Abstract
Acute decompensated heart failure (ADHF) is a leading cause of hospitalization amongst elderly in the U.S. The role of natriuretic peptides (NPs) is clear in the evaluation of acute dyspnea to help diagnose ADHF. NPs are quantitative markers of wall stress and reflect severity of heart failure. In the inpatient management of ADHF, serial sampling and recognition of down-trending NPs is a powerful indicator of patients who are at lower risk for future adverse events. Serial monitoring at least once during hospitalization (or if clinical uncertainty exists) and a pre-discharge NP should be checked to ensure patients are appropriate for discharge. If persistent elevation occurs, alternate etiologies for this should be considered and medications should be further up-titrated and optimized prior to discharge.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Natriuretic peptide
- Heart failure
- Prognosis in heart failure
- Monitoring in heart failure
- Mortality in heart failure
- Cardiac Biomarkers
- Acute decompensated heart failure
Background and Physiology
The natriuretic peptides (NPs), B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP), have an established role in the approach to undifferentiated dyspnea. As discussed in earlier chapters, BNP and NT-proBNP are secreted from cardiac myocytes in response to increased wall tension, usually from volume overload and expansion. While NPs have a powerful relationship with cardiac pressure, they have a relationship with valvular heart disease, pulmonary artery pressure, heart rhythm abnormalities and coronary ischemia. BNP is the biologically active hormone with a half-life of about 21 min, while NT-proBNP is not biologically active and has an estimated half-life of 70 min.
Signs and symptoms in the diagnosis of heart failure can be non-specific and the NPs have a role in the diagnosis of acute decompensated HF (ADHF). Patients with heart failure who suffer acute decompensations are admitted and decongested to a point where they appear near euvolemia with medication optimization and then discharged with outpatient follow up. Although these patients may feel symptomatically improved, they have high rates of readmission for heart failure, possibly due to inadequate decongestion and subclinical hypervolemia [1]. Risk stratification for future events in patients with ADHF is difficult since prognosis is often determined by factors such as New York Heart Association (NYHA) and functional class.
Readmissions for HF contribute greatly to the cost of medical care in the United States. Thus, if one could find ways to optimize medical therapy for those with ADHF, one could lower the rates of readmissions and decrease associated healthcare costs. The inpatient management of heart failure and incorporation of NPs is evolving. The rationale behind the routine use of NPs for inpatient HF monitoring is simple. Most proven HF therapies have been shown to decrease NP concentrations [2–5] and decreases in NP concentration over time have also demonstrated favorable outcomes. Therefore, strategies involving titration of therapy towards specific NP targets may improve outcomes (Fig. 17.1).
Value of NPS in ADHF
One of the difficulties in our ability to optimize patients admitted with ADHF is our limitations in clinical assessment of volume status. Accurate assessment of volume status is difficult. We rely on exam findings including jugular venous pressure, crackles on pulmonary exam, S3 gallop on cardiovascular exam, and lower extremity edema. Additionally, weights are often checked as an indicator of total body volume depletion with diuresis and decongestion. Therefore, accurate volume status can be considered to some extent an art with low inter-rater reliability. In contrast, NPs are quantitative, reproducible, and can serve as a potential surrogate for hypervolemia. In studies with invasive hemodynamic monitoring, NPs have had a positive relationship with pulmonary capillary wedge pressure (PCWP) [6]. However, placement of a pulmonary artery catheter has complications and as such is mainly reserved for patients with severe decompensations usually requiring inotrope therapy and/or those in undifferentiated shocks. Appropriate management can be difficult with over-diuresis running the risk of significant electrolyte abnormalities, acute kidney injury, orthostatic hypotension, syncope, and acute renal failure. Under-diuresis may lead to non-optimization of volume status prior to discharge with subsequent re-hospitalizations and increased cost, hypoxia, or cardiorenal syndrome. Thus, a more objective guide to management of fluid status would be beneficial. NPs may represent subclinical congestion that is difficult to assess on exam and persistently elevated concentrations may indicate mild hypervolemia.
In addition to volume status estimation, NPs also indicate persistent elevation of the renin-angiotensin aldosterone system (RAAS). The NPs are in fact the counter-regulatory measure to the deleterious overactivation of RAAS in ADHF [7]. Treatment of ADHF decreases NPs, endothelin, and circulating norepinephrine [8]. Therefore, the persistent elevation of the deleterious systems including RAAS and catecholamines are also indicated by elevations of NPs during treatment of ADHF. Therapies which lower NPs would indirectly indicate down-regulation of RAAS.
When assessing elevated concentrations of NPs, it is important to note that there is no cutoff that is 100 % diagnostic of HF. Alternate etiologies for elevation of NPs should always be considered. It should be noticed that heart failure with reduced ejection fraction (HFrEF) is known to have greater NP concentrations than in preserved ejection fraction (HFpEF). Alternate causes of NP elevation include dysrhythmias, cor pulmonale, pulmonary embolism, pulmonary hypertension and valvular heart diseases. Furthermore, renal dysfunction may cause higher concentrations of NPs and obesity may cause falsely lower concentrations. It is important to keep these caveats in mind when interpreting initial NP concentrations and during hospitalization when assessing response to therapy.
Prognostic Value of NPS
Knowledge of which patients admitted with ADHF are at highest risk for future adverse events is important in a disease with such morbidity and mortality. The NPs have a significant role in prognostication; many believe BNP values have two components. One represents the “dry” or euvolemic component, and the other represents the “wet” or hypervolemic component due to acute congestion.
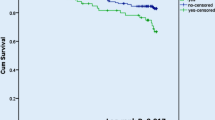
BNP measured on admission in ADHF is an independent predictor of in-hospital and future mortality and cardiovascular events in patients who presented with acute heart failure [9–12]. Given its association with reduced left ventricular ejection fraction (LVEF) and worsened NYHA functional status, this is not an unexpected finding but important for identifying high risk patients and establish closer follow up. Interestingly, NPs measured on discharge from HF hospitalization have been increasingly more useful. Multiple studies have demonstrated persistently elevated NPs on discharge (absolute and as compared to admission) portend poor outcomes [13–15]. Those with pre-discharge BNP less than 350 pg/mL have the lowest incidence of 6 month events [14] (Fig. 17.2). Incorporation of a pre-discharge NT-proBNP has also demonstrated similar prognostic ability as BNP [15]. Whether admission, discharge, or change in NP during hospitalization, is the most significant prognostic indicator has also been analyzed. The most important indicator in an analysis of 7,039 elderly patients with ADHF demonstrated discharge BNP was the most important characteristic for predicting 1-year mortality or re-hospitalization [16]. In addition to assessment of decrease prior to discharge, individuals have also studied whether an absolute versus percent change of NP has greater prognostic value. One study demonstrated that a percent change of NT-proBNP is more important for prediction of HF hospitalization-free survival than absolute value [15] while others demonstrated that a lower absolute BNP on discharge is more predictive than a percent decrease [16]. These data provide us the framework for creating an algorithm for how to utilize inpatient NP monitoring for those with ADHF (Fig. 17.3). Timely prognostic information by serial NP measurements allows clinicians to intensify treatment during hospitalization and improve prognosis.
BNP levels less than 350 pg/mL on discharge have the improved long-term outcomes (From Logeart et al. [14], with permission)
Case 1
A 63-year-old male with a history of ischemic cardiomyopathy (LVEF 48 %, dry BNP 180 pg/mL), chronic kidney disease (baseline serum creatinine 1.5 mg/dL), persistent atrial fibrillation (on novel anticoagulant rivaroxaban), presents with shortness of breath at rest, 8 lb of weight gain, and leg swelling. His exam is significant for elevated jugular venous pressure (14 cm H2O), bibasilar rales, S3 gallop and peripheral edema. His labs are unchanged from baseline except for BNP of 930 pg/mL.
The patient was admitted to general cardiology service and started on afterload reduction with his home dose of ACE inhibitor, beta blocker, and was diuresed with IV bumetanide with appropriate response. Patient’s symptoms and exam findings improved on hospital day 2, with a decrease in weight by 3 lb. His BNP on hospital day 2 was 860 pg/mL.
Patient’s current regimen was continued with mild improvement of signs and symptoms of congestion on hospital day 3. Patient was ambulating without symptoms and his BNP was 870 pg/mL.
Alternate etiologies for persistence of NP elevation were considered which included renal failure, pulmonary embolism, and dysrhythmia. There was no suggestion of any of these alternate causes, therefore it was concluded that the patient was far from optimization even though his symptoms and exam findings have improved. Patient’s medications were reviewed and his beta blocker dose was increased and addition of low-dose mineralocorticoid antagonist was initiated given this persistence of BNP elevation. His diuresis was continued and on discharge his BNP had decreased to 380 pg/mL with resolution of his initial symptoms and exam findings.
Conclusion
The natriuretic peptides have a solidified role in the diagnosis of ADHF in those with undifferentiated dyspnea. Their measurement at baseline in those with ADHF correlates with degree of HF severity and reflect long-term prognosis. While active treatment of HF ensues, NPs have been shown to downtrend reflecting improvement of hemodynamics and the RAAS system as well. During hospitalization, re-check of NP concentration during hospitalization should be considered if a patient’s clinical status is in question. More importantly, NPs should be checked prior to discharge and if a decrease is not observed these patients should be considered highest risk for adverse event and aggressive medical up-titration should be considered.
References
Maisel AS. Use of BNP levels in monitoring hospitalized heart failure patients with heart failure. Heart Fail Rev. 2003;8:339–44.
Troughton RW, Richards AM. Outpatient monitoring and treatment of chronic heart failure guided by amino-terminal Pro-B-type natriuretic peptide measurement. Am J Cardiol. 2008;101(Suppl):72A–5.
Tsutamoto T, Wada A, Maeda K, et al. Effect of spirinolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001;37:1228–33.
Rousseau MF, Gurne O, Duprez D, et al. Beneficial neurohormonal profile of spirinolactone in severe congestive heart failure: results from the rales neurohormonal substudy. J Am Coll Cardiol. 2002;40:1596–601.
Kohno M, Minami M, Kano H, et al. Effect of angiotensin-converting enzyme inhibitor on left ventricular parameters and circulating brain natriuretic peptide in elderly hypertensives with left ventricular hypertrophy. Metabolism. 2000;49:1356–60.
Kazanegra R, Cheng V, Garcia A, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. J Card Fail. 2001;7:21–9.
Munagala VK, Burnett Jr JC, Redfield MM. The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol. 2004;29:707–69.
Johnson W, Omland T, Hall C, Lucas C, Myking OL, Collins C, Pfeffer M, Rouleau JL, Stevenson LW. Neurohormonal activation rapidly decreases after intravenous therapy with diuretics and vasodilators for class IV heart failure. J Am Coll Cardiol. 2002;39(10):1623–9.
Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49:1943–50.
Yu CM, Sanderson JE. Plasma brain natriuretic peptide—an independent predictor of cardiovascular mortality in acute heart failure. Eur J Heart Fail. 1999;1:59–65.
Tamura K, Takahashi N, Nakatani Y, Onishi S, Iwasaka T. Prognostic impact of plasma brain natriuretic peptide for cardiac events in elderly patients with congestive heart failure. Gerontology. 2001;47:46–51.
Harrison A, Morrison LK, Krishnaswamy P, Kazanegra R, Clopton P, Dao Q, et al. B-type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea. Ann Emerg Med. 2002;39:131–8.
O’Brien RJ, Squire IB, Demme B, Davies JE, Ng LL. Pre-discharge, but not admission, levels of NT-proBNP predict adverse prognosis following acute LVF. Eur J Heart Fail. 2003;5(4):499–506.
Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, Bouvier E, Solal AC. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–41.
Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–74.
Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O’Connor CM, Felker GM, Hernandez AF. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail. 2011;4(5):628–36.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Shah, K., Iwaz, J. (2016). Heart Failure: Natriuretic Peptide Use in the Hospital. In: Maisel, A., Jaffe, A. (eds) Cardiac Biomarkers. Springer, Cham. https://doi.org/10.1007/978-3-319-42982-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-42982-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42980-9
Online ISBN: 978-3-319-42982-3
eBook Packages: MedicineMedicine (R0)