues are relatively poorly represented within trypanosomatid genomes as compared to humans. Yet, these are predicted to be active (Parsons et al. 2005). In T. brucei, the fine-tuned control of transient alteration from the “slender” (proliferative) to “stumpy” (quiescent) forms is performed by intracellular signaling pathways triggered by the “stumpy induction factor” (SIF). The slender state is retained until SIF accumulation reaches threshold levels triggering the nutritional stress-like response, which leads to concomitant repression of the “slender retainers” and activation of “stumpy inducers.” This dynamic transition leads to cell cycle arrest and prepares the cell for its next life cycle stage, in the insect, by reactivating mitochondrial functions required for oxidative phosphorylation (Mony and Matthews 2015). A potential role for AMPK in this developmental transition can be speculated. The signaling components that drives stumpy formation evaluated in a genome-wide RNA interference library screen identified a AMPK/SNF1/KIN11 homologue. Interestingly, the authors suggest that AMPK homologue, which acts as a SIF, could be a potential inhibitor of trypanosomes TORC4 (Mony et al. 2014). TORC4 activity is proposed to prevent stumpy formation, since the knockdown of TOR4 drove the cells to develop features characteristic of stumpy forms (Barquilla et al. 2012). TOR4 is one of the T. brucei TOR paralogues that retain the classical structure of mTOR kinase domains, yet displaying new features not described in other TORs (Barquilla et al. 2008). T. brucei procyclic forms are able to monitor glucose levels, through the surface molecule expression of procyclins, which are crucial for the parasite survive in the tsetse fly vector (Clemmens et al. 2009). Procyclins are glycoproteins that cover the surface of procyclic forms that are suspected to have a protective role against the action of insect proteases (Acosta-Serrano et al. 2001). Two major procyclin proteins EP and GPEET were found to be regulated by the beta and gamma subunits (TbAMPK beta and TbAMPK gamma, respectively), since the silencing of these genes leads to their upregulation. Interestingly, the latter procyclin is highly expressed in glucose-deficient environment within tsetse fly. Moreover, the localization of the scaffold beta subunit (glycosomes and flagellum) suggests a central positioning between surface molecule expression and glycolysis providing thus a molecular connection between these two mechanisms (Clemmens et al. 2009).
It is important to stress out that the role of AMPK/SNF1 protein kinase family are quite similar for all the organisms (extracellular or intracellular), playing a central positioning in its most relevant pathways. The described AMPK/SNF1 homologues have been implicated in the parasite developmental stages that occur within the same host or in different hosts in order to recover from a nutrition/energetic depletion status. Parasites have to deal with different microenvironments during proliferation and differentiation recurring to sensing mechanisms as the AMPK/SNF1 protein kinases that will have definitely a huge impact on parasite fitness.
5.2 SNF1/AMPK Pathways in Fungi
Over the past years, the incidence of fungal infections has increased in several countries. Compared to bacteria, infections caused by fungi are less frequent; nevertheless, their treatment is still complicated for systemic infections particularly due to the close functional similarity between fungal cells and the host mammalian cells (Navarro-Garcia et al. 2001). Human pathogenic fungi encounter a broad range of stress conditions in their natural environment as well as in the host during infection that challenge their ability to grow. The fungi ability to adapt to a variety of conditions has contributed to the ubiquitous nature in the environment and to the success of them as pathogens. In fact, adaptation to nutrient fluctuations is crucial for survival. Human pathogenic fungi must adapt to oxidant, pH, or nutritional stress, otherwise they are eliminated by the host defense system. Fungi survival, both in the environment or within the human host, requires the activation of signal transduction pathways that sense environmental or host stress cues (Hohmann 2002). During host nutritional deprivation, fungi pathogens are able to use alternative carbon sources, such mechanism being considered a virulence trait crucial to adapt to stress conditions (Lorenz and Fink 2001). Therefore, it is not surprising that genes that control several metabolic pathways may have a central role in fungal virulence (Navarro-Garcia et al. 2001).
5.3 Regulation and Function of Fungi SNF1 Protein Kinase
Snf1 protein kinase was initially identified by a screening performed with the budding yeast Saccharomyces cerevisiae (Celenza and Carlson 1986). Snf1 was identified as the yeast homolog of the mammalian AMPK acting as an energy sensor. This kinase, in fungi, is also able to reprogram the cellular metabolism through the energetic balance which is essential to sustain cell metabolism and to support the development of a stress response (Sampaio-Marques et al. 2014). S. cerevisiae Snf1 is a serine/threonine protein kinase (α subunit) complexed with other proteins, namely γ activating subunit Snf4 and β subunits Sip1, Sip2, or Gal83 depending on Snf1 localization (Celenza and Carlson 1989; Celenza et al. 1989; Estruch et al. 1992; Vincent and Carlson 1999). AMPK/Snf1 activation in fungi could be dependent or not on AMP/ATP cellular fluctuations. In the former, the allosteric regulation by AMP is not crucial for Snf1 activation while in the latter Snf1 function is regulated by cAMP-dependent protein kinase (PKA) (Ferretti et al. 2012).
A crucial activity on glucose balance has been also attributed to Snf1 kinase. This is of particular importance since glucose is one of the major carbon and energy source for the eukaryotic cells. Under carbon stress conditions, fungal cells required the activity of the Snf1 kinase in order to adapt to this harsh environment. Furthermore, Snf1 activity is requested for transcription of glucose repressed genes and increases with aging, even when glucose is abundant (Ashrafi et al. 2000; Hedbacker and Carlson 2008). Taken together, Snf1 allows cells to adapt to limitations on glucose availability using instead alternative carbon sources, such as sucrose, galactose and ethanol (Carlson 1999; Gancedo 1998). Additionally, Snf1 is highly important in many other cellular stress conditions helping to establish a response against sodium ion stress, heat shock, alkaline pH, and oxidative and genotoxic stress (Hedbacker and Carlson 2008). Other crucial biological impact of Snf1 has been described for glycogen, sterol, fatty acid biosynthesis, fatty acid-β oxidation, peroxisome biogenesis, and ultimately in the fungi sporulation developmental stage (Sanz 2003). The main functions of Snf1 kinase in pathogenic fungi are resumed in Fig. 12.4.
SNF1 protein kinase in pathogenic fungi. Snf1, AMPK homolog in fungi, functions as an energy sensor and allows for a proper response and adaptation to environmental stress. This kinase is activated during low ATP/AMP ratio and also by PKA, in an independent fashion. Snf1 activation allows the adaption to limitations on glucose availability and prompts the use of other alternative carbon sources, like sucrose, galactose, and ethanol. Snf1 ablation results in metabolic impairment and the loss of inability to use different carbon sources, with possible consequences in fungi pathogenicity, namely, regarding susceptibility to antifungal therapy and cell wall constitution. Furthermore, in glucose deprivation conditions, Snf1 promotes transcription of FLO11 gene, which encodes to a protein required for invasive growth and biofilms, thus impacting C. albicans invasion and consequent pathogenicity. Snf1 is also responsible for the regulation of melanin production by C. neoformans, which is an important cell wall-associated virulence factor. AMP adenosine monophosphate, AMPK AMP-activated protein kinase, ATP adenosine triphosphate, PKA cAMP-dependent protein kinase
5.4 SNF1 Protein Kinase in Pathogenic Fungi
In Candida spp., namely Candida glabrata, the importance of Snf1 kinase for the regulation of nutrient sources availability was already demonstrated. Snf1 ablation resulted in the loss of its capacity to utilize trehalose, which together with glucose are the carbon sources preferentially used by this organism (Petter and Kwon-Chung 1996). In the yeast Cryptococcus neoformans, a pathogenic fungus that infects via respiratory tract, particularly immunocompromised individual (Buchanan and Murphy 1998; Hull and Heitman 2002; Mitchell and Perfect 1995), the SNF1 abrogation leads to the incapacity to use alternative carbon sources such as acetate, ethanol, and sucrose. Furthermore, an increase on sensitivity to antifungal drugs such as amphotericin B and to nitrosative stress was also detected, manifested particularly at the host temperature of 37 °C (Hu et al. 2008; Yang et al. 2010a). In other fungi species such as Aspergillus spp., the absence of SnfA1 (Snf1 homologue) causes a defect on glucose-depression mechanisms and impairs the pathogen growth under various carbon sources. Together, data from the different pathogenic fungi suggest that Snf1 presents a conserved function in the regulation of catabolic repression. In alignment with this conserved function that allows the cells to adapt to adverse metabolic conditions, a role in fungus virulence is also attributed to Snf1, which evidences a regulatory connection between carbon source utilization and intracellular growth. In Candida albicans, an opportunistic pathogen that is able to invade different tissues of immunocompromised individuals causing disease, it was demonstrated that Snf1 is an essential protein. C. albicans cells survival depends on the functionality of Snf1 kinase even in the presence of glucose (Petter et al. 1997), which goes against what is observed in S. cerevisiae, suggesting a central role for Snf1 in C. albicans. However, since SNF1 is an essential gene, its functions in C. albicans are still few disclosed. In S. cerevisiae, it was unveiled that Snf1 is able to transcriptionally regulate flocullin (FLO11), which encodes to a protein required for invasive growth and biofilm formation. In addition, Snf1 inhibits the function of Nrg1 and Nrg2, two negative regulators of FLO11, in response to glucose depletion. This data suggests that pseudohyphal growth and invasive growth depend on Snf1 activity (Kuchin et al. 2002). Since SNF1 and NRG genes are sustained in C. albicans, it is hypothesized that this regulatory mechanism is functionally conserved. Thus, in this pathogenic fungus, Snf1 plays a role in the morphological transition from yeast form to filamentous growth, a process that is vital for the pathogenicity of C. albicans (Kuchin et al. 2002).
A role in C. neoformans virulence is also ascribed to Snf1. In this organism, the link of Snf1 with virulence might be also in part associated with the production of the pigment melanin, a cell wall-associated virulence factor, since snf1 mutant strains presented reduced melanin production at 37 °C (Hu et al. 2008). Melanin production is regulated by the activity of the copper-containing polyphenolic oxidase laccase, majority promoted by the LAC1 gene. LAC1 transcription is enhanced by glucose starvation, yet by undisclosed mechanisms; nevertheless, it is suggested that Snf1 participates in the LAC1 derepression by glucose depletion (Yang et al. 2010a).
Adhesion capacity is another important feature for fungus pathogenicity because it is able to mediate the interaction between colonies as well as host–pathogen interactions (Ramsook et al. 2010). Concerning this pathogenicity feature, it was demonstrated, in vitro, that SNF1 abrogation in C. neoformans impairs the cells ability to adhere to agar in comparison with control strains (Yang et al. 2011). Furthermore, the same study also demonstrated that snf1 mutant cells presented a phonotype compared with cell wall defects, since they are sensitive to SDS and Congo red (Yang et al. 2011). Thus, Snf1 apparently has also a contribution concerning the modulation of the cell wall integrity that functions as essential barrier to protect cells from harmful effects caused by the extreme conditions frequently face by fungal cells (Bermejo et al. 2008; Levin 2005; Waterman et al. 2007). Together, the data suggests that the observed cell wall impairment observed in the absence of Snf1 might cause changes of the cellular surface components such as glycoproteins which could lead to the loss of adhesion capacity; nevertheless, the adhesion regulation pathways in C. neoformans are yet undisclosed.
The kinase Snf1 functions are well characterized in the yeast S. cerevisiae, a model organism in which it was demonstrated that this kinase has a central metabolic function. At the same regulation level, several studies in important human pathogenic fungi showed that Snf1 homologs presented a conserved function, evidencing that this protein can be a potential antifungal target. More interestingly, several observations point out Snf1 as a regulator of virulence factors as well as a virulence factor. Concerning this aspect, Snf1 is able to modulate crucial fungal pathogenic mechanisms, as above discussed; nevertheless and due to its crucial role in the cells viability, new approaches must be developed in an attempt to disclose the precise pathogenic mechanism regulated by Snf1. Together, all the pieces of information highlighted that Snf1 might represent a promising alternative target of interest to drug development.
6 Final Remarks
Host–pathogen interactions are highly dynamic processes where both compete to acquire a myriad of essential nutrients, crucial to survive inside an infection niche. Although it seems well established that pathogens manipulate host metabolism in its own advantage, the preferred nutrient sources and the triggered metabolic pathways are still to be completely understood. In this context, the manipulation of host AMPK activity seems to be a valuable approach for controlling pathogen burden and infection progression.
The positive or negative impact on host AMPK protein during an infection could reflect the existence of distinct biological and nutritional needs for the distinct pathogens. Overall, two main factors contribute for the different responses observed concerning host AMPK modulation during infection. First, in vivo, microbial pathogens can find distinct niche/cells with different nutritional/immunological status. Secondly, an heterogeneity in host–pathogen interactions (various pathogens subsets) could occur simultaneously within the same host tissue reflecting disparate outcomes (Bumann 2015). We can then speculate that pathogen survival will depend on the ability to sense and find the most permissive cells without discarding the overall contribution of the different pathogen subsets within the same tissue. This vast heterogeneity during in vivo infections set the nutrient sensors, as AMPK, in a more privileged core to impact on the disease outcome. Moreover, an additional diversity in terms of AMPK/SNF1 orthologues function was described within parasites and fungal organisms. The particularities of each parasite or fungus life cycle and the pressure exerted by the different hosts could guide the evolutionary process of these orthologues in distinct branches of the phylogenetic tree that could account ultimately for their variability inside these organisms. In that sense, the parasite and fungal AMPK/SNF1 members could participate in the intracellular sensing of the nutritional status of the host. Thus, a rearrangement of their metabolic pathways may occur in order to acquire the sufficient amount of nutrients and energy from the host to survive. Nevertheless, the role for AMPK/SNF1 orthologues during the infectious process still remains uncomprehend.
The quite unexplored field of nutrient sensors open a new avenue to exploit AMPK as a possible drug target against microbial pathogens. The design of new drugs to activate or inhibit this kinase in the host during infection could be an interesting domain to explore. In fact, there are drugs currently used to treat metabolic diseases that could be considered an alternative approach to treat infectious diseases. A classic example is metformin, which is able to activate AMPK through mitochondria complex I inhibition that is currently the most common treatment for diabetes type 2. Although due to the large variation described in terms of niche/cells and the presence of various pathogen subsets during in vivo infections, several concerns are being raised. The described disparities may account for the unsuccessful treatment or resistance development found in conventional therapeutics. The possible addition/synergy of AMPK activators or inhibitors to the conventional treatment could represent a viable and important therapeutic alternative to overcome this infection issue. Despite the highly conservation among eukaryotes, AMPK orthologues described in parasites and fungi could be also explored as potential drug targets. To develop selective activators or inhibitors to these orthologues, the homology to mammals should be low, although relevant divergences in the catalytic domain or differences in the protein weight/conformation should be considered. As an example, we can speculate that PfKIN from P. falciparum could be considered a promising drug target due to the fact that its catalytic domain presents 40 % of homology with the SNF1 sequence of S. cerevisiae, and no significant amino acid sequence similarities to any other proteins were found outside the catalytic domain. AMPK orthologues have been more studied in Plasmodium; however, further work will be needed to characterize and define this protein as a possible drug target as well as in other pathogens.
Overall, the nutritional status of the host and the infection outcome can influence each other occupying the microbial pathogens a central position in this dynamic balance. The hallmark of the host metabolic status and infection outcome is the gut microbial communities, defined as microbiota, which is currently considered a hot research topic. In a healthy state, they contribute nutrients and energy to the host, and a balance is maintained with the host’s metabolism and immune system. On the other side, intestinal dysbiosis can act as a source of inflammation and in-fection and possible contributions to diabetes mellitus and obesity (Flint et al. 2012).This striking regulation could be essential to improve or trace new antimicrobial strategies through the modulation of host metabolism.
References
Abu Kwaik Y, Bumann D (2013) Microbial quest for food in vivo: ‘nutritional virulence’ as an emerging paradigm. Cell Microbiol 15(6):882–890. doi:10.1111/cmi.12138
Acosta-Serrano A, Vassella E, Liniger M, Kunz Renggli C, Brun R, Roditi I, Englund PT (2001) The surface coat of procyclic Trypanosoma brucei: programmed expression and proteolytic cleavage of procyclin in the tsetse fly. Proc Natl Acad Sci U S A 98(4):1513–1518. doi:10.1073/pnas.041611698
Adam RD (2000) The Giardia lamblia genome. Int J Parasitol 30(4):475–484
Al-Olayan EM, Williams GT, Hurd H (2002) Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito. Int J Parasitol 32(9):1133–1143
Ameisen JC, Idziorek T, Billaut-Mulot O, Loyens M, Tissier JP, Potentier A, Ouaissi A (1995) Apoptosis in a unicellular eukaryote (Trypanosoma cruzi): implications for the evolutionary origin and role of programmed cell death in the control of cell proliferation, differentiation and survival. Cell Death Differ 2(4):285–300
Appelberg R (2006) Macrophage nutriprive antimicrobial mechanisms. J Leukoc Biol 79(6):1117–1128. doi:10.1189/jlb.0206079
Artz JD, Wernimont AK, Allali-Hassani A, Zhao Y, Amani M, Lin YH, Senisterra G, Wasney GA, Fedorov O, King O, Roos A, Lunin VV, Qiu W, Finerty P Jr, Hutchinson A, Chau I, von Delft F, MacKenzie F, Lew J, Kozieradzki I, Vedadi M, Schapira M, Zhang C, Shokat K, Heightman T, Hui R (2011) The Cryptosporidium parvum kinome. BMC Genomics 12:478. doi:10.1186/1471-2164-12-478
Ashrafi K, Lin SS, Manchester JK, Gordon JI (2000) Sip2p and its partner snf1p kinase affect aging in S. cerevisiae. Genes Dev 14(15):1872–1885
Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA (2002) Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115(Pt 11):2433–2442
Barquilla A, Crespo JL, Navarro M (2008) Rapamycin inhibits trypanosome cell growth by preventing TOR complex 2 formation. Proc Natl Acad Sci U S A 105(38):14579–14584. doi:10.1073/pnas.0802668105
Barquilla A, Saldivia M, Diaz R, Bart JM, Vidal I, Calvo E, Hall MN, Navarro M (2012) Third target of rapamycin complex negatively regulates development of quiescence in Trypanosoma brucei. Proc Natl Acad Sci U S A 109(36):14399–14404. doi:10.1073/pnas.1210465109
Bermejo C, Rodriguez E, Garcia R, Rodriguez-Pena JM, Rodriguez de la Concepcion ML, Rivas C, Arias P, Nombela C, Posas F, Arroyo J (2008) The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol Biol Cell 19(3):1113–1124. doi:10.1091/mbc.E07-08-0742
Blagih J, Krawczyk CM, Jones RG (2012) LKB1 and AMPK: central regulators of lymphocyte metabolism and function. Immunol Rev 249(1):59–71. doi:10.1111/j.1600-065X.2012.01157.x
Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vazquez G, Yurchenko E, Raissi TC, van der Windt GJ, Viollet B, Pearce EL, Pelletier J, Piccirillo CA, Krawczyk CM, Divangahi M, Jones RG (2015) The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42(1):41–54. doi:10.1016/j.immuni.2014.12.030
Bonen A, Han XX, Habets DD, Febbraio M, Glatz JF, Luiken JJ (2007) A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am J Physiol Endocrinol Metab 292(6):E1740–E1749. doi:10.1152/ajpendo.00579.2006
Bracchi V, Langsley G, Thelu J, Eling W, Ambroise-Thomas P (1996) PfKIN, an SNF1 type protein kinase of Plasmodium falciparum predominantly expressed in gametocytes. Mol Biochem Parasitol 76(1–2):299–303
Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC (2008) Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112(7):2826–2835. doi:10.1182/blood-2008-05-159301
Brunton J, Steele S, Ziehr B, Moorman N, Kawula T (2013) Feeding uninvited guests: mTOR and AMPK set the table for intracellular pathogens. PLoS Pathog 9(10):e1003552. doi:10.1371/journal.ppat.1003552
Buchanan KL, Murphy JW (1998) What makes Cryptococcus neoformans a pathogen? Emerg Infect Dis 4(1):71–83. doi:10.3201/eid0401.980109
Bumann D (2015) Heterogeneous host-pathogen encounters: act locally, think globally. Cell Host Microbe 17(1):13–19. doi:10.1016/j.chom.2014.12.006
Burke JD, Platanias LC, Fish EN (2014) Beta interferon regulation of glucose metabolism is PI3K/Akt dependent and important for antiviral activity against coxsackievirus B3. J Virol 88(6):3485–3495. doi:10.1128/JVI.02649-13
Campillo-Gimenez L, Cumont MC, Fay M, Kared H, Monceaux V, Diop O, Muller-Trutwin M, Hurtrel B, Levy Y, Zaunders J, Dy M, Leite-de-Moraes MC, Elbim C, Estaquier J (2010) AIDS progression is associated with the emergence of IL-17-producing cells early after simian immunodeficiency virus infection. J Immunol 184(2):984–992. doi:10.4049/jimmunol.0902316
Caradonna KL, Engel JC, Jacobi D, Lee CH, Burleigh BA (2013) Host metabolism regulates intracellular growth of Trypanosoma cruzi. Cell Host Microbe 13(1):108–117. doi:10.1016/j.chom.2012.11.011
Carlson M (1999) Glucose repression in yeast. Curr Opin Microbiol 2(2):202–207. doi:10.1016/S1369-5274(99)80035-6
Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, Zaffiri L, Tryniszewska E, Tsai WP, Vaccari M, Parks RW, Venzon D, Douek DC, O’Shea JJ, Franchini G (2008) Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol 1(4):279–288. doi:10.1038/mi.2008.14
Celenza JL, Carlson M (1986) A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science 233(4769):1175–1180
Celenza JL, Carlson M (1989) Mutational analysis of the Saccharomyces cerevisiae SNF1 protein kinase and evidence for functional interaction with the SNF4 protein. Mol Cell Biol 9(11):5034–5044
Celenza JL, Eng FJ, Carlson M (1989) Molecular analysis of the SNF4 gene of Saccharomyces cerevisiae: evidence for physical association of the SNF4 protein with the SNF1 protein kinase. Mol Cell Biol 9(11):5045–5054
Cemma M, Brumell JH (2012) Interactions of pathogenic bacteria with autophagy systems. Curr Biol 22(13):R540–R545. doi:10.1016/j.cub.2012.06.001
Chakrabarti S, Liehl P, Buchon N, Lemaitre B (2012) Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila gut. Cell Host Microbe 12(1):60–70. doi:10.1016/j.chom.2012.06.001
Chantranupong L, Wolfson RL, Sabatini DM (2015) Nutrient-sensing mechanisms across evolution. Cell 161(1):67–83. doi:10.1016/j.cell.2015.02.041
Clemmens CS, Morris MT, Lyda TA, Acosta-Serrano A, Morris JC (2009) Trypanosoma brucei AMP-activated kinase subunit homologs influence surface molecule expression. Exp Parasitol 123(3):250–257. doi:10.1016/j.exppara.2009.07.010
Crawford A, Wilson D (2015) Essential metals at the host-pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens. FEMS Yeast Res 15(7):pii: fov071. doi:10.1093/femsyr/fov071
Cumont MC, Monceaux V, Viollet L, Lay S, Parker R, Hurtrel B, Estaquier J (2007) TGF-beta in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus. Cell Death Differ 14(10):1747–1758. doi:10.1038/sj.cdd.4402192
Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE (2004) Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol 186(15):5017–5030. doi:10.1128/JB.186.15.5017-5030.2004
Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE (2011) Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog 7(6):e1002093. doi:10.1371/journal.ppat.1002093
Deretic V, Saitoh T, Akira S (2013) Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13(10):722–737. doi:10.1038/nri3532
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331(6016):456–461. doi:10.1126/science.1196371
Escobar DA, Botero-Quintero AM, Kautza BC, Luciano J, Loughran P, Darwiche S, Rosengart MR, Zuckerbraun BS, Gomez H (2015) Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res 194(1):262–272. doi:10.1016/j.jss.2014.10.009
Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin AM, Venet A, Mehtali M, Muchmore E, Michel P, Mouton Y, Girard M, Ameisen JC (1994) Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci U S A 91(20):9431–9435
Estaquier J, Idziorek T, Zou W, Emilie D, Farber CM, Bourez JM, Ameisen JC (1995) T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med 182(6):1759–1767
Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen JC (1996) Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87(12):4959–4966
Estruch F, Treitel MA, Yang X, Carlson M (1992) N-terminal mutations modulate yeast SNF1 protein kinase function. Genetics 132(3):639–650
Ferretti AC, Larocca MC, Favre C (2012) Nutritional stress in eukaryotic cells: oxidative species and regulation of survival in time of scarceness. Mol Genet Metab 105(2):186–192. doi:10.1016/j.ymgme.2011.11.007
Flint HJ, Scott KP, Louis P, Duncan SH (2012) The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9(10):577–589. doi:10.1038/nrgastro.2012.156
Forbes JR, Gros P (2001) Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol 9(8):397–403
Francione L, Smith PK, Accari SL, Taylor PE, Bokko PB, Bozzaro S, Beech PL, Fisher PR (2009) Legionella pneumophila multiplication is enhanced by chronic AMPK signalling in mitochondrially diseased Dictyostelium cells. Dis Model Mech 2(9–10):479–489. doi:10.1242/dmm.003319
Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14(5):661–673. doi:10.1016/j.devcel.2008.02.004
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62(2):334–361
Ghosh D, Walton JL, Roepe PD, Sinai AP (2012) Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol 14(4):589–607. doi:10.1111/j.1462-5822.2011.01745.x
Gjini E, Haydon DT, Barry JD, Cobbold CA (2010) Critical interplay between parasite differentiation, host immunity, and antigenic variation in trypanosome infections. Am Nat 176(4):424–439. doi:10.1086/656276
Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Freche B (2008) Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci 65(3):493–507. doi:10.1007/s00018-007-7434-y
Gordon EB, Hart GT, Tran TM, Waisberg M, Akkaya M, Skinner J, Zinocker S, Pena M, Yazew T, Qi CF, Miller LH, Pierce SK (2015) Inhibiting the Mammalian target of rapamycin blocks the development of experimental cerebral malaria. MBio 6(3):e00725. doi:10.1128/mBio.00725-15
Guo Y, Zhang Y, Hong K, Luo F, Gu Q, Lu N, Bai A (2014) AMPK inhibition blocks ROS-NFkappaB signaling and attenuates endotoxemia-induced liver injury. PLoS One 9(1):e86881. doi:10.1371/journal.pone.0086881
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8(10):774–785. doi:10.1038/nrm2249
Hardie DG, Scott JW, Pan DA, Hudson ER (2003) Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546(1):113–120
Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG (2003) Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2(4):28. doi:10.1186/1475-4924-2-28
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2(1):9–19. doi:10.1016/j.cmet.2005.05.009
Hedbacker K, Carlson M (2008) SNF1/AMPK pathways in yeast. Front Biosci 13:2408–2420
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66(2):300–372
Holmes BF, Kurth-Kraczek EJ, Winder WW (1999) Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 87(5):1990–1995
Hood MI, Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10(8):525–537. doi:10.1038/nrmicro2836
Hoogendijk AJ, Pinhancos SS, van der Poll T, Wieland CW (2013) AMP-activated protein kinase activation by 5-aminoimidazole-4-carbox-amide-1-beta-D-ribofuranoside (AICAR) reduces lipoteichoic acid-induced lung inflammation. J Biol Chem 288(10):7047–7052. doi:10.1074/jbc.M112.413138
Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH (2006) Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem 281(9):5335–5340. doi:10.1074/jbc.M506850200
Horwitz MA (1983) The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med 158(6):2108–2126
Hu G, Cheng PY, Sham A, Perfect JR, Kronstad JW (2008) Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol Microbiol 69(6):1456–1475. doi:10.1111/j.1365-2958.2008.06374.x
Huang H, Kang R, Wang J, Luo G, Yang W, Zhao Z (2013) Hepatitis C virus inhibits AKT-tuberous sclerosis complex (TSC), the mechanistic target of rapamycin (MTOR) pathway, through endoplasmic reticulum stress to induce autophagy. Autophagy 9(2):175–195. doi:10.4161/auto.22791
Hull CM, Heitman J (2002) Genetics of Cryptococcus neoformans. Annu Rev Genet 36:557–615. doi:10.1146/annurev.genet.36.052402.152652
Hurtrel B, Petit F, Arnoult D, Muller-Trutwin M, Silvestri G, Estaquier J (2005) Apoptosis in SIV infection. Cell Death Differ 12(Suppl 1):979–990. doi:10.1038/sj.cdd.4401600
Hutterer C, Wandinger SK, Wagner S, Muller R, Stamminger T, Zeittrager I, Godl K, Baumgartner R, Strobl S, Marschall M (2013) Profiling of the kinome of cytomegalovirus-infected cells reveals the functional importance of host kinases Aurora A, ABL and AMPK. Antiviral Res 99(2):139–148. doi:10.1016/j.antiviral.2013.04.017
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115(5):577–590
Jeong KM, Choi JI, Lee SH, Lee HJ, Son JK, Seo CS, Song SW, Kwak SH, Bae HB (2014) Effect of sauchinone, a lignan from Saururus chinensis, on bacterial phagocytosis by macrophages. Eur J Pharmacol 728:176–182. doi:10.1016/j.ejphar.2014.01.039
Ji WT, Lee LH, Lin FL, Wang L, Liu HJ (2009) AMP-activated protein kinase facilitates avian reovirus to induce mitogen-activated protein kinase (MAPK) p38 and MAPK kinase 3/6 signalling that is beneficial for virus replication. J Gen Virol 90(Pt 12):3002–3009. doi:10.1099/vir.0.013953-0
Kang KY, Kim YK, Yi H, Kim J, Jung HR, Kim IJ, Cho JH, Park SH, Kim HY, Ju JH (2013) Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int Immunopharmacol 16(1):85–92. doi:10.1016/j.intimp.2013.03.020
Kelly B, O’Neill LA (2015) Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 25(7):771–784. doi:10.1038/cr.2015.68
Kim YW, Lee SM, Shin SM, Hwang SJ, Brooks JS, Kang HE, Lee MG, Kim SC, Kim SG (2009) Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radic Biol Med 47(7):1082–1092. doi:10.1016/j.freeradbiomed.2009.07.018
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141. doi:10.1038/ncb2152
Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM, Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, Shin SJ, Yoo H, Park YK, Park JB, Chung J, Yoshimori T, Jo EK (2012) Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 11(5):457–468. doi:10.1016/j.chom.2012.03.008
Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, Cheon HG (2014) Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem 289(33):23246–23255. doi:10.1074/jbc.M114.577908
Kloft N, Neukirch C, Bobkiewicz W, Veerachato G, Busch T, von Hoven G, Boller K, Husmann M (2010) Pro-autophagic signal induction by bacterial pore-forming toxins. Med Microbiol Immunol 199(4):299–309. doi:10.1007/s00430-010-0163-0
Kuchin S, Vyas VK, Carlson M (2002) Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol Cell Biol 22(12):3994–4000
Kudchodkar SB, Del Prete GQ, Maguire TG, Alwine JC (2007) AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J Virol 81(7):3649–3651. doi:10.1128/JVI.02079-06
Kumar SH, Rangarajan A (2009) Simian virus 40 small T antigen activates AMPK and triggers autophagy to protect cancer cells from nutrient deprivation. J Virol 83(17):8565–8574. doi:10.1128/JVI.00603-09
Kumar D, Nath L, Kamal MA, Varshney A, Jain A, Singh S, Rao KV (2010) Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell 140(5):731–743. doi:10.1016/j.cell.2010.02.012
Kwon HS, Brent MM, Getachew R, Jayakumar P, Chen LF, Schnolzer M, McBurney MW, Marmorstein R, Greene WC, Ott M (2008) Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe 3(3):158–167. doi:10.1016/j.chom.2008.02.002
Landfear SM (2011) Nutrient transport and pathogenesis in selected parasitic protozoa. Eukaryot Cell 10(4):483–493. doi:10.1128/EC.00287-10
Lee W, VanderVen BC, Fahey RJ, Russell DG (2013) Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J Biol Chem 288(10):6788–6800. doi:10.1074/jbc.M112.445056
Levin DE (2005) Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 69(2):262–291. doi:10.1128/MMBR.69.2.262-291.2005
Levine B, Mizushima N, Virgin HW (2011) Autophagy in immunity and inflammation. Nature 469(7330):323–335. doi:10.1038/nature09782
Li JV, Wang Y, Saric J, Nicholson JK, Dirnhofer S, Singer BH, Tanner M, Wittlin S, Holmes E, Utzinger J (2008) Global metabolic responses of NMRI mice to an experimental Plasmodium berghei infection. J Proteome Res 7(9):3948–3956. doi:10.1021/pr800209d
Lorenz MC, Fink GR (2001) The glyoxylate cycle is required for fungal virulence. Nature 412(6842):83–86. doi:10.1038/35083594
Lv G, Zhu H, Zhou F, Lin Z, Lin G, Li C (2014) AMP-activated protein kinase activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Biochem Biophys Res Commun 453(1):13–18. doi:10.1016/j.bbrc.2014.09.028
MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, Jones RG (2011) The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol 187(8):4187–4198. doi:10.4049/jimmunol.1100367
MacRae JI, Sheiner L, Nahid A, Tonkin C, Striepen B, McConville MJ (2012) Mitochondrial metabolism of glucose and glutamine is required for intracellular growth of Toxoplasma gondii. Cell Host Microbe 12(5):682–692. doi:10.1016/j.chom.2012.09.013
Mankouri J, Tedbury PR, Gretton S, Hughes ME, Griffin SD, Dallas ML, Green KA, Hardie DG, Peers C, Harris M (2010) Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc Natl Acad Sci U S A 107(25):11549–11554. doi:10.1073/pnas.0912426107
Marsin AS, Bouzin C, Bertrand L, Hue L (2002) The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem 277(34):30778–30783. doi:10.1074/jbc.M205213200
McArdle J, Schafer XL, Munger J (2011) Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny. J Virol 85(2):705–714. doi:10.1128/JVI.01557-10
McArdle J, Moorman NJ, Munger J (2012) HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication. PLoS Pathog 8(1):e1002502. doi:10.1371/journal.ppat.1002502
McKinney JD, Honer zu Bentrup K, Munoz-Elias EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR Jr, Russell DG (2000) Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406(6797):735–738. doi:10.1038/35021074
Meares GP, Qin H, Liu Y, Holdbrooks AT, Benveniste EN (2013) AMP-activated protein kinase restricts IFN-gamma signaling. J Immunol 190(1):372–380. doi:10.4049/jimmunol.1202390
Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, Nagarkatti P, Nagarkatti M, Chauhan A (2010) Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS One 5(6):e11160. doi:10.1371/journal.pone.0011160
Miranda-Saavedra D, Stark MJ, Packer JC, Vivares CP, Doerig C, Barton GJ (2007) The complement of protein kinases of the microsporidium Encephalitozoon cuniculi in relation to those of Saccharomyces cerevisiae and Schizosaccharomyces pombe. BMC Genomics 8:309. doi:10.1186/1471-2164-8-309
Mitchell TG, Perfect JR (1995) Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev 8(4):515–548
Monceaux V, Estaquier J, Fevrier M, Cumont MC, Riviere Y, Aubertin AM, Ameisen JC, Hurtrel B (2003) Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression towards AIDS. AIDS 17(11):1585–1596. doi:10.1097/01.aids.0000076286.54156.d6
Mony BM, Matthews KR (2015) Assembling the components of the quorum sensing pathway in African trypanosomes. Mol Microbiol 96(2):220–232. doi:10.1111/mmi.12949
Mony BM, MacGregor P, Ivens A, Rojas F, Cowton A, Young J, Horn D, Matthews K (2014) Genome-wide dissection of the quorum sensing signalling pathway in Trypanosoma brucei. Nature 505(7485):681–685. doi:10.1038/nature12864
Moreau K, Lacas-Gervais S, Fujita N, Sebbane F, Yoshimori T, Simonet M, Lafont F (2010) Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cell Microbiol 12(8):1108–1123. doi:10.1111/j.1462-5822.2010.01456.x
Moreira D, Rodrigues V, Abengozar M, Rivas L, Rial E, Laforge M, Li X, Foretz M, Viollet B, Estaquier J, Cordeiro da Silva A, Silvestre R (2015a) Leishmania infantum modulates host macrophage mitochondrial metabolism by hijacking the SIRT1-AMPK axis. PLoS Pathog 11(3):e1004684. doi:10.1371/journal.ppat.1004684
Moreira D, Silvestre R, Cordeiro-da-Silva A, Estaquier J, Foretz M, Viollet B (2015b) AMP-activated protein kinase as a target for pathogens: friends or foes? Curr Drug Targets. doi:10.1007/978-3-319-43589-3 [pii]
Moseley CE, Webster RG, Aldridge JR (2010) Peroxisome proliferator-activated receptor and AMP-activated protein kinase agonists protect against lethal influenza virus challenge in mice. Influenza Other Respi Viruses 4(5):307–311. doi:10.1111/j.1750-2659.2010.00155.x
Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S (2010) A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog 6(6):e1000954. doi:10.1371/journal.ppat.1000954
Moser TS, Schieffer D, Cherry S (2012) AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis. PLoS Pathog 8(4):e1002661. doi:10.1371/journal.ppat.1002661
Munoz-Elias EJ, McKinney JD (2005) Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med 11(6):638–644. doi:10.1038/nm1252 [pii]
Naderer T, Heng J, Saunders EC, Kloehn J, Rupasinghe TW, Brown TJ, McConville MJ (2015) Intracellular survival of leishmania major depends on uptake and degradation of extracellular matrix glycosaminoglycans by macrophages. PLoS Pathog 11(9):e1005136. doi:10.1371/journal.ppat.1005136
Nagajyothi F, Desruisseaux MS, Thiruvur N, Weiss LM, Braunstein VL, Albanese C, Teixeira MM, de Almeida CJ, Lisanti MP, Scherer PE, Tanowitz HB (2008) Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity (Silver Spring) 16(9):1992–1997
Nakashima K, Takeuchi K, Chihara K, Hotta H, Sada K (2011) Inhibition of hepatitis C virus replication through adenosine monophosphate-activated protein kinase-dependent and -independent pathways. Microbiol Immunol 55(11):774–782. doi:10.1111/j.1348-0421.2011.00382.x
Navarro-Garcia F, Sanchez M, Nombela C, Pla J (2001) Virulence genes in the pathogenic yeast Candida albicans. FEMS Microbiol Rev 25(2):245–268
Ng HC, Singh M, Jeyaseelan K (1995) Identification of two protein serine/threonine kinase genes and molecular cloning of a SNF1 type protein kinase gene from Toxoplasma gondii. Biochem Mol Biol Int 35(1):155–165
Ng HC, Singh M, Jeyaseelan K (1997) Nucleotide sequence of ToxPK1 gene from Toxoplasma gondii. DNA Seq 7(3-4):179–191
Nieves W, Oniskey T, Hung L, Herbert D (2014) Metabolic regulation of type 2 immunity controls tissue repair. In: 63rd annual ASTMH meeting, New Orleans, USA, 2–6 November 2014
Ong CW, Elkington PT, Brilha S, Ugarte-Gil C, Tome-Esteban MT, Tezera LB, Pabisiak PJ, Moores RC, Sathyamoorthy T, Patel V, Gilman RH, Porter JC, Friedland JS (2015) Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog 11(5):e1004917. doi:10.1371/journal.ppat.1004917
Pagans S, Pedal A, North BJ, Kaehlcke K, Marshall BL, Dorr A, Hetzer-Egger C, Henklein P, Frye R, McBurney MW, Hruby H, Jung M, Verdin E, Ott M (2005) SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol 3(2):e41. doi:10.1371/journal.pbio.0030041
Pandey AK, Sassetti CM (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A 105(11):4376–4380. doi:10.1073/pnas.0711159105
Park CY, Jun HJ, Wakita T, Cheong JH, Hwang SB (2009) Hepatitis C virus nonstructural 4B protein modulates sterol regulatory element-binding protein signaling via the AKT pathway. J Biol Chem 284(14):9237–9246. doi:10.1074/jbc.M808773200
Park DW, Jiang S, Tadie JM, Stigler WS, Gao Y, Deshane J, Abraham E, Zmijewski JW (2013) Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol Med 19:387–398. doi:10.2119/molmed.2013.00065
Parsons M, Worthey EA, Ward PN, Mottram JC (2005) Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics 6:127. doi:10.1186/1471-2164-6-127
Petter R, Kwon-Chung KJ (1996) Disruption of the SNF1 gene abolishes trehalose utilization in the pathogenic yeast Candida glabrata. Infect Immun 64(12):5269–5273
Petter R, Chang YC, Kwon-Chung KJ (1997) A gene homologous to Saccharomyces cerevisiae SNF1 appears to be essential for the viability of Candida albicans. Infect Immun 65(12):4909–4917
Plummer R, Lorigan P, Steven N, Scott L, Middleton MR, Wilson RH, Mulligan E, Curtin N, Wang D, Dewji R, Abbattista A, Gallo J, Calvert H (2013) A phase II study of the potent PARP inhibitor, Rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother Pharmacol 71(5):1191–1199. doi:10.1007/s00280-013-2113-1
Polge C, Thomas M (2007) SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci 12(1):20–28. doi:10.1016/j.tplants.2006.11.005
Pollitt LC, Mideo N, Drew DR, Schneider P, Colegrave N, Reece SE (2011) Competition and the evolution of reproductive restraint in malaria parasites. Am Nat 177(3):358–367. doi:10.1086/658175
Rabhi I, Rabhi S, Ben-Othman R, Rasche A, Daskalaki A, Trentin B, Piquemal D, Regnault B, Descoteaux A, Guizani-Tabbane L, Sysco C (2012) Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view. PLoS Negl Trop Dis 6(8):e1763. doi:10.1371/journal.pntd.0001763
Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ (2008) Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14(4):421–428. doi:10.1038/nm1743
Ramsook CB, Tan C, Garcia MC, Fung R, Soybelman G, Henry R, Litewka A, O’Meally S, Otoo HN, Khalaf RA, Dranginis AM, Gaur NK, Klotz SA, Rauceo JM, Jue CK, Lipke PN (2010) Yeast cell adhesion molecules have functional amyloid-forming sequences. Eukaryot Cell 9(3):393–404. doi:10.1128/EC.00068-09
Reece SE, Pollitt LC, Colegrave N, Gardner A (2011) The meaning of death: evolution and ecology of apoptosis in protozoan parasites. PLoS Pathog 7(12):e1002320. doi:10.1371/journal.ppat.1002320
Rey-Jurado E, Riedel CA, Gonzalez PA, Bueno SM, Kalergis AM (2015) Contribution of autophagy to antiviral immunity. FEBS Lett 589(22):3461–3470. doi:10.1016/j.febslet.2015.07.047
Rhee KY, de Carvalho LP, Bryk R, Ehrt S, Marrero J, Park SW, Schnappinger D, Venugopal A, Nathan C (2011) Central carbon metabolism in Mycobacterium tuberculosis: an unexpected frontier. Trends Microbiol 19(7):307–314. doi:10.1016/j.tim.2011.03.008
Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA (2013) AMPKalpha1: a glucose sensor that controls CD8 T-cell memory. Eur J Immunol 43(4):889–896. doi:10.1002/eji.201243008
Romero-Gomez M, Diago M, Andrade RJ, Calleja JL, Salmeron J, Fernandez-Rodriguez CM, Sola R, Garcia-Samaniego J, Herrerias JM, De la Mata M, Moreno-Otero R, Nunez O, Olveira A, Duran S, Planas R, Spanish Treatment of Resistance to Insulin in Hepatitis C Genotype 1 Group (2009) Treatment of insulin resistance with metformin in naive genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology 50(6):1702–1708. doi:10.1002/hep.23206
Rousset F, Roze D (2007) Constraints on the origin and maintenance of genetic kin recognition. Evolution 61(10):2320–2330. doi:10.1111/j.1558-5646.2007.00191.x
Sag D, Carling D, Stout RD, Suttles J (2008) Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 181(12):8633–8641. doi:10.4049/jimmunol.181.12.8633 [pii]
Sales-Dias J (2011) The role of glucose in Plasmodium liver infection. Biochemistry, University of Lisbon, Lisbon
Sampaio-Marques B, Burhans WC, Ludovico P (2014) Longevity pathways and maintenance of the proteome: the role of autophagy and mitophagy during yeast ageing. Microb Cell 1(4):118–127
Santarelli R, Granato M, Faggioni A, Cirone M (2015) Interference with the autophagic process as a viral strategy to escape from the immune control: lesson from gamma herpesviruses. J Immunol Res 2015:546063. doi:10.1155/2015/546063
Sanz P (2003) Snf1 protein kinase: a key player in the response to cellular stress in yeast. Biochem Soc Trans 31(Pt 1):178–181. doi:10.1042/bst0310178
Schubert-Unkmeir A, Sokolova O, Panzner U, Eigenthaler M, Frosch M (2007) Gene expression pattern in human brain endothelial cells in response to Neisseria meningitidis. Infect Immun 75(2):899–914. doi:10.1128/IAI.01508-06
Seo JY, Cresswell P (2013) Viperin regulates cellular lipid metabolism during human cytomegalovirus infection. PLoS Pathog 9(8):e1003497. doi:10.1371/journal.ppat.1003497
Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC (2004) The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6(1):91–99. doi:10.1016/j.ccr.2004.06.007
Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK (2010) Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol 12(11):1648–1665. doi:10.1111/j.1462-5822.2010.01497.x
Singhal A, Jie L, Kumar P, Hong GS, Leow MK, Paleja B, Tsenova L, Kurepina N, Chen J, Zolezzi F, Kreiswirth B, Poidinger M, Chee C, Kaplan G, Wang YT, De Libero G (2014) Metformin as adjunct antituberculosis therapy. Sci Transl Med 6(263):263ra159. doi:10.1126/scitranslmed.3009885
Sorbara MT, Girardin SE (2015) Emerging themes in bacterial autophagy. Curr Opin Microbiol 23:163–170. doi:10.1016/j.mib.2014.11.020
Steele S, Brunton J, Ziehr B, Taft-Benz S, Moorman N, Kawula T (2013) Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLoS Pathog 9(8):e1003562. doi:10.1371/journal.ppat.1003562
Stein SC, Woods A, Jones NA, Davison MD, Carling D (2000) The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345(Pt 3):437–443
Street A, Macdonald A, Crowder K, Harris M (2004) The Hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem 279(13):12232–12241. doi:10.1074/jbc.M312245200
Tang Y, Li F, Tan B, Liu G, Kong X, Hardwidge PR, Yin Y (2014) Enterotoxigenic Escherichia coli infection induces intestinal epithelial cell autophagy. Vet Microbiol 171(1-2):160–164. doi:10.1016/j.vetmic.2014.03.025
Tattoli I, Sorbara MT, Vuckovic D, Ling A, Soares F, Carneiro LA, Yang C, Emili A, Philpott DJ, Girardin SE (2012) Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11(6):563–575. doi:10.1016/j.chom.2012.04.012
Terry LJ, Vastag L, Rabinowitz JD, Shenk T (2012) Human kinome profiling identifies a requirement for AMP-activated protein kinase during human cytomegalovirus infection. Proc Natl Acad Sci U S A 109(8):3071–3076. doi:10.1073/pnas.1200494109
Tewari R, Straschil U, Bateman A, Bohme U, Cherevach I, Gong P, Pain A, Billker O (2010) The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8(4):377–387. doi:10.1016/j.chom.2010.09.006
Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR (2001) How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J Cell Sci 114(Pt 24):4637–4650
Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC (2011) Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol 162(7):1498–1508. doi:10.1111/j.1476-5381.2010.01126.x
van Zandbergen G, Luder CG, Heussler V, Duszenko M (2010) Programmed cell death in unicellular parasites: a prerequisite for sustained infection? Trends Parasitol 26(10):477–483. doi:10.1016/j.pt.2010.06.008
Vassella E, Reuner B, Yutzy B, Boshart M (1997) Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci 110(Pt 21):2661–2671
Vickerman K (1985) Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull 41(2):105–114
Vincent O, Carlson M (1999) Gal83 mediates the interaction of the Snf1 kinase complex with the transcription activator Sip4. EMBO J 18(23):6672–6681. doi:10.1093/emboj/18.23.6672
Wang Y, Utzinger J, Saric J, Li JV, Burckhardt J, Dirnhofer S, Nicholson JK, Singer BH, Brun R, Holmes E (2008) Global metabolic responses of mice to Trypanosoma brucei brucei infection. Proc Natl Acad Sci U S A 105(16):6127–6132. doi:10.1073/pnas.0801777105
Waterman SR, Hacham M, Panepinto J, Hu G, Shin S, Williamson PR (2007) Cell wall targeting of laccase of Cryptococcus neoformans during infection of mice. Infect Immun 75(2):714–722. doi:10.1128/IAI.01351-06
Winder WW, Hardie DG (1996) Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol 270(2 Pt 1):E299–E304
Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13(22):2004–2008
Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2(1):21–33. doi:10.1016/j.cmet.2005.06.005
Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ (2011) Structure of mammalian AMPK and its regulation by ADP. Nature 472(7342):230–233. doi:10.1038/nature09932
Yang K, Chi H (2015) AMPK helps T cells survive nutrient starvation. Immunity 42(1):4–6. doi:10.1016/j.immuni.2014.12.029
Yang J, Li D, Liu X, Pan J, Yan B, Zhu X (2010a) Regulation of virulence factors, carbon utilization and virulence by SNF1 in Cryptococcus neoformans JEC21 and divergent actions of SNF1 between cryptococcal strains. Fungal Genet Biol 47(12):994–1000. doi:10.1016/j.fgb.2010.08.002
Yang Z, Kahn BB, Shi H, Xue BZ (2010b) Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem 285(25):19051–19059. doi:10.1074/jbc.M110.123620
Yang J, Li D, Pan J, Zhu X (2011) Snf1/AMPK protein kinase modulates cell wall integrity in the human pathogenic yeast Cryptococcus neoformans. Wei Sheng Wu Xue Bao 51(6):740–746
Yang D, Xue B, Wang X, Yu X, Liu N, Gao Y, Liu C, Zhu H (2013) 2-octynoic acid inhibits hepatitis C virus infection through activation of AMP-activated protein kinase. PLoS One 8(5):e64932. doi:10.1371/journal.pone.0064932
Yang CS, Kim JJ, Lee HM, Jin HS, Lee SH, Park JH, Kim SJ, Kim JM, Han YM, Lee MS, Kweon GR, Shong M, Jo EK (2014) The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy 10(5):785–802. doi:10.4161/auto.28072
Yu JW, Sun LJ, Liu W, Zhao YH, Kang P, Yan BZ (2013) Hepatitis C virus core protein induces hepatic metabolism disorders through down-regulation of the SIRT1-AMPK signaling pathway. Int J Infect Dis 17(7):e539–e545. doi:10.1016/j.ijid.2013.01.027
Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK (2009) Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6(3):231–243. doi:10.1016/j.chom.2009.08.004
Zangger H, Mottram JC, Fasel N (2002) Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ 9(10):1126–1139. doi:10.1038/sj.cdd.4401071
Zhang HS, Wu MR (2009) SIRT1 regulates Tat-induced HIV-1 transactivation through activating AMP-activated protein kinase. Virus Res 146(1–2):51–57. doi:10.1016/j.virusres.2009.08.005
Zhang HS, Ruan Z, Sang WW (2011) HDAC1/NFkappaB pathway is involved in curcumin inhibiting of Tat-mediated long terminal repeat transactivation. J Cell Physiol 226(12):3385–3391. doi:10.1002/jcp.22691
Zhang Z, Wang S, Huang J, Liu L, Lu M, Li M, Sui Y, Xu L, Yan R, Song X, Li X (2015) Proteomic analysis of Eimeria acervulina sporozoite proteins interaction with duodenal epithelial cells by shotgun LC-MS/MS. Mol Biochem Parasitol 202(2):29–33. doi:10.1016/j.molbiopara.2015.09.006
Zhao Z, Hu J, Gao X, Liang H, Liu Z (2014) Activation of AMPK attenuates lipopolysaccharide-impaired integrity and function of blood-brain barrier in human brain microvascular endothelial cells. Exp Mol Pathol 97(3):386–392. doi:10.1016/j.yexmp.2014.09.006
Zhao H, Zhu H, Lin Z, Lin G, Lv G (2015) Compound 13, an alpha1-selective small molecule activator of AMPK, inhibits Helicobacter pylori-induced oxidative stresses and gastric epithelial cell apoptosis. Biochem Biophys Res Commun 463(4):510–517. doi:10.1016/j.bbrc.2015.05.059
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108(8):1167–1174. doi:10.1172/JCI13505
Funding Statement
This work was supported by grants to JE from the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) and from The Canadian HIV Cure Enterprise Team Grant HIG-13305 from the Canadian Institutes of Health Research (CIHR) in partnership with CANFAR and IAS. JE acknowledges the support of the Canada Research Chair program. It was also supported by FCT—Fundação para a Ciência e a Tecnologia/MEC—Ministério da Educação e Ciência através de fundos nacionais e quando aplicável cofinanciado pelo FEDER, no âmbito do Acordo de Parceria PT2020 referente à unidade de investigação n° 4293. DM is supported by SFRH/BD/91543/2012. RS is supported by the Fundação para a Ciência e Tecnologia (FCT) (IF/00021/2014).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mesquita, I. et al. (2016). AMPK in Pathogens. In: Cordero, M., Viollet, B. (eds) AMP-activated Protein Kinase. Experientia Supplementum, vol 107. Springer, Cham. https://doi.org/10.1007/978-3-319-43589-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-43589-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43587-9
Online ISBN: 978-3-319-43589-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)