Abstract
Cancer related mortality has been dramatically reduced in recent decades due to more effective cancer treatments, especially chemotherapy and radiation therapy. However, the use of these treatment modalities may be limited by the risk of significant cardiac damage. The current standard for cardiac safety assessment, in order to limit cardiotoxicity, predominantly focuses on serial cardiac imaging to identify changes in left ventricular ejection fraction (LVEF). Unfortunately, this method is imperfect and frequently is a late finding. Potentially permanent cardiac damage manifesting as a significantly reduced LVEF has to occur before any important change in management is undertaken. One alternative and complimentary approach is the appropriate use of cardiac biomarkers to identify subclinical cardiac damage allowing for earlier detection and institution of cardio-protective interventions. This chapter will highlight the clinical use of cardiac biomarkers, specifically natriuretic peptides, cardiac troponins, as well as emerging biomarkers, for the detection of cardiac injury in the context of cardio-oncology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Cancer and cardiovascular diseases are by far the most common diseases resulting in mortality in the developed world [1]. The last decade has seen a profound increase cancer therapeutic options and the efficacy of those treatments [2]. Consequently, there is an ever increasing cohort of patients who are long-term survivors of childhood and adult onset cancer [3]. As this patient population ages, there is an increasing overlap with concomitant cardiovascular disease (CVD) [4–6]. It appears that CVD in survivors may be an epidemiological consequence of aging but also is related to the toxicity of chemotherapy, radiation therapy or other treatments for cancer [6, 7]. Furthermore, a substantial portion of cancer patients may have pre-existing CVD which can be unmasked or exacerbated by increasingly specific chemotherapeutic agents with cardiotoxic effects. Cardiac damage may occur in a myriad of ways including arrhythmias, myocardial ischemia, hypertension, left ventricular (LV) dysfunction and heart failure (HF) [8–11]. Additionally, there are a host of vascular complications that may arise during and after treatment [12]. Encouragingly, there is also evidence that early detection of cardiovascular damage with initiation of cardiovascular based medical therapy can prevent and/or enhance cardiac recovery in the case of LV dysfunction but also prevention of toxicity with control of vascular complications [13–16]. The main limitation is related to detecting cardiovascular dysfunction at an early stage and initiating therapy before permanent damage occurs. The emphasis, thus, has been on cardiac imaging modalities including echocardiography with and without LV deformation (strain), multiple gated acquisition scan (MUGA) and cardiac magnetic resonance imaging (cMRI) to hopefully detect damage at an early point [17–21]. Unfortunately, the detection of a significant change in LVEF by any of these modalities is generally a late finding and usually indicates substantial underlying cardiac damage and remodeling [6, 22, 23]. The challenge at the present time is to be able to identify cardiac damage at the earliest stage prior to a reduction in LVEF and initiate therapy or modify dosing to prevent LV dysfunction. One way of achieving this goal is to utilize cardiac biomarkers to identify those at risk for developing cardiotoxicity. Overall, the advantage of cardiac biomarkers is that it is generally much less expensive, can be followed with ease in a serial fashion, and are less subject to interpretative variation [22]. In this chapter we will examine the data supporting the use of cardiac based biomarkers to enhance safety and cardio-protection during therapy for cancer.
B-type Natriuretic Peptide
B-type natriuretic peptide (BNP) is a neurohormone polypeptide secreted by the myocytes of the ventricles in response to increased wall stress from volume expansion and pressure overload and is secreted along with a 76-amino acid N-terminal pro BNP (NT-proBNP), that is biologically inactive, and eventually cleaved to the active 32 amino acid BNP [24]. The hemodynamic effects of BNP include a decrease in afterload and increase in natriuresis; thus, counteracting some of the pathophysiologic mechanisms responsible for the progression of HF. Robust data from the Breathing Not Properly trial (BNP trial) demonstrated that BNP was able to differentiate congestive heart failure (CHF) from non-CHF causes of dyspnea with good specificity and high negative predictive values. Subsequent studies also showed the utility of BNP for prognosis and risk stratification in the setting of HF [25, 26]. Additionally, NT-proBNP–guided optimal medical therapy is associated with a reduced incidence of cardiovascular death, new episodes of decompensated HF, and reduction in NT-proBNP that also correlates with LV remodeling and recovery [27]. Based on the aforementioned clinical utility, it comes as no surprise that the natriuretic peptides, BNP/NT-proBNP, can be useful in the setting of early detection of potential cardiotoxicity due to its ability to detect subclinical disease, direct medical therapy and assist with prognostication even prior to a decline in LVEF [28, 29].
Multiple studies have looked at the utility of perturbations in BNP/NT-proBNP levels in patients with cancer undergoing treatment with chemotherapy or radiation (Table 24.1). In one such study, patients receiving high dose anthracyclines for breast cancer, NT-proBNP was measured at baseline and immediately following each treatment cycle [30]. There was a high degree of correlation between a rise in NT-ProBNP and a reduction in LVEF. Similar findings with natriuretic peptides were replicated in other studies primarily with the use of anthracycline-based chemotherapeutic regimens [28, 29]. The utility of BNP to assist in identifying those patients at risk for cardiotoxicity and LV dysfunction goes beyond the acute setting. Persistently elevated BNP is predictive of late onset adriamycin-induced cardiotoxicity and correlates with cardiac dysfunction detected over time [6, 28–30]. Furthermore, a baseline elevation of BNP can mark a patient at high risk for the development of cardiotoxicity during subsequent rounds of chemotherapy [16].
Aside from predicting subsequent cardiotoxicity predominantly with anthracycline-based treatment, natriuretic peptide (NP) levels may indicate a potential therapeutic benefit. In one study, children with acute lymphoid leukemia (ALL) were randomized to receive doxorubicin with or without dexrazoxane (a cardioprotective free radical scavenger) and those patients given dexrazoxane tended to have reduced NT-proBNP concentrations indicating a cardioprotective effect (47 vs. 20 %, p = 0.07) [31]. It is especially important to have cardioprotective strategies in the pediatric population that have increased long-term survival into adulthood [5, 32]. Additionally, NT-proBNP levels were lower and the LV mass was reduced in a pediatric patient population nearly 4 years after anthracycline treatment (p =0.003) [32]. Consequently, NT-BNP/BNP levels appear to guide providers in identifying those specific patients at risk for toxicity as well as indicating what therapeutic interventions may reduce the impact of cardiac dysfunction with anthracyclines.
The utility of natriuretic peptides (NP) to assist in detecting cardiac damage during cancer therapy can extend to a broader population than just those receiving known substantial cardio-toxins like anthracyclines. For instance, those patients receiving chest radiation, those at risk for development of atrial fibrillation while receiving anti-VEGF based therapy, those at risk for HF with tyrosine kinase inhibitors, and potentially those receiving combination therapy for multiple myeloma all may be populations in which NP may be useful [16, 33–35]. Elevated BNP levels correlate with an increased risk for radiation induced cardiomyopathy (early and late) and are directly related to the amount of radiation delivered [33]. Furthermore, NT-proBNP levels are used to stage and predict outcomes in patients with AL amyloidosis as well as monitor response to therapy [36–38].
It should be noted that NP, although broadly useful and predictive, must be interpreted within the entire clinical context at the moment of sampling for any given patient. For example, a rapid increase in BNP/NT-proBNP in a patient undergoing chemotherapy easily could be related to a concomitant process, such acute kidney injury or volume overload, without evidence of cardiac dysfunction or toxicity. In this context, the clinician is encouraged to make a careful assessment of the volume status of the patient and attempt to define the presence of other prominent co-morbidity such as sepsis [39].
Troponin
Troponin I (TnI) and troponin T (TnT) are both cardiac specific proteins that form an integral part of the cardiac contractile unit [16, 22]. As biomarkers they are highly specific and sensitive for cardiac damage and are widely used in the diagnosis/treatment of acute coronary syndromes as currently supported by major guideline documents [40]. Elevations in troponin correlate with cardiac myocyte damage/death, however, it does not distinguish the mechanism of injury. As such, cardiac troponin has found utility in the screening of asymptomatic patients for cardiotoxicity during and after treatment [41–43]. A summary of the major clinical trials examining the utility of troponin in cardio-oncology is provided in Table 24.2 [6].
One of the larger studies to examine this topic enrolled 703 patients with various advanced malignances who were receiving high dose chemotherapy [41]. TnI was checked at initiation of therapy, and 1 month after. Cardiac function was measured and documented with echocardiography at baseline, and 1, 2, 6, and 12 months post therapy. Thirty percent of the patients had early TnI elevation, and a third of subsequently showed elevated TnI at 1 month. Reductions in ejection fraction were predicted by both early (r = 0.78, p <0.001) and persistent elevation at 1 month (r =0.92, p <0.001). Not only did elevated troponin predict decline in EF, persistent elevation was able to predict the development of symptomatic HF which suggests that troponin elevation closely correlates with the cardiotoxic effects of the chemotherapeutic agents [44]. Having a positive troponin at any time predicted future cardiovascular events with a positive predictive value of 84 % and negative predictive value of 99 %. There is a suggestion that troponin I elevation may be able to predict cardiac dysfunction with other cardio-toxic therapy, such as trastuzumab, but the data has not been as consistent as initially reported [16, 45, 46]. Additionally, troponin T has shown utility in the care of patients with light chain amyloidosis [37, 38, 47]. Troponin levels are predictive of outcomes and decreases correlate with response to therapy and can be used to monitor disease activity in the post therapy patient with amyloidosis.
The utility of troponin to detect cardiac damage in patients who survived prior treatment of childhood cancer is a hopeful goal but has not been well established to date [48–50]. However, a study in patients with ALL treated with anthracyclines and dexrazoxane had a reduced incidence of elevated troponin underscoring the protective effect during active treatment [31]. In another study elevated troponin correlated with lower LV mass at 4 years post treatment [44]. Although early troponin elevation during therapy was predictive of cardiac dysfunction, post therapy troponin did not correlate with risk of late onset cardiotoxicity [48].
Despite the utility of troponin as outlined above, there appears to be mixed data as it relates to the utility of troponin levels for predicting radiation induced cardiomyopathy [51, 52].
Emerging Biomarkers
There has been a desire to identify an effective biomarker to detect cardiac injury during cancer treatment and therefore other markers have been investigated.
Myeloperoxidase (MPO)
MPO is a proatherogenic enzyme produced by neutrophils that is indicative off oxidative stress and lipid peroxidation. Its prognostic role in acute coronary syndrome and heart failure has been suggested [46, 53–55]. In the context of cancer chemotherapy, a panel of biomarkers including NT-proBNP, growth differentiation factor (GDF)-15, placenta growth factor (PlGF), c reactive protein (crp), soluble fms-like tyrosine kinase receptor (sFlt)-1, and galectin (gal)-3 in breast cancer patients receiving antracyclines and herceptin were examined [46]. In patients with 90th percentile MPO interval change from baseline the probability of cardiotoxicity at 15 months was 34.2 %, and the risk of future cardiac toxicity increased with each standard deviation increase in MPO concentration (HR 1.34, p = 0.048). Although, the most useful biomarker tested was high sensitivity troponin I, MPO was also modestly useful in detection of cardiac damage.
C-reactive Protein (CRP)
CRP is an acute phase reactant produced in response to inflammation [56, 57]. Although its role in CAD and HF is well documented, the utility of CRP in the cardio-oncology patient population has mixed results [58, 59]. High-sensitivity CRP (hsCRP) concentrations ≥3 mg/l predicted impaired LVEF with 92.9 % sensitivity and 45.7 % specificity (PPV, 40.6 %; NPV, 94.1 %) in a cohort of breast cancer patients. HsCRP elevations occurred >70 days before echocardiographic changes were seen. As such, hsCRP maybe able to risk stratify patients and delineate who needs more stringent follow up [58]. Another study, in a survivorship cohort, found higher CRP values regardless of exposure to cardiotoxic treatment but poor correlation with LV mass, wall thickness, and dimension [50]. This suggests that hs-CRP may be a surrogate for overall inflammation or tumor burden in addition to drug effects.
Total Antioxidant Status (TAOS)
Total antioxidant status is a sum total of antioxidants in the blood and could potentially be used to monitor for cardiac toxicity in anthracycline based therapy [49]. A study of 29 children undergoing anthracycline based therapy for acute leukemia showed statistically significant decrease in TAOS which correlated with higher total doses of anthracyclines and subsequent reduction in LVEF.
Nitric Oxide (NO)
NO is generated by NO synthase from l-arginine in numerous cell types and is a key regulator of cardiomyocyte contractility [60]. Dysregulated NO synthesis is implicated in the pathophysiology of doxorubicin-induced cardiotoxicity [6, 60]. One study demonstrated significantly higher plasma levels of total nitrite, a stable product of NO, in children that received doxorubicin and in those with abnormal LVEF as compared to healthy controls and an increased NO may be an indicator of subclinical cardiotoxicity.
In addition to the markers discussed above, future directions include heart-type fatty acid-binding protein, cytochrome C, glycogen phosphorylase isoenzyme BB and circulating microRNAs deserve mention as potential targets.
Conclusion
With a dramatic improvement in the overall survival and outcomes of patients with cancer, cardiac damage or exacerbation of underlying cardiac disease by cancer therapy has become a critically important issue for cancer survivors and clinicians. Screening for cardiotoxicity, as per current guidelines, focuses predominantly on serial noninvasive imaging. This is costly, subject to variation in reader interpretation, and often detects changes when cardiac remodeling has already taken place. Cardiac biomarkers have emerged as an inexpensive means to serially follow patients and to potentially detect early subclinical cardiac toxicity. Biomarkers can delinate low versus high risk patients allowing for intensive screening in the later group. As such, biomarkers, can potentially reduce costs associated with unnecessary serial screening. Early, detection of subclinical cardiotoxic effects, facilitate changes in the chemotherapy regimen and/or the initiation of cardioprotective medical regimen (eg. Beta blocker) to prevent permanent cardiac remodeling.
In summary, biomarkers offer significant advantages in the detection, treatment and prognostication of cardiotoxicity. Multiple cardiac biomarkers have been studied and shown utility in this setting. However, as we look to the future with ever increasing array of available chemotherapy agents, further prospective randomized trials need to be conducted with the incorporation of cardiac biomarkers to improve our understanding of their optimal role. Eventually, cardiac biomarkers maybe implemented in every day practice and serve to replace or complement cardiac imaging.
Here we will present a patient recently seen at our medical center and illustrate how we applied biomarkers to their medical care.
Case
A 65 y/o Caucasian male, WL, with medical history of hypertension and dyslipidemia was referred to our heart failure clinic from an outside general cardiology clinic for evaluation of difficult to manage heart failure with preserved ejection fraction (HFpEF). Despite diuretic therapy, he continued to have orthopnea, edema and dyspnea with minimal activity. His echocardiogram showed mild concentric LVH, normal ejection fraction, grade II diastolic dysfunction and no significant valvular pathology. His electrocardiogram showed low voltage and a pseudoinfarct pattern raising suspicion for infiltrative cardiomyopathy. Laboratory evaluation showed a monoclonal protein spike and a bone marrow biopsy was notable for 15 % clonal plasma cells and no amyloid. A cMRI demonstrated global subendocardial delayed enhancement consistent with amyloidosis. A cardiac catheterization was negative for significant coronary disease with biopsy positive for congo red staining- confirming a diagnosis of AL cardiac amyloidosis. His diuretic regimen was adjusted and with careful attention to salt/fluid intake his HFpEF symptoms improved.
He was referred to the Oncology clinic for further evaluation. At that point his troponin I was 0.12 ng/ml (<0.03 ng/ml), BNP 569 pg/ml (<100 pg/ml) and serum lamda light chain 27.19 mg/dl (0.57-2.63 mg/dl). At this point he was started on induction therapy with 6 cycles of bortezomib and dexamethasone. During induction therapy he developed worsening heart failure symptoms and a repeat echocardiogram showed ejection fraction of 35 %. His troponin I and BNP increased to 0.21 ng/ml and 1006 pg/ml respectively. His cardiac dysfunction was presumed secondary to bortezomib. Based on prior reported studies this is generally reversible [52]. We adjusted his medical regimen with the addition of carvedilol, spironolactone and uptitration of his diuretic regimen. We continued and completed induction therapy. After approximately 6 months his LVEF was back to normal. Additionally, troponin I and BNP decreased to 0.13 ng/ml and 340 pg/ml respectively, signally cardiac recovery and reduction in disease activity. He subsequently underwent consolidation therapy with reduced dose melphalan and stem cell transplantation. During post-transplant follow up, troponin I normalized, BNP decreased to
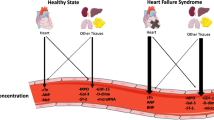
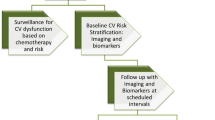
120 pg/ml and serum lambda light chain decreased to the normal range <1.66 mg/dl. Overall, the biomarker activity was consistent with no amyloid disease activity and no ongoing cardiac damage. We will continue to follow him closely checking BNP and troponin levels periodically (Figs. 24.1, 24.2, and 24.3).
References
Sanz J, Moreno PR, Fuster V. The year in atherothrombosis. J Am Coll Cardiol. 2007;49:1740–9.
Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–45.
Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82.
McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31:631–40.
Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–80.
Tian S, Hirshfield KM, Jabbour SK, et al. Serum biomarkers for the detection of cardiac toxicity after chemotherapy and radiation therapy in breast cancer patients. Front Oncol. 2014;4:277.
Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–27.
Ewer MS, Suter TM, Lenihan DJ, et al. Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: a comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur J Cancer (Oxford, England: 1990). 2014;50:2162–70.
Cheng H, Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res. 2010;106:21–34.
Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–9.
Lotrionte M, Biondi-Zoccai G, Abbate A, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–4.
Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol. 2015;66:1160–78.
Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–81.
Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–62.
Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. 2013;61:2355–62.
Stevens PL, Lenihan DJ. Cardiotoxicity due to Chemotherapy: the Role of Biomarkers. Curr Cardiol Rep. 2015;17:603.
Lotrionte M, Cavarretta E, Abbate A, et al. Temporal changes in standard and tissue Doppler imaging echocardiographic parameters after anthracycline chemotherapy in women with breast cancer. Am J Cardiol. 2013;112:1005–12.
Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109–18.
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–68.
Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2014;27:911–39.
Nousiainen T, Vanninen E, Jantunen E, et al. Concomitant impairment of left ventricular systolic and diastolic function during doxorubicin therapy: a prospective radionuclide ventriculographic and echocardiographic study. Leuk Lymphoma. 2002;43:1807–11.
Christenson ES, James T, Agrawal V, Park BH. Use of biomarkers for the assessment of chemotherapy-induced cardiac toxicity. Clin Biochem. 2015;48:223–35.
Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J Clin Oncol. 2008;26:1201–3.
Selvais PL, Donckier JE, Robert A, et al. Cardiac natriuretic peptides for diagnosis and risk stratification in heart failure: influences of left ventricular dysfunction and coronary artery disease on cardiac hormonal activation. Eur J Clin Invest. 1998;28:636–42.
McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–22.
Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7.
Januzzi JL, Troughton R. Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide measurements are useful in heart failure management. Circulation. 2013;127:500–7; discussion 8.
Nousiainen T, Vanninen E, Jantunen E, et al. Natriuretic peptides during the development of doxorubicin-induced left ventricular diastolic dysfunction. J Intern Med. 2002;251:228–34.
Feola M, Garrone O, Occelli M, et al. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol. 2011;148:194–8.
Kouloubinis A, Kaklamanis L, Ziras N, et al. ProANP and NT-proBNP levels to prospectively assess cardiac function in breast cancer patients treated with cardiotoxic chemotherapy. Int J Cardiol. 2007;122:195–201.
Lipshultz SE, Miller TL, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042–9.
Germanakis I, Kalmanti M, Parthenakis F, et al. Correlation of plasma N-terminal pro-brain natriuretic peptide levels with left ventricle mass in children treated with anthracyclines. Int J Cardiol. 2006;108:212–5.
Jingu K, Nemoto K, Kaneta T, et al. Temporal change in brain natriuretic Peptide after radiotherapy for thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1417–23.
Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604.
Grandin EW, Ky B, Cornell RF, Carver J, Lenihan DJ. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail. 2015;21:138–44.
Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–7.
Dispenzieri A, Gertz MA, Kyle RA, et al. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104:1881–7.
Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–95.
Burjonroppa SC, Tong AT, Xiao LC, Johnson MM, Yusuf SW, Lenihan DJ. Cancer patients with markedly elevated B-type natriuretic peptide may not have volume overload. Am J Clin Oncol. 2007;30:287–93.
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425.
Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–54.
Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–6.
Cardinale D, Sandri MT. Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog Cardiovasc Dis. 2010;53:121–9.
Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation. 1997;96:2641–8.
Ky B, Carver JR. Biomarker approach to the detection and cardioprotective strategies during anthracycline chemotherapy. Heart Fail Clin. 2011;7:323–31.
Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–16.
Gertz MA. Immunoglobulin light chain amyloidosis: 2014 update on diagnosis, prognosis, and treatment. Am J Hematol. 2014;89:1132–40.
Pongprot Y, Sittiwangkul R, Charoenkwan P, Silvilairat S. Use of cardiac markers for monitoring of doxorubixin-induced cardiotoxicity in children with cancer. J Pediatr Hematol Oncol. 2012;34:589–95.
Erkus B, Demirtas S, Yarpuzlu AA, Can M, Genc Y, Karaca L. Early prediction of anthracycline induced cardiotoxicity. Acta Paediatr. 2007;96:506–9.
Lipshultz SE, Landy DC, Lopez-Mitnik G, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30:1050–7.
Kozak KR, Hong TS, Sluss PM, et al. Cardiac blood biomarkers in patients receiving thoracic (chemo)radiation. Lung Cancer. 2008;62:351–5.
Nellessen U, Zingel M, Hecker H, Bahnsen J, Borschke D. Effects of radiation therapy on myocardial cell integrity and pump function: which role for cardiac biomarkers? Chemotherapy. 2010;56:147–52.
Tang WH, Tong W, Troughton RW, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–70.
Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–5.
Reichlin T, Socrates T, Egli P, et al. Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. 2010;56:944–51.
Arruda-Olson AM, Enriquez-Sarano M, Bursi F, et al. Left ventricular function and C-reactive protein levels in acute myocardial infarction. Am J Cardiol. 2010;105:917–21.
Windram JD, Loh PH, Rigby AS, Hanning I, Clark AL, Cleland JG. Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J. 2007;153:1048–55.
Onitilo AA, Engel JM, Stankowski RV, Liang H, Berg RL, Doi SA. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2012;134:291–8.
Morris PG, Chen C, Steingart R, et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin cancer Res Off J Am Assoc Cancer Res. 2011;17:3490–9.
Guler E, Baspinar O, Cekmen M, Kilinc M, Balat A. Nitric oxide: a new biomarker of Doxorubicin toxicity in children? Pediatr Hematol Oncol. 2011;28:395–402.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Egolum, U.O., Lenihan, D.J. (2016). Biomarkers in Specific Disease States: Cardio-Oncology. In: Maisel, A., Jaffe, A. (eds) Cardiac Biomarkers. Springer, Cham. https://doi.org/10.1007/978-3-319-42982-3_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-42982-3_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42980-9
Online ISBN: 978-3-319-42982-3
eBook Packages: MedicineMedicine (R0)