Abstract
Circulating cell-free DNA (ccfDNA) is a promising diagnostic tool and its size fractionation is of interest. However, kits for isolation of ccfDNA available on the market are designed for small volumes hence processing large sample volumes is laborious. We have tested a new method that enables enrichment of ccfDNA from large volumes of plasma and subsequently allows size-fractionation of isolated ccfDNA into two fractions with individually established cut-off levels of ccfDNA length. This method allows isolation of low-abundant DNA as well as separation of long and short DNA molecules. This procedure may be important e.g., in prenatal diagnostics and cancer research that have been already confirmed by our primary experiments. Here, we report the results of selective separation of 200- and 500-bp long synthetic DNA fragments spiked in plasma samples. Furthermore, we size-fractionated ccfDNA from the plasma of pregnant women and verified the prevalence of fetal ccfDNA in all fractions.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
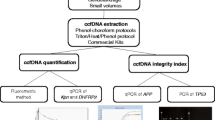
The crucial step for an effective genetic analysis of ccfDNA is its isolation in sufficient amounts, even if it is low-abundant and highly fragmented as in plasma (Schwarzenbach et al. 2011). Moreover, for some applications, like prenatal and tumor diagnostics, it may be crucial to separate short ccfDNA fragments from long ones (Chan et al. 2004; Schwarzenbach 2013). Currently, available kits and methods use standard nucleic acid extraction procedures based on sample lysis, binding the nucleic acids on a solid material and washing and elution of nucleic acids. When processing high sample volumes, the procedures are time- and work- consuming, and require large amounts of reagents. Moreover, none of these kits enable to separate ccfDNA in short and long molecules.
We have tested a kit, which differs from other kits by the capturing of biomolecules (DNA, RNA, exosomes, viruses) by a polymer prior to the lysis step and the separation of total ccfDNA in short and long DNA fragments. Large sample volumes can be processed in a fast and simple manner, increasing the probability of capturing low-abundant ccfDNA molecules. The polymer-trapped biomolecules are spun down and can be used either for nucleic acid extraction or can be dissolved in a small volume of a special buffer and used for other downstream applications (e.g. exosome detection by western blot, plaque assay to detect alive viruses). After the nucleic acid isolation, the total ccfDNA (Fraction X) can be selectively size-fractionated in long and short DNA molecules. The size cut-off can be set individually by adding a Binding Conditioner (BC) and should be determined empirically for each application. The amount of BC added to the sample influences the size of long DNA fragments that bind on the spin filter (Fraction Y) and short DNA fragments that pass through the spin filter (Fraction Z). Under special conditions, Fraction Z is subsequently bound to the second filter and both fractions (Y and Z) can be further cleaned up and separately eluted.
This kind of separation allows enrichment of the DNA of interest as well as removal of undesirable DNA, and is qualified for prenatal screening (Ashoor et al. 2013) and cancer research (Schwarzenbach et al. 2012).
Materials and Methods
Extraction and Size Fractionation of Synthetic DNA Spiked in Plasma Samples
Twenty ng of 200- and 500-bp synthetic DNA fragments (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) were spiked in 1 ml of plasma samples (Haema, Frankfurt Oder, Germany), extracted and size- fractionated using the PME and subsequent size-fractionized extraction of cell-free DNA kit (Analytik Jena, Jena, Germany). The DNA size cut-off was established by adding 8 μl of BC, resulting in a separation of total DNA (Fraction X) in long DNA fragments (Fraction Y), containing 500-bp DNA, and short DNA fragments (Fraction Z) containing 200-bp DNA. Samples of all three fractions (X, Y and Z) were analyzed on an Agilent 2100 Bioanalyzer using the Agilent DNA 1000 Kit (Agilent Technologies, Santa Clara, California, USA).
Enrichment of Fetal ccfDNA
ccfDNA from 1 ml of plasma from pregnant women bearing a male fetus was isolated and size-fractionized using the PME and subsequent size -fractionized extraction of cell-free DNA kit. The cut-off level was set to 350 bp. Total genome equivalent copies in all three fractions were determined by PCR with primers specific for the ß-globin (autosomal – maternal and fetal ) gene. The yield of fetal DNA in all three fractions (X, Y and Z) was quantified by PCR with primers specific for sex-determining region Y gene.
Results
Extraction and Size Fractionatio n of Synthetic DNA Spiked in Plasma
The isolated and fractionated synthetic 200- and 500-bp long DNA fragments were analyzed on an Agilent 2100 Bioanalyzer (Fig. 30.1). Total DNA fraction (X) contained equal yields of DNA of both lengths (upper graph). After size fractionation, Fraction Y included almost only 500-bp long DNA (middle graph) and Fraction Z mostly 200-bp long DNA fragments (lower graph). Fractions Y and Z contained negligible traces of 200-bp and 500-bp long DNA, respectively.
Percentage of Fetal ccfDNA in Fractions X, Y and Z
Total genome equivalent copies were calculated in all three fractions of ccfDNA from plasma of pregnant women (Fig. 30.2, red columns). We observed that most ccfDNA was longer than 350 bp. In addition, the yields (green columns) and the prevalence (%, blue columns) of fetal ccfDNA in all three fractions were calculated. The prevalence of fetal ccfDNA was highest in Fraction Z (<350 bp) and lowest in Fraction X (before fractionation). These results indicate the significant predominance of maternal ccfDNA in whole ccfDNA pool.
Discussion
Quantification of low- and high-molecular weight ccfDNA may be of relevance in prenatal screening (Ashoor et al. 2013) and tumor research (Schwarzenbach et al. 2012). Therefore, a fast, standardized and reliable method for size - depending separation of ccfDNA could be used as a diagnostic tool.
Depending on the cancer type, tumor ccfDNA can predominantly be of apoptotic or necrotic origin, and therefore, differently fragmented (Schwarzenbach 2013). Thus, the size-dependent ccfDNA selection could highlight its utility for further genetic and epigenetic analyses.
To date, the clinical relevance and application of ccfDN A have only been found in fetal medicine. Chan et al. (2004) discovered that maternal and fetal ccfDNA differ in their lengths. Low levels of small fragmented fetal ccfDNA are usually detected with a strong background of maternal ccfDNA. It has been reported, that at a 11–13 weeks’ gestation, the median prevalence of fetal ccfDN A in total ccfDNA from the mother’s plasma is around 10 % and may decrease along with e.g. the mother’s origin or body weight (Ashoor et al. 2013). Fetal DNA in maternal plasma has also been shown to be useful for the prenatal diagnosis of certain fetal disorders: fetal chromosomal aneuploidies, sex-linked disorders, cystic fibrosis, Huntington disease and 1-thalassemia (Chiu and Lo 2013). Therefore, the combination of the polymer-based enrichment and subsequent size fractionation of ccfDNA offers a perfect tool for prenatal diagnostics , allowing the enrichment of shorter, fe tal ccfDNA by removing longer, maternal ccfDNA from the total ccfDNA fraction.
To our knowledge, this is the first publication showing the separation of ccfDNA in nearly pure short and long ccfDNA fragments. Some faint DNA residues of redundant fractions were observed that should have no significant influence on any downstream applications. However, investigations on large patient cohorts are required to test the clinical relevance of our method.
References
Ashoor G, Syngelaki A, Poon LC et al (2013) Fetal fraction in maternal plasma cell- free DNA at 11–13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol 41:26–32. doi:10.1002/uog.12331
Chan KC, Zhang J, Hui AB et al (2004) Size distributions of maternal and fetal DNA in maternal plasma. Clin Chem 50(1):88–92
Chiu RW, Lo YM (2013) Clinical applications of maternal plasma fetal DNA analysis: translating the fruits of 15 years of research. Clin Chem Lab Med 51(1):197–204
Schwarzenbach H (2013) Circulating nucleic acids as biomarkers in breast cancer. Breast Cancer Res 15(5):211
Schwarzenbach H, Hoon DS, Pantel K (2011) Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 11(6):426–437
Schwarzenbach H, Eichelser C, Kropidlowski J et al (2012) Loss of heterozygosity at tumor suppressor genes detectable on fractionated circulating cell-free tumor DNA as indicator of breast cancer progression. Clin Cancer Res 18(20):5719–5730
Conflict of Interests
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Patel, V., Celec, P., Grunt, M., Schwarzenbach, H., Jenneckens, I., Hillebrand, T. (2016). Novel Technology for Enrichment of Biomolecules from Cell-Free Body Fluids and Subsequent DNA Sizing. In: Gahan, P., Fleischhacker, M., Schmidt, B. (eds) Circulating Nucleic Acids in Serum and Plasma – CNAPS IX. Advances in Experimental Medicine and Biology, vol 924. Springer, Cham. https://doi.org/10.1007/978-3-319-42044-8_30
Download citation
DOI: https://doi.org/10.1007/978-3-319-42044-8_30
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42042-4
Online ISBN: 978-3-319-42044-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)