Abstract
This review examines the function of the corpus luteum (CL) with emphasis on pregnancy in ruminant models and the possible impact of pregnancy in conferring luteal resistance to prostaglandin F2α (PGF2α). Critical processes involved with formation of the CL impact the capacity to secrete progesterone. Similarly, complete luteolysis is critically important in the event that pregnancy does not occur so that a new ovulation and opportunity for pregnancy is established. It is well known that serum progesterone must reach a critical nadir if ovulation and fertilization are to occur. Following fertilization, the function of the CL in providing adequate progesterone is critical in setting up an endometrial environment so that pregnancy is maintained. Benefits of supplemental progesterone during early pregnancy are inconsistent in ruminants. However, recent studies indicate that supplemental progesterone following artificial insemination (AI) may depend on the presence of the CL and the amount of progesterone released from the CL. The primary signal for maternal recognition of pregnancy, interferon-tau (IFNT), is secreted from the ruminant conceptus (embryo proper and extraembryonic membranes). IFNT disrupts release of PGF2α from the endometrium and is antiluteolytic through inhibiting uterine expression of the estradiol receptor (ESR1) or the oxytocin receptor (OXTR). Endocrine action of IFNT on peripheral blood mononuclear cells and on the CL may also contribute to immunomodulatory function and longer-term sustainability and function of the CL as pregnancy progresses.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Interferon
- Corpus luteum
- Pregnancy
- Ruminants
- Interferon-stimulated genes
- Peripheral blood mononuclear cells
- Leukocytes
11.1 Exposure to Progesterone Changes Gene Expression in the Uterus
Continuous exposure of the uterus to progesterone for 8–10 days during the estrous cycle causes downregulation of the progesterone receptor (PR) in the endometrium, allowing estradiol to bind to the estrogen receptor (ESR1), and resulting in the synthesis and insertion of the oxytocin receptor (OXTR) in the endometrium [1, 2]. Oxytocin (OXT) binds to its endometrial receptor, activating the synthesis and pulsatile release of PGF2α into the uterine ovarian vein [3, 4]. PGF2α crosses over into the ovarian artery from the ovarian vein via a countercurrent exchange mechanism [5, 6]. This unique mechanism allows PGF2α to be delivered directly to the CL without first passing through the systemic circulation. The local effects of PGF2α result in the demise of the CL, leading to a new estrous cycle if the mother/dam is not pregnant.
In ruminants, the utero-ovarian plexus provides an intimate association of the uterine vein and ovarian artery, which allows delivery of small molecules such as PGF2α from the uterus to the ovary to cause luteolysis [7–9]. Wiltbank and Casida [10] reported that hysterectomy, which disrupts the utero-ovarian plexus, delayed estrus in ewes for more than 100 days. The original CL at the time of hysterectomy was maintained until the time of necropsy, and the ovary had minimal follicular activity. If total hysterectomy was performed on days 13.5–15, then the CL had a lifespan of approximately 148 days, which was similar to the length of gestation [7, 11]. Other studies demonstrated that lifespan of the CL varies when one uterine horn is removed and is dependent on proximity of the removed horn to the CL [12]. Removal of the uterine horn that is ipsilateral to the CL-containing ovary extends luteal lifespan. However, if the uterine horn contralateral to the CL is removed, then the CL regresses in 15–17 days, which is similar to the normal cycle. These data are interpreted to mean that luteolysis requires an intimate connection between the CL and the nonpregnant ipsilateral uterine horn.
11.2 Downregulation of Progesterone Receptor Leads to Upregulation of Prostaglandin F2α (PGF2α) Pulses and Luteolysis in Sheep
Progesterone regulates expression of many receptors involved in coordinating the estrous cycle and secretion of prostaglandins. Progesterone prevents expression of the ESR1 and the oxytocin receptor (OXTR) in the endometrium, which generally precedes PGF2α release. Withdrawal of progesterone or interruption of PR activity results in an increase in the expression of these receptors [2]. Continuous exposure to progesterone causes a downregulation of the endometrial PR by day 11 of the estrous cycle [13]. Downregulation of endometrial PR is followed by an increase in ESR1 and OXTR expression and PGF2α release on days 13, 14, and 14–16, respectively [2, 13–16]. The onset of OXT and PGF2α pulses is concomitant with an increase in endometrial OXTR, suggesting that PGF2α synthesis and release can be driven by OXT signaling [16, 17]; this allows increased pulse amplitude and frequency of PGF2α secretion, which initiates luteal regression.
11.2.1 Nature of the Luteolysin and Luteolysis in Ruminants
PGF2α has been implicated as the initiator of luteolysis in many species including the ovine [6, 9, 18]. PGF2α-induced luteolysis appears to have three mechanisms of action: (1) auto-induction of PGF2α synthesis by the CL; (2) reduction of steroidogenesis; and (3) reduction of blood flow to the CL. In 1970, McCracken indicated that PGF2α was the luteolytic agent and would cause luteolysis when delivered to the CL [9]. Functional regression of the CL is strongly associated with a decrease in progesterone production [19]. Structural changes occur in the CL after the initial drop in progesterone concentrations. Binding of uterine-derived PGF2α to the CL induces several downstream effects in both large luteal cells (LLC) and small luteal cells (SLC). In LLCs, PGF2α interfaces with its receptor to induce a suicidal loop of production of PGF2α from the CL by upregulating the PTGS2 (prostaglandin synthase-2) pathway [20, 21]. PGF2α action also entails increased intake of calcium in LLC, which induces apoptosis, activates protein kinase C (PKC) and associated cellular responses, inhibits progesterone synthesis, and induces OXT release [20, 22]. In 1986, Moor demonstrated that uterine PGF2α release in ewes increases before OXT and oxytocin-associated neurophysin, indicating that PGF2α initiates release of OXT [4]. The OXT produced from LLC binds to the OXTR on the SLC, causing the release of calcium and activation of the PKC pathway, both of which lead to cell death via apoptosis [20, 23]. For a detailed description of gene expression during luteolysis in ruminants, see [24, 25].
11.3 Rescue of the CL During Pregnancy in Ruminants
Moor and Rowson [26], as well as Mapletoft and coworkers [27], used ovine embryo transfer experiments coupled with ligation of uterine horns to clarify the role of the conceptus in protecting the CL during establishment of early pregnancy. It was inferred from these studies that no systemic, conceptus-derived mediator rescued the CL, because ligation of the gravid horn protected the CL ipsilateral to the conceptus while the contralateral CL regressed. However, a systemic role of the conceptus in resistance for the CL to PGF2α and longer-term survival of the CL during early pregnancy cannot be excluded. For example, several investigators have described the CL of pregnancy to be more resistant to lytic effects of PGF2α [28–31]. Exactly why and how this resistance to PGF2α occurs in the CL during pregnancy is unknown.
There is no direct luteotropic counterpart to human chorionic gonadotropin in ruminants [32]. Rather, conceptus-derived IFNT regulates the antiluteolytic alteration of PGF2α release from the endometrium during pregnancy [33, 34]. IFNT may also have direct actions on the CL in context of conferring resistance to PGF2α [35, 36]. The latter possibility seems likely because the release of PGF2α from the endometrium is not ablated by pregnancy and the CL is capable of local production of PGF2α [37]. Actually, PGF2α is found in greater concentrations in uterine venous drainage from day 13 pregnant compared with estrous cycling ewes [38]. Also, the basal production of PGF2α is higher in pregnant compared to nonpregnant ewes, possibly because of the continued expression of PTGS2 in the uterine luminal and glandular epithelium and the production of prostaglandins by the conceptus [39].
11.4 Contribution of Conceptus to Lifespan of the CL
The conceptus must be present from day 12 through day 17 in the ewe for a successful pregnancy to be recognized and maintained [12, 40–42]. In support of this concept, infusion of IFNT into the uterine vein for only 3 days starting on day 12 of the estrous cycle was not sufficient to cause a delay in return to estrus (Antoniazzi and Hansen, unpublished data). IFNT was initially named protein X after its discovery on day 13 of pregnancy as the major conceptus secretory protein [33] and renamed as trophoblast protein-1 before being classified as an IFN and named IFNT [34, 43–45]. IFNT is the maternal recognition signal in ruminants that indicates the presence of a viable embryo(s), resulting in the differential expression of endometrial proteins [46–50], prevention of luteolysis, and continued production of progesterone [32, 36, 39]. IFNT can be detected in media from cultured ovine conceptuses by day 10 and increases in secretion through day 16 of pregnancy [51]. IFNT accumulates in uterine flushings to detectable concentrations between days 13 and 14 of pregnancy [52]. IFNT silences ESR1 transcription and consequently inhibits the production and insertion of OXTR into the endometrium, thus disrupting the pulsatile release of PGF2α [53]. The paracrine actions of IFNT alter PGF2α pulsatility in the ewe [54] and may actually reduce PGF2α concentrations in the cow [55].
Maternal recognition of pregnancy is clearly a paracrine mechanism, but it could also be sustained through endocrine induction of luteal resistance to prostaglandin through interferon-stimulated genes (ISGs) [25, 35, 36, 52, 56–58]. Even though the mechanism for maternal recognition of pregnancy varies among mammals, the upregulation of interferon-stimulated gene 15 (ISG15) in the endometrium appears to be a universal response to the presence of an embryo, as has been seen in humans and baboons [59, 60], cows [46, 61], sheep [62], swine [63] and mice [64–66]. IFNT stimulates production of ISGs in the glandular epithelium [48, 61, 62]. Several of these ISGs have been identified, such as ISG15 [46, 62, 67], myxovirus (influenza virus) resistance (MX1) [68], and 2′,5′-oligoadenylate synthetase (OAS) [48, 69, 70].
IFNT elicits its actions through the type 1 interferon receptor, which shares two subunits, IFNAR1 and IFNAR2. These subunits are expressed in the luminal epithelium, subglandular epithelium, and stroma of the ovine uterus during the estrous cycle and pregnancy in day 14–15 ewes [71]. In ovine endometrial cells, IFNT causes tyrosine phosphorylation and nuclear translocation of signal transduction and activator of transcription (STAT) -1, -2, -3, -5, and -6 as well as increased transcription of STAT1 and STAT2 [72, 73]. Interferon-stimulated gene factor 3 (ISGF3) and STAT1 form homodimers and bind to IFNT-stimulated response elements (ISRE) and gamma-activated sequences (GAS) to drive the expression of ISGs. In response to pregnancy (IFNT), mRNAs encoding these signal transducers and stimulated ISGs increase in concentration primarily in endometrial stroma and glandular epithelium in the ewe [74]. However, the ISGs are not strongly inducible in luminal epithelium and subluminal glandular epithelial cells because of expression of interferon regulatory factor 2 (IRF2) in these cells, which strongly inhibits IFN stimulatory response elements in ISGs. The consequences of a lack of induction of ISGs in ovine luminal epithelium have not been completely resolved in the context of regulation of release of PGF2α and antiluteolytic mechanisms of IFNT.
Several ISGs in addition to STATs and IRFs have been identified in the ruminant uterus. ISG15 can be found in its free 15-kDa form and conjugated to target proteins in the uteri of pregnant cows on days 17–45 [61]. ISG15 protein was localized to the glandular epithelium with light staining in the luminal epithelium and stroma during the timeframe of peak IFNT expression around day 18 of pregnancy [61]. IRF2 is a repressor of ISGs and is present in the luminal epithelium and subglandular epithelium, thus restricting the ability of IFNT to increase ISGs in the luminal epithelium, but not in the stroma and glandular epithelium regions of the ovine uterus [74].
ISGs such as STATs and IRFs and RNA helicases are upregulated in the endometrium and peripheral blood mononuclear cells (PBMC) [35] as well as in the CL [25, 52, 57, 58] of pregnant compared to nonpregnant ewes. IFNT and possibly ISGs enter the blood stream and condition T cells and macrophages, potentially activating a first line of defense against viruses to prevent early embryonic mortality or persistent viral infection of the embryo [35].
11.5 Progesterone and Early Conceptus Survival
The zona-enclosed embryo is thought not to be dependent on oviductal or uterine secretions. However, following hatching, the developing and elongating conceptus starts to release signals and the endometrium responds to these signals to provide a more complex and nourishing histotroph when compared to the progesterone-primed uterus of the estrous cycle. The mechanisms through which progesterone prepares the uterus for pregnancy and is associated with fertility have been extensively examined. The uterine epithelia secrete or selectively transport molecules into the uterine lumen that are collectively known as a histotroph. The uterine histotroph nourishes the free-floating conceptus and contains amino acids and glucose, cytokines, enzymes, growth factors, lymphokines, transport proteins for vitamins and minerals, and extracellular matrix molecules. Progesterone activates genes contributing to production of the histotroph, which supports the early pregnancy. However, crucial conceptus-derived signals such as IFNT work in concert with progesterone to fully engage a nurturing environment during elongation of the conceptus, formation of the placenta, and attachment/implantation to the luminal epithelium of the endometrium.
The benefits of exogenous supplementation of progesterone to pregnancy rates have been considered for decades (reviewed in [75–77]. Briefly, the actions of progesterone depend on the amount that is circulating, which also is regulated by the amount synthesized and metabolized. It is very clear that serum progesterone concentrations need to be low at the time of AI. Even very low (>0.9 ng/ml) serum concentrations of progesterone at the time of AI will significantly reduce fertility. Carvalho and coworkers [78] recently described an advantage to pregnancy rate by adding a second PGF2α treatment to ensure luteolysis just before induced ovulation using the Ovsynch gonadotropin-releasing hormone (GnRH) protocol (day −10, GnRH; day −3, PGF2α; day −0.7, GnRH; day 0, timed AI) in dairy cows. Following AI, supplementation of high serum progesterone concentrations has varied effects on fertility. When used with the Ovsynch protocol, supplementation of dairy cows with progesterone using CIDR (controlled internal drug release ) implants improved pregnancy rates in the cases in which no CL was present at the time of PGF2α injection [79]. There was no benefit of supplementation with progesterone when a functional CL was present at the time of PGF2α. However, if the CL was absent or producing subluteal-phase concentrations of serum progesterone at the first GnRH injection of the Ovsynch protocol [79, 80], then there was a benefit to supplementing with progesterone to improve fertility in dairy cows. The positive impact of serum progesterone supplementation during the estrous cycle depends on when during the luteal phase this is applied and may be most effective when concentrations of progesterone are impaired at the time of initiation of Ovsynch treatments . During pregnancy, there may be a greater advantage to supplementing serum progesterone during the late luteal phase when the conceptus is elongating and producing IFNT. For example, increasing serum progesterone concentrations during this period using human chorionic gonadotropin (hCG) [81] has a tendency to increase IFNT production from cultured day 18 bovine conceptuses. This finding was consistent with an earlier study describing a positive effect of hCG treatment on production of IFNT from cultured ovine embryos [82] but no effect of supplemental serum progesterone on the production of IFNT. Similarly, even though there was a positive effect of hCG treatment on day 5 of the estrous cycle in context of increased serum progesterone, treatment at that time before embryo transfer on day 7 had no impact on IFNT production by conceptuses flushed on day 14 and cultured for 24 h [83]. Although progesterone is critical for the establishment of pregnancy, the evidence for a benefit of supplementation of serum progesterone during early pregnancy is conflicting, and current interpretation suggests a moderate positive response in context of supporting development of more advanced conceptuses, which are typically correlated with increased production of IFNT.
In addition to progesterone, other intrauterine factors such as prostaglandins and cortisol influence conceptus responses to the endometrium. For example, PTGS2, the rate-limiting enzyme in endometrial prostaglandin synthesis, is upregulated in the bovine endometrium in response to pregnancy and IFNT [84, 85]. Thus, IFNT may stimulate greater endometrial PGE2 compared to PGF2α production, possibly providing local support for the CL during early pregnancy. A role for prostaglandins in elongation of the conceptus has been suggested in context of a local paracrine role during early pregnancy [86]. A direct role of PGE2 on the CL has not yet been described during early pregnancy in ruminants.
11.6 Development of Resistance of the CL to PGF2α During Pregnancy
In 1988, Zarco demonstrated that PGF2α is secreted in nonpregnant ewes in a pulsatile manner, whereas in pregnant ewes, PGF2α is released in a more constant pattern that steadily increases [87, 88], indicating that the pulsatile release of PGF2α, which is diminished in the pregnant ewe, may be required for luteolysis. Prostaglandin pulses associated with luteolysis of cyclic ewes occur every 7–8 h [89]. McCracken reported that uterine arterial infusion of OXT into ewes on day 16 of pregnancy did not elicit the same production of PGF2α that occurred during the estrous cycle in ewes at 16 days post estrus [90], and that this may be caused by the lower concentration of endometrial OXTR in pregnant versus cyclic ewes. The enzyme responsible for metabolizing PGF2α, prostaglandin dehydrogenase (PGDH), is elevated in the CL of pregnant ewes when compared to day 13 of the cyclic ewes, thus indicating higher metabolism of PGF2α [31, 37]. Furthermore, the dose of exogenous PGF2α necessary to cause luteal regression in pregnant ewes is elevated, especially surrounding the time of maternal recognition of pregnancy, compared to nonpregnant ewes [91].
The CL of pregnant ruminants is resistant to the luteolytic effects of PGF2α [31]. However, the biochemical mechanisms involved in luteal resistance to PGF2α are not well described. The lytic effects of intrafollicular injection of 200 μg PGF2α were tested in ewes on day 12 of pregnancy or the estrous cycle. In nonmated ewes and ewes without embryos present, serum progesterone declined, and 79–89 % of ewes returned to estrus within 2.4–2.9 days after PGF2α treatment. In contrast, 63 % of pregnant ewes with normal-sized embryos did not return to estrus after PGF2α treatment and also evidenced a decline in serum progesterone that then apparently rebounded after 24 h. It was concluded from these studies that an antiluteolytic factor from the conceptus might overcome the lytic action of PGF2α [28]. When examining the response to dose of PGF2α treatment, Silvia and Niswender [91] reported that CL from nonpregnant ewes responded (luteolysis) to lower doses of PGF2α on day 12 post estrus compared to CL from pregnant ewes.
It was concluded that the CL of pregnancy was more resistant to the luteolytic effect of PGF2α. When ewes were treated with PGF2α on days 13 or 16, serum progesterone concentrations declined regardless of pregnancy status. However, serum progesterone rebounded to levels before treatment with PGF2α in only day 13 and day 16 pregnant ewes [91]. This rebound in serum progesterone concentrations did not occur when the same experiment was repeated on days 16 and 26 of pregnancy. This result was interpreted to mean that the resistance of the CL to luteolytic effects of PGF2α may develop between day 10 and 13 of pregnancy and is lost between day 16 and 26 of pregnancy.
One obvious target for disruption of PGF2α-induced luteolysis is through its receptor. However, the numbers of receptors for PGF2α do not appear to change in response to pregnancy status on days 10 and 13 [92]. By day 15, there were actually increased concentrations of PGF2α receptors on the CL in response to pregnancy when compared to the estrous cycle. An increase in PGF2α-receptor mRNA concentrations also was observed in CL by day 16 of pregnancy when compared to the estrous cycle [93]; which was consistent with former PGF2α receptor-binding studies. Numbers of PGE2 receptors did not change in the CL in this study. These findings were interpreted as evidence that the CL of pregnant ewes is not protected from the actions of PGF2α at the time of maternal recognition of pregnancy by a reduction in numbers or affinity of PGF2α receptors but through another mechanism.
Alterations in expression of PGF2α-degrading and PGF2α-synthesizing enzymes have also been evaluated in the endometrium and CL. The manner in which PGF2α is secreted is dependent upon an animal’s physiological status. Pregnant and nonpregnant ewes have very different patterns of PGF2α release [89, 94]. Although peak production of PGF2α occurs at day 14–15, regardless of pregnancy status, ewes secrete PGF2α in a pulsatile fashion during the estrous cycle, whereas pregnant ewes have a more constant, slowly increasing pattern in the release of PGF2α based on the presence of PGF2α metabolites [87, 88]. More PGF2α is found exiting the uterus through the uterine vein in day 13 pregnant versus nonpregnant ewes [38]. The change in peaks of PGF2α during pregnancy may be associated with increases in metabolism by PGDH within the uterus and CL. Also, the presence of PGF2α in the uterine vein during pregnancy may necessitate a self-preservation mechanism for the CL to make PGDH. For example, PGDH mRNA is elevated in the CL on day 4 of the estrous cycle and day 13 of pregnancy when compared to day 13 of the estrous cycle in ewes [37]. Enzyme assays to determine PGDH activity revealed that production of PGFM was greater on day 4 in CL during the estrous cycle and on day 13 in CL from pregnant ewes compared to CL on day 13 of the estrous cycle. PTGS2 mRNA concentrations in the CL did not differ between pregnant and nonpregnant ewes on day 12 or 13 post estrus [37, 95]. Silva and colleagues [37] reported a significant increase in PGDH mRNA and enzyme activity in day 13 pregnant ewes compared to day 13 non-pregnant ewes, and Costine et al. [95] did not detect these differences on day 12. Similarly, Romero et al. [25, 95] saw no difference in PGDH mRNA concentrations using microarray approaches in CL on days 12 and 14 of pregnancy and the estrous cycle. Aside from a change in synthesis or degradation of PGF2α or numbers or sensitization of available receptors on luteal cells, there are other potential mechanisms by which the CL may become resistant to PGF2α during maternal recognition of pregnancy. Redundant or complementary mechanisms may be in place to maintain the CL during pregnancy. PGE1 [96] or PGE2 [97], in addition to other conceptus-derived factors such as IFNT [25, 35, 52, 56, 57], may act on the CL to confer increased resistance of the CL to PGF2α as pregnancy progresses.
11.7 Endocrine Release of IFNT into the Uterine Vein
When ovine [98] and bovine [99, 100] ISG15 mRNA concentrations were first described to be upregulated in PBMC in response to early pregnancy, it was hypothesized that IFNT or an IFNT-induced cytokine was released into the uterine vein and had endocrine action during early pregnancy. Data to further support the concept for an endocrine role of pregnancy was provided in cattle through examining genes expressed in PBMC compared to endometrium on day 18 of pregnancy in dairy cows [35]. In this study, several hundred endometrial (674 genes upregulated and 721 downregulated ~1.5 fold; P < 0.05) and PBMC (375 genes upregulated and 784 downregulated ~1.2 fold; P < 0.05) genes were differentially expressed based on pregnancy status on day 18 of pregnancy. Many of the genes upregulated in response to pregnancy in PBMC were the same as those upregulated in endometrium and were ISGs. Pregnancy also induced 55 genes in CL on day 12 and 734 genes on day 14. ISGs (i.e., ISG15 and MX1) represented many of the genes induced by pregnancy that also were induced when culturing luteal cells with IFNT, but not PGE2 [25].
Other groups also have described a pregnancy-associated increase in ISGs in peripheral tissues. For example, Green et al. [101] reported a significant increase in ISG mRNA concentrations in leukocytes in response to pregnancy on day 18 in dairy heifers, although this was not observed in lactating dairy cows. In contrast, on day 19 of pregnancy, lactating dairy cows supplemented with progesterone had no change in leukocyte ISG mRNA concentrations; however, pregnancy caused an upregulation in concentrations of ISG mRNA [102]. A more extensive analysis of ISGs in leukocytes was completed using beef cows, which demonstrated an increase in mRNA concentrations between days 15 and 22 with a peak concentration by day 20 of pregnancy [103]. Use of leukocyte ISG mRNA concentrations to predict pregnancy status was more accurate when coupled with ultrasound examination for the presence of a CL on day 20 of pregnancy. Also, upregulation of ISGs in the liver by day 18 of pregnancy was recently demonstrated in cattle [104], which is consistent with previous reports of upregulation of liver ISGs in response to pregnancy in sheep [52, 57]. Based on data from our laboratory in addition to these newer studies, one might make the inference that the detection of ISGs in blood is a reasonable indicator of pregnancy status in ruminants. However, there is a very large false-positive rate because of strong induction of innate immune responses, including upregulation of ISGs in response to infections and proinflammatory stressors in dairy cows (see [35, 105]).
To further delineate the mechanism by which ISGs were upregulated in extrauterine tissues, antiviral activity was evaluated in uterine vein blood from day 15 pregnant sheep. Significant amounts of IFNT (~200 μg/24 h) were released into the uterine vein on day 15 of pregnancy [58]. These results were actually similar to a previous paper that also described significant antiviral activity in uterine vein blood of pregnant sheep [106]. Preadsorption of uterine vein blood from day 15 pregnant ewes with a highly specific monoclonal antibody against recombinant ovine (ro) IFNT (provided by Dr. Fuller Bazer, Texas A&M University) significantly reduces antiviral activity [57]. By using a very specific and sensitive radioimmunoassay, IFNT has been detected in uterine flushings and uterine vein blood from pregnant sheep but was not detected in uterine flushings or uterine vein blood from nonpregnant sheep [52]. Similarly, by using mass spectroscopy, we have resolved IFNT in uterine vein serum from day 16 pregnant sheep based on detection of eight peptides ranging in size from 1147.75 to 2490.72 Da with a probability of amino acid match from 91 to 97 % (four peptides with >95 % match). Based on these studies, we suspect that IFNT is responsible for the bulk of the antiviral activity and is released in amounts large enough to elicit a peripheral endocrine response.
11.8 Infusion of IFNT into the Uterine Vein Provides Luteal Resistance to PGF2α
ISG mRNAs increased in the CL following intrauterine infusions or subcutaneous treatment with roIFNT [107]. In this study, intrauterine infusion of IFNT caused a delay of return to estrus whereas subcutaneous delivery did not, suggesting a primary paracrine role for IFNT on the endometrium. Endocrine actions induced by IFNT were further studied [57] using mini-osmotic pump infusion of roIFNT for 24 h or 7 days into the uterine vein. Utilizing the average weight of 60 kg and blood volume of 58 ml/kg for ewes, the blood volume was estimated to be 3.48 l. Oliveira and colleagues previously estimated that the release of IFNT into the uterine vein on day 15 is approximately 200 μg/day [58]. Based on these data, osmotic pumps were loaded to deliver 200 μg/day, which results in a release of 8.3 μg/h into the uterine vein. Employing the estimated blood volume as calculated here, systemic levels of IFNT in circulation would stabilize around 2.4 ng/ml/h. The calculated systemic level of IFNT in circulation is biologically relevant in the context of a dissociation constant (K d) for the receptor of 3.7 × 10−10 M [108] and estimated 50 % occupancy of the receptor at 6.3 ng IFNT/ml, although, based on the concept of spare receptors, only 1 % of IFNT receptors need to be occupied to elicit a biological response, which would reflect physiological levels of IFNT in the blood as low as 63 pg IFNT/ml. To achieve endocrine delivery of 200 μg/day of recombinant interferon tau (roIFNT ), osmotic pumps were surgically installed into the abdominal cavity to infuse either BSA or roIFNT via a catheter into the uterine vein on day 10 of the estrous cycle in sheep.
Seven days of infusion of roINFT into the uterine vein from day 10 to day 17 of the estrous cycle resulted in 80 % (four of five ewes) of ewes having extended estrous cycles lasting through day 32, whereas all ewes infused with BSA returned to estrus by day 19 [57]. The single ewe that did not respond to this delivery of IFNT through delay of return to estrus had low serum progesterone concentrations at the time of pump installation that continued to decline over time, meaning that this ewe presented a “short cycle” and had already started the luteolytic process at the start of delivery of IFNT into the uterine vein. This experiment was the first demonstrating that endocrine delivery of low concentrations of IFNT systemically can induce a significant (long-term) delay in returning to a normal estrous cycle. Subsequent studies demonstrated that IFNT action through the induction of ISG15 consistently occurred in CL ipsilateral and contralateral to the side of infusion of IFNT into the uterine vein [56]. This result was interpreted to mean that IFNT probably did not cross over from the uterine vein to ovarian artery to act on the CL. Rather, the action of IFNT on the CL is thought to be a systemic rather than a local utero-ovarian plexus transport. Also, serum progesterone concentrations did not differ in roIFNT - versus BSA-infused ewes, which was consistent with reports by others showing that IFNT does not have a luteotropic role in the context of increasing steroidogenesis and production of progesterone [54, 109, 110].
To further study the mechanisms associated with endocrine action of IFNT, a series of experiments with delivery of IFNT into the uterine vein and jugular vein, as well as subcutaneous delivery of IFNT, were completed in the presence or absence of an exogenous challenge with PGF2α [56, 57]. It was reasoned that if IFNT had a direct action on the CL, then this would be reflected in blocking the effect of exogenous challenge with PGF2α in causing a decline in serum progesterone . Delivery of 20–200 μg/day roIFNT starting on day 10 of the estrous cycle into the uterine vein, jugular vein, or through a subcutaneous route in the neck effectively blocked the decline in serum progesterone caused by PGF2α injection on day 10.5 or 11 (depending on the study) in the BSA-infused controls [56, 57].
To examine mechanisms of IFNT action on the CL, CLs were collected at various times following infusion with IFNT and treatment with PGF2α. Following a 24-h infusion of IFNT, there was an induction of ISG15 mRNA (as well as other ISGs) in endometrium and ipsilateral and contralateral CL relative to the side of the osmotic pump as well as the liver. These ISGs may contribue to development of resistance of the CL to luteolysis,
Because daily temporal responses to pregnancy (IFNT) had not been described in sheep in context of IFNT concentrations and then regulation of genes in endometrium and CL, we recently completed a study to see if ISGs induced by pregnancy [52] were similar to those induced by mini-osmotic pump infusion of roIFNT [56, 57]. By using an IFNT radioimmunoassay, IFNT was detected in uterine flushings by day 14 and in uterine vein serum by days 15–16 of pregnancy. ISG mRNA concentrations were detected in endometrium by day 13 of pregnancy, which was one day prior to upregulation of ESR1 and OXTR mRNA concentrations on day 14 of the estrous cycle. ISG mRNA concentrations in the CL and liver also were detected by day 14 and in peripheral blood mononuclear cells by day 15 in pregnant ewes. Concentrations of mRNAs for ISGs such as STAT1, STAT2, IRF7, IRF9 [52], and melanoma differentiation factor 5 (MDA5; DDX58), retinoic acid-inducible gene (RIGI/IFIHI), and ISG15 were greater in the CL on day 14 of pregnancy compared to the estrous cycle (Fig. 11.1). It was concluded that ISGs induced by pregnancy were similar to those induced by miniosmotic pump infusion of roIFNT.
Upregulation of ISGs mRNA concentration in corpora lutea as pregnancy progresses in sheep. Different superscripts represent differences (P < 0.05) across days of pregnancy. *Values differ because of pregnancy status on any given day. Pr pregnant, NP nonpregnant (not exposed to semen). Data reprinted with permission from [52]
To further examine the response of the CL to pregnancy, microarray studies were completed that demonstrated that pregnancy on day 14 was associated with differential expression of 734 genes, many of which were ISGs induced when culturing luteal cells with IFNT but not PGE2 [25]. In the CL of ewes, interleukin 6, luteinizing hormone/chorionic gonadotropin hormone receptor, pentraxin-related protein 3, and vascular endothelial growth factor gene expression was stabilized during early pregnancy, but diminished as the estrous cycle progressed and in response to culture of luteal cells with luteolytic hormones [25]. Culture of ovine LLC, SLC, and mixed luteal cells (MLC) with recombinant ovine (ov) IFNT [56] and bovine LLC and SLC with bovine (bo) IFNT (Fig. 11.2) resulted in increases in ISG15 mRNA (concentration-dependent) and protein (time-dependent) concentrations.
ISG15 mRNA concentrations in ovine small (SLC), large (LLC), and mixed (MLC) luteal cells cultured with varying concentrations of rovIFNT (left panel) and ISG15 protein induced in bovine SLC and LLC following culture with 10 ng/ml rboIFNT over time (right panel). The left panel represents quantitative real-time PCR. (Reprinted with permission from [56]). The right panel represents data from cultured bovine luteal cells prepared by J.S. Davis (University of Nebraska Medical Center). Bovine luteal cells were purified by centrifugal elutriation [111]. After overnight attachment in media containing 10 % fetal bovine serum, the cells were pre-equilibrated in serum-free media for 2 h and treated with IFNT (10 ng/ml) for up to 24 h. Western blot analysis was performed using 5F10 mouse monoclonal antibody against rbISG15 [61]. ISG15 becomes covalently bound to targeted proteins, which are represented by conjugated ISG15 [112]. Means with different superscripts differ (P < 0.05)
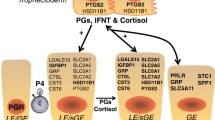
Based on these results, in addition to paracrine action on the endometrium to disrupt pulsatile release of PGF2α, pregnancy may also circumvent luteolytic responses through activation or stabilization of gene expression associated with interferon, chemokine, cell adhesion, cytoskeletal, and angiogenic pathways in the CL (Fig. 11.3).
Yang and coworkers [113] described upregulation of ISG15 in the bovine CL by day 16 of pregnancy, which continued to be upregulated through day 60 of pregnancy, which is consistent with other reports in cattle [114] and the induction of ISG15 in CL by pregnancy in the sheep [25, 52, 58]. However, attempts to induce ISG15 using bovine CL from day 15 of the estrous cycle through culture with rboIFNT did not succeed; whereas culture of ovarian stroma, endometrium and mammary cells all had greater ISG15 protein concentrations when cultured with rboIFNT. The lack of a bovine luteal cell response to culture with IFNT is in contrast to studies using day 10–12 ovine CL, where culture with IFNT strongly upregulated ISGs. For this reason, we used the same rboIFNT (provided by Dr. R.M. Roberts, University of Missouri) and antibody against boISG15 (5F10 [61]) and describe herein that 24 h culture of bovine luteal cells with rboIFNT does indeed cause an upregulation in ISG15 free and conjugated proteins in both SLC and LLC . The only difference in this study compared to the Yang study was use of a ten-fold-lower concentration of rboIFNT and a different source of bovine corpora lutea.
11.9 Conclusions
IFNT is released from the conceptus trophectoderm cells in significant amounts about the time that the blastocyst starts to expand. IFNT binds type 1 IFN receptors and stimulates STATs and IRFs that upregulate transcription of specific set of genes (i.e., the ISGs) or downregulate genes encoding stimulators of synthesis and release of PGF2α through binding to IFN stimulatory response elements. IFNT inhibits the upregulation of endometrial ESR1, and this is thought to occur through inhibitory action of induced and phosphorylated transcription factors directly on the promoter of this gene in sheep. In cattle, the action of IFNT might reside on the OXTR gene [115]. The bovine OXTR gene promoter region was cloned and found to contain an ISRE, ESR1 response element half-sites, and SP1 sites, and could only be transactivated by estrogen if cells were cotransfected with ESR1 and steroid receptor coactivator 1 [116]. Curiously, IRF2 overexpression, which is typically inhibitory in other systems, increased activity of the bovine OXTR promoter, but a direct effect of IFNT on promoter activity was not reported.
Regardless, in both sheep and cattle, the synthesis and pulsatile release of PGF2α is disrupted through paracrine action of IFNT on the endometrium, and this is considered to be an early maternal response to pregnancy and primary antiluteolytic mechanism that protects the CL in ruminants. As the conceptus develops and elongates, IFNT concentrations accumulate in the uterine lumen to the point where IFNT passes through the basement membrane of the endometrium and enters the endometrial venous drainage. Also, there may be variation in the expression of junctional complex proteins that allow the endometrial cells to become more or less leaky to movement of IFNT into the uterine venous blood [117]. IFNT responses, such as induction of ISG15 mRNA in the endometrium (paracrine action), can be detected as early as day 13 of pregnancy. This endometrial response to IFNT is followed 1–2 days later in extrauterine tissues (endocrine action) on days 14–15. The direct action of IFNT on the CL may confer resistance of the CL to the luteolytic pulses of PGF2α (see Antoniazzi et al. [34]). In addition, IFNT may control antiapoptotic mechanisms and cell survival genes to ensure luteal cell differentiation that prolongs luteal lifespan. Other studies remain to be done to determine where the lytic pathway downstream from binding of PGF2α to its receptor may be blocked during early pregnancy to confer resistance to luteolysis. Luteal resistance during early pregnancy may occur in response to ISGs as proximal to the PGF2α receptor as disruption of G-protein interaction to induce phospholipase C and consequent activation of protein kinase C; or as distal as possibly stabilizing cell survival gene expression and proteins to allow for recovery of the CL to any insults by PGF2α so that production of progesterone continues during early pregnancy. Whether IFNT acts alone or in concert with other CSP to directly protect the CL remains to be determined. Ultimately, longer-term luteal survival and resistance to lytic effects of PGF2α may be driven by pregnancy-induced ISGs, cell survival genes, and antiluteolytic mechanisms in the ruminant CL.
References
Spencer TE, Burghardt RC, Johnson GA, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Anim Reprod Sci. 2004;82-83:537–50.
Leavitt WW, Okulicz WC, McCracken JA, Schramm W, Robidoux Jr WF. Rapid recovery of nuclear estrogen receptor and oxytocin receptor in the ovine uterus following progesterone withdrawal. J Steroid Biochem. 1985;22(6):687–91.
Allison Gray C, Bartol FF, Taylor KM, Wiley AA, Ramsey WS, Ott TL, Bazer FW, Spencer TE. Ovine uterine gland knock-out model: effects of gland ablation on the estrous cycle. Biol Reprod. 2000;62(2):448–56.
Moore LG, Choy VJ, Elliot RL, Watkins WB. Evidence for the pulsatile release of PGF-2 alpha inducing the release of ovarian oxytocin during luteolysis in the ewe. J Reprod Fertil. 1986;76(1):159–66.
Banu SK, Arosh JA, Chapdelaine P, Fortier MA. Expression of prostaglandin transporter in the bovine uterus and fetal membranes during pregnancy. Biol Reprod. 2005;73(2):230–6.
McCracken JA, Carlson JC, Glew ME, Goding JR, Baird DT, Green K, Samuelsson B. Prostaglandin F2 identified as a luteolytic hormone in sheep. Nat New Biol. 1972;238(83): 129–34.
Inskeep EK, Butcher RL. Local component of utero-ovarian relationships in the ewe. J Anim Sci. 1966;25(4):1164–8.
Goding JR, Harrison FA, Heap RB, Linzell JL. Ovarian activity in the ewe after autotransplantation of the ovary or uterus to the neck. J Physiol. 1967;191(2):129P–30.
McCracken JA, Glew ME, Scaramuzzi RJ. Corpus luteum regression induced by prostaglandin F2-alpha. J Clin Endocrinol Metab. 1970;30(4):544–6.
Wiltbank J, Casida L. Alteration of ovarian activity by hysterectomy. J Anim Sci. 1956;15:134.
Senger PL. Pathways to pregnancy and parturition, 3rd edn. Pullman: Current Conceptions; 2012. 381 p.
Moor RM, Rowson LE. Local uterine mechanisms affecting luteal function in the sheep. J Reprod Fertil. 1966;11(2):307–10.
Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod. 1995;53(6):1527–43.
Barcikowski B, Carlson J, Wilson L, McCracken JA. The effect of endogenous and exogenous estradiol-17beta on the release of prostaglandin F2alpha from the ovine uterus. J Endocrinol. 1974;95(5):1340–9.
Hixon J, Flint A. Effects of a luteolytic dose of oestradiol benzoate on uterine oxytocin receptor concentrations, phosphoinositide turnover and prostaglandin F-2 alpha secretion in sheep. J Reprod Fertil. 1987;79:457–67.
Wathes D, Lamming G. The oxytocin receptor, luteolysis and the maintenance of pregnancy. J Reprod Fertil. 1995;49:53–67.
McCracken J, Schramm W, Baricikowski B, Wilson LJ. The identification of prostaglandin F2 alpha as a uterine luteolytic hormone and the hormonal control of its synthesis. Acta Vet Scand. 1981;(suppl 77):71–88.
Hansel W. Luteotrophic and luteolytic mechanisms in bovine corpora lutea. J Reprod Fertil Suppl. 1966;1:33–48.
Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17(3):221–44.
Niswender GD, Davis TL, Griffith RJ, Bogan RL, Monser K, Bott RC, Bruemmer JE, Nett TM. Judge, jury and executioner: the auto-regulation of luteal function. Soc Reprod Fertil Suppl. 2007;64:191–206.
Wiltbank MC, Ottobre JS. Regulation of intraluteal production of prostaglandins. Reprod Biol Endocrinol. 2003;1:91.
Wiltbank MC, Guthrie PB, Mattson MP, Kater SB, Niswender GD. Hormonal regulation of free intracellular calcium concentrations in small and large ovine luteal cells. Biol Reprod. 1989;41(4):771–8.
Vinatier D, Dufour P, Subtil D. Apoptosis: a programmed cell death involved in ovarian and uterine physiology. Eur J Obstet Gynecol Reprod Biol. 1996;67(2):85–102.
Mondal M, Schilling B, Folger J, Steibel JP, Buchnick H, Zalman Y, Ireland JJ, Meidan R, Smith GW. Deciphering the luteal transcriptome: potential mechanisms mediating stage-specific luteolytic response of the corpus luteum to prostaglandin F(2)alpha. Physiol Genomics. 2011;43(8):447–56.
Romero JJ, Antoniazzi AQ, Smirnova NP, Webb BT, Yu F, Davis JS, Hansen TR. Pregnancy-associated genes contribute to antiluteolytic mechanisms in ovine corpus luteum. Physiol Genomics. 2013;45(22):1095–108.
Moor RM, Rowson LE. The corpus luteum of the sheep: functional relationship between the embryo and the corpus luteum. J Endocrinol. 1966;34(2):233–9.
Mapletoft RJ, Lapin DR, Ginther OJ. The ovarian artery as the final component of the local luteotropic pathway between a gravid uterine horn and ovary in ewes. Biol Reprod. 1976;15(3):414–21.
Inskeep EK, Smutny WJ, Butcher RL, Pexton JE. Effects of intrafollicular injections of prostaglandins in non-pregnant and pregnant ewes. J Anim Sci. 1975;41(4):1098–104.
Mapletoft RJ, Del Campo MR, Ginther OJ. Local venoarterial pathway for uterine-induced luteolysis in cows. Proc Soc Exp Biol Med. 1976;153(2):289–94.
Pratt BR, Butcher RL, Inskeep EK. Antiluteolytic effect of the conceptus and of PGE2 in ewes. J Anim Sci. 1977;45(4):784–91.
Silvia WJ, Niswender GD. Maintenance of the corpus luteum of early pregnancy in the ewe. III. Differences between pregnant and nonpregnant ewes in luteal responsiveness to prostaglandin F2 alpha. J Anim Sci. 1984;59(3):746–53.
Roberts RM, Xie S, Mathialagan N. Maternal recognition of pregnancy. Biol Reprod. 1996;54(2):294–302.
Godkin JD, Bazer FW, Moffatt J, Sessions F, Roberts RM. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J Reprod Fertil. 1982;65(1):141–50.
Imakawa K, Anthony RV, Kazemi M, Marotti KR, Polites HG, Roberts RM. Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature (Lond). 1987;330(6146):377–9.
Hansen TR, Henkes LK, Ashley RL, Bott RC, Antoniazzi AQ, Han H. Endocrine actions of interferon-tau in ruminants. Soc Reprod Fertil Suppl. 2010;67:325–40.
Spencer TE, Hansen TR. Implantation and establishment of pregnancy in ruminants. Adv Anat Embryol Cell Biol. 2015;216:105–35.
Silva PJ, Juengel JL, Rollyson MK, Niswender GD. Prostaglandin metabolism in the ovine corpus luteum: catabolism of prostaglandin F(2alpha) (PGF(2alpha)) coincides with resistance of the corpus luteum to PGF(2alpha). Biol Reprod. 2000;63(5):1229–36.
Wilson Jr L, Butcher RL, Inskeep EK. Prostaglandin F2alpha in the uterus of ewes during early pregnancy. Prostaglandins. 1972;1(6):479–82.
Bazer FW, Ying W, Wang X, Dunlap KA, Zhou B, Johnson GA, Wu G. The many faces of interferon tau. Amino Acids. 2015;47(3):449–60.
Hansen PJ, Anthony RV, Bazer FW, Baumbach GA, Roberts RM. In vitro synthesis and secretion of ovine trophoblast protein-1 during the period of maternal recognition of pregnancy. Endocrinology. 1985;117(4):1424–30.
Moor RM, Rowson LE. Influence of the embryo and uterus on luteal function in the sheep. Nature (Lond). 1964;201:522–3.
Moor RM, Rowson LE. The corpus luteum of the sheep: effect of the removal of embryos on luteal function. J Endocrinol. 1966;34(4):497–502.
Bazer FW, Roberts RM. Biochemical aspects of conceptus–endometrial interactions. J Exp Zool. 1983;228(2):373–83.
Bazer FW, Thatcher WW, Hansen PJ, Mirando MA, Ott TL, Plante C. Physiological mechanisms of pregnancy recognition in ruminants. J Reprod Fertil Suppl. 1991;43:39–47.
Roberts RM, Cross JC, Leaman DW. Interferons as hormones of pregnancy. Endocr Rev. 1992;13(3):432–52.
Austin KJ, Ward SK, Teixeira MG, Dean VC, Moore DW, Hansen TR. Ubiquitin cross-reactive protein is released by the bovine uterus in response to interferon during early pregnancy. Biol Reprod. 1996;54(3):600–6.
Johnson GA, Austin KJ, Van Kirk EA, Hansen TR. Pregnancy and interferon-tau induce conjugation of bovine ubiquitin cross-reactive protein to cytosolic uterine proteins. Biol Reprod. 1998;58(4):898–904.
Johnson GA, Stewart MD, Gray CA, Choi Y, Burghardt RC, Yu-Lee LY, Bazer FW, Spencer TE. Effects of the estrous cycle, pregnancy, and interferon tau on 2′,5′-oligoadenylate synthetase expression in the ovine uterus. Biol Reprod. 2001;64(5):1392–9.
Staggs KL, Austin KJ, Johnson GA, Teixeira MG, Talbott CT, Dooley VA, Hansen TR. Complex induction of bovine uterine proteins by interferon-tau. Biol Reprod. 1998;59(2):293–7.
Teixeira MG, Austin KJ, Perry DJ, Dooley VD, Johnson GA, Francis BR, Hansen TR. Bovine granulocyte chemotactic protein-2 is secreted by the endometrium in response to interferon-tau (IFN-tau). Endocrine. 1997;6(1):31–7.
Ashworth CJ, Bazer FW. Changes in ovine conceptus and endometrial function following asynchronous embryo transfer or administration of progesterone. Biol Reprod. 1989;40(2):425–33.
Romero JJ, Antoniazzi AQ, Nett TM, Ashley RL, Webb BT, Smirnova NP, Bott RC, Bruemmer JE, Bazer FW, Anthony RV, Hansen TR. Temporal release, paracrine and endocrine actions of ovine conceptus-derived interferon-tau during early pregnancy. Biol Reprod. 2015;93(6):146.
Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW. Ovine interferon-tau inhibits estrogen receptor up-regulation and estrogen-induced luteolysis in cyclic ewes. Endocrinology. 1995;136(11):4932–44.
Ott TL, Fleming JG, Spencer TE, Joyce MM, Chen P, Green CN, Zhu D, Welsh Jr TH, Harms PG, Bazer FW. Effects of exogenous recombinant ovine interferon tau on circulating concentrations of progesterone, cortisol, luteinizing hormone, and antiviral activity; interestrous interval; rectal temperature; and uterine response to oxytocin in cyclic ewes. Biol Reprod. 1997;57(3):621–9.
Thatcher WW, Bartol FF, Knickerbocker JJ, Curl JS, Wolfenson D, Bazer FW, Roberts RM. Maternal recognition of pregnancy in cattle. J Dairy Sci. 1984;67(11):2797–811.
Antoniazzi AQ, Webb BT, Romero JJ, Ashley RL, Smirnova NP, Henkes LE, Bott RC, Oliveira JF, Niswender GD, Bazer FW, Hansen TR. Endocrine delivery of interferon tau protects the corpus luteum from prostaglandin F2 alpha-induced luteolysis in ewes. Biol Reprod. 2013;88(6):144.
Bott RC, Ashley RL, Henkes LE, Antoniazzi AQ, Bruemmer JE, Niswender GD, Bazer FW, Spencer TE, Smirnova NP, Anthony RV, Hansen TR. Uterine vein infusion of interferon tau (IFNT) extends luteal life span in ewes. Biol Reprod. 2010;82(4):725–35.
Oliveira JF, Henkes LE, Ashley RL, Purcell SH, Smirnova NP, Veeramachaneni DN, Anthony RV, Hansen TR. Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-tau release from the uterine vein. Endocrinology. 2008;149(3):1252–9.
Bebington C, Bell SC, Doherty FJ, Fazleabas AT, Fleming SD. Localization of ubiquitin and ubiquitin cross-reactive protein in human and baboon endometrium and decidua during the menstrual cycle and early pregnancy. Biol Reprod. 1999;60(4):920–8.
Bebington C, Doherty FJ, Fleming SD. Ubiquitin cross-reactive protein gene expression is increased in decidualized endometrial stromal cells at the initiation of pregnancy. Mol Hum Reprod. 1999;5(10):966–72.
Austin KJ, Carr AL, Pru JK, Hearne CE, George EL, Belden EL, Hansen TR. Localization of ISG15 and conjugated proteins in bovine endometrium using immunohistochemistry and electron microscopy. Endocrinology. 2004;145(2):967–75.
Johnson GA, Spencer TE, Hansen TR, Austin KJ, Burghardt RC, Bazer FW. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol Reprod. 1999;61(1):312–8.
Johnson GA, Bazer FW, Burghardt RC, Spencer TE, Wu G, Bayless KJ. Conceptus–uterus interactions in pigs: endometrial gene expression in response to estrogens and interferons from conceptuses. Soc Reprod Fertil Suppl. 2009;66:321–32.
Ashley RL, Henkes LE, Bouma GJ, Pru JK, Hansen TR. Deletion of the Isg15 gene results in up-regulation of decidual cell survival genes and down-regulation of adhesion genes: implication for regulation by IL-1beta. Endocrinology. 2010;151(9):4527–36.
Austin KJ, Bany BM, Belden EL, Rempel LA, Cross JC, Hansen TR. Interferon-stimulated gene-15 (Isg15) expression is up-regulated in the mouse uterus in response to the implanting conceptus. Endocrinology. 2003;144(7):3107–13.
Henkes LE, Pru JK, Ashley RL, Anthony RV, Veeramachaneni DN, Gates KC, Hansen TR. Embryo mortality in Isg15−/− mice is exacerbated by environmental stress. Biol Reprod. 2015;92(2):36.
Naivar KA, Ward SK, Austin KJ, Moore DW, Hansen TR. Secretion of bovine uterine proteins in response to type I interferons. Biol Reprod. 1995;52(4):848–54.
Ott TL, Yin J, Wiley AA, Kim HT, Gerami-Naini B, Spencer TE, Bartol FF, Burghardt RC, Bazer FW. Effects of the estrous cycle and early pregnancy on uterine expression of Mx protein in sheep (Ovis aries). Biol Reprod. 1998;59(4):784–94.
Mirando MA, Short Jr EC, Geisert RD, Vallet JL, Bazer FW. Stimulation of 2′,5′-oligoadenylate synthetase activity in sheep endometrium during pregnancy, by intrauterine infusion of ovine trophoblast protein-1, and by intramuscular administration of recombinant bovine interferon-alpha I1. J Reprod Fertil. 1991;93(2):599–607.
Schmitt RA, Geisert RD, Zavy MT, Short EC, Blair RM. Uterine cellular changes in 2′,5′-oligoadenylate synthetase during the bovine estrous cycle and early pregnancy. Biol Reprod. 1993;48(3):460–6.
Rosenfeld CS, Han CS, Alexenko AP, Spencer TE, Roberts RM. Expression of interferon receptor subunits, IFNAR1 and IFNAR2, in the ovine uterus. Biol Reprod. 2002;67(3):847–53.
Stewart DM, Johnson GA, Vyhlidal CA, Burghardt RC, Safe SH, Yu-Lee LY, Bazer FW, Spencer TE. Interferon-tau activates multiple signal transducer and activator of transcription proteins and has complex effects on interferon-responsive gene transcription in ovine endometrial epithelial cells. Endocrinology. 2001;142(1):98–107.
Perry DJ, Austin KJ, Hansen TR. Cloning of interferon-stimulated gene 17: the promoter and nuclear proteins that regulate transcription. Mol Endocrinol. 1999;13(7):1197–206.
Choi Y, Johnson GA, Burghardt RC, Berghman LR, Joyce MM, Taylor KM, Stewart MD, Bazer FW, Spencer TE. Interferon regulatory factor-two restricts expression of interferon-stimulated genes to the endometrial stroma and glandular epithelium of the ovine uterus. Biol Reprod. 2001;65(4):1038–49.
Lonergan P, Forde N. The role of progesterone in maternal recognition of pregnancy in domestic ruminants. Adv Anat Embryol Cell Biol. 2015;216:87–104.
Spencer TE, Forde N, Lonergan P. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J Dairy Sci. 2016;99(7):5941–50.
Wiltbank MC, Souza AH, Carvalho PD, Cunha AP, Giordano JO, Fricke PM, Baez GM, Diskin MG. Physiological and practical effects of progesterone on reproduction in dairy cattle. Animal. 2014;8 suppl 1:70–81.
Carvalho PD, Fuenzalida MJ, Ricci A, Souza AH, Barletta RV, Wiltbank MC, Fricke PM. Modifications to Ovsynch improve fertility during resynchronization: evaluation of presynchronization with gonadotropin-releasing hormone 6 d before initiation of Ovsynch and addition of a second prostaglandin F2alpha treatment. J Dairy Sci. 2015;98(12):8741–52.
Bisinotto RS, Castro LO, Pansani MB, Narciso CD, Martinez N, Sinedino LD, Pinto TL, Van de Burgwal NS, Bosman HM, Surjus RS, Thatcher WW, Santos JE. Progesterone supplementation to lactating dairy cows without a corpus luteum at initiation of the Ovsynch protocol. J Dairy Sci. 2015;98(4):2515–28.
Bisinotto RS, Lean IJ, Thatcher WW, Santos JE. Meta-analysis of progesterone supplementation during timed artificial insemination programs in dairy cows. J Dairy Sci. 2015;98(4):2472–87.
Kerbler TL, Buhr MM, Jordan LT, Leslie KE, Walton JS. Relationship between maternal plasma progesterone concentration and interferon-tau synthesis by the conceptus in cattle. Theriogenology. 1997;47(3):703–14.
Nephew KP, Cardenas H, McClure KE, Ott TL, Bazer FW, Pope WF. Effects of administration of human chorionic gonadotropin or progesterone before maternal recognition of pregnancy on blastocyst development and pregnancy in sheep. J Anim Sci. 1994;72(2):453–8.
Rizos D, Scully S, Kelly AK, Ealy AD, Moros R, Duffy P, Al Naib A, Forde N, Lonergan P. Effects of human chorionic gonadotrophin administration on day 5 after oestrus on corpus luteum characteristics, circulating progesterone and conceptus elongation in cattle. Reprod Fertil Dev. 2012;24(3):472–81.
Arosh JA, Banu SK, Kimmins S, Chapdelaine P, Maclaren LA, Fortier MA. Effect of interferon-tau on prostaglandin biosynthesis, transport, and signaling at the time of maternal recognition of pregnancy in cattle: evidence of polycrine actions of prostaglandin E2. Endocrinology. 2004;145(11):5280–93.
Emond V, MacLaren LA, Kimmins S, Arosh JA, Fortier MA, Lambert RD. Expression of cyclooxygenase-2 and granulocyte-macrophage colony-stimulating factor in the endometrial epithelium of the cow is up-regulated during early pregnancy and in response to intrauterine infusions of interferon-tau. Biol Reprod. 2004;70(1):54–64.
Brooks K, Burns G, Spencer TE. Conceptus elongation in ruminants: roles of progesterone, prostaglandin, interferon tau and cortisol. J Anim Sci Biotechnol. 2014;5(1):53.
Peterson AJ, Tervit HR, Fairclough RJ, Havik PG, Smith JF. Jugular levels of 13,14-dihydro-15-keto-prostaglandin F and progesterone around luteolysis and early pregnancy in the ewe. Prostaglandins. 1976;12(4):551–8.
Zarco L, Stabenfeldt GH, Quirke JF, Kindahl H, Bradford GE. Release of prostaglandin F-2 alpha and the timing of events associated with luteolysis in ewes with oestrous cycles of different lengths. J Reprod Fertil. 1988;83(2):517–26.
Zarco L, Stabenfeldt GH, Basu S, Bradford GE, Kindahl H. Modification of prostaglandin F-2 alpha synthesis and release in the ewe during the initial establishment of pregnancy. J Reprod Fertil. 1988;83(2):527–36.
McCracken JA. Hormone receptor control of prostaglandin F2 alpha secretion by the ovine uterus. Adv Prostaglandin Thromboxane Res. 1980;8:1329–44.
Silvia WJ, Niswender GD. Maintenance of the corpus luteum of early pregnancy in the ewe. IV. Changes in luteal sensitivity to prostaglandin F2 alpha throughout early pregnancy. J Anim Sci. 1986;63(4):1201–7.
Wiepz G, Wiltbank M, Nett T, Niswender GD, Sawyer H. Receptors for prostaglandins F2 alpha and E2 in ovine corpora lutea during maternal recognition of pregnancy. Biol Reprod. 1992;47(6):984–91.
Rueda BR, Botros IW, Pierce KL, Regan JW, Hoyer PB. Comparison of mRNA levels for the PGF(2alpha) receptor (FP) during luteolysis and early pregnancy in the ovine corpus luteum. Endocrine. 1995;3(11):781–7.
Thornburn G, Cox R, Currie W, Restall B, Schneider W. Prostaglandin F concentration in the utero-ovarian venous plasma of the ewe during the oestrous cycle. J Endocrinol. 1972;53:325–6.
Costine BA, Inskeep EK, Blemings KP, Flores JA, Wilson ME. Mechanisms of reduced luteal sensitivity to prostaglandin F2alpha during maternal recognition of pregnancy in ewes. Domestic Anim Endocrinol. 2007;32(2):106–21.
Weems YS, Nett TM, Rispoli LA, Davis TL, Johnson DL, Uchima T, Raney A, Lennon E, Pang J, Harbert T, Bowers G, Goto K, Ong A, Tsutahara N, Randel RD, Weems CW. Prostaglandin E1 (PGE1), but not prostaglandin E2 (PGE2), alters luteal and endometrial luteinizing hormone (LH) occupied and unoccupied LH receptors and mRNA for LH receptors in ovine luteal tissue to prevent luteolysis. Prostaglandins Other Lipid Mediat. 2010;91(1-2):42–50.
Lee J, McCracken JA, Stanley JA, Nithy TK, Banu SK, Arosh JA. Intraluteal prostaglandin biosynthesis and signaling are selectively directed towards PGF2alpha during luteolysis but towards PGE2 during the establishment of pregnancy in sheep. Biol Reprod. 2012;87(4):97.
Yankey SJ, Hicks BA, Carnahan KG, Assiri AM, Sinor SJ, Kodali K, Stellflug JN, Stellflug JN, Ott TL. Expression of the antiviral protein Mx in peripheral blood mononuclear cells of pregnant and bred, non-pregnant ewes. J Endocrinol. 2001;170(2):R7–11.
Han H, Austin KJ, Rempel LA, Hansen TR. Low blood ISG15 mRNA and progesterone levels are predictive of non-pregnant dairy cows. J Endocrinol. 2006;191(2):505–12.
Gifford CA, Racicot K, Clark DS, Austin KJ, Hansen TR, Lucy MC, Davies CJ, Ott TL. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J Dairy Sci. 2007;90(1):274–80.
Green JC, Okamura CS, Poock SE, Lucy MC. Measurement of interferon-tau (IFN-tau) stimulated gene expression in blood leukocytes for pregnancy diagnosis within 18-20d after insemination in dairy cattle. Anim Reprod Sci. 2010;121(1-2):24–33.
Monteiro Jr PL, Ribeiro ES, Maciel RP, Dias AL, Sole Jr E, Lima FS, Bisinotto RS, Thatcher WW, Sartori R, Santos JE. Effects of supplemental progesterone after artificial insemination on expression of interferon-stimulated genes and fertility in dairy cows. J Dairy Sci. 2014;97(8):4907–21.
Pugliesi G, Miagawa BT, Paiva YN, Franca MR, Silva LA, Binelli M. Conceptus-induced changes in the gene expression of blood immune cells and the ultrasound-accessed luteal function in beef cattle: how early can we detect pregnancy? Biol Reprod. 2014;91(4):95.
Meyerholz MM, Mense K, Knaack H, Sandra O, Schmicke M. Pregnancy-induced ISG-15 and MX-1 gene expression is detected in the liver of Holstein-Friesian heifers during late peri-implantation period. Reprod Domestic Anim. 2015;10(8):e0133377.
Hansen TR, Smirnova NP, Webb BT, Bielefeldt-Ohmann H, Sacco RE, Van Campen H. Innate and adaptive immune responses to in utero infection with bovine viral diarrhea virus. Animal Health Research Reviews/Conference of Research Workers in Animal Diseases. 2015;16(1):15–26.
Schalue-Francis TK, Farin PW, Cross JC, Keisler D, Roberts RM. Effect of injected bovine interferon-alpha I1 on estrous cycle length and pregnancy success in sheep. J Reprod Fertil. 1991;91(1):347–56.
Spencer TE, Stagg AG, Ott TL, Johnson GA, Ramsey WS, Bazer FW. Differential effects of intrauterine and subcutaneous administration of recombinant ovine interferon tau on the endometrium of cyclic ewes. Biol Reprod. 1999;61(2):464–70.
Li J, Roberts RM. Interferon-tau and interferon-alpha interact with the same receptors in bovine endometrium. Use of a readily iodinatable form of recombinant interferon-tau for binding studies. J Biol Chem. 1994;269(18):13544–50.
Helmer SD, Hansen PJ, Thatcher WW, Johnson JW, Bazer FW. Intrauterine infusion of highly enriched bovine trophoblast protein-1 complex exerts an antiluteolytic effect to extend corpus luteum lifespan in cyclic cattle. J Reprod Fertil. 1989;87(1):89–101.
Wiltbank MC, Wiepz GJ, Knickerbocker JJ, Belfiore CJ, Niswender GD. Proteins secreted from the early ovine conceptus block the action of prostaglandin F2 alpha on large luteal cells. Biol Reprod. 1992;46(3):475–82.
Mao D, Hou X, Talbott H, Cushman R, Cupp A, Davis JS. ATF3 expression in the corpus luteum: possible role in luteal regression. Mol Endocrinol. 2013;27(12):2066–79.
Hansen TR, Pru JK. ISGylation: a conserved pathway in mammalian pregnancy. Adv Exp Med Biol. 2014;759:13–31.
Yang L, Wang XL, Wan PC, Zhang LY, Wu Y, Tang DW, Zeng SM. Up-regulation of expression of interferon-stimulated gene 15 in the bovine corpus luteum during early pregnancy. J Dairy Sci. 2010;93(3):1000–11.
Magata F, Shirasuna K, Struve K, Herzog K, Shimizu T, Bollwein H, Miyamoto A. Gene expressions in the persistent corpus luteum of postpartum dairy cows: distinct profiles from the corpora lutea of the estrous cycle and pregnancy. J Reprod Dev. 2012;58(4):445–52.
Robinson RS, Hammond AJ, Wathes DC, Hunter MG, Mann GE. Corpus luteum-endometrium-embryo interactions in the dairy cow: underlying mechanisms and clinical relevance. Reprod Domestic Anim. 2008;43(suppl 2):104–12.
Telgmann R, Bathgate RA, Jaeger S, Tillmann G, Ivell, R. Transcriptional regulation of the bovine oxytocin receptor gene. Biol Reprod. 2003;68:1015-26.
Satterfield MC, Dunlap KA, Hayashi K, Burghardt RC, Spencer TE, Bazer FW. Tight and adherens junctions in the ovine uterus: differential regulation by pregnancy and progesterone. Endocrinology. 2007;148(8):3922–31.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hansen, T.R., Bott, R., Romero, J., Antoniazzi, A., Davis, J.S. (2017). Corpus Luteum and Early Pregnancy in Ruminants. In: Meidan, R. (eds) The Life Cycle of the Corpus Luteum. Springer, Cham. https://doi.org/10.1007/978-3-319-43238-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-43238-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43236-6
Online ISBN: 978-3-319-43238-0
eBook Packages: MedicineMedicine (R0)