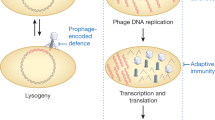
Abstract
The ubiquity of bacteria-phage interactions across biomes on earth has resulted in a diverse suite of adaptations conferring either bacterial resistance or phage infectivity. Understanding the mechanisms underlying these adaptations has important implications for the use of phages as therapeutic agents, but also offers key insights into how bacterial populations and communities are structured across time and space. In this chapter, we provide, first, an overview of coevolutionary theory relevant to bacteria-phage interactions. Next, we summarize the findings of experimental coevolution studies, focusing on the insights provided into the bacteria-phage coevolutionary processes. Although most experimental studies of bacteria-phage coevolution focus on mutational resistance and counter-resistance, we next survey the variety of resistance and counter-resistance strategies described in nature and consider their implications for bacteria-phage coevolution. We conclude by considering the implications of coevolution for developing phage therapies.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
The Coevolutionary Process and the Red Queen’s Race
Coevolution , the reciprocal evolution of adaptation and counter-adaptation between ecologically interacting species, is an important evolutionary process, which is believed to play a role, among many other effects, in the emergence and maintenance of diversity , the evolution of parasite virulence, and species extinction (Thompson 1994, 2005). In particular, antagonistic coevolution , whereby adaptation by each species reduces the fitness of the other (as between bacteria and bacteriophages), provides a potent and pervasive source of natural selection thought to be responsible for some of the fastest rates of evolutionary change yet observed (Brockhurst et al. 2014). This idea, that interspecific biotic conflict is a prime driving force of evolution, is best encapsulated in the Red Queen hypothesis, proposed by Van Valen (1973). The eponymous Red Queen refers to the character in Lewis Carroll’s Through the Looking Glass, with whom Alice has a race but soon realizes that despite running as fast as they can, neither of them is moving anywhere. This metaphor neatly captures the hypothesized situation in biological communities: Due to antagonistic ecological interactions, species must continually evolve simply to keep up with their evolving biological enemies, with the result that despite continual evolutionary adaptation, each species’ fitness never improves because any temporary gains are rapidly nullified by counteracting adaptation(s) (Lively 2010; Brockhurst et al. 2014). Correspondingly, much research has attempted to understand the dynamics and outcomes of the coevolutionary races between bacteria and lytic bacteriophages (other phage life-histories, such as temperate and filamentous phages, remain far less studied from a coevolutionary perspective) (Koskella and Brockhurst 2014).
Before coming to the empirical data, we first provide a brief overview of the theory underpinning our current view of host-parasite coevolutionary processes. Most models of host-parasite coevolution assume a genetic basis of infection, whereby infection is a product of the interaction between parasite and host alleles at loci encoding infectivity and resistance, respectively. (Although, see Nuismer et al. (2005) and Best et al. (2010) for examples of models where infection is determined by quantitative traits.) The most commonly used models of infection genetics are matching allele (MA) and gene-for-gene (GFG) (Agrawal and Lively 2002), although variants of these (inverse matching allele, inverse gene-for-gene) as well as alternatives (e.g., lock and key) exist. Which model of infection genetics best applies to bacteria-phage interactions is a matter of debate, with authors variously arguing the merits of MA, GFG, multilocus-GFG, modified-GFG, IGFG, and relaxed lock and key (Hurst et al. 2008; Fenton et al. 2009; Williams 2013). However, it is likely that none accurately describes any specific mechanism of bacteria-phage interaction and, moreover, that within many bacteria-phage infection processes, multiple different resistance mechanisms corresponding to different infection genetics operate sequentially or simultaneously (Fenton et al. 2012). In the absence of detailed mechanistic understanding of the appropriate infection genetics, most researchers have instead focused on determining the mode(s) of coevolution operating in bacteria-phage interactions. Thus, we next describe the modes of coevolutionary dynamic that emerge from models of the best-studied infection genetics, MA and GFG.
Under MA, parasites cause infection when their infectivity allele matches the host resistance allele, such that each parasite genotype is a specialist that can only infect a single corresponding host genotype (Agrawal and Lively 2002); this mode of infection genetics was derived from interactions between pathogens and invertebrate innate immunity (Luijckx et al. 2013). Under GFG, hosts carry either a Resistant or a Susceptible allele, while parasites carry either a Virulent or an Avirulent allele, A-allele parasites can infect only S-allele hosts whereas V-allele parasites can infect both R- and S-allele hosts; as such GFG allows for the evolution of generalist parasites (i.e., carrying the V-allele) and hosts (i.e., carrying the R-allele), and was derived from plant-pathogen interactions (Thompson and Burdon 1992). The contrasting properties of MA and GFG infection genetics give rise to different selection dynamics in coevolutionary models: MA models typically display time-lagged allele frequency oscillations driven by negative frequency-dependent selection (nFDS) (Agrawal and Lively 2002). Here, parasite allele frequencies track host allele frequencies such that at any given time, selection favors rare host alleles, which are less prone to infection by the prevailing, common parasite genotype(s); under these circumstances, high levels of allelic diversity in both hosts and parasites is maintained over time. GFG models typically display directional selection favoring fixation of the generalist host and parasite alleles (R and V, respectively), where coevolution corresponds to an arms race for increasing resistance and infectivity over time (Agrawal and Lively 2002). (However, readers should note that more complex GFG models, incorporating pleiotropic costs of resistance and infectivity, can instead give rise to balanced polymorphism or sustained coevolutionary cycling, similar to that seen in MA models (Sasaki 2000).)
Coevolutionary theory therefore suggests two predominant modes of dynamical antagonistic coevolution; these have been named: (i) Fluctuating Selection Dynamics , where host and parasite allele frequencies undergo time-lagged oscillations driven by nFDS ; (ii) Arms Race Dynamics , where host and parasite alleles undergo time-lagged selective sweeps driven by directional selection for increased resistance and infectivity, respectively, over time (Gandon et al. 2008). Distinguishing these modes of coevolution in host-parasite populations has been achieved using time-shift experiments (Gandon et al. 2008; Gaba and Ebert 2009). These are cross-infection experiments where hosts (or parasites) from a given point in time are exposed to parasites (or hosts) from past, contemporary, and future time-points. Plots of infectivity or resistance against time-shift, i.e., the difference in time between the host and the parasite sample, can then be used to infer the mode of coevolution (Brockhurst and Koskella 2013). Whereas ARD gives rise to monotonic relationships of resistance or infectivity against time-shift, more complex patterns are predicted for FSD depending on the phase of the coevolutionary cycle, including monotonic, V-shaped, and unimodal relationships; as such, it is essential that studies of coevolutionary mode perform time-shift assays for multiple sampling points over time (Gandon et al. 2008).
Experimental Studies of Bacteria-Bacteriophage Antagonistic Coevolution
Coculture studies across a range of bacteria-bacteriophage associations reveal the potential for rapid evolutionary responses to phage-imposed selection in bacterial populations, and the potential for evolutionary counter-adaptation in bacteriophage infectivity (Koskella and Brockhurst 2014). Early studies using the classic laboratory model bacterium, Escherichia coli B, and various T-even, T-odd, and λ-vir bacteriophages suggested that while bacteria-bacteriophage coevolution occurred, it was limited to one or two cycles of adaptation and counter-adaptation (Dennehy 2012). To detail one example, coculture of E. coli and T7 in chemostats led to a predictable coevolutionary sequence: The evolution of a resistant bacterial mutants, followed by the evolution of a bacteriophage host-range mutant infecting both the ancestral bacterium and the resistant mutant, followed by the further evolution of bacterial mutants to resist both the ancestral phage and the host-range phage mutant, whereupon coevolution ceased (i.e., 1.5 cycles of coevolution) (Chao et al. 1977). Despite the limited nature of coevolution in this experiment, it nevertheless revealed that the evolution of generalist resistance and generalist infectivity can occur, suggesting therefore that, at least under laboratory conditions, E. coli – T7 coevolution followed the ARD scenario. Similar patterns of limited ARD coevolution have been reported in the other lab studies of E. coli – lytic phage interactions, where resistance and infectivity evolved through spontaneous de novo mutation (Bohannan and Lenski 2000, Dennehy 2012).
E. coli B has a long history of adaptation to the lab environment (Daegelen et al. 2009) and is known to harbor defects in cell wall moieties and lipopolysaccharides (Yoon et al. 2012) commonly used by bacteriophages as targets for adsorption (Lenski and Levin 1985). Therefore, its coevolutionary relationship with bacteriophages may not be representative of bacteria-bacteriophage associations less far-removed from the natural environment. Indeed, lab coculture studies of a range of other bacteria-bacteriophage interactions have revealed the potential for dynamical coevolutionary change to be sustained over many 100 s of bacterial generations (reviewed in Koskella and Brockhurst (2014)). The best-studied interaction is that between the plant-associated soil bacterium Pseudomonas fluorescens SBW25 and the T7-like bacteriophage φ2 (Buckling and Rainey 2002; Brockhurst et al. 2007). Over the first approx. 300 bacterial generations, P. fluorescens – φ2 coevolution conforms to ARD: For multiple sampled time-points, bacterial resistance and phage infectivity displayed positive monotonic relationships with time-shift, consistent with recurrent time-lagged selective sweeps of de novo adaptive mutations driven by directional selection (Brockhurst et al. 2003; Paterson et al. 2010; Hall et al. 2011). By contrast, after approx. 300 bacterial generations, the coevolutionary mode shifts toward FSD: No further increases in bacterial resistance or phage infectivity ranges were observed, indicating a weakening of the response to directional selection, yet coevolution proceeded as sustained oscillations of bacterial resistance and phage infectivity types of approximately equal breadth but different specificity (Hall et al. 2011). The transition, from ARD to FSD, appears to be driven by costs associated with increased resistance and infectivity in this system, which, past some threshold, act to impede further responses to directional selection (Hall et al. 2011).
The balance of costs and benefits of resistance/infectivity traits seems to play a key role in determining the dynamics of bacteria-bacteriophage coevolution. Manipulation of environmental parameters can alter this balance, with predictable effects on coevolutionary dynamics. For example, population mixing elevates bacteria-phage encounter rates, which increases the benefit of resistance and thereby accelerates the tempo of coevolution (Brockhurst et al. 2003). Similarly, increasing the supply of resources to P. fluorescens has demographic (increased bacteria-phage population sizes, and therefore higher encounter rates), population genetic (increased mutational supply), and physiological (reduced costs of resistance) effects, which reduce the costs and increase the benefits of resistance, and thereby accelerates the tempo of coevolution (Lopez-Pascua and Buckling 2008). The abiotic environment can also affect the mode of coevolution: P. fluorescens – φ2 coculture experiments in a soil environment demonstrate that coevolution conformed to FSD, with bacteria showing highest resistance against their contemporary phages. This was the case even during the early stages of coevolution, which show ARD in rich liquid media, and was caused by high costs of bacterial resistance in soil environments (Gomez and Buckling 2011).
Mechanisms of Bacterial Resistance to Lytic Bacteriophage and Phage Counter-Resistance
The adaptive race between bacteria and their phages has resulted in a striking diversity of mechanisms for infection and resistance. Current understanding of these mechanisms has been reviewed elsewhere (Nechaev and Severinov 2008; Hyman and Abedon 2010; Labrie et al. 2010; Westra et al. 2012; Molineux and Panja 2013; Samson et al. 2013; Young 2013), so here we aim to update and summarize the breadth of this work by highlighting examples of each mechanism and discussing the potential impact each has on the interaction. Bacterial cells can resist phages by blocking phage infection, reproduction, and/or transmission using a variety of resistance mechanisms (Hyman and Abedon 2010). These mechanisms differ from one another in many ways, from the ease at which phages can overcome them (Samson et al. 2013), to the potential fitness costs a host pays for employing them (Bohannan and Lenski 2000), to the breadth of phage resistance attained (Hyman and Abedon 2010), and these differences have the potential to influence the way in which bacteria coevolve with different phages in the environment (Jessup and Bohannan 2008; Gomez and Buckling 2011; Hall et al. 2011; Betts et al. 2014).
As a first line of defense, bacteria can become resistant to particular phages by preventing phage adsorption through either: the loss or alteration of target receptors; the production of extracellular polysaccharide matrices; or the production of competitive inhibitors that bind to the phage attachment site (reviewed in Hyman and Abedon (2010) and Labrie et al. (2010)). Common phage attachment sites include bacterial pili, flagella, and lipopolysaccharides (LPS) from the outer membrane (Lindberg 1973). Intriguingly, there is also recent evidence that phage attachment might require the presence of multiple receptors for successful attachment (Reyes-Cortés et al. 2012), and some phages are known to use motility appendages such as flagella and pili to move toward the bacterial cell surface (Bender et al. 1989; Guerrero-Ferreira et al. 2011). Loss or alteration of these key structures in the cell wall are predicted to lead to decreased bacterial fitness, and evidence for such costs of resistance is now substantial. Phage-resistant mutants of Yersinia pestis were found to have decreased production of LPS core biosynthesis enzymes, and this lead to slower growth rates and increased death rate (Filippov et al. 2011). In this case, loss of fitness resulted in attenuated virulence when tested on mice suggesting that phage resistance critically alters the interaction between the pathogenic bacterium and its host. Similarly, modification of a capsular polysaccharide (CPS) resulting in a nonfunctional gene product was found to confer phage resistance in mutant Campylobacter jejuni (Sørensen et al. 2011). Phage resistance of this food-borne pathogen found in the avian gut has also been associated with decreased fitness in the host (Scott et al. 2007). Such attenuated virulence of bacterial pathogens as a result of phage-mediated selection is proving to be a common feature of bacteria-phage systems (Le et al. 2014; Capparelli et al. 2010; Meaden and Koskella 2013). Thus, although receptor loss or modification offers a rapid acquisition of resistance to circulating phages, it is also likely to be among the most costly mechanisms of resistance and therefore may well be lost over time in favor of less costly mechanisms. Alternatively, bacteria are also capable of varying expression of receptors either stochastically or in response to particular stimuli, therefore only paying the cost when risk of infection is high. This “phase variation” can confer resistance of a subset of genetically identical bacterial cells to phage. For example, Vibrio cholerae exhibit intrastrain heterogeneity for expression of the lipopolysaccharide O1 antigen, a known phage receptor (Seed et al. 2012), and variable expression of O antigens in Salmonella enterica has also been associated with phage resistance (Kim and Ryu 2012). Finally, some bacteria are able to block the entry of phage DNA into host cells through the acquisition of prophages (Labrie et al. 2010). These superinfection exclusion mechanisms have been demonstrated for prophages of both Gram-negative (Maillou and Dreiseikelmann 1990) and Gram-positive bacteria (Mahony et al. 2008), and are generally thought to involve phage-encoded proteins that are found in the inner membrane of cells and block the injection of DNA from a subset of other lytic phages.
Like loss or modification of receptors, production of extracellular polysaccharides has long been thought to interfere with phage adsorption, although the effect is not universal (Gu et al. 2011; Roach et al. 2013). For example, phage-resistant mutants of Bacillus anthracis were found to have a mucoid colony phenotype, with increased production of extracellular matrix, as a result of single mutational steps in the csaB gene encoding a cell surface anchoring protein (Bishop-Lilly et al. 2012). However, while the production of exopolysaccharides by the plant pathogen, Erwinia amylovora, has been found to be negatively associated with infection by Myoviridae phages, it seems to be essential for infection and reproduction of Podoviridae phages (Roach et al. 2013), highlighting that this resistance mechanism is not yet clear cut. Finally, there is some evidence for competitive inhibition of phage binding due to the production of particular molecules by the host cell. For example, there is evidence that the siderophore Ferrichrome interferes with binding of phage T5 to its receptor on the surface of Escherichia coli (Luckey et al. 1975). Similarly, coincubation of Staphylococcus aureus with immunoglobulin G from either human and rabbit serum was found to block absorption of phage (Nordström et al. 1974), and host cell-produced protein A was also found to inhibit phage absorption (Nordström and Forsgren 1974). In addition, support for the potential of competitive inhibition as a mechanism for increasing resistance to phages comes from studies in which synthetic peptides designed to block receptors are used to confirm phage binding sites (Killmann et al. 1995). However, this latter mechanism is currently less well supported as a widespread natural mechanism of resistance.
Phages are able to counter-adapt to adsorption-blocking resistance mechanisms of their hosts by altering their tail fibers to either recognize newly altered receptors or by switching to entirely new receptors (Hyman and Abedon 2010; Meyer et al. 2012; Munsch-Alatossava and Alatossava 2013), by degrading extracellular polysaccharide matrices (Yan et al. 2014), or by stochastically altering receptor recognition (Samson et al. 2013). For example, analysis of phage genomes after experimental evolution with Pseudomonas fluorescens identified a number of independent mutations in the tail fiber genes required for adsorption (Scanlan et al. 2011). As a more extreme response, experimental evolution of a lytic derivative of phage λ that attaches to the LamB outer membrane protein of E. coli was found to evolve the ability to also use OmpF as a receptor in a glucose-limited environment (Meyer et al. 2012). Furthermore, phages infective to Bordetella spp. have been shown to use diversity-generating retroelements that direct mutagenesis to specific sites in order to vary their recognition of bacterial surface receptors (Doulatov et al. 2004). In response to bacterial production of extracellular polysaccharide matrices, phages are known to produce hydrolyzing enzymes that breakdown the matrix and allow the phage to come into contact with the receptor. For example, phage infection of P. aeruginosa strains isolated from patients with cystic fibrosis was recently shown to involve hydrolysis of the bacterial exopolysaccharide secretion, which resulted in production of clear halos around phage plaques as a result of overproduction of polysaccharide-degrading enzymes leading to bacteria without surrounding capsules (Glonti et al. 2010). Similarly, a number of phages have recently been characterized that are capable of degrading biofilms produced by Escherichia coli strains associated with urinary tract infections (Chibeu et al. 2012).
If the phage is able to successfully adsorb to its bacterial host and inject its own genetic material into the host cell, there are still a number of possible routes to resistance for the cell; phage DNA entering the host cell can be degraded by host-encoded enzymes and/or replication of DNA can be blocked by the host directly. First, a number of restriction-modification systems have been found to be widespread across bacterial taxa. These systems act to recognize and degrade unmethylated DNA in the cell, including that of phages, through production of restriction enzymes (Wilson and Murray 1991). Restriction endonucleases are generally effective against DNA phages as well as plasmids, but phages that are successfully methylated within the cell, thus evading degradation, are not only able to reproduce but also produce progenies that can evade recognition by future host cells with the same restriction-modification system. Although restriction-modification systems are often considered quite specific, recent work has demonstrated that bacteria harboring highly promiscuous restriction-modification systems are better able to deal with phage anti-restriction mechanisms than those carrying high-fidelity systems (Vasu et al. 2012). In addition, there is evidence that restriction-modification-mediated resistance may be temperature dependent; phage resistance of Listeria monocytogenes across varying temperatures was found to be due to differential expression of the restriction endonuclease (Kim et al. 2012). Phages can overcome restriction-modification systems by accumulating mutations at endonuclease recognition sites (Krüger et al. 1987), by acquiring their own methylase genes (Hill et al. 1991, McGrath et al. 1999), by switching to more unusual nucleic acids (Krüger and Bickle 1983), or by modifying the host’s restriction-modification system directly (King and Murray 1995). In addition to restriction-modification systems, the majority of bacterial and archaeal genomes examined to date contain arrays of clustered, regularly interspaced short palindromic repeats (CRISPRs) which have been linked to, among other functions, resistance against phages (Barrangou et al. 2007; Bondy-Denomy and Davidson 2014). In contrast to the restriction-modification systems, the CRISPR-Cas system is highly specific to infecting phages, as the bacterial host must match a short segment of the phage genome in order to block replication. In order to achieve such specific recognition, bacteria incorporate small segments of foreign DNA into their own genomes (referred to as “spacers”), and these sequences are used to generate RNA that is carried by the ribonucleoprotein Cas complex to cleave the matching phage DNA (Bhaya et al. 2011; Westra et al. 2012). The acquisition of spacers is known to be highly biased away from sequences that match the host DNA and also toward specific phage genome locations (Paez-Espino et al. 2013). Interestingly, there is building evidence that the presence of “old” spacers (i.e., those which no longer directly match the foreign genetic elements in the local environment) might “prime” the CRISPR-cas system for more rapid acquisition of new spacers in response to mutations in the DNA targets (the protospacers), which could help explain the rapid acquired immunity observed (Fineran et al. 2014). As with each of the previous resistance mechanisms discussed, there is building evidence that coevolving phages can rapidly escape recognition by the CRISPR-Cas system (Samson et al. 2013). A number of “anti-CRISPR” genes have already been found within the genomes of Pseudomonas aeruginosa phages (Bondy-Denomy et al. 2012), and some phages have even been found to carry their own CRISPR-cas system to target a chromosomal island of the bacterial host (Seed et al. 2013). Furthermore, experimentally evolved phages infecting Streptococcus thermophiles hosts were found to rapidly escape CRISPR-Cas based immunity via mutations in the proto-spacer adjacent motif (PAM) (Sun et al. 2013). Finally, there is recent evidence that the CRISPR-Cas and restriction-modification systems can work synergistically to increase bacterial resistance to phages, perhaps going some way to explain the ubiquity of the two systems across the bacterial tree of life (Dupuis et al. 2013).
A final known mechanism of bacterial resistance is the abortive infection (Abi) system. This mechanism of cell suicide, encoded by a toxin-antitoxin system, has been shown to protect bacterial hosts against infection by multiple phages and seems to be relatively difficult for phages to circumvent (Fineran et al. 2009). Most Abi systems appear to be plasmid-encoded (Chopin et al. 2005), suggesting that they can move readily among bacterial species. Furthermore, despite the great diversity of Abi systems uncovered to date, there do seem to be some common features. In most cases, phage infection leads to the activation of dormant enzymes, and this activation results in cleavage of highly conserved and essential components of the cellular translational apparatus (which itself is known to be hijacked by RNA viruses for replication; Bushell and Sarnow (2002)). This response has recently been demonstrated in Escherichia coli where it was found to be a low cost and effective strategy at the population level (Refardt et al. 2013) that is particularly favored in spatially structured environments where protection is conferred primarily to highly related neighbors (Berngruber et al. 2013). Not surprisingly, there is evidence that phages are able to adapt in response to Abi systems. Isolation of mutant lytic phages that showed resistance against one or two Abi systems, AbiK and/or AbiT, in Lactococcus lactis were found to have acquired this resistance via extensive homologous and nonhomologous recombination with prophages within the bacterial genome, exchanging as much as 79% of their genomes (Labrie and Moineau 2007). More recently, evidence for phage production of a “pseudotoxin” that mimics ToxI in order to suppress ToxN has been uncovered (Blower et al. 2012). Abi escape mutants of the lytic phage ΦTE, infecting Pectobacterium atrosepticum, were found to have acquired a noncoding RNA sequence with expanded repeats that allowed the phage to replicate in the presence of ToxIN. Finally, comparative genomic approaches to explore bacterial and archaeal defense systems has uncovered an intriguing hypothesis: that the immunity systems whereby bacteria target and either degrade or block replication of phage DNA are functionally coupled to systems that lead to cell death or dormancy upon phage infection (Makarova et al. 2013). Under this model, the latter defense pathway would only be triggered upon failure of the first, thereby both minimizing the probability of cell death and maximizing protection at the bacterial population level.
The variety of resistance and infectivity mechanisms observed so far represents the result of an ancient, obligate association and ongoing coevolution. Although the exploration of how these mechanisms might differentially affect the outcome of coevolution is in its infancy (Hall et al. 2011; Betts et al. 2014), the data thus far provide intriguing support for the translation of mechanistic understanding into predictive power. One aspect of bacterial resistance and phage counter-adaptation that warrants further research is in regard to how such mechanisms might vary across environments and time, regardless of bacterial genetics. Many bacterial resistance mechanisms are remarkably labile and can often be regulated by reversible switching of phenotypic expression in response to environmental cues (reviewed in Hoskisson and Smith (2007)), and this variation will have key implications for the coevolutionary trajectory of the two players. The known resistance mechanisms are of course not mutually exclusive, and there is now good reason to think that multiple mechanisms will be acting simultaneously in a population, even within the same pairwise interaction. Moving forward, it will also become important to determine when and how these defenses interact to protect bacterial populations and communities against circulating phages (Tables 1 and 2).
Effects of Resistance and Counter-Resistance on Fitness and Phenotype
Since phage receptors are often components of the bacterial cell membrane like LPS or membrane-associated proteins like transporters, bacterial evolution of phage resistance often has deleterious side-effects. As such, bacterial resistance mutations often reduce bacterial fitness in the absence of phages through antagonistic pleiotropy, wherein the molecular changes that cause resistance by, for instance, altering a phage-binding site impair the normal biological function of the molecule. Furthermore, the costs of multiple resistance mutations in an individual bacterial host may interact through epistasis. Epistasis may constrain resistance evolution if the combined costs are greater than the sum of the costs of each individual mutation (negative epistasis ), or conversely could promote resistance evolution if the cost of each subsequent mutation is less than additive (positive epistasis ). Examples of both forms of epistasis have been observed in bacteria-phage experiments (Bohannan et al. 1999; Buckling et al. 2006; Koskella et al. 2012). In addition to the direct effects of resistance mutations, the evolution of resistance can affect the bacterial phenotype due to linkage between the primary resistance mutation and coincident mutations at other loci in the genome. Here, selective sweeps of resistance mutations could also lead to fixation of other mutations by hitchhiking. This has been observed in populations of P. fluorescens wherein mutations causing colony morphology variants can hitchhike with resistance, leading to phenotypic divergence between populations due to the chance associations between linked mutations (Buckling and Rainey 2003, Brockhurst et al. 2004).
On the other side of the coin, the consequence of coevolution for phage fitness and phenotypic traits has been less well studied. However, this is particularly relevant under the ARD coevolution scenario: fitness cost of infectivity is a most parsimonious explanation for the absence of phage phenotypes with “universal infectivity” (one that can infect all host genotypes) (Agrawal and Lively 2002). Empirical evidence has mainly come from two model systems. In the E. coli – T7 system, the more infective phage phenotype (T71) often reaches a lower population size than the ancestral phenotype (T70) (Forde et al. 2007). In the P. fluorescens – φ2 system, phage phenotypes with broader infectivity ranges have lower growth rate than those with narrow infectivity ranges when infecting the ancestral, susceptible, bacterial genotype (Poullain et al. 2008); and fixation of phage phenotypes that form tiny plaques on bacterial lawn were occasionally observed in phage populations coevolving with bacteria but not in those always infecting the ancestral, susceptible, bacterial phenotype (Q.-G. Zhang, personal observations). The evolution of infectivity may also show pleiotropic effect on phage traits. In recent work with the P. fluorescens – φ2 system, phages with broader infectivity showed higher sensitivity to temperature elevation, with a negative consequence for the population-level adaptation: phages coevolved with bacteria were less able to evolutionarily adapt to higher temperature compared with those which always infected the ancestral, susceptible, bacterial phenotype (Zhang and Buckling 2011). This supports a notion that reciprocal coevolutionary adaptation may come at the expense of adaptation to the physical environments (Thompson 2013), and highlight the importance to consider coevolutionary dynamics when studying evolutionary rescue effects.
(Co)Evolutionary Considerations of Phage Therapy
Therapeutic uses of phages for treating bacterial infections have received a resurgent attention in the past two decades, due to the challenge from the unprecedented level of bacterial antibiotic resistance . As bacteria may readily evolve resistance to phages too, a naturally arising question is whether or not we will also be faced with a “tragedy of the commons” in phage applications: overuse of phages results in the spread of phage-resistant bacteria, leaving us with no weapon to use to combat bacterial pathogens (Meaden and Koskella 2013). Parallel with the debate about single-drug versus multiple-drug uses, there are different opinions in the design of phage treatments: the conventional wisdom is to use cocktails of different types of phages infecting the same species or strains to reduce the chance of emergence of resistant bacteria (Gu et al. 2012); and the opposite opinion is to use only a single type of phage to treat a specific infection in order to prevent the emergence of bacteria that are resistant to broad-host range cocktails of phages (Krylov et al. 2012).
One positive aspect with phage therapy is that phages can evolve novel infectivity phenotypes at a speed much faster than humans can develop new antibiotic drugs. Considering what we have learned about bacteria-phage coevolution, it is reasonable to be more optimistic about phage therapy than antibiotic uses. For either antibiotic or phage therapy, the emergence of resistant bacteria is unlikely to be preventable (Levin and Bull 2004). But the spread and persistence of bacterial resistance in natural environments matter a lot. The cost of antibiotic resistance in bacteria is often relatively small and may often be compensated for by further compensatory evolution, leading to long-term persistence of such resistance in nature (Andersson and Hughes 2011). The situation with bacterial resistance to phages is likely to be different; bacteria have been continuously attacked by phages during their evolutionary history (in most habitats the virus-to-bacterium ratio is over 10; Srinivasiah et al. (2008)), but such strong selection did not result in fixation of broadly phage-resistant bacteria: biogeography studies of phage infection patterns show that phages often be infective to bacterial host cells sampled from distant locations (Clokie et al. 2011). Experimental evolution work with conventional lab environment (nutrient-rich liquid medium) suggests that broadly phage-resistant bacteria can emerge, and such broad resistance may not necessarily confer a large fitness cost (Buckling and Rainey 2002; Forde et al. 2008b; Koskella et al. 2012). However, when such evolution experiments were done in more natural environments such as soil or tree leaves (Koskella 2014), bacteria-phage coevolution is more likely to follow an FSD pattern, and the bacteria do not maintain broad resistance to phages (Gomez and Buckling 2011). In these more naturalistic environments, it may be very costly for the bacteria to maintain resistance to phage types no longer present in their local environments. This suggests that even the broadly phage-resistant bacteria do emerge in clinic settings, their spread and persistence in natural environments may be limited.
Meanwhile, the evolution of phages against bacteria does not guarantee desirable therapeutic effectiveness. What we hope phages do in therapeutic applications (driving bacteria to very low density or even extinct) is often not what phages manage to do in nature (Wommack and Colwell 2000; Danovaro et al. 2011). Appropriate human interventions are often necessary for better therapeutic practice. In fact, we may make use of experimental evolution protocols for producing more effective phage preparations. First, many phages isolated from natural environments do not kill bacteria very efficiently, e.g., due to low growth rate but not a lack of infectivity, because selection for other traits such as lower decay rates may have limited the evolution of high virulence of phages in nature. Here, simple evolutionary training, repeated serial passages on susceptible bacterial types in the lab, may significantly increase the phage virulence (Betts et al. 2013). Second, under the FSD scenario, every phage type and the bacterial type susceptible to it undergo time-lagged oscillations in frequency and population size, leading to limited effect of phages on the total population size of bacteria due to temporal mismatches. In this case, repeated introduction of cocktails of phages infective against different bacterial types may achieve much better control of bacterial density. Third, we may adopt an experimental approach for “breeding” very broadly infective phages (and unavoidably broadly resistant bacteria) that may have not emerged or not widely persisted in nature, and use them for therapeutic use. Of course cautions are needed here to deal with the broadly resistant bacteria yielded in such breeding work, although as mentioned above, such broadly phage-resistant bacteria are unlikely to persist in nature. Previous experimental work in lab environments have provided several clues for ideal culture conditions for such breeding work: (i) large culture volumes, as larger population sizes increase the rate of supply of novel mutations; (ii) nutrient-rich medium that promotes ARD-like coevolution by increasing phage population sizes as well as decreasing the fitness cost of broad resistance in bacteria (Forde et al. 2008a; Lopez-Pascua and Buckling 2008; Lopez Pascua et al. 2014); (iii) population mixing that increases the rate of encounter between phages and susceptible bacteria and thus leads to stronger selection for bacterial defense which in turn increases the strength of selection for phage infectivity (Brockhurst et al. 2003); and (iv) repeated immigration of susceptible bacteria at an appropriate migration rate; here the bacterial immigration needs to be strong enough to increase the population sizes of the phages but not so strong to select against the evolution of broad infectivity ranges in the phages (Benmayor et al. 2009).
An important difference between phage treatment of bacteria and biocontrol of higher organisms (such as weeds or insects) is that phage uses can be combined with chemical treatments. Phages are usually insensitive to antibiotics, a recent study with the P. fluorescens – φ2 system showed that simultaneous use of the phage and an antibiotic (kanamycin) greatly reduce the chance of bacterial survival that requires resistance evolution; and furthermore, in the very rare cases where the bacteria survived the combined treatments, the resistant bacteria had very low fitness (Zhang and Buckling 2012). In another study with the same bacterium-phage system, under simultaneous treatments of the phage and an antibiotic (rifampicin), bacteria evolved to be resistant and survived, but later some biofilm-forming bacterial genotypes in the populations showed some levels of reversion to antibiotic susceptibility; but this did not happen in the populations treated with the antibiotic only. Presumably this biofilm-forming genotype can, through forming a biofilm on the surface of liquid medium, protect a portion of its cells from antibiotic stress. Moreover, because antibiotic resistance had been very costly when the populations were also exposed to phages, this fitness cost drove the resistance reversion at the population level (Escobar-Páramo et al. 2012). However, the fitness cost conferred by resistance to phages may not generally augment that of resistance to antibiotics. In a more recent study also with P. fluorescens – φ2, bacteria were exposed to phage treatment first, and then evolved on concentration gradients of three single antibiotics (cefotaxime, chloramphenicol, and kanamycin), with migration along the gradients (mimicking the situations where bacteria evolve in more realistic heterogeneous drug environment), additive or antagonistic, but not synergistic, interaction between fitness costs of resistance to the phage and those of resistance to antibiotics were observed (Zhang 2014). It is possible that in more realistic environments, migration of bacteria from low-drug environments can promote compensatory evolution that reduces the fitness cost of antibiotic resistance in the high-drug environments.
Therefore, phage therapy does provide a very promising alternative to antibiotic uses, particularly because the evolution of bacterial resistance to phages might be a less serious problem compared with bacterial antibiotic resistance. Studies of bacteria-phages coevolution can provide much valuable knowledge for improving the practice of phage therapy.
References
Agrawal A, Lively CM (2002) Infection genetics: gene-for-gene versus matching-alleles models and all points in between. Evol Ecol Res 4:79–90
Andersson DI, Hughes D (2011) Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol Rev 35:901–911
Avrani S, Wurtzel O, Sharon I, Sorek R, Lindell D (2011) Genomic island variability facilitates Prochlorococcus-virus coexistence. Nature 474:604–608
Baker J, Dong S, Pritchard D (2002) The hyaluronan lyase of Streptococcus pyogenes bacteriophage H4489A. Biochem J 365:317–322
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712
Bender RA, Refson CM, O'Neill EA (1989) Role of the flagellum in cell-cycle-dependent expression of bacteriophage receptor activity in Caulobacter crescentus. J Bacteriol 171:1035–1040
Benmayor R, Hodgson DJ, Perron GG, Buckling A (2009) Host mixing and disease emergence. Curr Biol 19:764–767
Berngruber TW, Lion S, Gandon S (2013) Evolution of suicide as a defence strategy against pathogens in a spatially structured environment. Ecol Lett 16:446–453
Best A, White A, Kisdi E, Antonovics J, Brockhurst MA, Boots M (2010) The evolution of host-parasite range. Am Nat 176:63–71
Betts A, Vasse M, Kaltz O, Hochberg ME (2013) Back to the future: evolving bacteriophages to increase their effectiveness against the pathogen Pseudomonas aeruginosa PAO1. Evol Appl 6:1054–1063
Betts A, Kaltz O, Hochberg ME (2014) Contrasted coevolutionary dynamics between a bacterial pathogen and its bacteriophages. Proc Natl Acad Sci USA 111:11109–11114
Bhaya D, Davison M, Barrangou R (2011) CRISPR-Cas Systems in Bacteria and Archaea: versatile small RNAs for adaptive defense and regulation. Ann Rev Genet 45:273–297
Bishop-Lilly K, Plaut R, Chen P, Akmal A, Willner K, Butani A, Dorsey S, Mokashi V, Mateczun A, Chapman C, George M, Luu T, Read T, Calendar R, Stibitz S, Sozhamannan S (2012) Whole genome sequencing of phage resistant Bacillus anthracis mutants reveals an essential role for cell surface anchoring protein CsaB in phage AP50c adsorption. Virol J 9:246
Blower TR, Evans TJ, Przybilski R, Fineran PC, Salmond GP (2012) Viral evasion of a bacterial suicide system by RNA-based molecular mimicry enables infectious altruism. PLoS Genet 8:e1003023
Bohannan BJM, Lenski RE (2000) Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol Lett 3:362–377
Bohannan BJM, Travisano M, Lenski RE (1999) Epistatic interactions can lower the cost of resistance to multiple consumers. Evolution 53:292–295
Bondy-Denomy J, Davidson AR (2014) To acquire or resist: the complex biological effects of CRISPR–Cas systems. Trends Microbiol 22:218–225
Bondy-Denomy J, Pawluk A, Maxwell KL, Davidson AR (2012) Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 493:429–432
Brockhurst MA, Koskella B (2013) Experimental coevolution of species interactions. Trends Ecol Evol 28(6):367–375
Brockhurst MA, Morgan AD, Rainey PB, Buckling A (2003) Population mixing accelerates coevolution. Ecol Lett 6:975–979
Brockhurst MA, Rainey PB, Buckling A (2004) The effect of spatial heterogeneity and parasites on the evolution of host diversity. Proc R Soc B Biol Sci 271:107–111
Brockhurst MA, Morgan AD, Fenton A, Buckling A (2007) Experimental coevolution with bacteria and phage the Pseudomonas fluorescens – phi 2 model system. Infect Genet Evol 7:547–552
Brockhurst MA, Chapman T, King KC, Mank JE, Paterson S, Hurst GD (2014) Running with the red queen: the role of biotic conflicts in evolution. Proc R Soc B Biol Sci 281:20141382
Buckling A, Rainey PB (2002) Antagonistic coevolution between a bacterium and a bacteriophage. Proc R Soc B Biol Sci 269:931–936
Buckling A, Rainey PB (2003) The role of parasites in sympatric and allopatric host diversification (vol 420, pg 496, 2002). Nature 421:294–294
Buckling A, Wei Y, Massey RC, Brockhurst MA, Hochberg ME (2006) Antagonistic coevolution with parasites increases the cost of host deleterious mutations. Proc R Soc B Biol Sci 273:45–49
Bushell M, Sarnow P (2002) Hijacking the translation apparatus by RNA viruses. J Cell Biol 158:395–399
Capparelli R, Nocerino N, Lanzetta R, Silipo A, Amoresano A, Giangrande C, Becker K, Blaiotta G, Evidente A, Cimmino A, Iannaccone M, Parlato M, Medaglia C, Roperto S, Roperto F, Ramunno L, Iannelli D (2010) Bacteriophage-resistant Staphylococcus aureus mutant confers broad immunity against staphylococcal infection in mice. PLoS One 5:e11720
Chao L, Levin BR, Stewart FM (1977) Complex Community in a Simple Habitat – experimental-study with bacteria and phage. Ecology 58:369–378
Chibeu A, Lingohr EJ, Masson L, Manges A, Harel J, Ackermann H-W, Kropinski AM, Boerlin P (2012) Bacteriophages with the ability to degrade uropathogenic Escherichia coli biofilms. Viruses 4:471–487
Chopin M-C, Chopin A, Bidnenko E (2005) Phage abortive infection in lactococci: variations on a theme. Curr Opin Microbiol 8:473–479
Clokie MRJ, Millard AD, Letarov AV, Heaphy S (2011) Phages in nature. Bacteriophage 1:31–45
Daegelen P, Studier FW, Lenski RE, Cure S, Kim JF (2009) Tracing ancestors and relatives of Escherichia coli B, and the derivation of B strains REL606 and BL21(DE3). J Mol Biol 394:634–643
Danovaro R, Corinaldesi C, Dell'Anno A, Fuhrman JA, Middelburg JJ, Noble RT, Suttle CA (2011) Marine viruses and global climate change. FEMS Microbiol Rev 35:993–1034
Dennehy JJ (2012) What can phages tell us about host-pathogen coevolution? Int J Evol Biol 2012:396165
Destoumieux-Garzón D, Duquesne S, Peduzzi J, Goulard C, Desmadril M, Letellier L, Rebuffat S, Boulanger P (2005) The iron-siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25: role of the microcin Val11-Pro16 beta-hairpin region in the recognition mechanism. Biochem J 389:869–876
Doulatov S, Hodes A, Dai L, Mandhana N, Liu M, Deora R, Simons RW, Zimmerly S, Miller JF (2004) Tropism switching in Bordetella bacteriophage defines a family of diversity-generating retroelements. Nature 431:476–481
Dupuis M-È, Villion M, Magadán AH, Moineau S (2013) CRISPR-Cas and restriction–modification systems are compatible and increase phage resistance. Nat Commun 4:2087
Escobar-Páramo P, Gougat-Barbera C, Hochberg ME (2012) Evolutionary dynamics of separate and combined exposure of Pseudomonas fluorescens SBW25 to antibiotics and bacteriophage. Evol Appl 5:583–592
Fenton A, Antonovics J, Brockhurst MA (2009) Inverse-gene-for-gene infection genetics and Coevolutionary dynamics. Am Nat 174:E230–E242
Fenton A, Antonovics J, Brockhurst MA (2012) Two-step infection processes can lead to coevolution between functionally independent infection and resistance pathways. Evolution 66:2030–2041
Filippov AA, Sergueev KV, He Y, Huang X-Z, Gnade BT, Mueller AJ, Fernandez-Prada CM, Nikolich MP (2011) Bacteriophage-resistant mutants in Yersinia pestis: identification of phage receptors and attenuation for mice. PLoS One 6:e25486
Fineran PC, Blower TR, Foulds IJ, Humphreys DP, Lilley KS, Salmond GPC (2009) The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antitoxin pair. Proc Natl Acad Sci USA 106:894–899
Fineran PC, Gerritzen MJH, Suárez-Diez M, Künne T, Boekhorst J, van Hijum SAFT, Staals RHJ, Brouns SJJ (2014) Degenerate target sites mediate rapid primed CRISPR adaptation. Proc Natl Acad Sci USA 111:E1629–E1638
Forde S, Thompson J, Bohannan BM (2007) Gene flow reverses an adaptive cline in a coevolving host-parasitoid interaction. Am Nat 169:794–801
Forde SE, Beardmore RE, Gudelj I, Arkin SS, Thompson JN, Hurst LD (2008a) Understanding the limits to generalizability of experimental evolutionary models. Nature 455:220–223
Forde SE, Thompson JN, Holt RD, Bohannan BJM (2008b) Coevolution drives temporal changes in fitness and diversity across environments in a bacteria-bacteriophage interaction. Evolution 62:1830–1839
Gaba S, Ebert D (2009) Time-shift experiments as a tool to study antagonistic coevolution. Trends Ecol Evol 24:226–232
Gandon S, Buckling A, Decaestecker E, Day T (2008) Host-parasite coevolution and patterns of adaptation across time and space. J Evol Biol 21:1861–1866
Glonti T, Chanishvili N, Taylor P (2010) Bacteriophage-derived enzyme that depolymerizes the alginic acid capsule associated with cystic fibrosis isolates of Pseudomonas Aeruginosa. J Appl Microbiol 108:695–702
Gomez P, Buckling A (2011) Bacteria-phage antagonistic coevolution in soil. Science 332:106–109
Gu E, Nguyen D, Shah N (2011) Capsular polysaccharide has a minor role on streptomycin-induced reduction of T7 phage adsorption to Escherichia coli. J Exp Microbiol Immunol (JEMI) 15:47–51
Gu J, Liu X, Li Y, Han W, Lei L, Yang Y, Zhao H, Gao Y, Song J, Lu R, Sun C, Feng X (2012) A method for generation phage cocktail with great therapeutic potential. PLoS One 7:e31698
Guerrero-Ferreira RC, Viollier PH, Ely B, Poindexter JS, Georgieva M, Jensen GJ, Wright ER (2011) Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proc Natl Acad Sci USA 108:9963–9968
Hall AR, Scanlan PD, Morgan AD, Buckling A (2011) Host-parasite coevolutionary arms races give way to fluctuating selection. Ecol Lett 14:635–642
Hill C, Miller L, Klaenhammer T (1991) In vivo genetic exchange of a functional domain from a type II A methylase between lactococcal plasmid pTR2030 and a virulent bacteriophage. J Bacteriol 173:4363–4370
Hoskisson PA, Smith MCM (2007) Hypervariation and phase variation in the bacteriophage ‘resistome’. Curr Opin Microbiol 10:396–400
Hurst LD, Forde SE, Beardmore RE, Gudelj I, Arkin SS, Thompson JN (2008) Understanding the limits to generalizability of experimental evolutionary models. Nature 455:220–U244
Hyman P, Abedon ST (2010) Bacteriophage host range and bacterial resistance. Adv Appl Microbiol 70:217–248
Jessup CM, Bohannan BJ (2008) The shape of an ecological trade-off varies with environment. Ecol Lett 11:947–959
Killmann H, Braun V (1992) An aspartate deletion mutation defines a binding site of the multifunctional FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol 174:3479–3486
Killmann H, Videnov G, Jung G, Schwarz H, Braun V (1995) Identification of receptor binding sites by competitive peptide mapping: phages T1, T5, and phi 80 and colicin M bind to the gating loop of FhuA. J Bacteriol 177:694–698
Kim M, Ryu S (2012) Spontaneous and transient defence against bacteriophage by phase-variable glucosylation of O-antigen in Salmonella enterica serovar Typhimurium. Mol Microbiol 86:411–425
Kim J-W, Dutta V, Elhanafi D, Lee S, Osborne JA, Kathariou S (2012) A novel restriction-modification system is responsible for temperature-dependent phage resistance in Listeria monocytogenes ECII. Appl Environ Microbiol 78:1995–2004
King G, Murray NE (1995) Restriction alleviation and modification enhancement by the Rac prophage of Escherichia coli K-12. Mol Microbiol 16:769–777
Koskella B (2014) Bacteria-phage interactions across time and space: merging local adaptation and time-shift experiments to understand phage evolution. Am Nat 184:S9–S21
Koskella B, Brockhurst MA (2014) Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol Rev 38:916–931
Koskella B, Lin DM, Buckling A, Thompson JN (2012) The costs of evolving resistance in heterogeneous parasite environments. Proc R Soc B Biol Sci 279:1896–1903
Krüger D, Bickle TA (1983) Bacteriophage survival: multiple mechanisms for avoiding the deoxyribonucleic acid restriction systems of their hosts. Microbiol Rev 47:345
Krüger D, Barcak G, Smith H (1987) Abolition of DNA recognition site resistance to the restriction endonuclease EcoRII. Biomed Biochim Acta 47:K1–K5
Krylov V, Shaburova O, Krylov S, Pleteneva E (2012) A genetic approach to the development of new therapeutic phages to fight Pseudomonas aeruginosa in wound infections. Viruses 5:15–53
Labrie SJ, Moineau S (2007) Abortive infection mechanisms and prophage sequences significantly influence the genetic makeup of emerging lytic Lactococcal phages. J Bacteriol 189:1482–1487
Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327
Le S, Yao X, Lu S, Tan Y, Rao X, Li M, Jin X, Wang J, Zhao Y, Wu NC et al (2014) Chromosomal DNA deletion confers phage resistance to Pseudomonas aeruginosa. Sci Rep 4:4738. https://doi.org/10.1038/srep04738
Lenski RE, Levin BR (1985) Constraints on the coevolution of bacteria and virulent phage – a model, some experiments, and predictions for natural communities. Am Nat 125:585–602
Levin BR, Bull JJ (2004) Population and evolutionary dynamics of phage therapy. Nat Rev Microbiol 2:166–173
Lindberg AA (1973) Bacteriophage receptors. Ann Rev Microbiol 27:205–241
Liu M, Deora R, Doulatov SR, Gingery M, Eiserling FA, Preston A, Maskell DJ, Simons RW, Cotter PA, Parkhill J (2002) Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 295:2091–2094
Lively CM (2010) A review of red queen models for the persistence of obligate sexual reproduction. J Hered 101:S13–S20
Lopez Pascua L, Hall AR, Best A, Morgan AD, Boots M, Buckling A (2014) Higher resources decrease fluctuating selection during host–parasite coevolution. Ecol Lett 17:1380–1388
Lopez-Pascua LDC, Buckling A (2008) Increasing productivity accelerates host-parasite coevolution. J Evol Biol 21:853–860
Luckey M, Wayne R, Neilands J (1975) In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun 64:687–693
Luijckx P, Fienberg H, Duneau D, Ebert D (2013) A matching-allele model explains host resistance to parasites. Curr Biol 23:1085–1088
Mahony J, McGrath S, Fitzgerald GF, van Sinderen D (2008) Identification and characterization of lactococcal-prophage-carried superinfection exclusion genes. Appl Environ Microbiol 74:6206–6215
Maillou J, Dreiseikelmann B (1990) The sim gene of Escherichia coli phage P1: nucleotide sequence and purification of the processed protein. Virology 175:500–507
Makarova KS, Wolf YI, Koonin EV (2013) Comparative genomics of defense systems in archaea and bacteria. Nucleic Acids Res 41:4360–4377
McGrath S, Seegers JF, Fitzgerald GF, van Sinderen D (1999) Molecular characterization of a phage-encoded resistance system in Lactococcus lactis. Appl Environ Microbiol 65:1891–1899
Meaden S, Koskella B (2013) Exploring the risks of phage application in the environment. Front Microbiol 4:358
Meyer JR, Dobias DT, Weitz JS, Barrick JE, Quick RT, Lenski RE (2012) Repeatability and contingency in the evolution of a key innovation in phage lambda. Science 335:428–432
Molineux IJ, Panja D (2013) Popping the cork: mechanisms of phage genome ejection. Nat Rev Microbiol 11:194–204
Munsch-Alatossava P, Alatossava T (2013) The extracellular phage-host interactions involved in the bacteriophage LL-H infection of Lactobacillus delbrueckii ssp. lactis ATCC 15808. Front Microbiol 4:408
Nechaev S, Severinov K (2008) The elusive object of desire – interactions of bacteriophages and their hosts. Curr Opin Microbiol 11:186–193
Nordström K, Forsgren A (1974) Effect of protein a on adsorption of bacteriophages to Staphylococcus aureus. J Virol 14:198–202
Nordström K, Forsgren A, Cox P (1974) Prevention of bacteriophage adsorption to Staphylococcus aureus by immunoglobulin G. J Virol 14:203–206
Nuismer SL, Doebeli M, Browning D (2005) The coevolutionary dynamics of antagonistic interactions mediated by quantitative traits with evolving variances. Evolution 59:2073–2082
Paez-Espino D, Morovic W, Sun CL, Thomas BC, Ueda K, Stahl B, Barrangou R, Banfield JF (2013) Strong bias in the bacterial CRISPR elements that confer immunity to phage. Nat Commun 4:1430
Parma DH, Snyder M, Sobolevski S, Nawroz M, Brody E, Gold L (1992) The Rex system of bacteriophage lambda: tolerance and altruistic cell death. Genes Dev 6:497–510
Paterson S, Vogwill T, Buckling A, Benmayor R, Spiers AJ, Thomson NR, Quail M, Smith F, Walker D, Libberton B, Fenton A, Hall N, Brockhurst MA (2010) Antagonistic coevolution accelerates molecular evolution. Nature 464:275–U154
Poullain V, Gandon S, Brockhurst MA, Buckling A, Hochberg ME (2008) The evolution of specificity in evolving and coevolving antagonistic interactions between a bacteria and its phage. Evolution 62:1–11
Refardt D, Bergmiller T, Kümmerli R (2013) Altruism can evolve when relatedness is low: evidence from bacteria committing suicide upon phage infection. Proc R Soc B Biol Sci 280:20123035
Reyes-Cortés R, Martínez-Peñafiel E, Martínez-Pérez F, de la Garza M, Kameyama L (2012) A novel strategy to isolate cell-envelope mutants resistant to phage infection: bacteriophage mEp213 requires lipopolysaccharides in addition to FhuA to enter Escherichia coli K-12. Microbiology 158:3063–3071
Roach DR, Sjaarda DR, Castle AJ, Svircev AM (2013) Host exopolysaccharide quantity and composition impact Erwinia amylovora bacteriophage pathogenesis. Appl Environ Microbiol 79:3249–3256
Samson JE, Magadán AH, Sabri M, Moineau S (2013) Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol 11:675–687
Sasaki A (2000) Host-parasite coevolution in a multilocus gene-for-gene system. Proc R Soc B Biol Sci 267:2183–2188
Scanlan PD, Hall AR, Lopez-Pascua LDC, Buckling A (2011) Genetic basis of infectivity evolution in a bacteriophage. Mol Ecol 20:981–989
Scott AE, Timms AR, Connerton PL, Loc Carrillo C, Adzfa Radzum K, Connerton IF (2007) Genome dynamics of campylobacter jejuni in response to bacteriophage predation. PLoS Pathog 3:e119
Seed KD, Faruque SM, Mekalanos JJ, Calderwood SB, Qadri F, Camilli A (2012) Phase variable O antigen biosynthetic genes control expression of the major protective antigen and bacteriophage receptor in Vibrio cholerae O1. PLoS Pathog 8:e1002917
Seed KD, Lazinski DW, Calderwood SB, Camilli A (2013) A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 494:489–491
Sørensen MCH, van Alphen LB, Harboe A, Li J, Christensen BB, Szymanski CM, Brøndsted L (2011) Bacteriophage F336 recognizes the capsular phosphoramidate modification of Campylobacter jejuni NCTC11168. J Bacteriol 193:6742–6749
Sørensen MCH, Van Alphen LB, Fodor C, Crowley SM, Christensen BB, Szymanski CM, Brøndsted L (2012) Phase variable expression of capsular polysaccharide modifications allows Campylobacter jejuni to avoid bacteriophage infection in chickens. Front Cell Infect Microbiol 2:11
Srinivasiah S, Bhavsar J, Thapar K, Liles M, Schoenfeld T, Wommack KE (2008) Phages across the biosphere: contrasts of viruses in soil and aquatic environments. Res Microbiol 159:349–357
Sun CL, Barrangou R, Thomas BC, Horvath P, Fremaux C, Banfield JF (2013) Phage mutations in response to CRISPR diversification in a bacterial population. Environ Microbiol 15:463–470
Thompson JN (1994) The coevolutionary process. University of Chicago Press, Chicago
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Thompson JN (2013) Relentless evolution. University of Chicago Press, Chichago and london
Thompson JN, Burdon JJ (1992) Gene-for-gene coevolution between plants and parasites. Nature 360:121–125
Van Valen L (1973) A new evolutionary law. Evol Theory 1:1–30
Vasu K, Nagamalleswari E, Nagaraja V (2012) Promiscuous restriction is a cellular defense strategy that confers fitness advantage to bacteria. Proc Natl Acad Sci USA 109:E1287–E1293
Westra ER, Swarts DC, Staals RH, Jore MM, Brouns SJ, van der Oost J (2012) The CRISPRs, they are a-changin': how prokaryotes generate adaptive immunity. Annu Rev Genet 46:311–339
Williams HT (2013) Phage-induced diversification improves host evolvability. BMC Evol Biol 13:17
Wilson GG, Murray NE (1991) Restriction and modification systems. Annu Rev Genet 25:585–627
Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69–114
Yan J, Mao J, Xie J (2014) Bacteriophage polysaccharide Depolymerases and biomedical applications. BioDrugs 28:265–274
Yoon SH, Han MJ, Jeong H, Lee CH, Xia XX, Lee DH, Shim JH, Lee SY, Oh TK, Kim JF (2012) Comparative multi-omics systems analysis of Escherichia coli strains B and K-12. Genome Biol 13(5):R37
Young R (2013) Phage lysis: do we have the hole story yet? Curr Opin Microbiol 16:790–797
Zaleski P, Wojciechowski M, Piekarowicz A (2005) The role of Dam methylation in phase variation of Haemophilus influenzae genes involved in defence against phage infection. Microbiology 151:3361–3369
Zhang Q-G (2014) Exposure to phages has little impact on the evolution of bacterial antibiotic resistance on drug concentration gradients. Evol Appl 7(3):394–402
Zhang Q-G, Buckling A (2011) Antagonistic coevolution limits population persistence of a virus in a thermally deteriorating environment. Ecol Lett 14:282–288
Zhang Q-G, Buckling A (2012) Phages limit the evolution of bacterial antibiotic resistance in experimental microcosms. Evol Appl 5:575–582
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry
Brockhurst, M.A., Koskella, B., Zhang, QG. (2021). Bacteria-Phage Antagonistic Coevolution and the Implications for Phage Therapy. In: Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L. (eds) Bacteriophages. Springer, Cham. https://doi.org/10.1007/978-3-319-41986-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-41986-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41985-5
Online ISBN: 978-3-319-41986-2
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences