Abstract
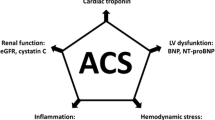
We have seen dramatic improvements of the acute cardiac care in the last 20 years, which have led to a substantial reduction in short and long term mortality after acute myocardial infarction (AMI) as well as after heart failure (HF) [1, 2]. These impressive successes have been achieved mainly by improved treatments but also through improved diagnostic methods. During this time period we have got access to some very strong new biomarkers, cardiac troponins (cardiac troponin I and cardiac troponin I) and natriuretic peptides (BNP and NT-proBNP). Their success and widespread use are based upon their excellent diagnostic properties rather than their similarly excellent prognostic properties. The introduction and development of more and more sensitive cTn assays have revolutionized the diagnosis of AMI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biomarkers in cardiology
- Novel biomarkers in cardiology
- Acute myocardial infarction and biomarkers
- Cardiac troponins
- Heart failure and biomarkers
We have seen dramatic improvements of the acute cardiac care in the last 20 years, which have led to a substantial reduction in short and long term mortality after acute myocardial infarction (AMI) as well as after heart failure (HF) [1, 2]. These impressive successes have been achieved mainly by improved treatments but also through improved diagnostic methods. During this time period we have got access to some very strong new biomarkers, cardiac troponins (cardiac troponin I and cardiac troponin I) and natriuretic peptides (BNP and NT-proBNP). Their success and widespread use are based upon their excellent diagnostic properties rather than their similarly excellent prognostic properties. The introduction and development of more and more sensitive cTn assays have revolutionized the diagnosis of AMI. AMI can now be ruled-in or ruled-out within a few hours, rather than within a day in the pre-troponin era. Furthermore, in a large proportion of patients previously classified as having unstable angina, elevated levels of cardiac troponin can be detected, and hence, these patients are now classified as having AMI. Similarly, the natriuretic peptides, BNP and NT-proBNP, markers increasing in response to pressure and/or volume overload of the heart, have been established as a first line diagnostic test both in acute and chronic HF.
However, despite these impressive advances, there are many remaining gaps in our diagnostic arsenal for acute cardiac patients. Numerous new biomarkers have been suggested during the last decades to close these gaps. However, very few of those, if any, are in routine clinical use, why is that? The key question for every biomarker is: will knowledge of the level of the marker in an individual patient lead to a change in the treatment or management of the patient?
A biomarker does not exist in isolation; the clinical usefulness of the marker is heavily dependent on available therapeutic options. That a marker provides independent prognostic (independent of already available risk markers) information is important; it tells that the level of the marker reflects some pathophysiological process of importance. However, that is seldom enough for a marker to be clinically useful. It must be possible to implement measures, based on the biomarker information, that improve the outcome for the patient. Unfortunately, that is seldom the case. That is why so many proposed markers in cardiology have failed to reach widespread use. It stands in contrast to in oncology where a number of biomarkers (mostly genetic markers) are decisive for the selection of adequate treatment because they classify the tumors into therapeutically meaningful subgroups [3].
Another important aspect of biomarkers is that there is a world of difference between being able to show statistical differences between disease and non-disease at group level, and being able to reliably identify or exclude the disease in an individual patient. There is an important difference in thinking between markers for diagnostic use and prognostic use. For diagnosis we tend to think in a dichotomous way – you either have, or not have the disease; or belong, or not belong to a diagnostic subgroup (e.g., ischemic or non-ischemic heart failure). In contrast, for prognosis we think in probabilities, e.g., the risk of dying within 1 year increases from 5 to 20 %. Many of the “new” biomarkers have been evaluated regarding their prognostic capacity and shown to be prognostic. However, more seldom are the studies appropriately designed to be able to show added value above established clinical risk markers and biomarkers, nor are proper diagnostic studies or prospective treatment studies stratified on the biomarker results performed.
Gaps in Our Biomarker Armamentarium: Patients with Suspicion of an Acute Coronary Syndrome
With the introduction of more sensitive cTn assays it has become painfully evident for clinicians that myocardial injury, and thereby cTn elevation, is present also in many acute and chronic conditions other than classical AMI [4]. Only a small minority of patients seeking acute care for symptoms indicative of an acute coronary syndrome will eventually be diagnosed with classical (type 1) AMI. Typically, less than 10 % of chest pain patients seen in the emergency department (ED) will have AMI [5] and in this population the positive predictive value for AMI of an elevation of cTn above the 99th percentile of healthy individuals is less than 50 %. Including also patients with other more vague symptoms causing enough suspicion to lead to cardiac troponin testing, an even lower proportion will be diagnosed with AMI. Therefore, it is important to be able to separate cTn elevation due to acute myocardial injury from cTn elevation due to chronic myocardial injury; and cTn elevation due to myocardial ischemia from cTn elevation due to non-ischemic causes. Furthermore, it is important to distinguish between ischemic myocardial injury caused by a athero-thrombo-embolic coronary event (type 1 AMI) from an ischemic myocardial injury secondary to oxygen supply–demand imbalance of other reasons (type 2 AMI) [4].
Gap 1: Separation of Acute and Chronic Myocardial Injury (Case 1)
Chronic elevations of cTn, as well as ST-T changes in the ECG, are common among patients with stable angina, stable congestive heart failure or atrial fibrillation. These patients often visit the emergency department and have cTn measured and therefore represent a diagnostic challenge. In one study of stable CAD patients without any acute symptoms, as much as 7 % would have been labeled AMI following the Universal Definition of AMI when diagnostic classification had been based on a single cTn result and ECG changes [6]. The obvious solution is to repeat cTn measurements and look for an increase or decrease in cTn levels. However, also patients with elevated cTn due to an acute AMI sometimes lack significant changes over a 3–6 h period, e.g., late presenters [7]. Therefore, a biomarker that could tell whether the cTn elevation represents an acute injury or a chronic condition would be very helpful. Simultaneous elevation of Copeptin and cTn indicates that the damage is acute and has started within the last few hours [8]. However, the opposite is not true, a non-elevated Copeptin level in conjunction with an elevated cTn do not exclude an acute injury, especially in cases with small acute AMIs or when the injury occurred for more than a few hours ago. Copeptin is therefore of limited value for this purpose. In the future, measuring different posttranslational modified subforms of cTn may be a way to determine the age of the myocardial injury [9]. Another future possibility may be measurement of single microRNAs or clusters of microRNAs (see below).
Gap 2: Separation of Acute Myocardial Infarction (=Acute Ischemic Injury) from Acute Non-ischemic Myocardial Injury
To differentiate myocardial damage and cTn elevation of ischemic from non-ischemic origin is a common problem facing clinicians, especially in the emergency department or in the intensive care unit (ICU). cTn is very often elevated in critically ill patients, e.g., studies of patients in the ICU show that the majority of the patients will have elevated cTn, although ischemia or a classical AMI (type 1) will only be possible to prove in a minority of these cases [10]. However, this problem is also frequent outside the ICU, e.g., myocarditis with an atypical presentation, especially when lacking a history of recent viral infection, can be very difficult to separate in the acute phase from a non-ST elevation myocardial infarction. Cardiac magnetic resonance imaging at a later stage is often required to separate the two entities.
A reliable marker of myocardial ischemia would also be valuable for identifying the remaining, but rare, cases of true unstable angina, i.e., patients with new onset of myocardial ischemia at rest or at minimal exertion and without concomitant elevation of cTn.
Therefore, a biomarker able to detect myocardial ischemia would be of great value to complement measurement of cTn. However, the proposed markers of ischemia, have so far been a disappointment. There was great hope around ischemia modified albumin (IMA) some 10 years ago, but studies failed to prove IMA as a reliable marker of ischemia and IMA is no longer on the market. Since microRNAs seem to be involved in the myocardium’s response to ischemia certain microRNA may emerge as a useful marker of myocardial ischemia in the future.
Gap 3: Separation of Type 1 AMI from Type 2 AMI (Case 2)
In type 1 AMI the ischemia and necrosis are caused by an insufficient blood supply due to a primary athero-thrombo-embolic event in the coronary artery completely or partially blocking the blood flow. In contrast, in type 2 AMI the myocardial ischemia and necrosis arise secondary to a non-coronary, triggering factor that provokes an imbalance in the supply and demand of oxygen to the myocardium [4]. In the former case the immediate therapeutic measures are focused on restoring and maintaining sufficient blood supply and in the latter case the immediate therapeutic measures are focused on removing the triggering factor causing the imbalance in the supply and demand of oxygen. Type 2 AMI is not uncommon, especially in more unselected populations. Depending on the type of population, the proportion of AMI patients with type-2 AMI varies tremendously in the literature, from 2 to over 30 % [11]. However, it may be very difficult to separate a type 1 and type 2 AMI from each other just from the clinical presentation and history. Therefore, it would be of great value to have a biomarker able to separate type 1 and 2 AMI already on admission. Possible candidates would be markers able to detect the underlying plaque rupture or plaque erosion, which will be a formidable challenge given the minimal size of the ruptured plaque. Alternative, and possibly more likely, candidates would be markers of the thrombotic process in the coronary artery. However, at present the markers of activation of the coagulation system have severe preanalytical limitations, are too insensitive or have very large inter individual variability, making them not useful for use on individual patients in clinical routine.
Gaps in Our Biomarker Armamentarium–Patients with Suspected or Verified Heart Failure
HF is a heterogeneous syndrome, with many different underlying causes and manifests itself in everything from mild unspecific symptoms and signs to very severe and typical symptoms and signs. Consequently, no single diagnostic test provides 100 % accuracy, e.g., natriuretic peptides provide a very high negative predictive value, but an only modest positive predictive value for the diagnosis. Once diagnosed, etiological considerations include ischemic (post myocardial infarction and chronic coronary artery disease) and nonischemic causes (e.g., hypertensive, valvular, genetic cardiomyopathies, metabolic, infiltrative, toxins, infectious, arrhythmic, and pericardial). Furthermore, comorbidities such as chronic kidney disease or chronic obstructive pulmonary disease are common. Obviously, knowing the underlying cause and any significant comorbidity has large prognostic and therapeutic consequences. It is therefore important to develop new diagnostic methods, and not least new biomarkers, that improve the diagnosis of HF, identify underlying causes, enhance risk assessment and guide therapy.
The natriuretic peptides (discussed in depth in Chaps. 13, 14, 15. 16, 17, 18, 19) have gained widespread use the last 15–20 years, and are undoubtedly valuable for diagnosis, prognosis and to some extent for guiding therapy. However, there are several areas in which additional biomarkers would be valuable. Several biomarkers, reflecting other pathophysiological mechanisms than the natriuretic peptides, have been suggested and evaluated, such as Soluble ST2 (sST2) (Chap. 21), Growth differentiation factor (GDF)-15, Mid-regional pro adrenomedullin (MR-proADM), Galectin-3 and cardiac troponins (Chaps. 7, 8, 9). All these markers have been shown to provide prognostic information in heart failure patients, in some studies independent of the natriuretic peptides and thus, providing additional prognostic information. However, the main limitations of these markers are that no one has shown any clinically meaningful added value for diagnosis of heart failure or selection of heart failure treatment. Hence, it is doubtful that these markers will gain widespread clinical use in heart failure patients, except cardiac troponins. Cardiac troponins are routinely used in patients with suspected acute heart failure to verify or exclude concomitant myocardial infarction or injury.
Gap 1: Heart Failure in Patients with Comorbidities
Patients with heart failure often have coexisting diseases that complicate the diagnostic process, e.g., the interpretation of the level of BNP or NT-proBNP is difficult in patients with depressed renal function. Other examples of diagnostic difficulties are patients with known chronic heart failure and chronic obstructive pulmonary disease, or with known chronic heart failure and signs of a respiratory infection that comes to the ED with worsening symptoms of dyspnea. In these situation multimarker approaches probably is the way forward; intelligent combinations of new and existing markers.
Gap 2: Heart Failure with Reduced Ejection Fraction Versus Preserved Ejection Fraction (Case 3)
Approximately, half of the heart failure population has heart failure with reduced ejection fraction (HFrEF), and the other half has heart failure with preserved ejection fraction (HFpEF). However, there is a gender difference; HFpEF is more common than HFrEF in women and vice versa in men. Both patients with HFpEF and HFrEF still have a poor prognosis, although patients with HFpEF have a slightly lower long term mortality compared to HFrEF, hazard ratio (95 % confidence interval) 0.86 (0.77-0.97) [12]. There is a strong evidence based foundation for the treatment recommendations in HFrEF. In contrast, there is no specific evidence based treatment for HFpEF. Hence, it is clinically important to separate these two entities. Furthermore, it may be challenging to diagnose heart failure in patients with HFpEF, especially in patients with an abnormal relaxation filling pattern in whom the levels of natriuretic peptides may be normal. Consequently, a limited utility of natriuretic peptides for the detection of mild systolic and diastolic dysfunction was found in a large unselected population [13].
Gap 3: Heart Failure of Ischemic Origin Versus Non-ischemic Origin (Case 3)
Identification of the underlying cause of the heart failure has important therapeutical consequences. In case of underlying coronary artery disease it is critical to determine whether there are signs of hibernating myocardium and thus a potentially reversible cause of the depressed ventricular function. Heart failure of nonischemic origin includes a wide variety of causes such as hypertension, genetic cardiomyopathies, valvular, congenital, metabolic, infiltrative, infectious, arrhythmic diseases, all with different treatment and different prognosis. It can sometimes be difficult and cumbersome to identify the cause. However, to make it even more complex, the causes of heart failure are not mutually exclusive; not seldom two or more causes exist in parallel and it can be very difficult to determine their relative importance. Therefore, biomarkers capable of identifying specific underlying causes would potentially be clinically useful.
What Novel Biomarkers Might Be Synergistic in Patients with Acute Disease
The Road to a Clinically Useful and Widely Adopted Biomarker
As discussed previously, to be clinically useful a novel biomarker must provide information that ultimately improves the management and treatment of the individual patient.
-
The new biomarker improves the diagnostic accuracy compared to an already established marker or any other diagnostic test, provides the diagnosis faster (if time is critical in the decision making process) or provides similar accuracy but to lower costs.
-
The new biomarker makes it possible to diagnose an entity where there currently is no useful biomarker or test, or makes it possible to subclassify the disease in a clinically meaningful way.
-
The new biomarker makes it possible to select treatment or monitor treatment based on the biomarker levels.
-
The new biomarker gives prognostic information of such magnitude that it directly affects the management of the patient, e.g., identifies a subgroup of such low risk that specific treatment can be avoided.
In the following a number of biomarkers will be discussed, biomarkers which may have the potential to meet any of the above claims. However, it is important to stress that all these markers still have a long way to go to prove their clinical usefulness. Appropriately sized prospective studies in appropriate populations and with robust endpoints and best available gold standards and comparators will be needed.
Micro RNA
MicroRNAs (miRNAs) are small, non-coding, RNA molecules consisting of approximately 22 nucleotides. Currently more than 2,500 miRNAs have been described in humans. miRNAs act as post-transcriptional regulators of gene expression and are key regulators of complex biological processes. Since some of the miRNAs are possible to measure in the circulation, they have been investigated as novel biomarkers for cardiovascular diseases, especially acute myocardial infarction and heart failure. The levels of miRNAs in patients are either elevated or decreased as compared to in healthy individuals. Measuring clusters of miRNAs, instead of single miRNA, identifying characteristic signatures of miRNA might increase the diagnostic and prognostic potential.
For quantification of miRNA, high throughput sequencing, real time PCR (qPCR) or microarrays is used, all time consuming methods not particularly suitable for measurements in daily practice. Furthermore, there is a lack of standardized protocols and automatized work flows.
For comprehensive reviews of miRNA in cardiovascular disease see reference [14, 15].
Acute Myocardial Infarction and Coronary Artery Disease
A number of circulating miRNAs have shown promising results for diagnosis of AMI in small studies (e.g., miR-1, miR-133a, miR-133b, miR-208a, miR-499, miR-499-5p); some studies have suggested that measurement of miRNA may allow earlier diagnosis of AMI compared to measurement of cardiac troponins. However, in one of the few larger clinical studies [16], none of the six evaluated miRNAs was able to out-perform, or add to, cardiac troponin. Other small studies have suggested that signatures of circulating miRNAs might be useful for separating patients with unstable angina from patients with stable angina pectoris. Maybe of potentially more importance is that a number of miRNAs has been linked to a diagnosis of coronary artery disease, a condition where we currently lack useful biomarkers. However, these initial results need to be verified in larger studies.
Heart Failure
An increasing number of miRNAs have been shown to exhibit altered circulating levels in the peripheral blood of patients with heart failure (e.g., miR-122, miR-210, miR-423-5p, miR-499 and miR-622). Particularly interesting is the possibility to subclassify heart failure patients according to underlying etiology. The expression of miRNA is a highly dynamic process; with different expression of miRNAs in different stages of the conditions leading to HF. Furthermore, different expression of miRNAs have been related to HF-associated pathologies, such as hypertrophy, hypertrophic cardiomyopathy, dilated cardiomyopathy and ischemic cardiomyopathy. Consequently, some small studies have verified different expression of miRNAs in patients suffering from heart failure of ischemic and nonischemic origin. In addition several miRNAs have shown potential as prognostic indicators.
However, the evaluation of miRNAs as clinically useful biomarkers in acute coronary syndromes/coronary artery disease as well in heart failure is still in its infancy; larger and appropriately designed trials are required to establish whether current candidates provide additional benefit, over and above those of existing biomarkers. Furthermore, to be clinically useful substantial technological development are required to enable rapid, reliable and reproducible results for the absolute quantification of circulating miRNAs.
Metabolomics
Metabolomics is a term used to describe the measurement of multiple small-molecule metabolites in biological specimens. The Human Metabolome Database contains to date over 41,000 metabolites (http://www.hmdb.ca/). Metabolites represent the end-products of multiple reactions and interactions in biological processes. Therefore, determination of metabolites may allow an integrated and dynamic measurement of phenotype and medical condition.
Although there is an increasing interest in metabolomics profiling for identification of novel biomarkers or signatures of biomarkers in cardiovascular diseases, metabolomics profiling may have its largest potential for identification of novel disease mechanisms.
Metabolomic profiling is most often performed with either nuclear magnetic resonance or mass spectrometry in combination with liquid chromatography or gas chromatography. Nuclear magnetic resonance is a simpler technique not requiring chemical manipulation of samples when working with biological fluids, while mass spectrometry offers a much better sensitivity. For comprehensive reviews of metabolomics in cardiovascular disease see references [17–19].
Acute Myocardial Ischemia and Coronary Artery Disease
Since it is well known that acute restrictions of coronary flow induce dramatic and immediate shifts in cardiac metabolism there are hopes that it will be possible to diagnose cardiac ischemia by metabolomics profiling. In a small study, 32 metabolites measured in serum showed dynamic changes after ischemia, of which four (Creatine, Glucose + taurine, Lactate and Triglycerides) displayed statistical significant differences 2 h after the induction of myocardial ischemia [20]. When testing this biosignature based on the 32 metabolites, on patients with acute chest pain, it was possible to reliably discriminate patients with myocardial ischemia from those with non-coronary ischemic chest pain. However, these results need to be confirmed in much larger studies. Other studies have shown a strong association of arginine and its downstream metabolites ornithine and citrulline with coronary artery disease and with future adverse cardiovascular events.
Heart Failure
Heart failure is associated with metabolic dysfunction. Therefore, metabolomics has been applied to studies of heart failure to discover valuable biomarkers. The studies so far are small and relatively few; and have reported a diverse array of molecular profiles, perhaps reflecting the heterogeneous nature of the heart failure syndrome, but also differences in selection of patients, study design, assays used, and statistical methodology. A recent study identified a cluster of 4 metabolites (histidine, phenylalanine, spermidine, and phosphatidylcholine C34:4) that separated stage C HF patients from healthy control subjects. The discriminatory ability of this combination was similar to that of B-type natriuretic peptide alone. A different combination of 4 metabolite parameters (dimethylarginine/arginine ratio, spermidine, butyrylcarnitine, and total essential amino acids) was associated with outcome after 1.3 years, and classified patients according to risk better than B-type natriuretic peptide [21].
Protein Markers
Several new protein markers of interest for diagnosis or prognosis in acute myocardial infarction or heart failure are described elsewhere in this book, e.g., Copeptin, ST2, and Galactin 3.
Growth Differentiation Factor 15 (GDF-15) is an additional marker of potential interest that has been evaluated extensively in recent years [22, 23]. GDF-15 is a member of the transforming growth factor β superfamily; it is also known as macrophage inhibitory cytokine-1, placental transforming growth factor-beta, gene placental bone morphogenic protein, prostate-derived factor, NSAID-activated gene 1, and placental transforming growth factor Beta. GDF-15 is expressed in virtually all tissues. The exact biological functions of GDF-15 are still poorly understood, however, its expression is up-regulated with many different pathological conditions including inflammation, cancer, cardiovascular diseases, pulmonary disease and renal disease.
Owing to the lack of tissue specificity, GDF-15 has a limited potential as a diagnostic marker. However, GDF-15 has been shown to be a strong and independent predictor of mortality and disease progression in patients with established disease such as acute coronary syndromes, angina pectoris, heart failure, stroke, chronic kidney disease, and different types of cancer. Also in community-dwellers, higher concentrations of GDF-15 have been associated with increased cardiovascular as well as non-cardiovascular mortality, and development and progression of a broad range of diseases including coronary artery disease and heart failure. Especially for prediction of mortality, GDF-15 has emerged as one of the strongest biomarker (“death marker”). There is however, no strong support so far that GDF-15 is useful for selection of specific treatment or for monitoring of treatment effects in cardiovascular diseases.
Concluding Remarks
The biomarker research is a rapidly evolving field and it is hard to predict exactly where the next break through will occur in cardiovascular diseases. However, undoubtedly we will get new useful biomarkers in our diagnostic and prognostic arsenal within the next few years. The more biomarkers we get, the more important it becomes for the diagnosing physician to use them properly and interpret the results wisely. Therefore, it will be very important that adequate training in using and interpreting of new biomarkers are conducted in parallel with the introduction of the markers.
Case 1
A woman, 84 years of age, with paroxysmal atrial fibrillation, congestive heart failure, renal dysfunction and chronic back pain, was admitted due to a new episode of atrial fibrillation and chest discomfort, but no typical ischemic chest pain. She has previously been hospitalized with an AMI 5 years ago and an episode of atrial fibrillation and worsening of heart failure 3 years ago.
On admission she had an oxygen saturation of 95 % on O2 2 L/min, had no peripheral oedema, a blood pressure of 165/105 mmHg and a heart rate of 110 beats/min. The ECG showed atrial fibrillation, minor unspecific lateral ST-depression and was otherwise unchanged compared to previous recordings. Laboratory values on admission: cTnI 0.15 μg/L (Upper reference limit 0.022 μg/L); Hb 118 g/L, Creatinine 277 μmol/L.
Clinically relevant questions for the emergency physican: Is the elevation of troponin I caused by an acute ischemic event, e.g., a type 1 or type 2 AMI? Or is the elevation of troponin I expression of a chronic condition that causes a constant rise of troponin, like CHF and atrial fibrillation, or just high age? Does the elevation of troponin prompt any specific treatment?
The patient was given extra medication temporarily to control the heart rate. Sinus rhythm was restored spontaneously within a few hours and the chest discomfort disappeared. cTnI after 3 and 9 h were 0.19 and 0.14 μg/L, respectively. The patient’s medical records revealed that the corresponding cTnI values during the previous hospitalization for atrial fibrillation and heart failures were 0.24, 0.22 and 0.19 μg/L.
The cTnI levels were interpreted as chronic elevation of cTnI in an elderly patient with atrial fibrillation, CHF and renal dysfunction and not as expression of an AMI. Therefore, no coronary angiogram was performed. She was discharged in her habitual state and with unchanged medication after a couple of days to her home.
A biomarker able to detect already on admission, ongoing ischemia or to separate acutely released cardiac troponin from cardiac troponin chronically released would be helpful in this situation for the immediate management of the patient.
Case 2
A man, 75 years of age, with known hypertension and diabetes mellitus, was admitted due to 1 h of chest pain, dyspnea and palpitations. On admission he had no peripheral oedema, a blood pressure of 140/60 mmHg and a heart rate of 150 beats/min. The ECG showed atrial fibrillation and ST segment depression in inferior and lateral leads. On admission cTnI was 0.02 (upper reference limit 0.022 μg/L), Creatinine 85 μmol/L and Hb 139 g/L.
Clinically relevant questions for the emergency physician: Has the patient ongoing ischemia? In case of ischemia, is it a type 1 or a type 2 AMI? Is there an indication of acute or subacute coronary angiography?
Cardiac TnI values after 6 and 24 h were 1.9 and 7.8 μg/L, respectively. After restoring sinus rhythm the patient was without symptoms. A coronary angiogram performed day three showed minor atherosclerotic changes in proximal and mid LAD and RCA, and a stenosis of around 50 % in LCX. No PCI was attempted. Warfarin was added to the previous medication for hypertension and diabetes mellitus and the patient was discharged home. Interpretation: Type 2 AMI in a man with moderate coronary artery disease but no culprit lesion, in which the acute ischemia was triggered by an episode of rapid atrial fibrillation.
In this patient biomarkers capable of detecting ischemia and to early differentiate between type 1 and type 2 AMI, respectively, would have been valuable for the further management, e.g., for determining the need and timing of coronary angiography.
Case 3
A woman, 60 years of age, with chronic treatment with warfarin due to repeated episodes of deep venous thrombosis, was examined at the outpatient clinic because of episodes of chest pain and palpitations, and dyspnea. The chest pain and dyspnea occur at moderate exertion. The examination showed no dyspnea at rest; no oedema; heart rate 80, ordinary first and second heart sound, a systolic murmur grade 2 with maximum in I3 sinister; blood pressure 110/60; no pulmonary crepitations. ECG showed sinus rhythm, supra ventricular ectopic beats, no Q wave or ischemic ST-T changes. Laboratory values: NT-proBNP 3790 ng/L, cardiac troponin I, potassium, sodium and creatinine within normal limits. Chest X-ray showed minor to moderate amount of pleural effusion and minor signs of interstitial pulmonary oedema.
The condition was interpreted as heart failure, possibly on the basis of ischemic heart disease. The patient was referred for echocardiography and myocardial scintigraphy.
The myocardial scintigraphy showed moderately reduced working capacity and chest pain during exercise that slowly vanished at rest. No significantly reduced myocardial uptake of the isotope, neither at rest nor during exercise. The left ventricular had normal size and a calculated ejection fraction of 62 %. Echocardiography showed also normal ejection fraction but clear signs of diastolic dysfunction and moderate pulmonary hypertension. No evidence of significant valvular engagement.
The condition was interpreted as heart failure with preserved ejection fraction (HFpEF), ischemic heart disease or hypertension unlikely causes. Despite medication the patient showed progression of the symptoms and with continuously highly elevated NT-proBNP values. The patient was referred for further investigation with right sided catheterization including myocardial biopsy.
The right sided catheterization showed a picture consistent with restrictive cardiomyopathy and secondary pulmonary hypertension. The biopsy verified cardiac amyloidosis. The patient was treated with autologous stem cell transplantation.
References
Jernberg T, Johanson P, Held C, Svennblad B, Lindbäck J, Wallentin L. Association between adoption of evidence-based treatment and survival for patients with st-elevation myocardial infarction. JAMA. 2011;305(16):1677–84.
Gabet A, Juillière Y, Lamarche-Vadel A, Vernay M, Olié V. National trends in rate of patients hospitalized for heart failure and heart failure mortality in France, 2000–2012. Eur J Heart Fail. 2015;17(6):583–90.
Rodríguez-Antona C, Taron M. Pharmacogenomic biomarkers for personalized cancer treatment. J Intern Med. 2015;277(2):201–17.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–67.
Backus BE, Six AJ, Kelder JC, et al. A Prospective validation of the HEART score for chest pain in the emergency department: a multinational validation study. Int J Cardiol. 2013;168(3):2153–8.
Eggers KM, Lind L, Venge P, Lindahl B. Will the universal definition of myocardial infarction criteria result in an overdiagnosis of myocardial infarction? Am J Cardiol. 2009;103(5):588–91.
Bjurman C, Larsson M, Johanson P, Petzold M, Lindahl B, Fu MLX, et al. Small changes in troponin T levels Are common in patients with non–ST-segment elevation myocardial infarction and are linked to higher mortality. J Am Coll Cardiol. 2013;62(14):1231–8.
Möckel M, Searle J. Copeptin—marker of acute myocardial infarction. Curr Atheroscler Rep. 2014;16(7):1–8. English.
Streng AS, de Boer D, van der Velden J, van Dieijen-Visser MP, Wodzig WKWH. Posttranslational modifications of cardiac troponin T: an overview. J Mol Cell Cardiol. 2013;63:47–56.
Ostermann M, Lo J, Toolan M, Tuddenham E, Sanderson B, Lei K, et al. A prospective study of the impact of serial troponin measurements on the diagnosis of myocardial infarction and hospital and six-month mortality in patients admitted to ICU with non-cardiac diagnoses. Crit Care. 2014;18(2):R62. PubMed PMID: doi:10.1186/cc13818.
Collinson PO, Lindahl B. Type 2 myocardial infarction – the chimaera of cardiology? Heart. 2015;101(21):1697–703. In Press.
Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996–1004.
Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109(25):3176–81.
Romaine SPR, Tomaszewski M, Condorelli G, Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101(12):921–8.
Schulte C, Zeller T. microRNA-based diagnostics and therapy in cardiovascular disease—Summing up the facts. Cardiovasc Diagn Ther. 2015;5(1):17–36.
Devaux Y, Mueller M, Haaf P, Goretti E, Twerenbold R, Zangrando J, et al. Diagnostic and prognostic value of circulating microRNAs in patients with acute chest pain. J Intern Med. 2015;277(2):260–71.
Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126(9):1110–20.
Senn T, Hazen SL, Tang WHW. Translating metabolomics to cardiovascular biomarkers. Prog Cardiovasc Dis. 2012;55(1):70–6.
Bodi V, Marrachelli V, Husser O, Chorro F, Viña J, Monleon D. Metabolomics in the diagnosis of acute myocardial ischemia. J Cardiovasc Transl Res. 2013;6(5):808–15. English.
Bodi V, Sanchis J, Morales JM, Marrachelli VG, Nunez J, Forteza MJ, et al. Metabolomic profile of human myocardial ischemia by nuclear magnetic resonance spectroscopy of peripheral blood serum: a translational study based on transient coronary occlusion models. J Am Coll Cardiol. 2012;59(18):1629–41.
Cheng ML, Wang C-H, Shiao M-S, Liu M-H, Huang Y-Y, Huang C-Y, et al. Metabolic disturbances identified in plasma Are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol. 2015;65(15):1509–20.
Xu X, Li Z, Gao W. Growth differentiation factor 15 in cardiovascular diseases: from bench to bedside. Biomarkers. 2011;16(6):466–75.
Lindahl B. The story of growth differentiation factor 15: another piece of the puzzle. Clin Chem. 2013;59(11):1550–2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lindahl, B. (2016). Gaps in Our Biomarker Armamentarium: What Novel Biomarkers Might Be Synergistic in Patients with Acute Disease. In: Maisel, A., Jaffe, A. (eds) Cardiac Biomarkers. Springer, Cham. https://doi.org/10.1007/978-3-319-42982-3_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-42982-3_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42980-9
Online ISBN: 978-3-319-42982-3
eBook Packages: MedicineMedicine (R0)