Abstract
Many allergens from grasses and trees share structural similarities that result in IgE cross-reactivity. Some of these allergens are defined as marker allergens because immunoreactivity indicates sensitization to specific botanical families of grasses or trees, whereas other allergens are classified as panallergens because they elicit immunoreactivity to many unrelated allergen sources. These allergens are presented and discussed. It is concluded that the use of recombinant marker allergens in clinical routine work improves the accurate diagnosis of allergy for the prescription of immunotherapy.
The present chapter is based on and modified from an article by the authors published in 2015 in Allergo Journal International (Gangl K, Niederberger V, Valenta R, Nandy A: Marker allergens and pan allergens in tree and grass pollen allergy. Allergo J Int 2015, 24:158–169).
The authors gratefully thank Greg Plunkett PhD, ALK Inc. Headquarters and Analytical Laboratories, Round Rock, TX, USA, for reviewing the manuscript and for his editorial assistance and helpful suggestions including a North American perspective.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Many allergens from botanically related sources share structural similarities resulting in IgE cross-reactivity. As a consequence, allergens sharing similar structures are often also related on an immunological level, and patients sensitized to one specific allergen may show clinical or in vitro reactivity to structurally similar allergenic proteins of other allergen sources. Different IgE sensitization profiles can be identified in allergic patients according to reactivity to certain allergens. These allergens are defined as marker allergens (Kazemi-Shirazi et al. 2002; Suphioglu 2000; Valenta et al. 2007).
Today, genuine allergic sensitization can be differentiated from cross-reactivity using modern allergen component resolved diagnostics (CRD) (Valenta et al. 1999; Hiller et al. 2002; Lupinek et al. 2014). In grass and tree pollen allergy, CRD can identify the appropriate immunotherapy in poly-sensitized patients. Since specific immunotherapy is time-consuming (taking up to several years) and burdensome, early identification of patients suffering from genuine sensitization to grass or tree pollen who should benefit from a specific immunotherapy is important for clinical management of patients.
Allergens that pinpoint genuine sensitization and may be defined as marker allergens for specific tree and grass pollen allergies, will be described in this article.
2 Allergen Sources in Trees and Grasses
Tree and grass pollen from wind-pollinated plants are a frequent source of allergens. Between 12 and 17 % of the general population in Europe suffer from pollen allergy with almost 10 % suffering from tree pollen allergy (Blomme et al. 2013; Wüthrich et al. 1995). After hydration, tree and grass pollen rapidly release large amounts of allergens, i.e., defined IgE-binding proteins and glycoproteins. Upon contact with the mucosal surfaces of the respiratory tract, these allergens trigger allergic symptoms in susceptible patients (Grote et al. 2001; Vrtala et al. 1993).
2.1 Grasses
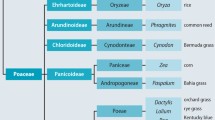
Most allergenic grasses belong to a botanical family of grasses called Poaceae, which is mainly found in temperate climate zones. As examples, timothy grass (Phleum pratense), perennial ryegrass (Lolium perenne), orchard grass (Dactylis glomerata), and Kentucky bluegrass (Poa pratensis) belong to the Pooideae subfamily and are closely related. Other grasses, such as Bermuda grass (Cynodon dactylon), Rhodes grass (Chloris gayana), love grass (Eragrostis tenella), rice (Oryza sativa), common reed (Phragmites communis), Bahia grass (Paspalum notatum), Johnson grass (Sorghum halepense), corn (Zea mays), and buffel grass (Cenchrus ciliaris), belong to the Chloridoideae, Ehrhartoideae, Arundinoideae, and Panicoideae subfamilies, respectively, found in subtropical and tropical climate zones (Andersson and Lidholm 2003; Hejl et al. 2009; Johansen et al. 2009; Simon et al. 2011; Davies 2014). An overview of the botanical relationship between grasses is shown in ⦿ Fig. 10.1.
Phylogenetic botanical relationship between important allergenic grasses (Adapted from Simon et al. (2011))
2.2 Trees
Unlike grass pollen, allergenic tree pollen originates from different botanical groups of spermatophytes occurring in different geographical regions (Mothes et al. 2004; Swoboda et al. 2008; Marth et al. 2014). The following overview and ⦿ Fig. 10.2 have been compiled according to the principles of phylogenetic classification (The Angiosperm Phylogeny Group 2009; Christenhusz et al. 2011).
The majority of trees are flowering plants (angiosperms). An important group of cross-reactive allergenic tree pollen originates from two families of the order Fagales:
-
The Betulaceae family (birch, Betula verrucosa; alder, Alnus glutinosa; hazelnut, Corylus avellana; and hornbeam, Carpinus betulus)
-
The Fagaceae family (oak, Quercus alba; common beech, Fagus sylvatica; and chestnut, Castanea sativa)
These trees are mainly found in temperate climate zones of Northern Europe, North America, and other continents (Wuthrich et al. 1995; Mothes et al. 2004; Asam et al. 2015).
Trees of the family Oleaceae (order Lamiales) are the source of a second important group of cross-reactive allergenic pollen. They are endemic all over Europe, but are also found in North America and other continents (Marth et al. 2014; Asam et al. 2015). The olive tree (Olea europaea) is the most widely spread species, especially in the Mediterranean region (Bousquet et al. 1984), but Olea ssp. is also found in other continents in areas with a Mediterranean climate, e.g., the Southwestern USA. Other allergenic members of the Oleaceae family are privet (Ligustrum vulgare), lilac (Syringa vulgaris), and ash trees (Fraxinus excelsior).
In some areas of the Mediterranean, different species of plane tree (order Proteales, family Platanaceae) represent a locally important source of allergens originating from angiosperms. Plane trees are also increasingly planted for ornamental purposes in many regions of Europe and North America and may cause allergic symptoms there (Asam et al. 2015).
Another important source of cross-reactive allergenic pollen originates from the botanical group of gymnosperms. The most important trees belong to the order of Cupressales (family Cupressaceae), such as the Arizona cypress (Cupressus arizonica), mountain cedar (Juniperus ashei), and the Japanese cedar (Cryptomeria japonica) (Swoboda et al. 2008; Di Felice et al. 2001; Marth et al. 2014). While the Arizona cypress has been widely exported from its native southwest of North America to Europe, and is frequently found in regions around the Mediterranean, the mountain cedar is of particular importance in southwestern North America, especially in Texas.
3 Important Grass Pollen Allergens
⦿ Table 10.1 gives an overview of the most important grass pollen allergens.
3.1 Allergens Found in all Poaceae Grasses
3.1.1 Marker Allergen for all Poaceae Grasses: Group 1 (Phl p 1)
Group-1 allergens have been isolated and/or cloned from more than 20 Poaceae species (Andersson and Lidholm 2003; Griffith et al. 1991; Johnson and Marsh 1965; Laffer et al. 1994a; Perez et al. 1990). Phl p 1 is the group-1 allergen of timothy grass. It has a sequence identity of between 85 and 95 % with other members of the Pooideae subfamily. Most amino acid substitutions found in isoforms and in group-1 allergens of other Pooideae species (e.g., Hol l 1, Poa p 1, und Lol p 1) do not significantly alter allergenicity of the molecule (Andersson and Lidholm 2003; Johansen et al. 2009; Laffer et al. 1994a, b). Most IgE epitopes of Phl p 1 cluster at the c-terminus (Flicker et al. 2006), and grass pollen-specific IgE antibodies have been shown to bind with high density to the Phl p 1 molecule (Madritsch et al. 2015).
Up to 90 % of all grass pollen allergic patients show IgE reactivity to group-1 allergens of other grass species (Andersson and Lidholm 2003; Johansen et al. 2009; Laffer et al. 1994a, 1996; Van Ree et al. 1992). Phl p 1 is the most important group-1 allergen and represents an important cross-reactive major allergen. Cross-reactivity of group-1 allergens has been demonstrated in many studies with natural extracts of different Pooideae species and other Poaceae subfamilies (Johansen et al. 2009; Laffer et al. 1994b; Van Ree et al. 1992). Purified recombinant Phl p 1 inhibited binding of patient sera to natural extracts of eight different grasses (timothy grass, Phleum pratense; sweet vernal grass, Anthoxanthum odoratum; oat, Avena sativa; Bermuda grass, Cynodon dactylon; perennial ryegrass, Lolium perenne; common reed, Phragmites communis; Kentucky bluegrass, Poa pratensis; and rye, Secale cereale) inducing an average inhibition of 76 % (Laffer et al. 1996). Monoclonal antibodies raised against Phl p 1 and defining four distinct epitopes as well as recombinant human Phl p 1-specific IgE-Fabs (antigen-binding fragments) recognize and bind to a panel of natural group-1 allergens of different Pooideae grasses (Flicker et al. 2006; Duffort et al. 2008).
Sequence homologies and cross-reactivity between Phl p 1 and group-1 allergens of subtropical grasses such as Bermuda grass (Cynodon dactylon; 67–70 % sequence identity) or Bahia grass (Paspalum notatum) are less pronounced (Andersson and Lidholm 2003; Johansen et al. 2009; Davies 2014; Timbrell et al. 2014). There is no complete cross inhibition between group-1 allergens of grasses originating in temperate climate zones and group-1 allergens of grasses originating in subtropical climate zones, especially with patient sera from subtropical climate zones (overview presented in Davies (2014)). However, there are indications that these species-specific IgE epitopes are not protein epitopes but carbohydrate epitopes without clinical relevance (Cabauatan et al. 2014).
Phl p 1 is the most important marker allergen for genuine sensitization to grasses belonging to all subfamilies of Poaceae for the following reasons:
-
Approximately 90 % of grass pollen allergic patient sera contain specific IgE against Phl p 1.
-
Group-1 allergens have been found in all Poaceae grasses, but not in other taxonomically unrelated plants.
-
There is widespread cross-reactivity between group-1 allergens from different grass species (Niederberger et al. 1998a).
3.1.2 Group 13
The group-13 grass pollen allergen, a 55-kDa protein, has also been described in all grasses examined to date (Suck et al. 2000). Although over 50 % of grass pollen allergic patients display IgE reactivity against Phl p 13, it has only little clinical relevance as it showed only low allergenic reactivity in clinical and in vitro studies (Westritschnig et al. 2008).
3.2 Allergens Found Only in Pooideae Grasses
3.2.1 Marker Allergen for Pooideae: Group 5 (Phl p 5)
Group-5 allergens are marker allergens for Pooideae grasses. Homologous allergens have been found in all grasses of the Pooideae subfamily, such as timothy grass (Phleum pratense), rye (Secale cereale), Kentucky bluegrass (Poa pratense), and perennial ryegrass (Lolium perenne). Group-5 allergens are not found in grasses belonging to the Panicoideae, Chloridoideae, Ehrhartoideae, or Arundinoideae subfamilies, which are mainly distributed in the southern hemisphere and are highly prevalent in tropical and subtropical climate zones (Davies 2014). Group-5 allergens are not found in corn (Zea mays), Bermuda grass (Cynodon dactylon), or rice (Oryza sativa), for example (Niederberger et al. 1998a).
Phl p 5, one of the best characterized group-5 allergens, is one of several allergens to occur in different isoallergenic forms as Phl p 5a (i.e., Phl p 5.01) and Phl p 5b (i.e., Phl p 5.02). The overall sequence identity between Phl p 5a and Phl p 5b is approximately 65 % but is higher (70–77 %) in important parts of the molecule. Multiple independent IgE epitopes have been identified on both isoforms of Phl p 5 (Levin et al. 2014).
Between 65 and 85 % of grass pollen allergic patients in temperate climate zones display IgE reactivity to group-5 allergens, and the clinical allergenic activity of Phl p 5a is very high (Andersson and Lidholm 2003; Westritschnig et al. 2008; Flicker et al. 2000; Vrtala et al. 1993).
Most patients display extensive IgE cross-reactivity to the Phl p 5 isoallergens as well as to different group-5 allergens from Pooideae grasses (Andersson and Lidholm 2003; Niederberger et al. 1998a; van Ree 2002).
Phl p 5 is therefore an important marker allergen for sensitization to grasses of the Pooideae subfamily.
3.2.2 Other Pooideae-Specific Allergens
Group-2/group-3 and group-6 allergens are also only found in the pollen of Pooideae grasses. In some populations more than 50 % of grass pollen allergic patients display IgE reactivity to these molecules, yet the overall rate of patient sensitization is not high enough to give them the status of marker allergens (for an overview, see Andersson and Lidholm 2003; Gangl et al. 2013). Although patient IgE titers against group-2/group-3 allergens are often rather low, Phl p 2 shows high allergenic activity in skin tests (Westritschnig et al. 2008). The allergenic activity of Phl p 6 has not been tested yet in clinical studies.
Group-11 allergens are not very important in the clinic. Although few patients react with these allergens, they have been found in Phleum pratense and Lolium perenne (Marknell DeWitt et al. 2002), and homologs from other plants, e.g., olive (Ole e 1), corn (Zea m 13), and tomato, have been identified. Cross-reactivity between homologs from taxonomically unrelated allergen sources is very limited.
3.3 Allergens from Tropical and Subtropical Grasses
Subtropical grasses of the Panicoideae (e.g., Paspalum notatum (Bahia grass), Sorghum halepense (Johnson grass), Imperata cylindrica (cogon grass), Cenchrus sp. (buffel grass)) and Chloridoideae (e.g., Cynodon dactylon (Bermuda grass), Eragrostis sp. (e.g., Boer love), Chloris gayana (Rhodes grass)) subfamilies are abundant in regions adjacent to the equator (Esch 2004; Seidel et al. 2008) and appear to be clinically important for pollen allergy in subtropical regions of Africa, Asia, Central America, and southern parts of the USA (e.g., Florida, Texas) (Prescott and Potter 2001; Liang et al. 2010; Sam et al. 1998; Phillips et al. 1989; Calabria and Dice 2007). Allergens of Panicoideae (Pas n 1 and Pas n 13 of Bahia grass pollen and Sor h 1, Sor h 2, Sor h 13, and Sor h 23 of Johnson grass) and Chloridoideae (Cyn d 1, Cyn d 4, and Cyn d 22) species have potential to serve as diagnostic markers, but studies in relevant patient populations with recombinant allergens devoid of CCD are needed to determine the clinical relevance (Cabauatan et al. 2014; Davies et al. 2011; Davies 2014; Campbell et al. 2015). Notably, studies from Zimbawe show that tropical grasses are more prevalent than temperate grasses (Westritschnig et al. 2003).
Subjects with and without allergic symptoms from the Philippines contained IgE antibodies against tropical grasses and were mainly sensitized against carbohydrate epitopes which did not induce basophil activation. In this study it is likely that sensitization to tropical grasses is of low clinical relevance, but this needs to be investigated in other populations (Cabauatan et al. 2014). Recombinant Pas n 1 of Bahia grass pollen activated basophils of grass pollen allergic patients indicating clinically relevant sensitization in the Australian population exposed to and allergic to Bahia grass pollen (Davies et al. 2008).
3.4 Marker Allergens for Grass Pollen Allergy: Summary
Group-1 and group-5 allergens account for 60–80 % of grass pollen allergic patient IgE in different populations from different geographic areas (Laffer et al. 1996). Extensive cross inhibition of IgE binding of patients to nine different grass pollen extracts (sweet vernal grass, Anthoxanthum odoratum; oat, Avena sativa; Bermuda grass, Cynodon dactylon; perennial ryegrass, Lolium perenne; common reed, Phragmites australis; Kentucky bluegrass, Poa pratensis; rye, Secale cereale; wheat, Triticum sativum; and corn, Zea mays) was achieved with a small panel of purified, recombinant, grass pollen allergens (Phl p 1, Phl p 2, and Phl p 5) and profilin (Bet v 2) (Niederberger et al. 1998a). In a clinical vaccination study involving 64 subjects, patients were successfully treated with a mixture of recombinant Phl p 1, Phl p 2, Phl p 5a + b, and Phl p 6 (Jutel et al. 2005). A proof of principle was thus established that successful therapy of temperate grass pollen allergy in patients from Europe is possible using a combination of distinct grass pollen-specific and clinically important allergens. Recently, a novel recombinant hypoallergenic grass pollen allergy vaccine based on allergen peptides derived from the abovementioned grass pollen allergens has been developed for safe immunotherapy of grass pollen allergy (Focke-Tejkl et al. 2015) and, in clinical trials, has been shown to be hypoallergenic (Niederberger et al. 2015) as well as clinically effective for treatment (Zieglmayer et al. 2016; Cornelius et al. 2016; Gerlich and Glebe 2016).
Group-1 and group-5 allergens, such as Phl p 1 and Phl p 5, are the most suitable marker allergens for diagnosis of grass pollen allergy in temperate climate zones.
3.5 Carbohydrate Sensitivity in Grass Pollen Allergic Patients
Phl p 1, Phl p 4, Phl p 11, Phl p 13 and their subtropical orthologs (e.g., Cyn d 1, Cyn d 4, and Pas n 13) are glycoproteins carrying cross-reactive carbohydrate determinants (CCD). Using CCD-free recombinant allergens in allergen CRD has the advantage that only functional IgE (i.e., capable of IgE aggregation) directed against protein epitopes is detected. For instance, up to 85 % of grass pollen allergic patients have detectable group-4 allergen-specific IgE. Group-4 allergens are glycoproteins with a molecular weight of 50–67 kDa. However, specific IgE in patient sera is often rather low, and despite in vitro reactivity, no clinical reactivity has been described (Andersson and Lidholm 2003; Westritschnig et al. 2008; Niederberger et al. 2001; Zafred et al. 2013). In tropical regions, IgE cross-reactivity is based nearly exclusively on CCD of these glycoproteins (Cabauatan et al. 2014), and in temperate climate zones, the frequency of sensitization seen in patient sera is less than 60 % if recombinant Phl p 4 is used for diagnosis (Tripodi et al. 2012).
Phl p 4 homologous proteins are found in Ambrosia sp. and birch pollen, as well as in peanut, apple, celery, and carrot, but clinical significance remains unclear (Grote et al. 2002).
4 Important Tree Pollen Allergens
⦿ Table 10.2 gives an overview of the most important tree pollen allergens.
4.1 Allergens of Trees of the Order Fagales
4.1.1 Marker Allergen for Fagales: Bet v 1
The complementary deoxyribonucleic acid (cDNA) of Bet v 1, the major allergen of birch, was isolated in 1989 (Breiteneder et al. 1989), the 17-kDa protein allergen was produced using recombinant gene technology, and IgE reactivity in up to 95 % of birch pollen allergic patients was detected (Valenta et al. 1991b; Menz et al. 1996).
The major allergens of other tree pollen in the order of Fagales from the families of Betulaceae, (alder, Alnus glutinosa, Aln g 1; hornbeam, Carpinus betulus, Car p 1; hazelnut, Corylus avellana, Cor a 1) and Fagaceae (oak, Quercus alba, Que a 1; chestnut, Castanea sativa, Cas s 1; common beech, Fagus sylvatica, Fag s 1) all show pronounced cross-reactivities and sequence homologies within the group and to Bet v 1 (Mothes et al. 2004; Valenta et al. 1991b; Ipsen and Hansen 1991; Marth et al. 2014). Together, they form a group known as pathogenesis-related proteins (PR-10). Recombinant Bet v 1 inhibits IgE reactivity of patient sera with other tree pollen of the Fagales order (Niederberger et al. 1998b). A great number of proteins from different plant foods (nuts, vegetables and spices) display homology and cross-reactivity to Bet v 1, e.g., apple (Malus domestica, Mal d 1), hazelnut (Corylus avellana, Cor a 1), sweet cherry (Prunus avium, Pru av 1), apricot (Prunus armeniaca, Pru ar 1), peach (Prunus persica, Pru p 1), pear (Pyrus communis, Pyr c 1), carrot (Daucus carota, Dau c 1), celery (Apium graveolens, Api g 1), and soybean (Glycine max, Gly m 4) (Mothes et al. 2004; Swoboda et al. 2008; Heiss et al. 1996; Kazemi-Shirazi et al. 2000), and are responsible for birch pollen-related oral allergy syndrome (see Chap. 2).
Due to the high number of IgE-binding epitopes, Bet v 1 is thought to be the original sensitizing protein in clinically manifest allergy to Fagales pollen or in oral allergy syndrome (Kazemi-Shirazi et al. 2002; Swoboda et al. 2008; Moverare et al. 2002). In birch pollen allergic patients, exposure to birch pollen primarily increased Bet v 1-specific IgE without increasing IgE to other birch pollen allergens such as Bet v 2 (Birkner et al. 1990). Allergy patients in Central Africa reacting with natural birch pollen extracts did not display IgE antibodies against Bet v 1 but against other birch pollen allergens (Westritschnig et al. 2003; Odongo et al. 2015).
Several studies have shown that subcutaneous immunotherapy with birch pollen extract alone is equally effective as therapy with a mixture of different Fagales tree pollen extracts in tree pollen allergic patients (Henzgen et al. 1989; Petersen et al. 1988). Allergy diagnosis (skin test, specific IgE) with recombinant Bet v 1 is as effective in detecting birch pollen allergic patients as diagnosis using natural birch pollen extracts (Tresch et al. 2003).
As a consequence of these in vitro and in vivo data, Bet v 1 represents the marker allergen for sensitization to Fagales tree pollen and indicates the possibility of an associated oral allergy syndrome.
4.1.2 Other Fagales-Specific Minor Allergens
Bet v 6 (formerly known as Bet v 5), an isoflavone reductase, is a minor allergen which is cross-reactive with pollen and proteins from several edible plants (fruits, vegetables, and spices); Bet v 7 is a cyclophilin. Both are recognized by less than 20 % of birch pollen allergic patients. Bet v 8 is a pectinesterase with a clinical significance that has yet to be determined (for an overview, see (Mothes et al. 2004; Marth et al. 2014) and Table 10.2).
4.2 Allergens of Trees of the Order Lamiales
4.2.1 Marker Allergen for Lamiales: Ole e 1
Ole e 1, the most important olive pollen allergen, exists in a non-glycosylated (19 kDa) and a glycosylated (21 kDa) form and is recognized by more than 70 % of olive pollen allergic patients (Villalba et al. 1993). It displays substantial sequence homologies with other members of the Ole-e-1-like protein family. This protein family derives from pollen of other Oleaceae species (for an overview, see Rodriguez et al. 2007) such as:
-
Ash (Fraxinus excelsior, Fra e 1)
-
Privet (Ligustrum vulgare, Lig v 1)
-
Lilac (Syringa vulgaris, Syr v 1)
Moreover this protein family comprises Pla l 1 from plantain (Plantago lanceolata, family of Plantaginaceae) as well as allergens from taxonomically unrelated species such as Lol p 11 from Lolium perenne (perennial ryegrass), Phl p 11 from Phleum pratense (timothy grass), and Che a 1 from Chenopodium album (white goosefoot).
There is extensive cross-reactivity between Ole e 1 homologous allergens of the Oleaceae (Overview in Valenta et al. 2007). IgE from sera of two different groups of European patients either sensitized to olive or ash pollen was inhibited from binding to extracts of different Oleaceae pollen by Ole e 1. Birch pollen, grass pollen, and weed pollen extracts did not inhibit patient IgE binding to Ole e 1 (Palomares et al. 2006) showing the existence of specific epitopes for Oleaceae pollen in Ole e 1.
In patients from regions without local sources of olive pollen such as Austria, Germany, or Northern Italy, specific IgE against Ole e 1 indicates a sensitization to ash pollen (Fraxinus excelsior, Fra e 1) (Asero 2011; Niederberger et al. 2002). This is relevant in patients showing clinical symptoms during the birch pollen season, but who are not sensitized to birch or any other member of the Fagales order (Palomares et al. 2006).
Ole e 1 is the marker allergen for sensitization to olive pollen and is important in this respect in the Mediterranean region. In regions without olive pollen, Ole e 1 can be used as a marker allergen for sensitization to ash pollen.
The group-11 grass pollen allergens Phl p 11 and Lol p 11 are members of the Ole-e-1-like protein family due to structural homologies and sequence homologies (e.g., approximately 30 % sequence identity between Ole e 1 and Phl p 11). However, they do not share any IgE epitopes with Ole e 1, and no significant cross-reactivity was detected between Ole e 1 and Phl p 11 or Lol p 11 (Palomares et al. 2006).
4.2.2 Other Lamiales-Specific Allergens
Other specific minor allergens of olive pollen have been described (for an overview, see (Rodriguez et al. 2007) and ⦿ Table 10.2). Ole e 7 is a member of the nonspecific lipid transfer protein (nsLTP) family. Sensitization to Ole e 7 is associated with a tendency for severe allergic reactions; however, cross-reactivity with other nonspecific LTP seems to be limited (Tordesillas et al. 2011). In some regions of southern Spain, an elevated prevalence of sensitization against Ole e 7 and Ole e 9 was seen, and in some regions, up to 40 % of Ole-e-1-negative allergic patients are sensitized to Ole e 7 (Barber et al. 2007). Ole e 9 und Ole e 10 are also possibly associated with cross-reactivity to birch, tomato, potato, bell pepper, banana, and latex (Palomares et al. 2005; Quiralte et al. 2007).
4.3 Allergens of Trees of the Order Proteales
Tree pollen from trees of the Platanaceae family, genus Platanus, comprising about ten species (e.g., London plane tree, Platanus acerifolia), are highly cross-reactive and induce severe symptoms in a small number of sensitized patients. In regions with many plane trees, such as Spain, peaks of allergy symptoms are seen during the wind pollination season (Varela et al. 1997). Pla a 1 from the London plane tree, an invertase inhibitor, is recognized by up to 90 % of all plane tree allergic patients and is therefore considered a major allergen of plane trees (Asturias et al. 2002). Pla a 1 is used as a marker allergen for plane tree allergy (⦿ Table 10.2); however, the allergen Pla a 2, a polygalacturonase, may also be important in this respect (Asturias et al. 2002, 2006).
4.4 Allergens of Trees of the Order Cupressales
Pollen from trees of the Cupressaceae family (e.g., Arizona cypress, Cupressus arizonica; Japanese cedar, Cryptomeria japonica; mountain cedar, Juniperus ashei) are highly cross-reactive (for an overview, see Di Felice et al. 2001; Marth et al. 2014). The prevalence of allergy to different Cupressaceae pollen has increased in Central Europe, even though Cupressaceae trees are distributed mainly in the Mediterranean region (Panzner et al. 2014). It is possible that allergy to Cupressales pollen was underestimated for a long time, because the flowering season is in winter (January to March/April), and clinical symptoms of allergy to Cupressales may have been mistaken for the common cold or thought to have been caused by perennial allergens such as from house dust mite (D’Amato et al. 2007).
Cry j 1 (from Japanese cedar) is a 40-kDa protein and was the first Cupressaceae allergen to be described (Yasueda et al. 1983). Cry j 1 displays high sequence homology and IgE cross-reactivity with other Cupressaceae allergens such as Cup a 1 from the Arizona cypress (Aceituno et al. 2000) and Jun a 1 from the mountain cedar (Midoro-Horiuti et al. 1999). These allergens are glycosylated pectate lyases. Although the major allergen of ragweed (Ambrosia artemisiifolia, Amb a 1) is also a pectate lyase, there is only very limited cross-reactivity with the above mentioned allergens (Pichler et al. 2015). Cup a 1 and Cry j 1 are used as marker allergens for Cupressales allergy.
5 Panallergens: Markers for Cross-Reactivity
Panallergens are found in grass and tree pollen as well as in many other botanically unrelated plants. They belong either to the polcalcin (calcium-binding allergens carrying two, three, or four binding sites for calcium, so-called EF-hands) or profilin protein families. Amino acid sequences of both protein families are highly conserved regardless of the taxonomical relationship of allergenic plant species leading to extensive immunological cross-reactivity. Therefore, they are considered marker allergens for cross-reactivity in the diagnosis of grass and tree pollen allergy (see Chap. 3).
5.1 Polcalcins
Members of the polcalcin protein family (approximately 9-kDa proteins) from tree and grass pollen include:
-
2-EF-hand-proteins Bet v 4, Aln g 4, Ole e 3, Cyn d 7, Phl p 7, Cyn d 7
-
3-EF-hand-protein Bet v 3
-
4-EF-hand-protein Ole e 8
Polcalcins have only been found in the pollen of trees, grasses, and weeds. Almost 10 % of grass pollen allergic patients in temperate regions have specific IgE to Phl p 7, but in sensitized patients, Phl p 7 displays a high allergenic activity (see Chap. 3) (Kazemi-Shirazi et al. 2002; Niederberger et al. 1999).
5.2 Profilins
Profilin (Bet v 2, 15 kDa) was first identified in birch pollen (Valenta et al. 1991a) and has since been found in the pollen of many grasses (e.g., Phl p 12, Cyn d 12), trees (e.g., Ole e 2), and weeds, but also in plant-derived food and latex (for an overview. see Kazemi-Shirazi et al. 2002; Radauer et al. 2006). The amount of specific patient IgE varies according to geographical region and allergen source and is found in approximately 10–30 % of pollen allergic patients.
5.3 Panallergens: Summary
Cross-inhibition experiments between polcalcins from different sources and between profilins from different sources have confirmed extensive cross-reactivity of allergens within these two protein families; the highest IgE reactivity is observed with the grass pollen allergens, i.e., Phl p 7 for the polcalcins and Phl p 12 for profilins (Tinghino et al. 2002; Radauer et al. 2006).
Phl p 7 and Phl p 12 are considered marker allergens for cross-reactivity, and the presence of specific IgE to either of these allergens in patient sera may explain clinical symptoms upon exposure to a range of different allergen sources. Sensitization to either of these panallergens, Phl p 7 or Phl p 12, may result in apparent polysensitization presenting as multiple positive skin tests to pollen extracts and potential symptoms with unrelated allergen sources.
In patients with grass pollen allergy, sensitizations to Phl p 7 and Phl p 12 are often seen in later stages of allergic disease after sensitization to Phl p 1 and Phl p 5 (Hatzler et al. 2012) and may be considered as markers for clinically manifest grass pollen allergy.
6 Conclusions for Clinical Routine Work
Structured Approach to Routine Clinical Work
Diagnostic tests with marker allergens, Phl p 1/Phl p 5 (marker for grass pollen), Bet v 1 (marker for beech and birch trees as well as other Fagales trees), Ole e 1 (marker for olive trees and other Oleaceae trees including ash), Pla a 1 (marker for plane trees), and Cup a 1/Cry j 1 (marker for cypress trees), and with the panallergens (e.g., timothy grass, polcalcin/profilin), Phl p 7/Phl p 12 (indicators for cross-reactivity), establish a patient allergen-specific sensitization profile to tree and grass pollen allergens.
Genuine sensitization to grass pollen in Europe is reliably diagnosed with a combination of the major grass pollen allergens Phl p 1 and Phl p 5 (⦿ Fig. 10.3). If sensitization to Phl p 1 without IgE reactivity to Phl p 5 (and in addition, no reactivity to Phl p 2/Phl p 3 and Phl p 6) is found, this may be due to sensitization to one of the tropical/subtropical grass subfamilies.
Specific IgE to Bet v 1 characterizes sensitization to Fagales trees (birch, alder, hornbeam, hazelnut, common beech, oak, chestnut) and possible related oropharyngeal symptoms (oral allergy syndrome) due to reactions with cross-reactive plant-derived foods (e.g., apple, hazelnut, pear, sweet cherry, peach, carrot, celery, soybean) (see Chap. 2).
Ole e 1 is the major allergen in olive pollen. It displays extensive sequence identity and cross-reactivity with other major allergens of the Oleaceae family such as ash, privet, and lilac. In the Mediterranean region, genuine sensitization to olive pollen is diagnosed with Ole e 1; in more temperate climate zones such as Central Europe, Ole e 1 can be used to prove sensitization to ash pollen and to distinguish it from the clinical symptoms of birch pollen allergy occurring in the same season.
Sensitization to tree pollen of the Platanaceae family is diagnosed with Pla a 1 (possibly including Pla a 2), sensitization to pollen of trees of the Cupressaceae family with Cup a 1/Cry j 1.
Association of the abovementioned marker allergens with specific clinically relevant sensitization profiles was confirmed in clinical studies (Canis et al. 2011; Jahn-Schmid et al. 2003; Twardosz-Kropfmuller et al. 2010; Moreno et al. 2014; Darsow et al. 2014).
If no clear-cut sensitization to one of the abovementioned marker allergens can be detected, the following rules apply:
-
Low or no IgE reactivity to genuine marker allergens indicates that a patient is not sensitized to the corresponding allergen source. An allergen extract from this source is not suitable for specific immunotherapy.
-
Exclusive sensitization to the panallergens profilin and polcalcin (e.g., Phl p 7 and Phl p 12 from timothy grass pollen) is very rare. This sensitization profile often cannot be attributed to one specific allergen source. Patients therefore are not suited for specific immunotherapy.
-
The presence of specific IgE to profilin and/or polcalcin by nature of their cross-reactivity rules out further diagnosis with natural (pollen) extracts, as sensitization to panallergens abolishes analytical specificity (selectivity) of natural extracts.
In these cases, allergy CRD together with a detailed patient history should be used to reach a therapeutic decision. This will ensure that the correct decision for or against specific immunotherapy and its correct composition is taken (Douladiris et al. 2013; Letrán et al. 2013).
Conclusions
Detection of specific IgE using component resolved diagnostics (CRD) identifies the underlying allergen source in suspected cases of tree and grass pollen allergy. Suitable marker allergens can be used to distinguish genuine sensitization to tree or grass pollen from cross-reactivity to pollen panallergens (e.g., profilin and polcalcins) and to overcome the lack of analytical specificity of natural allergen extracts. In allergic patients suspected of polysensitization who react with a variety of pollen extracts, CRD allows specific allergen diagnosis regardless of the confounding effect of panallergenic cross-reactivity and prescription of tailored, specific immunotherapy.
Allergens defined as marker allergens for tree and grass pollen allergy are:
-
Bet v 1 (birch pollen major allergen) for birch, beech, and other trees from the Fagales order
-
Ole e 1 (olive tree major allergen) for olive and other trees including ash from the Oleaceae family
-
Pla a 1 (major allergen of the London plane) for plane trees
-
Cry j 1 (major allergen of the Japanese cedar)
-
Cup a 1 (major allergen of the Arizona cypress) for cypress trees
-
Jun a 1 (major allergen of mountain cedar) for juniper trees
-
Phl p 1 (timothy grass group-1 major allergen) for most grasses including rye (Secale sp.)
-
Phl p 5 (timothy grass group-5 major allergen) for “temperate climate” grasses (Pooideae)
Grass and tree pollen allergens with serological and clinical cross-reactivity to a great number of allergen sources are also identified as possible confounding factors in allergen-specific diagnosis with natural extracts. Structured diagnostic procedures for clinical routine work are needed to improve the appropriate selection of allergen sources for AIT.
Abbreviations
- CCD:
-
Cross-reactive carbohydrate determinants
- cDNA:
-
Complementary deoxyribonucleic acid
- CRD:
-
Component resolved diagnostics
- Fab:
-
Antigen-binding fragment
- IgE:
-
Immunoglobulin E
- LTP:
-
Lipid transfer protein
- nsLTP:
-
Nonspecific lipid transfer protein
- OAS:
-
Oral allergy syndrome
- PR-10:
-
Pathogenesis-related proteins
References
Aceituno E, Del Pozo V, Minguez A, Arrieta I, Cortegano I, Cardaba B, Gallardo S, Rojo M, Palomino P, Lahoz C. Molecular cloning of major allergen from Cupressus arizonica pollen: Cup a 1. Clin Exp Allergy. 2000;30:1750–8.
Andersson K, Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol. 2003;130:87–107. doi:69013.
Asam C, Hofer H, Wolf M, Aglas L, Wallner M. Tree pollen allergens – an update from a molecular perspective. Allergy. 2015;70:1201–11.
Asero R. Analysis of hypersensitivity to oleaceae pollen in an olive-free and ash-free area by commercial pollen extracts and recombinant allergens. Eur Ann Allergy Clin Immunol. 2011;43:77–80.
Asturias JA, Ibarrola I, Amat P, Tella R, Malet A, Cistero-Bahima A, Enrique E, Malek T, Martinez A. Purified allergens vs. complete extract in the diagnosis of plane tree pollen allergy. Clin Exp Allergy. 2006;36:1505–12.
Asturias JA, Ibarrola I, Bartolome B, Ojeda I, Malet A, Martinez A. Purification and characterization of Pla a 1, a major allergen from Platanus acerifolia pollen. Allergy. 2002;57:221–7.
Barber D, Moreno C, Ledesma A, Serrano P, Galan A, Villalba M, Guerra F, Lombardero M, Rodriguez R. Degree of olive pollen exposure and sensitization patterns. Clinical implications. J Investig Allergol Clin Immunol. 2007;17 Suppl 1:11–6.
Birkner T, Rumpold H, Jarolim E, Ebner H, Breitenbach M, Skvaril F, Scheiner O, Kraft D. Evaluation of immunotherapy-induced changes in specific IgE, IgG and IgG subclasses in birch pollen allergic patients by means of immunoblotting. Correlation with clinical response. Allergy. 1990;45:418–26.
Blomme K, Tomassen P, Lapeere H, Huvenne W, Bonny M, Acke F, Bachert C, Gevaert P. Prevalence of allergic sensitization versus allergic rhinitis symptoms in an unselected population. Int Arch Allergy Immunol. 2013;160:200–7.
Bousquet J, Cour P, Guerin B, Michel FB. Allergy in the Mediterranean area. I. Pollen counts and pollinosis of Montpellier. Clin Allergy. 1984;14:249–58.
Breiteneder H, Pettenburger K, Bito A, Valenta R, Kraft D, Rumpold H, Scheiner O, Breitenbach M. The gene coding for the major birch pollen allergen Bet v 1, is highly homologous to a pea disease resistance response gene. Embo J. 1989;8:1935–8.
Cabauatan CR, Lupinek C, Scheiblhofer S, Weiss R, Focke-Tejkl M, Bhalla PL, Singh MB, Knight PA, van Hage M, Ramos JD, Valenta R. Allergen microarray detects high prevalence of asymptomatic IgE sensitizations to tropical pollen-derived carbohydrates. J Allergy Clin Immunol. 2014;133:910–4.
Calabria CW, Dice J. Aeroallergen sensitization rates in military children with rhinitis symptoms. Ann Allergy Asthma Immunol. 2007;99:161–9.
Campbell BC, Gilding EK, Timbrell V, Guru P, Loo D, Zennaro D, Mari A, Solley G, Hill MM, Godwin ID, Davies JM. Total transcriptome, proteome, and allergome of Johnson grass pollen, which is important for allergic rhinitis in subtropical regions. J Allergy Clin Immunol. 2015;135:133–42.
Canis M, Groger M, Becker S, Klemens C, Kramer MF. Recombinant marker allergens in diagnosis of patients with allergic rhinoconjunctivitis to tree and grass pollens. Am J Rhinol Allergy. 2011;25:36–9.
Christenhusz MJM, Reveal JL, Farjon A, Gardner MF, Mill RR, Chase MW. A new classification and linear sequence of extant gymnosperms. Phytotaxa. 2011;19:55–70.
Cornelius C, Schöneweis K, Georgi F, Weber M, Niederberger V, Zieglmayer P, Niespodziana K, Trauner M, Hofer H, Urban S, Valenta R. Immunotherapy With the PreS-based Grass Pollen Allergy Vaccine BM32 Induces Antibody Responses Protecting Against Hepatitis B Infection. EBioMedicine. 2016;11:58–67.
D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, Liccardi G, Popov T, van Cauwenberge P. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–90.
Darsow U, Brockow K, Pfab F, Jakob T, Petersson CJ, Borres MP, Ring J, Behrendt H, Huss-Marp J. Allergens. Heterogeneity of molecular sensitization profiles in grass pollen allergy – implications for immunotherapy? Clin Exp Allergy. 2014;44:778–86.
Davies JM, Mittag D, Dang TD, Symons K, Voskamp A, Rolland JM, O’Hehir RE. Molecular cloning, expression and immunological characterisation of Pas n 1, the major allergen of Bahia grass Paspalum notatum pollen. Mol Immunol. 2008;46:286–93.
Davies JM, Voskamp A, Dang TD, Pettit B, Loo D, Petersen A, Hill MM, Upham JW, Rolland JM, O’Hehir RE. The dominant 55kDa allergen of the subtropical Bahia grass (Paspalum notatum) pollen is a group 13 pollen allergen, Pas n 13. Mol Immunol. 2011;48:931–40.
Davies JM. Grass pollen allergens globally: the contribution of subtropical grasses to burden of allergic respiratory diseases. Clin Exp Allergy. 2014;44:790–801.
Di Felice G, Barletta B, Tinghino R, Pini C. Cupressaceae pollinosis: identification, purification and cloning of relevant allergens. Int Arch Allergy Immunol. 2001;125:280–9.
Douladiris N, Savvatianos S, Roumpedaki I, Skevaki C, Mitsias D, Papadopoulos NG. A molecular diagnostic algorithm to guide pollen immunotherapy in southern Europe: towards component-resolved management of allergic diseases. Int Arch Allergy Immunol. 2013;162:163–72.
Duffort O, Quintana J, Ipsen H, Barber D, Polo F. Antigenic similarity among group 1 allergens from grasses and quantitation ELISA using monoclonal antibodies to Phl p 1. Int Arch Allergy Immunol. 2008;145:283–90.
Flicker S, Steinberger P, Ball T, Krauth MT, Verdino P, Valent P, Almo S, Valenta R. Spatial clustering of the IgE epitopes on the major timothy grass pollen allergen Phl p 1: importance for allergenic activity. J Allergy Clin Immunol. 2006;117:1336–43.
Esch RE. Grass pollen allergens. Clin Allergy Immunol. 2004;18:185–205.
Flicker S, Vrtala S, Steinberger P, Vangelista L, Bufe A, Petersen A, Ghannadan M, Sperr WR, Valent P, Norderhaug L, Bohle B, Stockinger H, Suphioglu C, Ong EK, Kraft D, Valenta R. A human monoclonal IgE antibody defines a highly allergenic fragment of the major timothy grass pollen allergen, Phl p 5: molecular, immunological, and structural characterization of the epitope-containing domain. J Immunol. 2000;165:3849–59.
Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, Stegfellner G, Maderegger B, Hauer M, Stolz F, Niederberger V, Marth K, Eckl-Dorna J, Weiss R, Thalhamer J, Blatt K, Valent P, Valenta R. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 135:1207–7.e1201–11.
Gangl K, Niederberger V, Valenta R. Multiple grass mixes as opposed to single grasses for allergen immunotherapy in allergic rhinitis. Clin Exp Allergy. 2013;43:1202–16.
Gerlich WH, Glebe D. Development of an Allergy Immunotherapy Leads to a New Type of Hepatitis B Vaccine. EBioMedicine. 2016;11:5–6.
Griffith IJ, Smith PM, Pollock J, Theerakulpisut P, Avjioglu A, Davies S, Hough T, Singh MB, Simpson RJ, Ward LD, et al. Cloning and sequencing of Lol pI, the major allergenic protein of rye-grass pollen. FEBS Lett. 1991;279:210–5.
Grote M, Stumvoll S, Reichelt R, Lidholm J, Rudolf V. Identification of an allergen related to Phl p 4, a major timothy grass pollen allergen, in pollens, vegetables, and fruits by immunogold electron microscopy. Biol Chem. 2002;383:1441–5.
Grote M, Vrtala S, Niederberger V, Wiermann R, Valenta R, Reichelt R. Release of allergen-bearing cytoplasm from hydrated pollen: a mechanism common to a variety of grass (Poaceae) species revealed by electron microscopy. J Allergy Clin Immunol. 2001;108:109–15.
Hatzler L, Panetta V, Lau S, Wagner P, Bergmann RL, Illi S, Bergmann KE, Keil T, Hofmaier S, Rohrbach A, Bauer CP, Hoffman U, Forster J, Zepp F, Schuster A, Wahn U, Matricardi PM. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J Allergy Clin Immunol. 2012;130:894–901.
Heiss S, Fischer S, Muller WD, Weber B, Hirschwehr R, Spitzauer S, Kraft D, Valenta R. Identification of a 60 kd cross-reactive allergen in pollen and plant-derived food. J Allergy Clin Immunol. 1996;98:938–47.
Hejl C, Wurtzen PA, Kleine-Tebbe J, Johansen N, Broge L, Ipsen H. Phleum pratense alone is sufficient for allergen-specific immunotherapy against allergy to Pooideae grass pollens. Clin Exp Allergy. 2009;39:752–9. .
Henzgen M, Wenz W, Strumpfel R. Experiences with desensitization of early spring pollen allergy using 2 tree pollen extracts. Z Gesamte Inn Med. 1989;44:691–3.
Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, Barletta B, Becker WM, Blaser K, Breiteneder H, Chapman M, Crameri R, Duchene M, Ferreira F, Fiebig H, Hoffmann-Sommergruber K, King TP, Kleber-Janke T, Kurup VP, Lehrer SB, Lidholm J, Muller U, Pini C, Reese G, Scheiner O, Scheynius A, Shen HD, Spitzauer S, Suck R, Swoboda I, Thomas W, Tinghino R, Van Hage-Hamsten M, Virtanen T, Kraft D, Muller MW, Valenta R. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. Faseb J. 2002;16:414–6.
Ipsen H, Hansen OC. The NH2-terminal amino acid sequence of the immunochemically partial identical major allergens of Alder (Alnus glutinosa) Aln g I, birch (Betula verrucosa) Bet v I, hornbeam (Carpinus betulus) Car b I and oak (Quercus alba) Que a I pollens. Mol Immunol. 1991;28:1279–88.
Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, Mueller MW. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003;33:1443–9.
Johansen N, Weber RW, Ipsen H, Barber D, Broge L, Hejl C. Extensive IgE cross-reactivity towards the Pooideae grasses substantiated for a large number of grass-pollen-sensitized subjects. Int Arch Allergy Immunol. 2009;150:325–34.
Johnson P, Marsh DG. ‘Isoallergens’ from rye grass pollen. Nature. 1965;206:935–7.
Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–13.
Kazemi-Shirazi L, Niederberger V, Linhart B, Lidholm J, Kraft D, Valenta R. Recombinant marker allergens: diagnostic gatekeepers for the treatment of allergy. Int Arch Allergy Immunol. 2002;127:259–68.
Kazemi-Shirazi L, Pauli G, Purohit A, Spitzauer S, Froschl R, Hoffmann-Sommergruber K, Breiteneder H, Scheiner O, Kraft D, Valenta R. Quantitative IgE inhibition experiments with purified recombinant allergens indicate pollen-derived allergens as the sensitizing agents responsible for many forms of plant food allergy. J Allergy Clin Immunol. 2000;105:116–25.
Laffer S, Duchene M, Reimitzer I, Susani M, Mannhalter C, Kraft D, Valenta R. Common IgE-epitopes of recombinant Phl p I, the major timothy grass pollen allergen and natural group I grass pollen isoallergens. Mol Immunol. 1996;33:417–26.
Laffer S, Valenta R, Vrtala S, Susani M, van Ree R, Kraft D, Scheiner O, Duchene M. Complementary DNA cloning of the major allergen Phl p I from timothy grass (Phleum pratense); recombinant Phl p I inhibits IgE binding to group I allergens from eight different grass species. J Allergy Clin Immunol. 1994a;94:689–98.
Laffer S, Vrtala S, Duchene M, van Ree R, Kraft D, Scheiner O, Valenta R. IgE-binding capacity of recombinant timothy grass (Phleum pratense) pollen allergens. J Allergy Clin Immunol. 1994b;94:88–94.
Letrán A, Espinazo M, Moreno F. Measurement of IgE to pollen allergen components is helpful in selecting patients for immunotherapy. Ann Allergy Asthma Immunol. 2013;111:295–7.
Levin M, Rotthus S, Wendel S, Najafi N, Kallstrom E, Focke-Tejkl M, Valenta R, Flicker S, Ohlin M. Multiple independent IgE epitopes on the highly allergenic grass pollen allergen Phl p 5. Clin Exp Allergy. 2014;44:1409–19.
Liang KL, Su MC, Shiao JY, Wu SH, Li YH, Jiang RS. Role of pollen allergy in Taiwanese patients with allergic rhinitis. J Formosan Med Assoc. 2010;109:879–85.
Lupinek C, Wollmann E, Baar A, Banerjee S, Breiteneder H, Broecker BM, Bublin M, Curin M, Flicker S, Garmatiuk T, Hochwallner H, Mittermann I, Pahr S, Resch Y, Roux KH, Srinivasan B, Stentzel S, Vrtala S, Willison LN, Wickman M, Lodrup-Carlsen KC, Anto JM, Bousquet J, Bachert C, Ebner D, Schlederer T, Harwanegg C, Valenta R. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19.
Madritsch C, Gadermaier E, Roder UW, Lupinek C, Valenta R, Flicker S. High-density IgE recognition of the major grass pollen allergen Phl p 1 revealed with single-chain IgE antibody fragments obtained by combinatorial cloning. J Immunol. 2015;194:2069–78.
Marknell DeWitt A, Niederberger V, Lehtonen P, Spitzauer S, Sperr WR, Valent P, Valenta R, Lidholm J. Molecular and immunological characterization of a novel timothy grass (Phleum pratense) pollen allergen, Phl p 11. Clin Exp Allergy. 2002;32:1329–40.
Marth K, Garmatiuk T, Swoboda I, Valenta R. Tree pollen allergens. In: Lockey RF, Ledford DK, editors. Allergens and allergen immunotherapy. 5th ed. London: CRC Press; 2014. p. 113–32.
Menz G, Dolecek C, Schonheit-Kenn U, Ferreira F, Moser M, Schneider T, Suter M, Boltz-Nitulescu G, Ebner C, Kraft D, Valenta R. Serological and skin-test diagnosis of birch pollen allergy with recombinant Bet v I, the major birch pollen allergen. Clin Exp Allergy. 1996;26:50–60.
Midoro-Horiuti T, Goldblum RM, Kurosky A, Goetz DW, Brooks EG. Isolation and characterization of the mountain Cedar (Juniperus ashei) pollen major allergen, Jun a 1. J Allergy Clin Immunol. 1999;104:608–12.
Moreno C, Justicia JL, Quiralte J, Moreno-Ancillo A, Iglesias-Cadarso A, Torrecillas M, Labarta N, Garcia MA, Davila I. Olive, grass or both? Molecular diagnosis for the allergen immunotherapy selection in polysensitized pollinic patients. Allergy. 2014;69:1357–63.
Mothes N, Horak F, Valenta R. Transition from a botanical to a molecular classification in tree pollen allergy: implications for diagnosis and therapy. Int Arch Allergy Immunol. 2004;135:357–73.
Moverare R, Westritschnig K, Svensson M, Hayek B, Bende M, Pauli G, Sorva R, Haahtela T, Valenta R, Elfman L. Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol. 2002;128:325–35.
Niederberger V, Hayek B, Vrtala S, Laffer S, Twardosz A, Vangelista L, Sperr WR, Valent P, Rumpold H, Kraft D, Ehrenberger K, Valenta R, Spitzauer S. Calcium-dependent immunoglobulin E recognition of the apo- and calcium-bound form of a cross-reactive two EF-hand timothy grass pollen allergen, Phl p 7. Faseb J. 1999;13:843–56.
Niederberger V, Laffer S, Froschl R, Kraft D, Rumpold H, Kapiotis S, Valenta R, Spitzauer S. IgE antibodies to recombinant pollen allergens (Phl p 1, Phl p 2, Phl p 5, and Bet v 2) account for a high percentage of grass pollen-specific IgE. J Allergy Clin Immunol. 1998a;101:258–64.
Niederberger V, Pauli G, Gronlund H, Froschl R, Rumpold H, Kraft D, Valenta R, Spitzauer S. Recombinant birch pollen allergens (rBet v 1 and rBet v 2) contain most of the IgE epitopes present in birch, alder, hornbeam, hazel, and oak pollen: a quantitative IgE inhibition study with sera from different populations. J Allergy Clin Immunol. 1998b;102:579–91.
Niederberger V, Purohit A, Oster JP, Spitzauer S, Valenta R, Pauli G. The allergen profile of ash (Fraxinus excelsior) pollen: cross-reactivity with allergens from various plant species. Clin Exp Allergy. 2002;32:933–41.
Niederberger V, Stubner P, Spitzauer S, Kraft D, Valenta R, Ehrenberger K, Horak F. Skin test results but not serology reflect immediate type respiratory sensitivity: a study performed with recombinant allergen molecules. J Invest Dermatol. 2001;117:848–51.
Niederberger V, Marth K, Eckl-Dorna J, Focke-Tejkl M, Weber M, Hemmer W, Berger U, Neubauer A, Stolz F, Henning R, Valenta R. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol. 2015;136:1101-3.e8.
Odongo L, Mulyowa G, Goebeler M, Trautmann A. Bet v 1- and Bet v 2-Associated Plant Food Sensitization in Uganda and Germany: differences and similarities. Int Arch Allergy Immunol. 2015;167:264–9.
Palomares O, Swoboda I, Villalba M, Balic N, Spitzauer S, Rodriguez R, Valenta R. The major allergen of olive pollen Ole e 1 is a diagnostic marker for sensitization to Oleaceae. Int Arch Allergy Immunol. 2006;141:110–8.
Palomares O, Villalba M, Quiralte J, Polo F, Rodriguez R. 1,3-beta-glucanases as candidates in latex-pollen-vegetable food cross-reactivity. Clin Exp Allergy. 2005;35:345–51.
Panzner P, Vachova M, Vitovcova P, Brodska P, Vlas T. A comprehensive analysis of middle-European molecular sensitization profiles to pollen allergens. Int Arch Allergy Immunol. 2014;164:74–82. .
Perez M, Ishioka GY, Walker LE, Chesnut RW. cDNA cloning and immunological characterization of the rye grass allergen Lol p I. J Biol Chem. 1990;265:16210–5.
Petersen BN, Janniche H, Munch EP, Wihl JA, Bowadt H, Ipsen H, Lowenstein H. Immunotherapy with partially purified and standardized tree pollen extracts. I. Clinical results from a three-year double-blind study of patients treated with pollen extracts either of birch or combinations of alder, birch and hazel. Allergy. 1988;43:353–62.
Phillips JW, Bucholtz GA, Fernandez-Caldas E, Bukantz SC, Lockey RF. Bahia grass pollen, a significant aeroallergen: evidence for the lack of clinical cross-reactivity with timothy grass pollen. Ann Allergy. 1989;63:503–7.
Pichler U, Hauser M, Wolf M, Bernardi ML, Gadermaier G, Weiss R, Ebner C, Yokoi H, Takai T, Didierlaurent A, Rafaiani C, Briza P, Mari A, Behrendt H, Wallner M, Ferreira F. Pectate lyase pollen allergens: sensitization profiles and cross-reactivity pattern. PLoS One. 2015;10, e0120038.
Prescott RA, Potter PC. Allergenicity and cross-reactivity of buffalo grass (Stenotaphrum secundatum). South African Med J. 2001;91:237–43.
Quiralte J, Palacios L, Rodriguez R, Cardaba B, Arias de Saavedra JM, Villalba M, Florido JF, Lahoz C. Modelling diseases: the allergens of Olea europaea pollen. J Investig Allergol Clin Immunol. 2007;17 Suppl 1:24–30.
Radauer C, Willerroider M, Fuchs H, Hoffmann-Sommergruber K, Thalhamer J, Ferreira F, Scheiner O, Breiteneder H. Cross-reactive and species-specific immunoglobulin E epitopes of plant profilins: an experimental and structure-based analysis. Clin Exp Allergy. 2006;36:920–9.
Rodriguez R, Villalba M, Batanero E, Palomares O, Quiralte J, Salamanca G, Sirvent S, Castro L, Prado N. Olive pollen recombinant allergens: value in diagnosis and immunotherapy. J Investig Allergol Clin Immunol. 2007;17 Suppl 1:4–10.
Sam CK, Kesavan P, Liam CK, Soon SC, Lim AL, Ong EK. A study of pollen prevalence in relation to pollen allergy in Malaysian asthmatics. Asian Pac J Allergy Immunol. 1998;16:1–4.
Seidel DJ, Fu Q, Randel WJ, Riechler TJ. Widening of the tropical belt in a changing climate. Nat Geosci. 2008;1:21–4.
Simon BK, Clayton W, Harman K, Vorontsova M, Brake I, Healy D, Alfonso Y. 2011. Last accessed 22 Sept 2014.
Suck R, Hagen S, Cromwell O, Fiebig H. The high molecular mass allergen fraction of timothy grass pollen (Phleum pratense) between 50-60 kDa is comprised of two major allergens: Phl p 4 and Phl p 13. Clin Exp Allergy. 2000;30:1395–402.
Suphioglu C. What are the important allergens in grass pollen that are linked to human allergic disease? Clin Exp Allergy. 2000;30:1335–41.
Swoboda I, Twaroch T, Valenta R, Grote M. Tree pollen allergens. Clin Allergy Immunol. 2008;21:87–105.
The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical J Linnean Soc. 2009;161:105–21.
Timbrell VL, Riebelt L, Simmonds C, Solley G, Smith WB, McLean-Tooke A, van Nunen S, Smith PK, Upham JW, Langguth D, Davies JM. An immunodiagnostic assay for quantitation of specific IgE to the major pollen allergen component, Pas n 1, of the subtropical Bahia grass. Int Arch Allergy Immunol. 2014;165:219–28.
Tinghino R, Twardosz A, Barletta B, Puggioni EM, Iacovacci P, Butteroni C, Afferni C, Mari A, Hayek B, Di Felice G, Focke M, Westritschnig K, Valenta R, Pini C. Molecular, structural, and immunologic relationships between different families of recombinant calcium-binding pollen allergens. J Allergy Clin Immunol. 2002;109:314–20.
Tordesillas L, Sirvent S, Diaz-Perales A, Villalba M, Cuesta-Herranz J, Rodriguez R, Salcedo G. Plant lipid transfer protein allergens: no cross-reactivity between those from foods and olive and Parietaria pollen. Int Arch Allergy Immunol. 2011;156:291–6.
Tresch S, Holzmann D, Baumann S, Blaser K, Wuthrich B, Crameri R, Schmid-Grendelmeier P. In vitro and in vivo allergenicity of recombinant Bet v 1 compared to the reactivity of natural birch pollen extract. Clin Exp Allergy. 2003;33:1153–8.
Tripodi S, Frediani T, Lucarelli S, Macri F, Pingitore G, Di Rienzo BA, Dondi A, Pansa P, Ragusa G, Asero R, Faggian D, Plebani M, Matricardi PM. Molecular profiles of IgE to Phleum pratense in children with grass pollen allergy: implications for specific immunotherapy. J Allergy Clin Immunol. 2012;129:834–9.e838.
Twardosz-Kropfmuller A, Singh MB, Niederberger V, Horak F, Kraft D, Spitzauer S, Valenta R, Swoboda I. Association of allergic patients’ phenotypes with IgE reactivity to recombinant pollen marker allergens. Allergy. 2010;65:296–303.
Valenta R, Duchene M, Pettenburger K, Sillaber C, Valent P, Bettelheim P, Breitenbach M, Rumpold H, Kraft D, Scheiner O. Identification of profilin as a novel pollen allergen; IgE autoreactivity in sensitized individuals. Science. 1991a;253:557–60.
Valenta R, Duchene M, Vrtala S, Birkner T, Ebner C, Hirschwehr R, Breitenbach M, Rumpold H, Scheiner O, Kraft D. Recombinant allergens for immunoblot diagnosis of tree-pollen allergy. J Allergy Clin Immunol. 1991b;88:889–94.
Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin Exp Allergy. 1999;29:896–904.
Valenta R, Twaroch T, Swoboda I. Component-resolved diagnosis to optimize allergen-specific immunotherapy in the Mediterranean area. J Investig Allergol Clin Immunol. 2007;17 Suppl 1:36–40.
van Ree R. Isoallergens: a clinically relevant phenomenon or just a product of cloning? Clin Exp Allergy. 2002;32:975–8.
Van Ree R, Driessen MN, Van Leeuwen WA, Stapel SO, Aalberse RC. Variability of crossreactivity of IgE antibodies to group I and V allergens in eight grass pollen species. Clin Exp Allergy. 1992;22:611–7.
Varela S, Subiza J, Subiza JL, Rodriguez R, Garcia B, Jerez M, Jimenez JA, Panzani R. Platanus pollen as an important cause of pollinosis. J Allergy Clin Immunol. 1997;100:748–54.
Villalba M, Batanero E, Lopez-Otin C, Sanchez LM, Monsalve RI, Gonzalez de la Pena MA, Lahoz C, Rodriguez R. The amino acid sequence of Ole e I, the major allergen from olive tree (Olea europaea) pollen. Eur J Biochem. 1993;216:863–9.
Vrtala S, Sperr WR, Reimitzer I, van Ree R, Laffer S, Muller WD, Valent P, Lechner K, Rumpold H, Kraft D, et al. cDNA cloning of a major allergen from timothy grass (Phleum pratense) pollen; characterization of the recombinant Phl pV allergen. J Immunol. 1993;151:4773–81.
Westritschnig K, Horak F, Swoboda I, Balic N, Spitzauer S, Kundi M, Fiebig H, Suck R, Cromwell O, Valenta R. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur J Clin Invest. 2008;38:260–7.
Westritschnig K, Sibanda E, Thomas W, Auer H, Aspock H, Pittner G, Vrtala S, Spitzauer S, Kraft D, Valenta R. Analysis of the sensitization profile towards allergens in central Africa. Clin Exp Allergy. 2003;33:22–7.
Wuthrich B, Schindler C, Leuenberger P, Ackermann-Liebrich U. Prevalence of atopy and pollinosis in the adult population of Switzerland (SAPALDIA study). Swiss Study on Air Pollution and Lung Diseases in Adults. Int Arch Allergy Immunol. 1995;106:149–56.
Yasueda H, Yui Y, Shimizu T, Shida T. Isolation and partial characterization of the major allergen from Japanese cedar (Cryptomeria japonica) pollen. J Allergy Clin Immunol. 1983;71:77–86.
Zafred D, Nandy A, Pump L, Kahlert H, Keller W. Crystal structure and immunologic characterization of the major grass pollen allergen Phl p 4. J Allergy Clin Immunol. 2013;132:696–703.e610.
Zieglmayer P, Focke-Tejkl M, Schmutz R, Lemell P, Zieglmayer R, Weber M, Kiss R, Blatt K, Valent P, Stolz F, Huber H, Neubauer A, Knoll A, Horak F, Henning R, Valenta R. Mechanisms, safety and efficacy of a B cell epitope-based vaccine for immunotherapy of grass pollen allergy. EBioMedicine. 2016;11:43–57.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gangl, K., Niederberger, V., Davies, J.M., Valenta, R., Nandy, A. (2017). Marker Allergens and Panallergens in Tree and Grass Pollen Allergy. In: Kleine-Tebbe, J., Jakob, T. (eds) Molecular Allergy Diagnostics. Springer, Cham. https://doi.org/10.1007/978-3-319-42499-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-42499-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42498-9
Online ISBN: 978-3-319-42499-6
eBook Packages: MedicineMedicine (R0)